A Starch- and Sucrose-Reduced Diet in Irritable Bowel Syndrome Leads to Lower Circulating Levels of PAI-1 and Visfatin: A Randomized Controlled Study
Abstract
1. Introduction
2. Materials and Methods
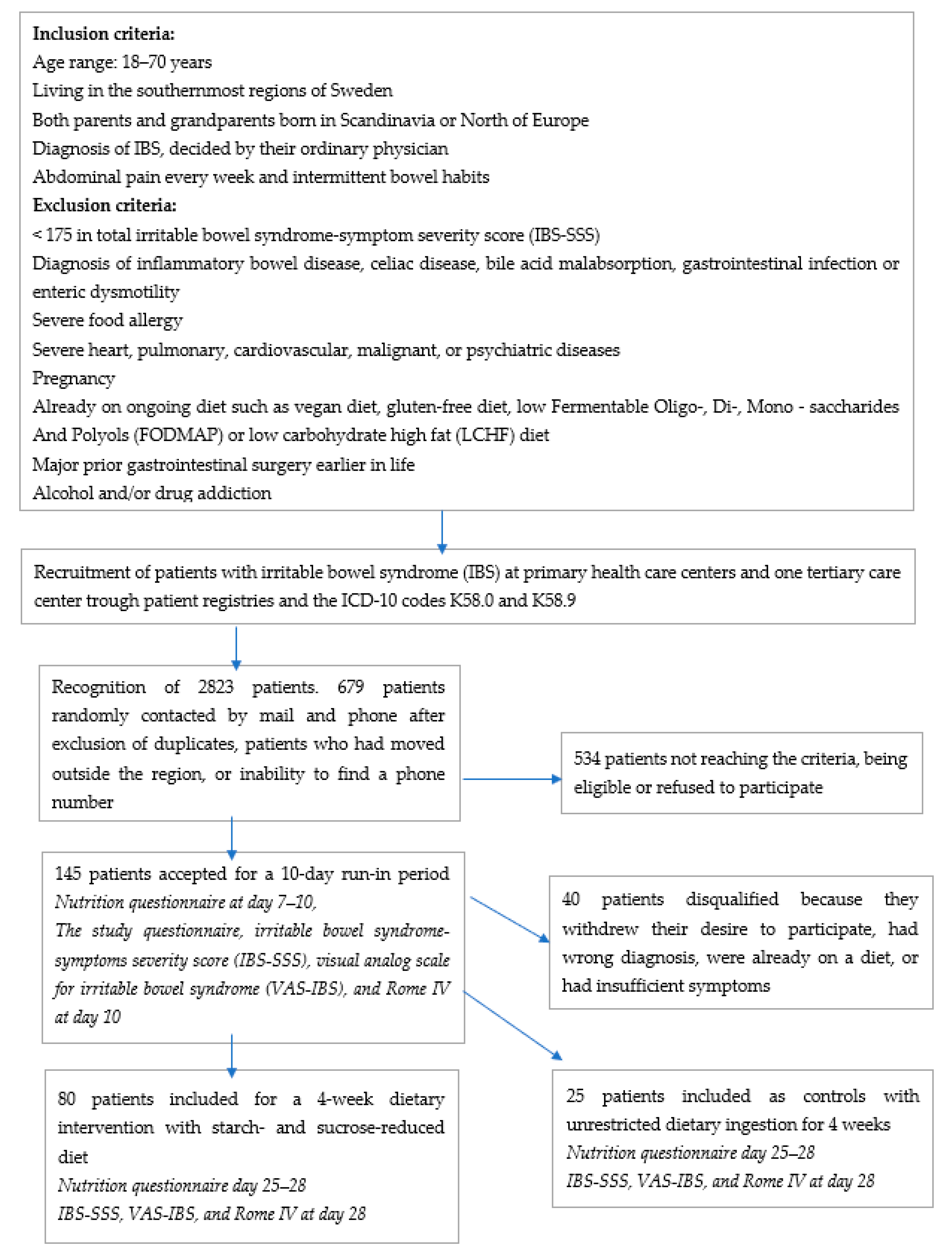
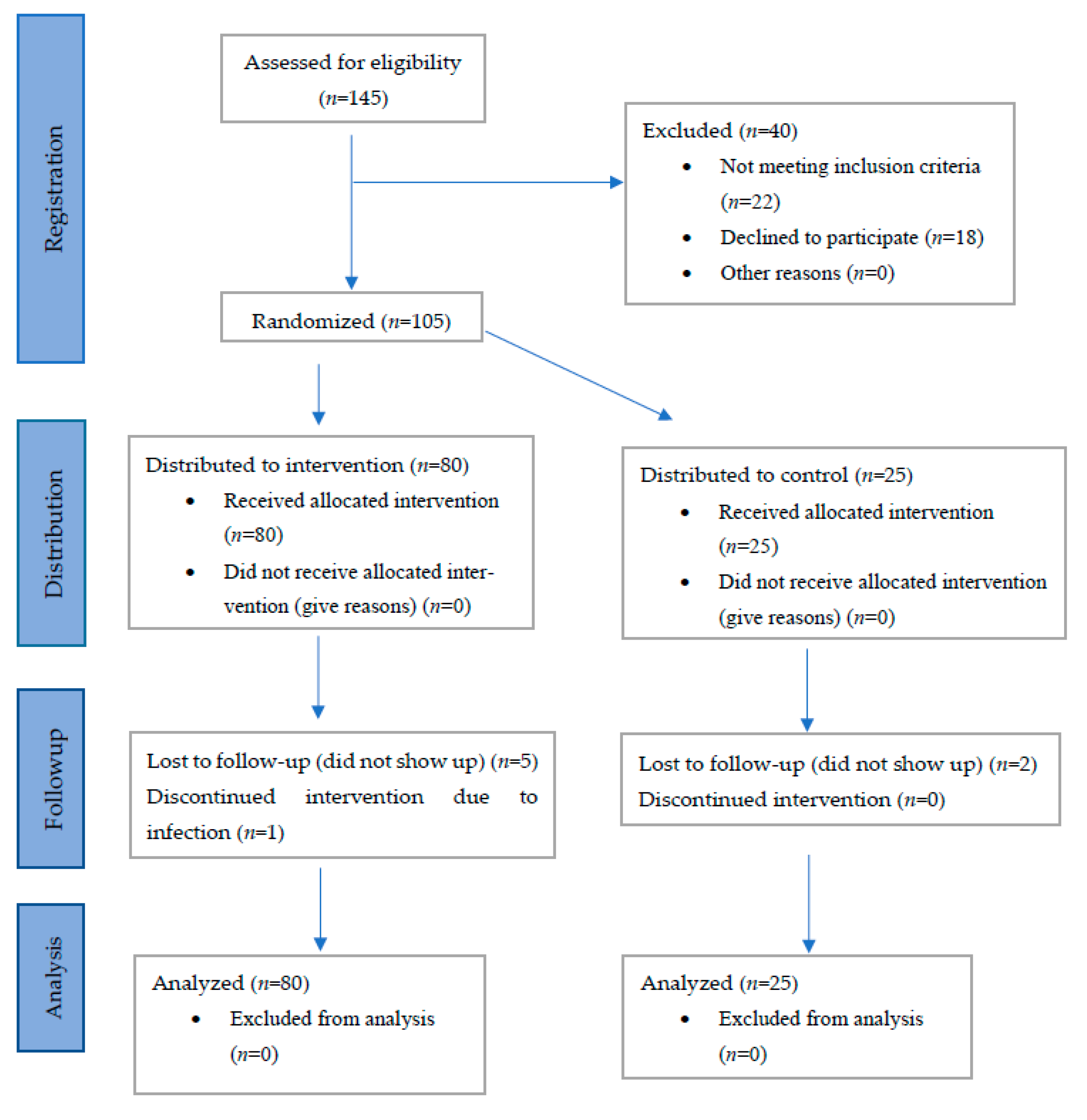
2.1. Study Design and Subjects
2.2. Dietary Advice
2.3. Questionnaires
2.4. Healthy Volunteers
2.5. Hormonal Analyses
2.6. AXIN1 Analysis
2.7. Statistical Analyses
3. Results
3.1. Basic Characteristics
3.2. Dietary Intake
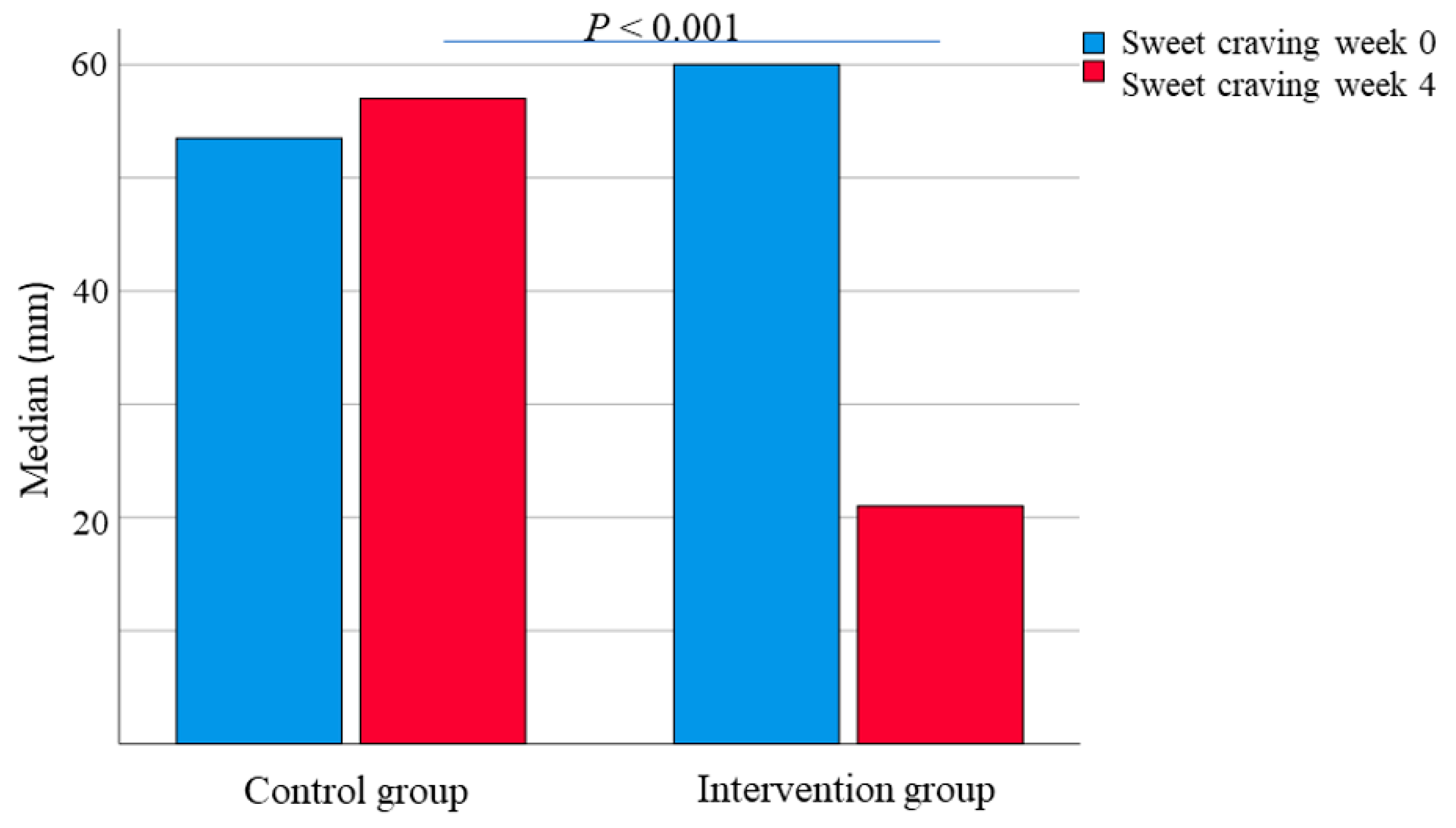
3.3. Gastrointestinal Symptoms and Sweet Craving
3.4. AXIN1 Levels
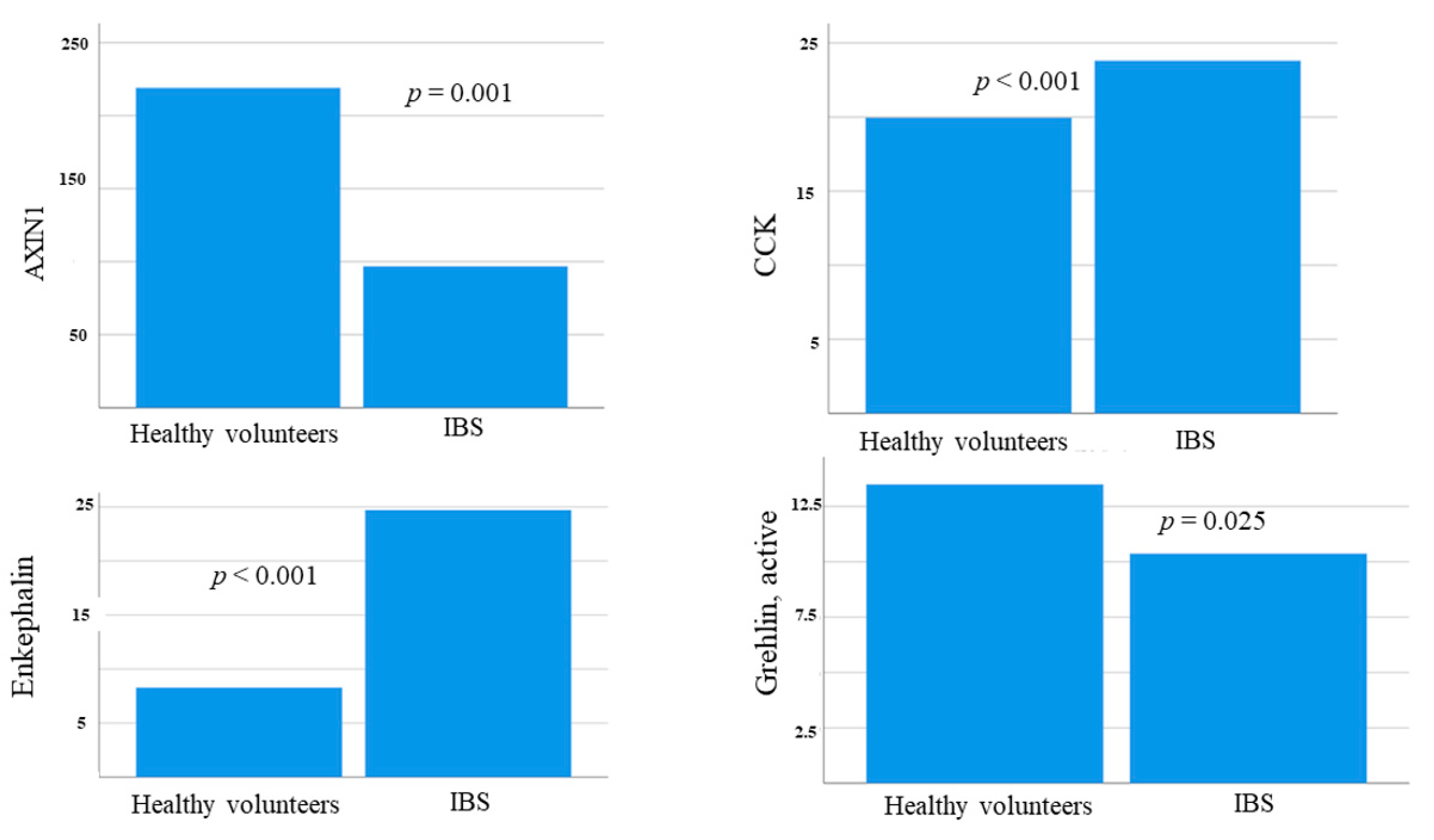
3.5. Hormonal Levels at Baseline
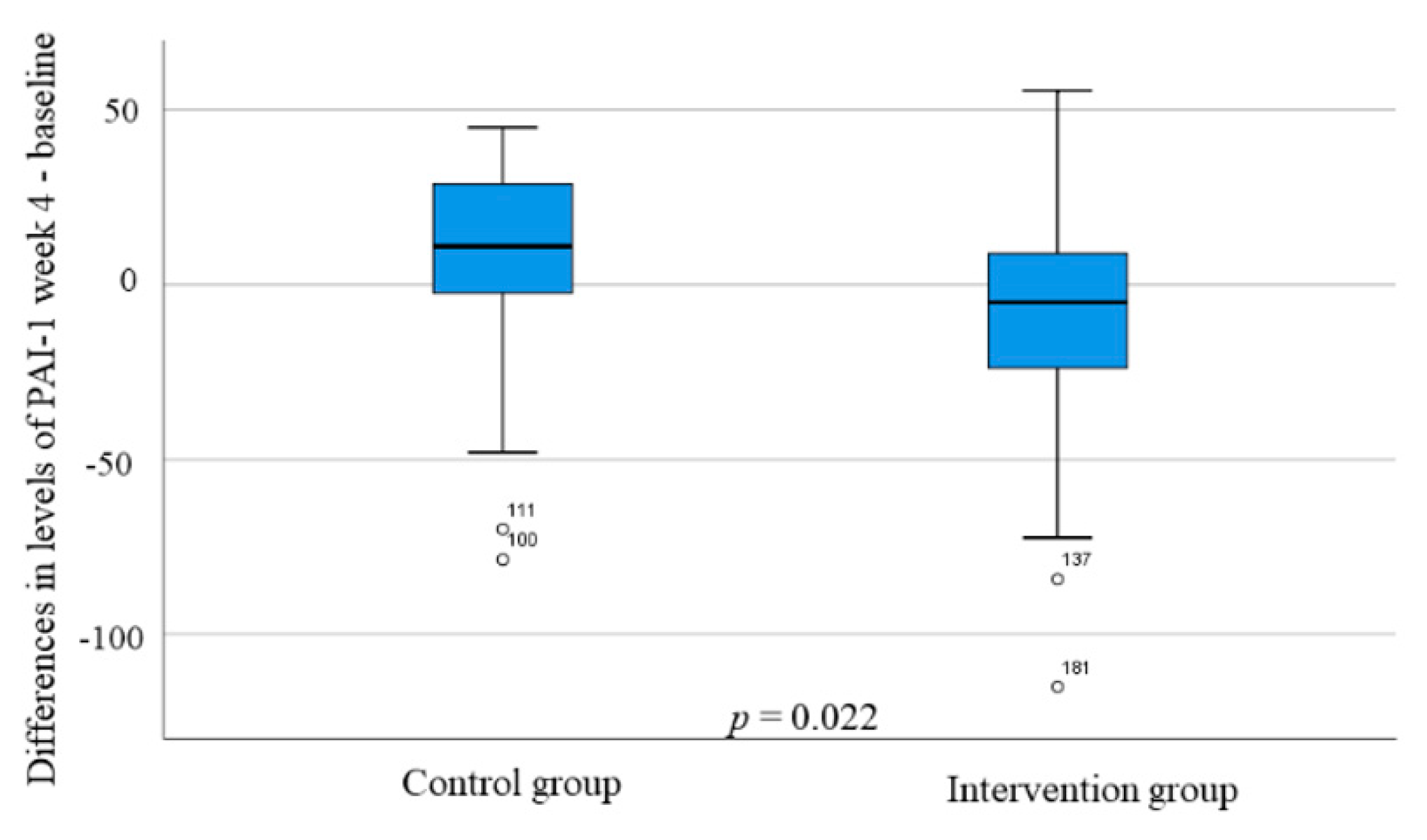
3.6. Hormonal Levels during the SSRD Intervention
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CCK | Cholecystokinin |
| DGBI | Disorder of gut-brain interaction |
| ELISA | Enzyme-linked immunosorbent assay |
| GIP | Gastric inhibitory peptide |
| IBS | Irritable bowel syndrome |
| IBS-SSS | Irritable bowel syndrome-symptoms severity score |
| ICD-10 | International Classification of Diseases, tenth revision |
| MSD | Meso Scale Discovery |
| NPY | Neuropeptide Y |
| PAI-1 | Plasminogen activator inhibitor-1 |
| SSRD | Starch- and sucrose-reduced diet |
| tPA | Tissue plasminogen activator |
| VAS-IBS | Visual analog scale for irritable bowel syndrome |
References
- Lacy, B.E.; Mearin, F.; Chang, L.; Chey, W.D.; Lembo, A.J.; Simren, M.; Spiller, R. Bowel disorders. Gastroenterology 2016, 150, 1393–1407. [Google Scholar] [CrossRef] [PubMed]
- Sperber, A.D.; Dumitrascu, D.; Fukudo, S.; Gerson, C.; Ghoshal, U.; Gwee, K.A.; Hungin, A.P.S.; Kang, J.Y.; Minhu, C.; Schmulson, M.; et al. The global prevalence of IBS in adults remains elusive due to the heterogeneity of studies: A Rome Foundation working team literature review. Gut 2017, 66, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Palsson, O.S.; Whitehead, W.; Törnblom, H.; Sperber, A.D.; Simren, M. Prevalence of Rome IV functional bowel disorders among adults in the United States, Canada, and the United Kingdom. Gastroenterology 2020, 158, 1262–1273. