What about TSH and Anti-Thyroid Antibodies in Patients with Autoimmune Thyroiditis and Celiac Disease Using a Gluten-Free Diet? A Systematic Review
Abstract
:1. Introduction
2. Methods
2.1. Conduction of Review
2.2. Search Strategy
2.3. Data Extraction
2.4. Study Quality Assessment
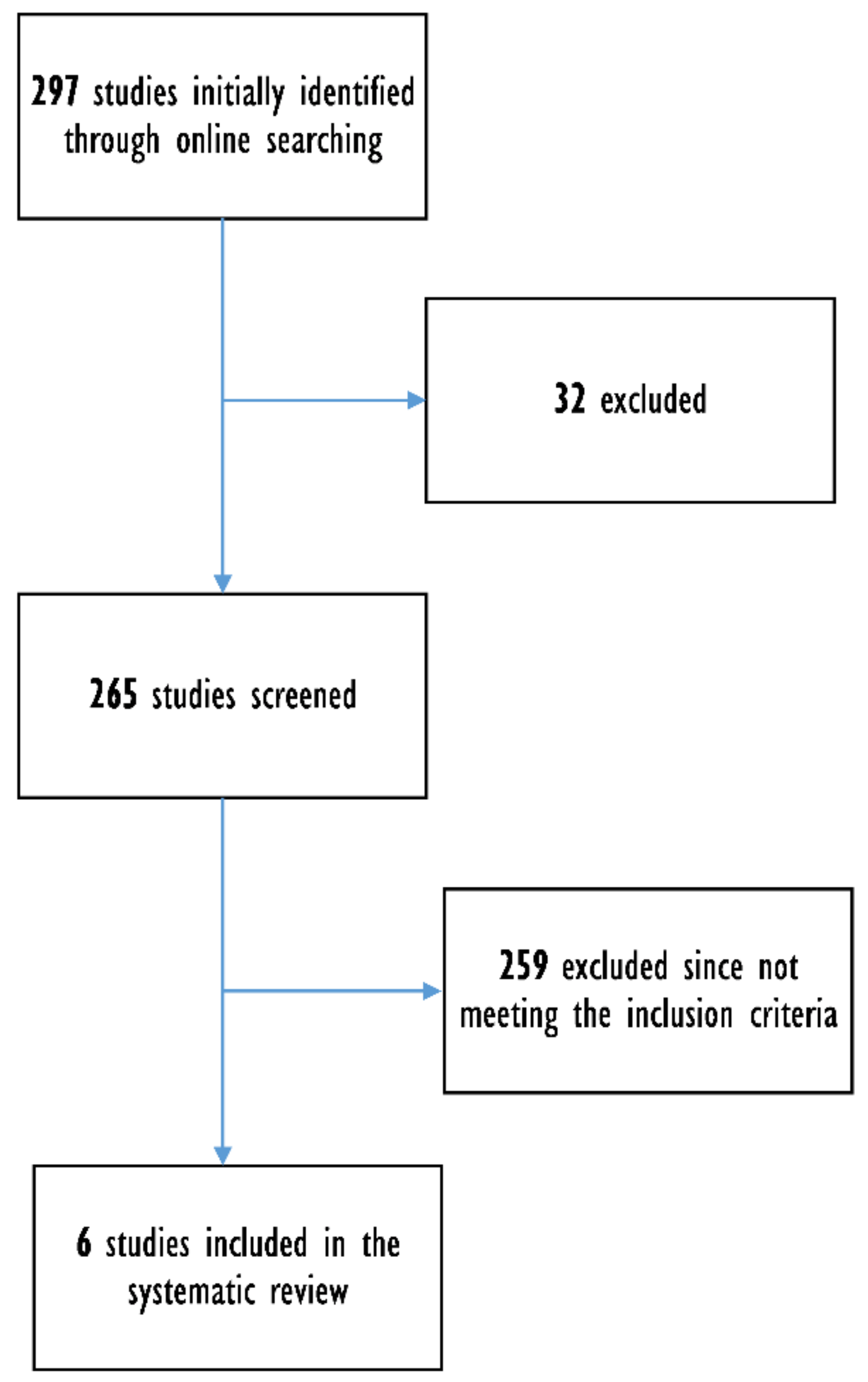
3. Results
3.1. Articles Retrieved
3.2. Qualitative Analysis (Systematic Review)
3.3. Quality Assessment
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wiersinga, W.M. Hashimoto’s thyroiditis. In Endocrinology; Springer Science and Business Media LLC: Berlin, Germany, 2018; pp. 205–247. [Google Scholar]
- Fallahi, P.; Ferrari, S.M.; Ruffilli, I.; Elia, G.; Biricotti, M.; Vita, R.; Benvenga, S.; Antonelli, A. The association of other autoimmune diseases in patients with autoimmune thyroiditis: Review of the literature and report of a large series of patients. Autoimmun. Rev. 2016, 15, 1125–1128. [Google Scholar] [CrossRef] [PubMed]
- Boelaert, K.; Newby, P.R.; Simmonds, M.; Holder, R.L.; Carr-Smith, J.D.; Heward, J.M.; Manji, N.; Allahabadia, A.; Armitage, M.; Chatterjee, K.V.; et al. Prevalence and Relative Risk of Other Autoimmune Diseases in Subjects with Autoimmune Thyroid Disease. Am. J. Med. 2010, 123, 183.e1–183.e9. [Google Scholar] [CrossRef] [PubMed]
- Pobłocki, J.; Pańka, T.; Szczuko, M.; Telesiński, A.; Syrenicz, A. Whether a Gluten-Free Diet Should Be Recommended in Chronic Autoimmune Thyroiditis or Not?—A 12-Month Follow-Up. J. Clin. Med. 2021, 10, 3240. [Google Scholar] [CrossRef]
- Kahaly, G.J.; Frommer, L. Polyglandular autoimmune syndromes. J. Endocrinol. Investig. 2014, 41, 91–98. [Google Scholar] [CrossRef]
- Naiyer, A.J.; Shah, J.; Hernandez, L.; Kim, S.-Y.; Ciaccio, E.J.; Cheng, J.; Manavalan, S.; Bhagat, G.; Green, P.H.R. Tissue transglutaminase antibodies in individuals with celiac disease bind to thyroid follicles and extracellular matrix and may contribute to thyroid dysfunction. Thyroid 2008, 18, 1171–1178. [Google Scholar] [CrossRef] [Green Version]
- Stazi, A.V.; Trinti, B. Selenium status and over-expression of interleukin-15 in celiac disease and autoimmune thyroid diseases. Ann. Ist. Super. Sanita 2010, 46, 389–399. [Google Scholar]
- Fisher, A.H.; Lomasky, S.J.; Fisher, M.J.; Oppenheim, Y.L. Celiac disease and the endocrinologist: A diagnostic opportunity. Endocr. Pract. 2008, 14, 381–388. [Google Scholar] [CrossRef]
- Virili, C.; Bassotti, G.; Santaguida, M.G.; Iuorio, R.; Del Duca, S.C.; Mercuri, V.; Picarelli, A.; Gargiulo, P.; Gargano, L.; Centanni, M. Atypical celiac disease as cause of increased need for thyroxine: A systematic study. J. Clin. Endocrinol. Metab. 2012, 97, E419–E422. [Google Scholar] [CrossRef] [Green Version]
