Bacillus licheniformis Reshapes the Gut Microbiota to Alleviate the Subhealth
Abstract
1. Introduction
2. Materials and Methods
2.1. Experience Design
2.2. Subhealthy Rat Model
2.3. Preparation of B. licheniformis Suspension and Gavage
2.4. Behavioral Testing
2.4.1. Forced Swimming Test (FST)
2.4.2. Elevated plus Maze (EPM) Test
2.5. Measurement of Cytokines and Neurotransmitters
2.6. Measurement of SCFAs
2.7. 16S rDNA Sequencing and Analysis
2.8. Statistical Analysis
3. Results
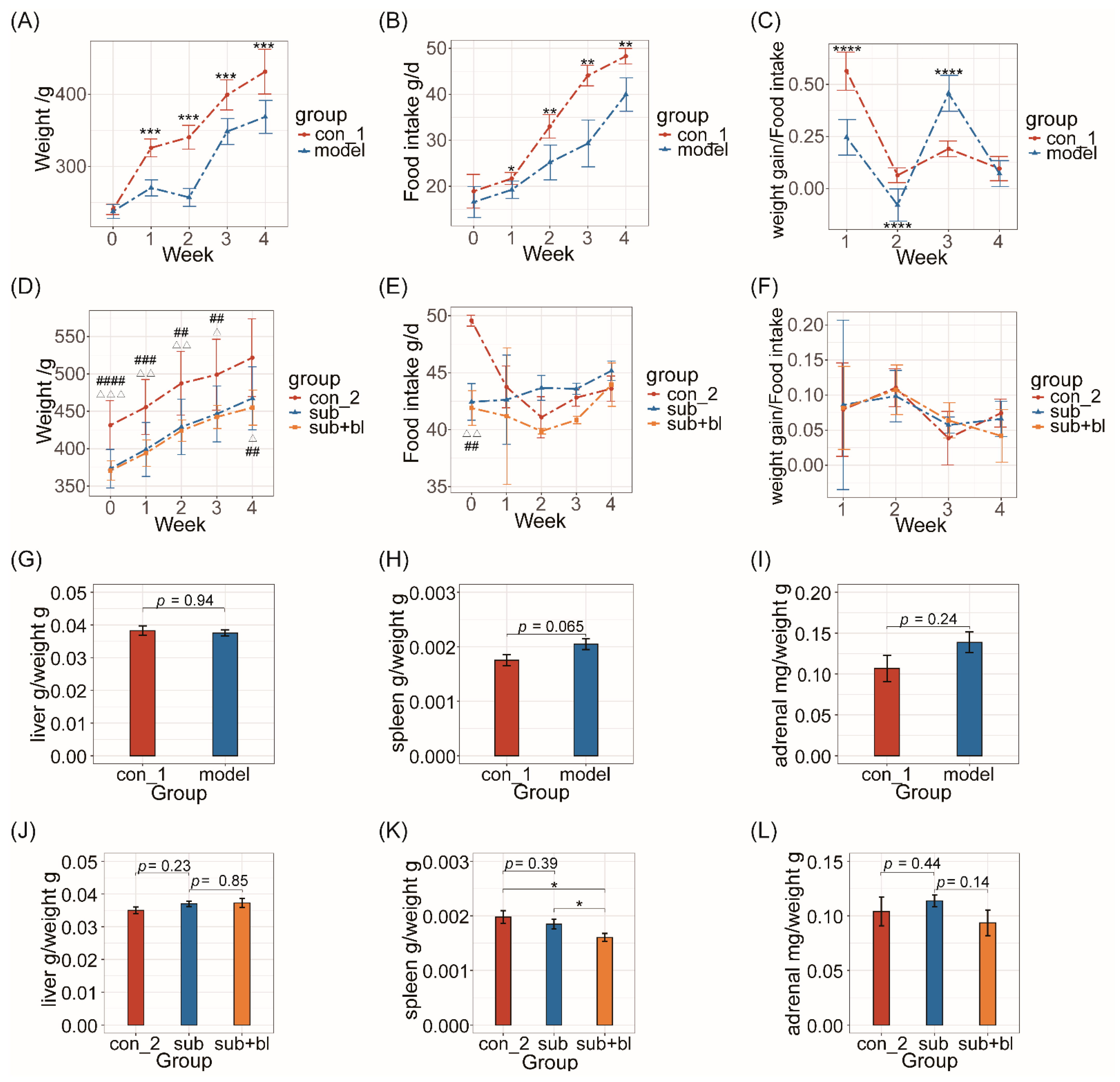
3.1. Changes in Body Weight, Food Intake, and Organ Indices
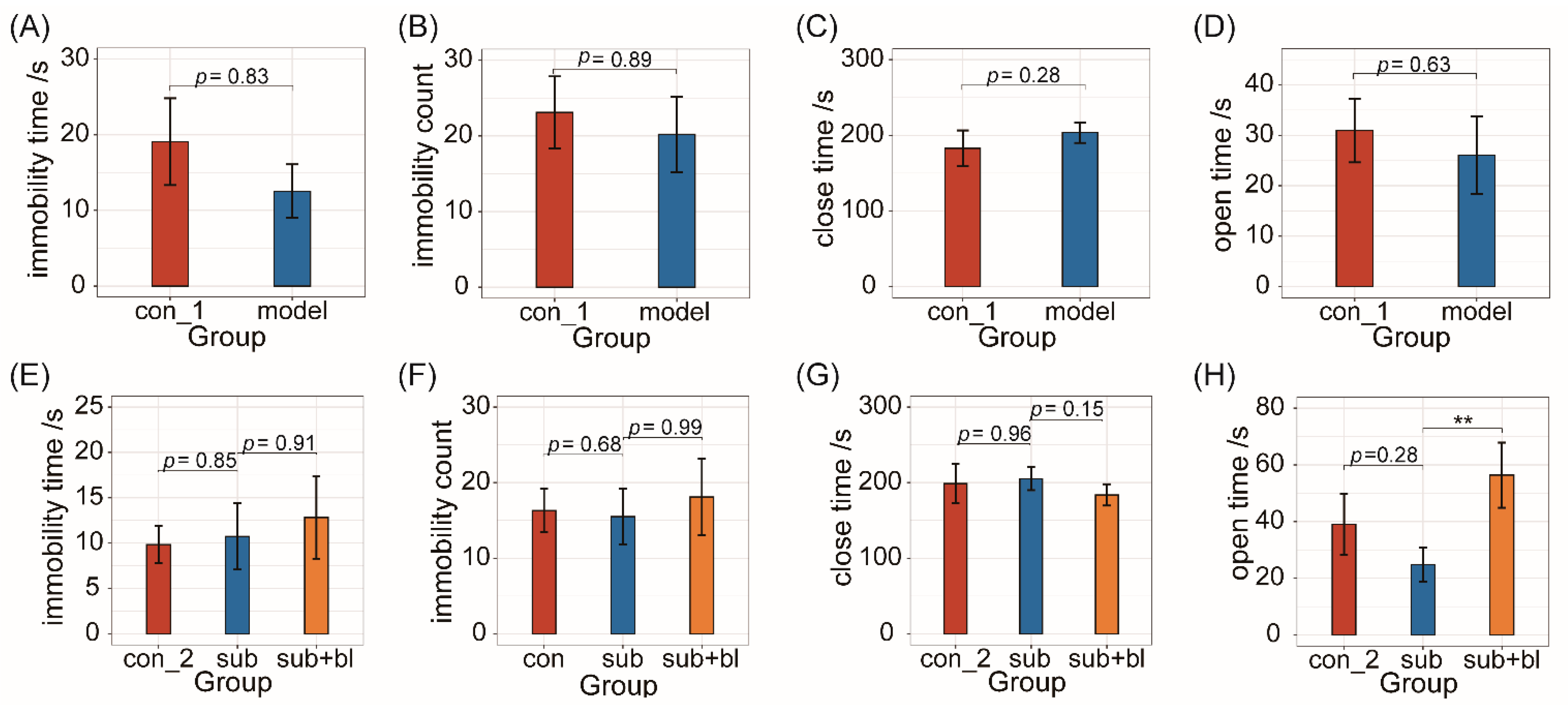
3.2. Behavioral Changes
3.3. Changes in Cytokines and Nervous System-Related Metabolites