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, H.; Porter, J. Review article: Implementation of a diet low in FODMAPs for patients with irritable bowel syndrome-directions for future research. Aliment. Pharmacol. Ther. 2019, 49, 124–139. [Google Scholar] [CrossRef] [PubMed]
- Besterman, H.S.; Sarson, D.L.; Rambaud, J.C.; Stewart, J.S.; Guerin, S.; Bloom, S.R. Gut Hormone responses in the irritable bowel syndrome. Digestion 1981, 21, 219–224. [Google Scholar] [CrossRef] [PubMed]
- El-Salhy, M.; Gilja, O.H.; Gundersen, D.; Hatlebakk, J.G.; Hausken, T. Interaction between ingested nutrients and gut endocrine cells in patients with irritable bowel syndrome. Int. J. Mol. Med. 2014, 34, 363–371. [Google Scholar] [CrossRef]
- Saidi, K.; Nilholm, C.; Roth, B.; Ohlsson, B. A carbohydrate-restricted diet for patients with irritable bowel syndrome lowers serum C-peptide, insulin, and leptin without any correlation with symptom reduction. Nutr. Res. 2021, 86, 23–36. [Google Scholar] [CrossRef]
- Eriksson, E.M.; Andrén, K.I.; Eriksson, H.T.; Kurlberg, G.K. Irritable bowel syndrome subtypes differ in body awareness, psychological symptoms and biochemical stress markers. World J. Gastroenterol. 2008, 14, 4889. [Google Scholar] [CrossRef]
- Gulcan, E.; Taser, F.; Toker, A.; Korkmaz, U.; Alcelik, A. Increased frequency of prediabetes in patients with irritable bowel syndrome. Am. J. Med. Sci. 2009, 338, 116–119. [Google Scholar] [CrossRef]
- Mazur, M.; Furgała, A.; Jabłoński, K.; Mach, T.; Thor, P. Autonomic nervous systemactivity in constipation-predominant irritable bowel syndrome patients. Med. Sci. Monit. 2012, 18, CR493–CR499. [Google Scholar] [CrossRef]
- Bayrak, M. Metabolic syndrome, depression, and fibromyalgia syndrome prevalence in patients with irritable bowel syndrome: A case-control study. Medicine 2020, 99, e20577. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, G.; Muscogiuri, G.; Barrea, L.; Laudisio, D.; Savastano, S.; Colao, A. Irritable bowel syndrome: A new therapeutic target when treating obesity? Hormones 2019, 18, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Henström, M.; Diekmann, L.; Hadizadeh, F.; Zheng, F.B.T.; Assadi, G.; Kuech, E.M.; Dierks, C.; Heine, M.; Philipp, U.; Distl, O.; et al. Functional variants in the sucrase-isomaltase gene associate with increased risk of irritable bowel syndrome. Gut 2018, 67, 263–270. [Google Scholar] [CrossRef]
- Nilholm, C.; Roth, B.; Ohlsson, B. A Dietary Intervention with Reduction of Starch and Sucrose Leads to Reduced Gastrointestinal and Extra-Intestinal Symptoms in IBS Patients. Nutrients 2019, 11, 1662. [Google Scholar] [CrossRef] [PubMed]
- Nilholm, C.; Larsson, E.; Sonestedt, E.; Roth, B.; Ohlsson, B. Assessment of a 4-Week Starch- and Sucrose-Reduced Diet and Its Effects on Gastrointestinal Symptoms and Inflammatory Parameters among Patients with Irritable Bowel Syndrome. Nutrients 2021, 13, 416. [Google Scholar] [CrossRef]
- Ek, M.; Roth, B.; Ekström, P.; Valentin, L.; Bengtsson, M.; Ohlsson, B. Gastrointestinal symptoms among endometriosis patients—A case-cohort study. BMC Womens Health 2015, 15, 59. [Google Scholar] [CrossRef] [PubMed]
- Dihm, K.; Ek, M.; Roth, B.; Ohlsson, B. Plasma AXIN1 expression exhibit negative correlations with inflammatory biomarkers and is associated with gastrointestinal symptoms in endometriosis. Biomed. Rep. 2020, 12, 211–221. [Google Scholar] [CrossRef]
- Pilichiewicz, A.N.; Papadopoulos, P.; Brennan, I.M.; Little, T.J.; Meyer, J.H.; Wishart, J.M.; Horowitz, M.; Feinle-Bisset, C. Load-dependent effects of duodenal lipid on antropyloroduodenal motility, plasma CCK and PYY, and energy intake in healthy men. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R2170–R2178. [Google Scholar] [CrossRef]
- Steinert, R.E.; Feinle-Bisset, C.; Asarian, L.; Horowitz, M.; Beglinger, C.; Geary, N.; Ghrelin, C.C.K. GLP-1, and PYY (3–36): Secretory Controls and Physiological Roles in Eating and Glycemia in Health, Obesity, and After RYGB. Physiol. Rev. 2017, 97, 411–463. [Google Scholar] [CrossRef]