- Itzlinger, A.; Branchi, F.; Elli, L.; Schumann, M. Gluten-free diet in celiac disease forever and for all? Nutrients 2018, 10, 1796. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Lu, L.; Yang, R.; Li, Y.; Shan, L.; Wang, Y. Increased Incidence of Thyroid Disease in Patients with Celiac Disease: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0168708. [Google Scholar] [CrossRef]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef] [PubMed]
- Ryan, D.; Giele, H. The scratch collapse test: A QUADAS-2 assessment of a systematic review. J. Plast. Reconstr. Aesthet. Surg. 2019, 72, 1418–1433. [Google Scholar] [CrossRef] [PubMed]
- Metso, S.; Hyytia-Ilmonen, H.; Kaukinen, K.; Huhtala, H.; Jaatinen, P.; Salmi, J.; Taurio, J.; Collin, P. Gluten-free diet and autoimmne thyroiditis in patients with celiac disease. A prospective controllend study. Scand. J. Gastroenterol. 2012, 47, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Krysiak, R.; Szkrobka, W.; Okopien, B. The effects of gluten-gree diet on thyroid autoimmunity indrug-naive women with Hashimoto’s thyroiditis: A pilot study. Exp. Clin. Endocrinol. Diabetes 2019, 127, 417–422. [Google Scholar]
- Asamoah, E. Levothyroxine sodium oral solution to control thyroid function in a patient with hypothyroidism and celiac disease. Clin. Case Rep. 2021, 9, e04170. [Google Scholar] [CrossRef]
- Mainardi, E.; Montanelli, A.; Dotti, M.; Nano, R.; Moscato, G. Thyroid-Related Autoantibodies and Celiac Disease. J. Clin. Gastroenterol. 2002, 35, 245–248. [Google Scholar] [CrossRef]
- Stramazzo, I.; Centanni, M.; Virili, C. Unilateral Thyroid-Associated Orbitopathy as the Only Sign of Occult Celiac Disease: Effective Treatment with a Gluten-Free Diet. Endocrinol. Metab. 2021, 36, 466–467. [Google Scholar] [CrossRef]
- Valentino, R.; Savastano, S.; Tommaselli, A.P.; Dorato, M.; Scarpitta, M.T.; Gigante, M.; Micillo, M.; Paparo, F.; Petrone, E.; Lombardi, G.; et al. Prevalence of coeliac disease in patients with thyroid autoimmunity. Horm. Res. 1999, 51, 124–127. [Google Scholar] [CrossRef]
- Biesiekierski, J. What is gluten? J. Gastroenterol. Hepatol. 2017, 32, 78–81. [Google Scholar] [CrossRef] [Green Version]
- Fasano, A. Leaky Gut and Autoimmune Diseases. Clin. Rev. Allergy Immunol. 2011, 42, 71–78. [Google Scholar] [CrossRef]
- Kinashi, Y.; Hase, K. Partners in Leaky Gut Syndrome: Intestinal Dysbiosis and Autoimmunity. Front. Immunol. 2021, 12, 673708. [Google Scholar] [CrossRef] [PubMed]