- Van Der Veek, P.P.; Biemond, I.; Masclee, A.A. Proximal and distal gut hormonesecretion in irritable bowel syndrome. Scand. J. Gastroenterol. 2006, 41, 170–177. [Google Scholar] [CrossRef]
- Zhang, H.; Yan, Y.; Shi, R.; Lin, Z.; Wang, M.; Lin, L. Correlation of gut hormones with irritable bowel syndrome. Digestion 2008, 78, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Holzer, P. Opioid receptors in the gastrointestinal tract. Regul. Pept. 2009, 155, 11–17. [Google Scholar] [CrossRef]
- Szymaszkiewicz, A.; Storr, M.; Fichna, J.; Zielinska, M. Enkephalinase inhibitors, potential therapeutics for the future treatment of diarrhea predominant functional gastrointestinal disorders. Neurogastroenterol. Motil. 2019, 31, e13526. [Google Scholar] [CrossRef] [PubMed]
- Shevchouk, O.T.; Tufvesson-Alm, M.; Jerlhag, E. An Overview of Appetite-Regulatory Peptides in Addiction Processes; From Bench to Bed Side. Front. Neurosci. 2021, 15, 774050. [Google Scholar] [CrossRef] [PubMed]
- Rabal, P.M.; Coveñas, R. Regulation of homeostasis by neuropeptide Y: Involvement in food intake. Curr. Med. Chem. 2021. [Google Scholar] [CrossRef] [PubMed]
- Şahin-Eryılmaz, G.; Başak, K.; Çakır-Madenci, Ö.; Koç, H.; Tüzün, S.; Dolapçıoğlu, C.; Ahıshalı, E.; Dabak, M.R. Relationship between irritable bowel syndrome and plasma and tissue ghrelin levels. Turk. J. Gastroenterol. 2018, 29, 631–635. [Google Scholar] [CrossRef] [PubMed]
- El-Salhy, M.; Gilja, O.H.; Gundersen, D.; Hausken, T. Endocrine cells in the oxyntic mucosa of the stomach in patients with irritable bowel syndrome. World J. Gastrointest. Endosc. 2014, 6, 176–185. [Google Scholar] [CrossRef]
- Al-Suhaimi, E.A.; Shehzad, A. Leptin, resistin, and visfatin: The missing link between endocrine metabolic disorders and autoimmunity. Eur. J. Med. Res. 2013, 18, 12. [Google Scholar] [CrossRef]
- Yarmolinsky, J.; Bordin Barbieri, N.; Weinmann, T.; Ziegelmann, P.K.; Duncan, B.B.; Schmidt, M.I. Plasminogen activator inhibitor-1 and type 2 diabetes: A systematic review and meta-analysis of observational studies. Sci. Rep. 2016, 6, 17714. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R. Role of adiponectin and PBEF/visfatin as regulator of inflammation: Involvement in obesity-associated diseases. Clin. Sci. 2008, 114, 275–288. [Google Scholar] [CrossRef]
- Eliasson, M.C.; Jansson, J.H.; Lindahl, B.; Stegmayr, B. High levels of tissue plasminogen activator (tPA) antigen precede the development of type 2 diabetes in a longitudinal population study. Cardiovasc. Diabetol. 2003, 2, 19. [Google Scholar] [CrossRef] [PubMed][Green Version]
- QOL Medical. Sucrose Intolerance. Genetic Sucrase-Isomaltase Deficiency. Available online: https://www.sucroseintolerance.com/choosing-your-foods/ (accessed on 25 September 2019).
- Palsson, O.S.; Whitehead, W.E.; Van Tilburg, M.A.L.; Chang, L.; Chey, W.; Crowell, M.D.; Keefer, L.; Lembo, A.J.; Parkman, H.P.; Rao, S.S. Development and validation of the Rome IV diagnostic questionnaire for adults. Gastroenterology 2016, 150, 1481–1491. [Google Scholar] [CrossRef] [PubMed]
- Mashie FoodTech Solutions. The AIVO Diet Computer Program. Available online: https://www.matildafoodtech.com/page/aivo (accessed on 25 March 2021).
- Francis, C.Y.; Morris, J.; Whorwell, P.J. The irritable bowel severity scoring system: A Simple method of monitoring irritable bowel syndrome and its progress. Aliment. Pharmacol. Ther. 1997, 11, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson, M.; Ohlsson, B.; Ulander, K. Development and psychometric testing of the Visual Analogue Scale for Irritable Bowel Syndrome (VAS-IBS). BMC Gastroenterol. 2007, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson, B.; Darwiche, G.; Roth, B.; Bengtsson, M.; Hoglund, P. High Fiber Fat and Protein Contents Lead to Increased Satiety Reduced Sweet Cravings and Decreased Gastrointestinal Symptoms Independently of Anthropometric Hormonal and Metabolic Factors. J. Diabetes Metab. 2017, 8, 3. [Google Scholar] [CrossRef]
- Meso Scale Diagnostics. Mesoscale Discovery® U-PLEX Metabolic Group 1 HumanIndividual Assays Product Insert. Available online: https://www.mesoscale.com/~/media/files/productinserts/u-plex-metabolic-group-1-human-product-insert-singleplex.pdf (accessed on 15 October 2019).