- Mu, Q.; Kirby, J.; Reilly, C.M.; Luo, X.M. Leaky Gut As a Danger Signal for Autoimmune Diseases. Front. Immunol. 2017, 8, 598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonder, M.J.; Tigchelaar, E.F.; Cai, X.; Trynka, G.; Cenit, M.C.; Hrdlickova, B.; Zhong, H.; Vatanen, T.; Gevers, D.; Wijmenga, C.; et al. The influence of a short-term gluten-free diet on the human gut microbiome. Genome Med. 2016, 8, 45. [Google Scholar] [CrossRef] [PubMed]
- Akdis, C.A. Does the epithelial barrier hypothesis explain the increase in allergy, autoimmunity and other chronic conditions? Nat. Rev. Immunol. 2021, 21, 739–751. [Google Scholar] [CrossRef] [PubMed]
- Larsen, J.; Dall, M.; Antvorskov, J.C.; Weile, C.; Engkilde, K.; Josefsen, K.; Buschard, K. Dietary gluten increases natural killer cell cytotoxicity and cytokine secretion. Eur. J. Immunol. 2014, 44, 3056–3067. [Google Scholar] [CrossRef] [PubMed]
- Antvorskov, J.C.; Fundová, P.; Buschard, K.; Funda, D.P. Impact of Dietary Gluten on Regulatory T Cells and Th17 Cells in BALB/c Mice. PLoS ONE 2012, 7, e33315. [Google Scholar] [CrossRef]
- Hansen, C.H.; Larsen, C.S.; Zachariassen, L.F.; Mentzel, C.M.; Laigaard, A.; Krych, L.; Nielsen, D.S.; Gobbi, A.; Haupt-Jorgensen, M.; Buschard, K.; et al. Gluten-free diet reduces autoimmune diabetes mellitus in mice across multiple generations in a microbiota-independent manner. J. Autoimmun. 2022, 127, 102795. [Google Scholar] [CrossRef]
- Bellastella, G.; Maiorino, M.I.; Cirillo, P.; Longo, M.; Pernice, V.; Costantino, A.; Annunziata, C.; Bellastella, A.; Esposito, K.; De Bellis, A. Remission of Pituitary Autoimmunity Induced by Gluten-Free Diet in Patients With Celiac Disease. J. Clin. Endocrinol. Metab. 2020, 105, 2252–2261. [Google Scholar] [CrossRef]
- Mische, L.J.; Mowry, E.M. The Evidence for Dietary Interventions and Nutritional Supplements as Treatment Options in Multiple Sclerosis: A Review. Curr. Treat. Options Neurol. 2018, 20, 8. [Google Scholar] [CrossRef]
- Sategna-Guidetti, C.; Volta, U.; Ciacci, C.; Usai, P.; Carlino, A.; De Franceschi, L.; Camera, A.; Pelli, A.; Brossa, C. Prevalence of thyroid disorders in untreated adult celiac disease patients and effect of gluten withdrawal: An Italian multicenter study. Am. J. Gastroenterol. 2001, 96, 751–757. [Google Scholar] [CrossRef]
- Lerner, A.; Freire de Carvalho, J.; Kotrova, A.; Shoenfeld, Y. Gluten-free diet can ameliorate the symptoms of non-celiac autoimmune diseases. Nutr. Rev. 2022, 80, 525–543. [Google Scholar] [CrossRef] [PubMed]
- Cardo, A.; Churruca, I.; Lasa, A.; Navarro, V.; Vázquez-Polo, M.; Perez-Junkera, G.; Larretxi, I. Nutritional Imbalances in Adult Celiac Patients Following a Gluten-Free Diet. Nutrients 2021, 13, 2877. [Google Scholar] [CrossRef] [PubMed]
| First Author, Year [Ref] | Country | Design | Population | Case (n) | Control (n) |
|---|---|---|---|---|---|
| Metso, 2012 [14] | Finland | Prospective controlled study | Adult | 27 | 27 |
| Krysiak, 2019 [15] | Poland | Prospective non-randomized study | Adult | 16 | 18 |
| Asamoah, 2021 [16] | USA | Case report | Adult | 1 | 0 |
| Mainardi, 2002 [17] | Italy | Prospective study | Adult | 2 | 0 |
| Stramazzo, 2021 [18] | Italy | Letter to editor | Adult | 1 | 0 |
| Valentino, 1999 [19] | Italy | Prospective study | Adult | 3 | 0 |
| First Author [Ref] | Year | TSH Gluten-Free (mIU/L, Mean (SD)) | TSH Normal Diet (mIU/L, Mean (SD)) | fT3 Gluten-Free (pmol/L, Mean (SD)) | fT3 Normal Diet (pmol/L, Mean (SD)) | fT4 Gluten-Free (pmol/L, Mean (SD)) | fT4 Normal Diet (pmol/L, Mean (SD)) | Tg-Ab Gluten-Free (U/mL; Mean (SD)) | Tg-Ab Normal Diet (U/mL; Mean (SD)) | TPO-Ab Gluten-Free (U/mL; Mean (SD)) | TPO-Ab Normal Diet (U/mL; Mean (SD)) | 25-Hydroxyvitamin D Gluten-Free (ng/mL, Mean (SD)) | 25-Hydroxyvitamin D Normal Diet (ng/mL, Mean (SD)) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Krysiak [15] | 2019 | Baseline 2.7 (1.0), after 6 months 2.4 (0.8), change -0.3 (0.2) | Baseline 2.9 (0.8), after 6 months 2.6 (0.9), change -0.3 (0.2) | Baseline 3.2 (0.6), after 6 months 3.6 (0.7), change 0.4 (0.3) | Baseline 3.1 (0.6), after 6 months 3.2 (0.7), change 0.1 (0.3) | Baseline 14.9 (2.3), after 6 months 16.1 (2.4), change 1.2 (1.4) | Baseline 15.3 (2.7), after 6 months 15.0 (2.3), change 0.3 (0.6) | Baseline 832 (311), after 6 months 629 (240), change -203 (120) | Baseline 792 (274), after 6 months 845 (324), change 53 (58) | Baseline 925 (265), after 6 months 705 (206), change -200 (105) | Baseline 891 (242), after 6 months 920 (280), change 29 (25) | Baseline 20 (6), after 6 months 25 (6), change 5 (3) | Baseline 21 (5), after 6 months 20 (5), change -1 (2) |
| Metso [14] | 2012 | Median baseline 1.7, follow-up 12 months 1.7 | Median baseline 1.5, follow-up 12 months 1.7 | / | / | Median baseline 12.9 mU/L, follow-up 12 months 12.1 mU/L | Median baseline 12.1 mU/L, follow-up 12 months 12.7 mU/L | / | / | / | / | / | / |
| Asamoah [16] | 2021 | Baseline 3.57, after 3 months 0.77, after 12 months 0.45 | / | / | / | Baseline 11.97, after 3 months 13.13, after 12 months 14.80 | / | / | / | / | / | / | / |
| Mainardi [17] | 2002 | Baseline 13.5, after 10 months 7.65, after 18 months 0.65 | / | Baseline 2.7 pg/mL, after 10 months 2.5 | / | Baseline 0.85 ng/dL, after 10 months 0.86, after 18 months 1.18 | / | Baseline 222, after 10 months 888, after 18 months 305 | / | Baseline 265, after 10 months 716, after 18 months 289 | / | / | / |