- Ma, L.J.; Mao, S.L.; Kevin, L.; Taylor, K.L.; Kanjanabuch, T.; Guan, Y.F.; Zhang, Y.H.; Brown, N.J.; Swift, L.L.; McGuinness, O.P.; et al. Prevention of obesity and insulin resistance in mice lacking plasminogen activator inhibitor 1. Diabetes 2004, 53, 336–346. [Google Scholar] [CrossRef]
- Fukuhara, A.; Matsuda, M.; Nishizawa, M.; Segawa, K.; Tanaka, M.; Kishimoto, K.; Matsuki, Y.; Murakami, M.; Ichisaka, T.; Murakami, H.; et al. Visfatin: A protein secreted by visceral fat that mimics the effects of insulin. Science 2005, 307, 426–430. [Google Scholar] [CrossRef]
- De Gennaro, G.; Palla, G.; Battini, L.; Simoncini, T.; Del Prato, S.; Bertolotto, A.; Bianchi, C. The role of adipokines in the pathogenesis of gestational diabetes mellitus. Gynecol. Endocrinol. 2019, 35, 737–751. [Google Scholar] [CrossRef]
- Pagano, C.; Pilon, C.; Olivieri, M.; Mason, P.; Fabris, R.; Serra, R.; Milan, G.; Rossato, M.; Federspil, G.; Vettor, R. Reduced plasma visfatin/pre-B cell colony-enhancing factor in obesity is not related to insulin resistance in humans. J. Clin. Endocrinol. Metab 2006, 91, 3165–3170. [Google Scholar] [CrossRef]
- Haider, D.G.; Holzer, G.; Schaller, G.; Weghuber, D.; Widhalm, K.; Wagner, O.; Kapiotis, S.; Wolzt, M. The adipokine visfatin is markedly elevated in obese children. J. Pediatr. Gastroenterol. Nutr. 2006, 43, 548–549. [Google Scholar] [CrossRef]
- Bilski, J.; Jaworek, J.; Pokorski, J.; Nitecki, J.; Nitecka, E.; Pokorska, J.; Mazur-Bialy, A.; Szklarczyk, J. Effects of time of day and the wingate test on appetite perceptions, food intake and plasma levels of adipokines. J. Physiol. Pharmacol. 2016, 67, 667–676. [Google Scholar] [PubMed]
- Ohlsson, B.; Darwiche, G.; Roth, B.; Höglund, P. Alignments of endocrine, anthropometric, and metabolic parameters in type 2 diabetes after intervention with an Okinawa-based Nordic diet. Food Nutr. Res. 2018, 62. [Google Scholar] [CrossRef] [PubMed]
- Nilholm, C.; Larsson, E.; Roth, B.; Gustafsson, R.; Ohlsson, B. Irregular Dietary Habits with a High Intake of Cereals and Sweets Are Associated with More Severe Gastrointestinal Symptoms in IBS Patients. Nutrients 2019, 11, 1279. [Google Scholar] [CrossRef]
- Hossain, M.J.; Kendig, M.D.; Wild, B.M.; Issar, T.; Krishnan, A.V.; Morris, M.J.; Arnold, R. Evidence of altered peripheral nerve function in a rodent model of diet-induced prediabetes. Biomedicines 2020, 8, 313. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson, B. Theories behind the effect of starch- and sucrose-reduced diets on gastrointestinal symptoms in irritable bowel syndrome. Mol. Med. Rep. 2021, 24, 732. [Google Scholar] [CrossRef] [PubMed]
- Gordon, E.L.; Ariel-Donges, A.H.; Bauman, V.; Merlo, L.J. What is the evidence for “Food Addiction”? A systematic review. Nutrients 2018, 10, 477. [Google Scholar] [CrossRef]
- Vasiliu, O. Current status of evidence for a new diagnosis: Food addiction-A. Literature review. Front. Psychiatry 2022, 12, 824936. [Google Scholar] [CrossRef]
- Ruderstam, H.; Ohlsson, B. Self-reported IBS and gastrointestinal symptoms in the general population are associated with asthma, drug consumption, and a family history of gastrointestinal diseases. Scand. J. Gastroenterol. 2022, 1–11, Online ahead of print. [Google Scholar] [CrossRef]
- Nordic Council of Ministers. Nordic Nutrition Recommendations 2012. Available online: https://norden.diva-portal.org/smash/get/diva2:704251/FULLTEXT01.pdf (accessed on 7 November 2021).
- Bowen, J.; Noakes, M.; Trenerry, C.; Clifton, P.M. Energy intake, ghrelin, and cholecystokinin after different carbohydrate and protein preloads in overweight men. J. Clin. Endocrinol. Metab. 2006, 91, 1477–1483. [Google Scholar] [CrossRef]
- Berna, M.J.; Tapia, J.A.; Sancho, V.; Jensen, R.T. Progress in developing cholecystokinin (CCK)/gastrin receptor ligands that have therapeutic potential. Curr. Opin. Pharmacol. 2007, 7, 583–592. [Google Scholar] [CrossRef]
- Roth, B.; Larsson, E.; Ohlsson, B. Poor intake of vitamins and minerals among patients with irritable bowel syndrome is associated with symptoms. JGH 2022. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Chang, G.Q.; Karatayev, O.; Ahsan, R.; Gaysinskaya, V.; Marwil, Z.; Leibowitz, S.F. Dietary Fat stimulates endogenous en-kephalin and dynorphin in the paraventricular nucleus: Role of circulating triglycerides. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E561–E570. [Google Scholar] [CrossRef] [PubMed]
- Chang, G.Q.; Karatayev, O.; Barson, J.R.; Chang, S.Y.; Leibowitz, S.F. Increased encephalin in brain of rats prone to overcon-suming a fat-rich diet. Physiol. Behav. 2010, 101, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Segarra, A.B.; Prieto, I.; Martinez-Canamero, M.; Ruiz-Sanz, J.I.; Ruiz-Larrea, M.B.; De Gasparo, M.; Banegas, I.; Zorad, S.; Ramirez-Sanchez, M. Enkephalinase activity is modified and correlates with fatty acids in frontal cortex depending on fish, olive or coconut oil used in the diet. Endocr. Regul. 2019, 53, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, A. Roles of axin in the Wnt signalling pathway. Cell. Signal. 1999, 11, 777–788. [Google Scholar] [CrossRef]
- Shi, J.; Chi, S.; Xue, J.; Yang, J.; Li, F.; Liu, X. Review Article: Emerging Role andTherapeutic Implication of Wnt Signaling Pathways in Autoimmune Diseases. J. Immunol. Res. 2016, 674, 57–69. [Google Scholar]
- Katoh, M. Multi-layered prevention and treatment of chronic inflammation, organ fibrosis and cancer associated with canonical WNT/β-catenin signaling activation. Int. J. Mol. Med. 2018, 42, 713–725. [Google Scholar] [CrossRef]
- Moparthi, L.; Koch, S. Wnt signaling in intestinal inflammation. Differentiation 2019, 108, 24–32. [Google Scholar] [CrossRef]
- Talley, N.J. What Causes Functional Gastrointestinal Disorders? A Proposed Disease Model. Am. J. Gastroenterol. 2020, 115, 41–48. [Google Scholar] [CrossRef]
- Moraes, L.; Magnusson, M.K.; Mavroudis, G.; Polster, A.; Jonefjäll, B.; Törnblom, H.; Sundin, J.; Simrén, M.; Strid, H.; Öhman, L. Systemic Inflammatory Protein Profiles Distinguish Irritable Bowel Syndrome (IBS) and Ulcerative Colitis, Irrespective of Inflammation or IBS-Like Symptoms. Inflamm. Bowel Dis. 2020, 26, 874–884. [Google Scholar] [CrossRef]
- Stenlund, H.; Nilholm, C.; Chorell, E.; Roth, B.; D’Amato, M.; Ohlsson, B. Metabolic Profiling of Plasma in Patients with Irritable Bowel Syndrome after a 4-Week Starch- and Sucrose-Reduced Diet. Metabolites 2021, 11, 440. [Google Scholar] [CrossRef] [PubMed]
| Highly Tolerated |
| Berries and fruit: avocado, blackberries, blueberries, boysenberries, cherries, cranberries, currants, figs, gooseberries, grapes, kiwi fruits, lemons, limes, loganberries, olives, papayas, pears, pomegranates, prunes, raspberries, rhubarbs, and strawberries. |
| Vegetables and legumes: alfalfa sprouts, artichokes*, arugulas, asparagus, bamboo shoots, bok choy, broccoli *, brussel sprouts *, cabbages *, cauliflower *, celery, chard, chicories, chives, collard greens, cress, cucumbers, eggplants, endive, green beans, kale, lettuces, mung bean sprout, mushrooms, peppers, radishes, spaghetti squash, spinach, tomatoes, turnips, yellow squash, zucchini. |
| Tolerated by a Few |
| Berries and fruit: persimmons, plums, raisins, watermelon. |
| Vegetables and legumes: edamame soybeans, jicamas, leeks, okra, pumpkins, snow peas, tempeh, tofu, yellow wax beans. |
| Not Tolerated |
| Berries and fruit: apples, apricots, bananas, cantaloupe, dates, grapefruits, guava, honeydew melon, mangos, nectarines, oranges, passion fruits, peaches, pineapples, tangelos, tangerines |
| Vegetables and legumes: beets, black beans, black-eyed peas, butternut, carrots, cassavas, chickpeas, corn, garlic, green peas, lentils, kidney beans, lima beans, navy beans, onion, parsnips, pinto beans, potatoes, soybeans, split peas, sweet potatoes, yams. |
| Analysis Description | Catalog Number of Antibody Set Used | Detection Range |
|---|---|---|
| hu CCK | CEA802Hu | 12.35–1000 pg/mL |
| hu Ghrelin, total | MSD U-PLEX | 1.3–5180 pg/mL |
| hu Ghrelin, active | MSD U-PLEX | 2.2–9070 pg/mL |
| hu NPY | MSD R-PLEX | 122–500,000 pg/mL |
| hu PAI-1 | BMS2033 and BMS2033TEN | 78–5000 pg/mL |
| hu Proenkephalin | CSB-EL017781HU | 15.6–1000 pg/mL |
| hu Visfatin | MSD R-PLEX | 0.6–2500 ng/mL |
| Intra-Assay CV | Inter-Assay CV | |
|---|---|---|
| CCK CV (%) | n = 8 <10 | n = 24 <12 |
| PAI-1 CV (%) | n = 6 4.7 | n = 18 5.0 |
| Proenkephalin CV (%) | n = 20 <8 | n = 20 <10 |
| Visfatin CV (%) | n = 8 4.5 | n = 5 14.4 |
| Characteristics | Control Group (n = 25) | p Value * | Intervention Group (n = 80) | p Value * | p Value ** |
|---|---|---|---|---|---|
| Sex (women/men; n, %) | 22 (88)/3 (12) | 60 (75)/20 (25) | 0.267 | ||
| Age (years) | 41.4 ± 14.5 | 47.5 ± 12.4 | 0.064 | ||
| Carbohydrate intake (g) | |||||
| Baseline | 177 (112–207) | 185 (144–223) | 0.402 | ||
| Week 4 | 182 (89–224) | 0.235 | 88 (66–128) | <0.001 | <0.001 |
| Fat intake (g) | |||||
| Baseline | 61 (46–72) | 65 (44–94) | 0.394 | ||
| Week 4 | 69 (46–96) | 0.566 | 72 (56–104) | 0.049 | 0.264 |
| Protein intake (g) | |||||
| Baseline | 59 (46–71) | 72 (55–83) | 0.046 | ||
| Week 4 | 65 (53–81) | 0.571 | 82 (58–99) | 0.023 | 0.127 |
| Sucrose intake (g) | |||||
| Baseline | 20 (13–43) | 23 (13–38) | 0.823 | ||
| Week 4 | 19 (5–36) | 0.144 | 5 (2–13) | <0.001 | <0.001 |
| Starch intake (g) | |||||
| Baseline | 71 (43–90) | 76 (49–116) | 0.448 | ||
| Week 4 | 82 (37–101) | 0.849 | 22 (3–48) | <0.001 | <0.001 |