| Mainardi [17] | 2002 | Baseline 1.78, after 8 months 0.01 | / | After 8 months 3.6 pg/ml | / | Baseline 0.73 ng/dL, after 8 months 1.4 | / | Baseline 134, after 8 months 255 | / | Baseline 474, after 8 months 586 | / | / | / |
| Stramazzo [18] | 2021 | Baseline 3.5, after 3 months 3.98, after 6 months 3.45 | / | / | / | Baseline 0.8 ng/dL, after 3 months 1, after 6 months 1.2 | / | Baseline 756, after 3 months 1533 | / | Baseline 549, after 3 months 651 | / | / | / |
| Valentino [19] | 1999 | Baseline 9.8, after 6 months 1.5 | / | / | / | Baseline 7.5 pg/mL, after 6 months 12 | / | Baseline 212, after 6 months 206 | / | Baseline 2580, after 6 months 1976 | / | / | / |
| Valentino [19] | 1999 | Baseline 9.1, after 6 months 1.3 | / | / | / | Baseline 8.2 pg/mL, after 6 months 10.9 | / | Baseline 450, after 6 months 380 | / | Baseline 1230, after 6 months 1115 | / | / | / |
| Valentino [19] | 1999 | Baseline 7.9, after 6 months 1.1 | / | / | / | Baseline 7.3 pg/mL, after 6 months 11.8 | / | Baseline 312, after 6 months 210 | / | Baseline 1580, after 6 months 1395 | / | / | / |
| Risk of Bias | Feasibility | ||||||
|---|---|---|---|---|---|---|---|
| First Author | Patient’s Selection | Index Test | Reference Standard | Flow and Timing | Patient’s Selection | Index Test | Reference Standard |
| Krysiak | Low | Low | Low | Low | Low | Low | Low |
| Metso | Low | Unclear | Low | Low | Low | Low | Low |
| Mainardi | Low | Unclear | Unclear | Low | Low | Unclear | Low |
| Valentino | High | Unclear | Unclear | Low | Low | Unclear | Low |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malandrini, S.; Trimboli, P.; Guzzaloni, G.; Virili, C.; Lucchini, B. What about TSH and Anti-Thyroid Antibodies in Patients with Autoimmune Thyroiditis and Celiac Disease Using a Gluten-Free Diet? A Systematic Review. Nutrients 2022, 14, 1681. https://doi.org/10.3390/nu14081681
Malandrini S, Trimboli P, Guzzaloni G, Virili C, Lucchini B. What about TSH and Anti-Thyroid Antibodies in Patients with Autoimmune Thyroiditis and Celiac Disease Using a Gluten-Free Diet? A Systematic Review. Nutrients. 2022; 14(8):1681. https://doi.org/10.3390/nu14081681
Chicago/Turabian StyleMalandrini, Sabrina, Pierpaolo Trimboli, Gabriele Guzzaloni, Camilla Virili, and Barbara Lucchini. 2022. "What about TSH and Anti-Thyroid Antibodies in Patients with Autoimmune Thyroiditis and Celiac Disease Using a Gluten-Free Diet? A Systematic Review" Nutrients 14, no. 8: 1681. https://doi.org/10.3390/nu14081681
APA StyleMalandrini, S., Trimboli, P., Guzzaloni, G., Virili, C., & Lucchini, B. (2022). What about TSH and Anti-Thyroid Antibodies in Patients with Autoimmune Thyroiditis and Celiac Disease Using a Gluten-Free Diet? A Systematic Review. Nutrients, 14(8), 1681. https://doi.org/10.3390/nu14081681