| Weight (kg) | |||||
| Baseline | 68.3 ± 14.8 | 75.8 ± 14.9 | 0.037 | ||
| Week 4 | 70.0 ± 14.7 | 0.141 | 73.7 ± 14.8 | <0.001 | 0.304 |
| Total IBS-SSS | |||||
| Baseline | 310 (247–351) | 306 (250–356) | 0.820 | ||
| Week 4 | 300 (233–331) | 0.248 | 156 (88–250) | <0.001 | <0.001 |
| Abdominal pain (mm) | |||||
| Baseline | 49 (27–63) | 52 (37–65) | 0.441 | ||
| Week 4 | 50 (32–63) | 0.650 | 24 (6–43) | <0.001 | <0.001 |
| Diarrhea (mm) | |||||
| Baseline | 47 (5–70) | 57 (18–76) | 0.312 | ||
| Week 4 | 24 (1–49) | 0.300 | 14 (1–33) | <0.001 | 0.269 |
| Constipation (mm) | |||||
| Baseline | 54 (30–69) | 47 (1–73) | 0.763 | ||
| Week 4 | 28 (1–68) | 0.045 | 18 (0–36) | <0.001 | 0.146 |
| Bloating and flatulence (mm) | |||||
| Baseline | 78 (68–89) | 78 (60–85) | 0.429 | ||
| Week 4 | 69 (56–80) | 0.001 | 28 (11–54) | <0.001 | <0.001 |
| Vomiting and nausea (mm) | |||||
| Baseline | 29 (6–50) | 12 (1–37) | 0.134 | ||
| Week 4 | 12 (2–56) | 0.112 | 3 (0–24) | 0.002 | 0.043 |
| Psychological well-being (mm) | |||||
| Baseline | 47 (24–71) | 50 (24–69) | 0.950 | ||
| Week 4 | 48 (32–60) | 0.732 | 36 (13–53) | <0.001 | 0.092 |
| Influence on daily life (mm) | |||||
| Baseline | 72 (52–86) | 0.539 | |||
| Week 4 | 0.732 | 35 (20–66) | <0.001 | <0.001 |
| Healthy Volunteers | IBS Patients (n = 105) | β | 95% CI | p-Value | |
|---|---|---|---|---|---|
| P-AXIN1 (pg/mL) | 219.0 (176.0–281.2) | 96.9 (64.8–158.9) | −117.477 | −189.484–(−45.470) | 0.001 |
| S-CCK (pg/mL) Missing value: 13 in patients | 20.0 (17.7–23.0) | 23.8 (20.4–28.0) | 4.621 | 2.368–6.874 | <0.001 |
| P-Enkephalin (pg/mL) Missing value: 21 and 11 | 8.3 (5.3–11.7) | 24.7 (18.3–32.3) | 15.410 | 9.131–21.689 | <0.001 |
| P-Ghrelin active (pg/mL) Missing value: 23 and 12 | 13.5 (6.8–24.4) | 10.4 (3.4–19.2) | −6.036 | −11.307–(−0.765) | 0.025 |
| P-Ghrelin total (pg/mL) Missing value: 33 and 56 | 408.5 (275.5–789.0) | 385.0 (240.5–701.5) | −109.548 | −307.153–88.056 | 0.277 |
| S-PAI-1 (ng/mL) Missing value: 2 and 13 | 124.0 (105.2–156.8) | 113.8 (84.3–142.5) | −13.990 | −46.356–18.377 | 0.397 |
| S-Visfatin (ng/mL) Missing value: 29 and 12 | 13.5 (5.2–55.5) | 14.6 (5.0–107.4) | 758,732 | −810,652–2,328,117 | 0.343 |
| Hormones | Control Group (n = 25) | p Value * | Intervention Group (n = 80) | p Value * | p Value ** |
|---|---|---|---|---|---|
| P-AXIN1 (pg/mL) | |||||
| Baseline | 142.1 (99.4–292.6) | 81.1 (56.7–134.0) | <0.001 | ||
| Week 4 | 124.4 (96.4–321.1) | 0.899 | 88.4 (60.8–149.0) | 0.491 | 0.006 |
| S-CCK (pg/mL) | |||||
| Baseline | 21.6 (17.8–26.3) | 24.0 (21.6–28.3) | 0.027 | ||
| Week 4 | 26.6 (18.1–28.0) | 0.031 | 25.0 (21.9–29.5) | 0.275 | 0.209 |
| P-Enkephalin (pg/mL) | |||||
| Baseline | 26.4 (18.3–35.3) | 24.6 (17.9–31.8) | 0.420 | ||
| Week 4 | 24.9 (19.4–30.7) | 0.482 | 26.5 (16.2–32.8) | 0.999 | 0.984 |
| P-Ghrelin, active (pg/mL) | |||||
| Baseline | 6.9 (3.5–12.4) | 12.7 (3.89–22.0) | 0.107 | ||
| Week 4 | 6.8 (3.7–14.9) | 0.266 | 9.7 (3.5–22.6) | 0.944 | 0.456 |
| P-Ghrelin, total (pg/mL) | |||||
| Baseline | 462.0 (270.0–804.5) | 369.0 (223.5–682.0) | 0.382 | ||
| Week 4 | 484.0 (221.5–805.0) | 0.664 | 393.0 (250.5–665.8) | 0.171 | 0.907 |
| S-PAI-1 (ng/mL) | |||||
| Baseline | 116.6 (67.0–161.6) | 113.8 (88.4–141.8) | 0.822 | ||
| Week 4 | 133.0 (106.8–160.8) | 0.129 | 107.9 (83.1–134.0) | 0.066 | 0.113 |
| S-Visfatin (ng/mL) | |||||
| Baseline | 20.2 (7.8–104.5) | 12.0 (4.6–117.6) | 0.382 | ||
| Week 4 | 19.2 (8.8–105.0) | 0.273 | 10.3 (5.2–114.1) | 0.007 | 0.327 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roth, B.; Myllyvainio, J.; D’Amato, M.; Larsson, E.; Ohlsson, B. A Starch- and Sucrose-Reduced Diet in Irritable Bowel Syndrome Leads to Lower Circulating Levels of PAI-1 and Visfatin: A Randomized Controlled Study. Nutrients 2022, 14, 1688. https://doi.org/10.3390/nu14091688
Roth B, Myllyvainio J, D’Amato M, Larsson E, Ohlsson B. A Starch- and Sucrose-Reduced Diet in Irritable Bowel Syndrome Leads to Lower Circulating Levels of PAI-1 and Visfatin: A Randomized Controlled Study. Nutrients. 2022; 14(9):1688. https://doi.org/10.3390/nu14091688
Chicago/Turabian StyleRoth, Bodil, Julia Myllyvainio, Mauro D’Amato, Ewa Larsson, and Bodil Ohlsson. 2022. "A Starch- and Sucrose-Reduced Diet in Irritable Bowel Syndrome Leads to Lower Circulating Levels of PAI-1 and Visfatin: A Randomized Controlled Study" Nutrients 14, no. 9: 1688. https://doi.org/10.3390/nu14091688
APA StyleRoth, B., Myllyvainio, J., D’Amato, M., Larsson, E., & Ohlsson, B. (2022). A Starch- and Sucrose-Reduced Diet in Irritable Bowel Syndrome Leads to Lower Circulating Levels of PAI-1 and Visfatin: A Randomized Controlled Study. Nutrients, 14(9), 1688. https://doi.org/10.3390/nu14091688