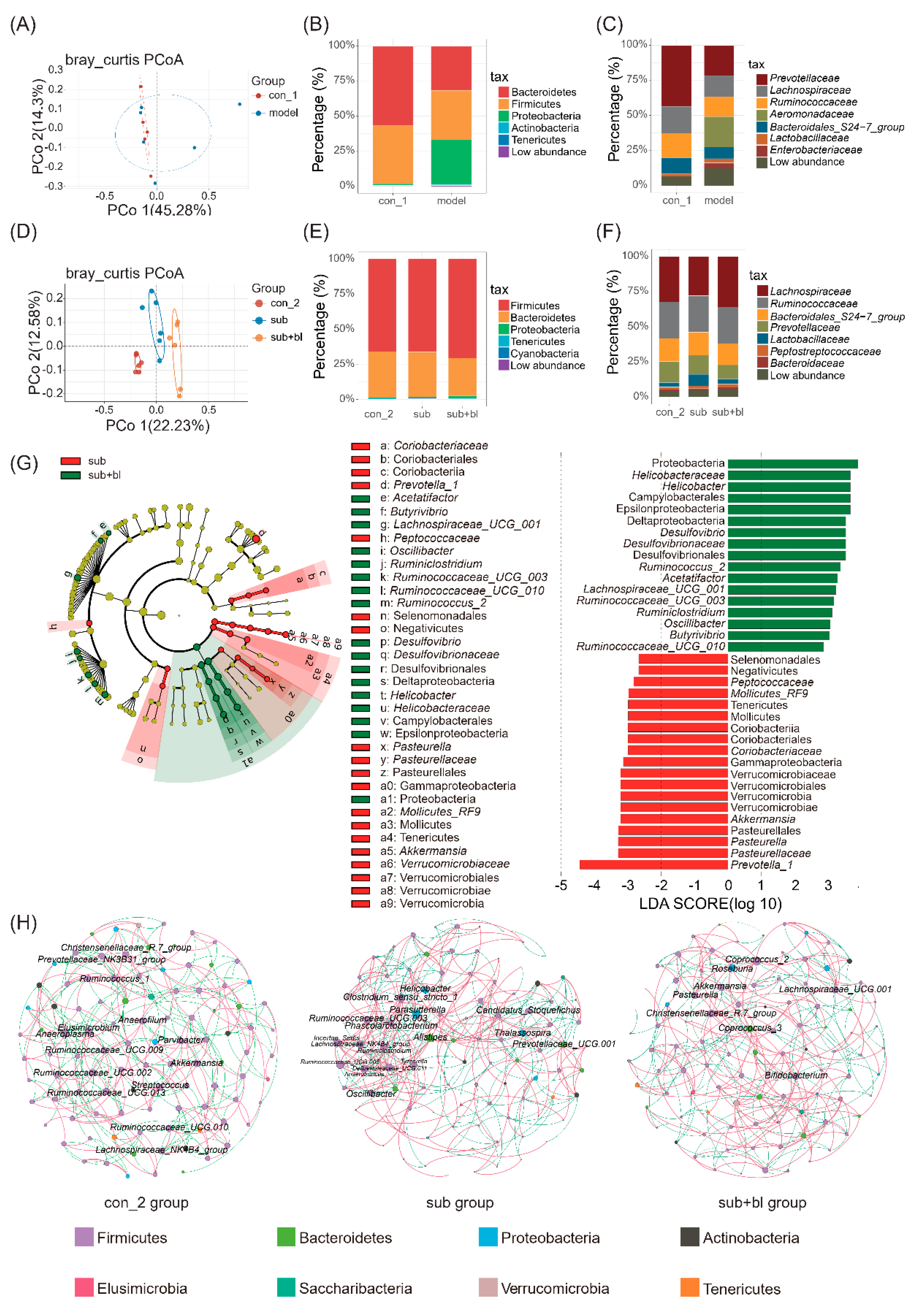
3.4. Changes in the Gut Microbiota
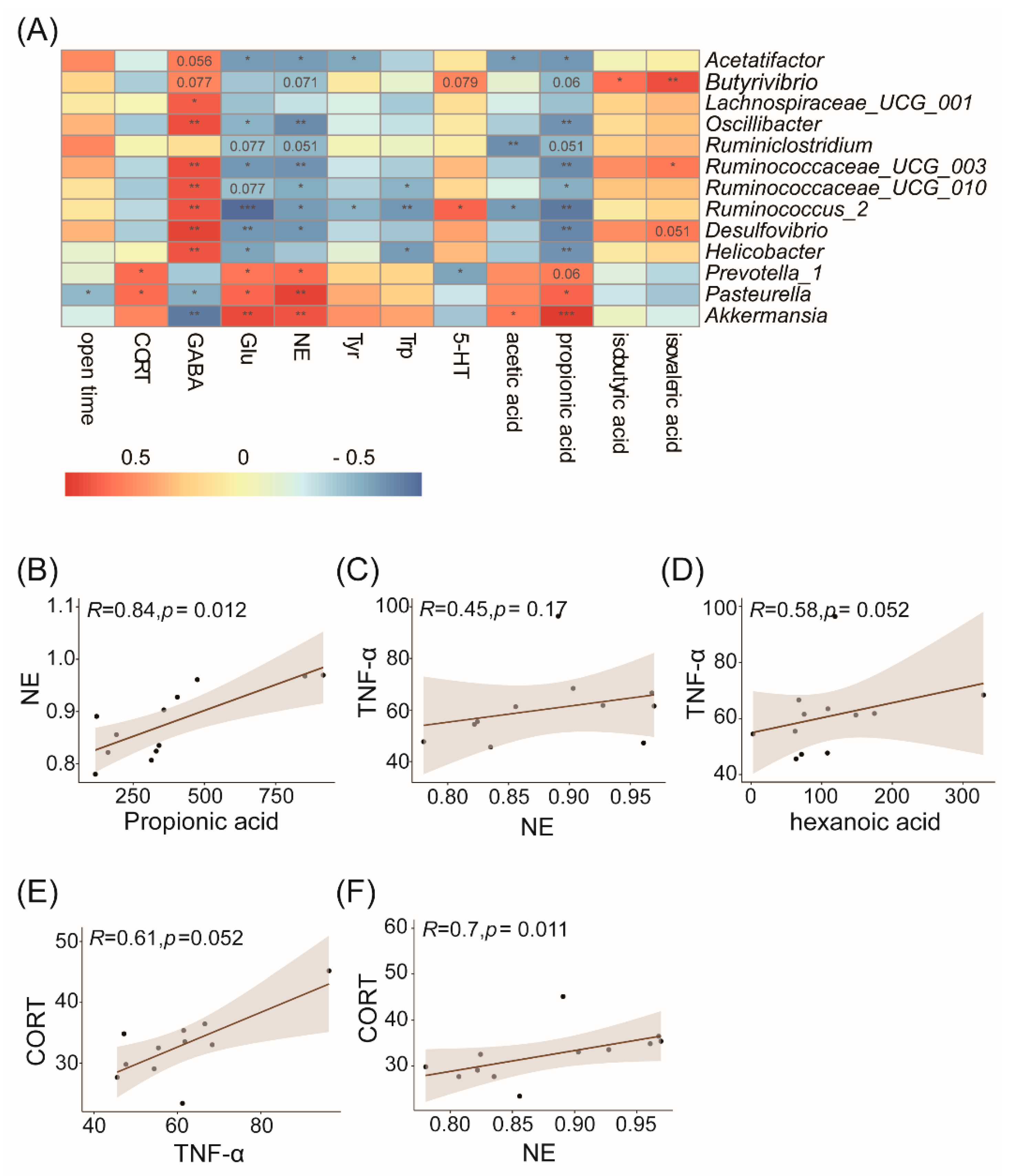
3.5. Correlation Analysis of the Gut Microbiota and Physiological or Behavioral Indices
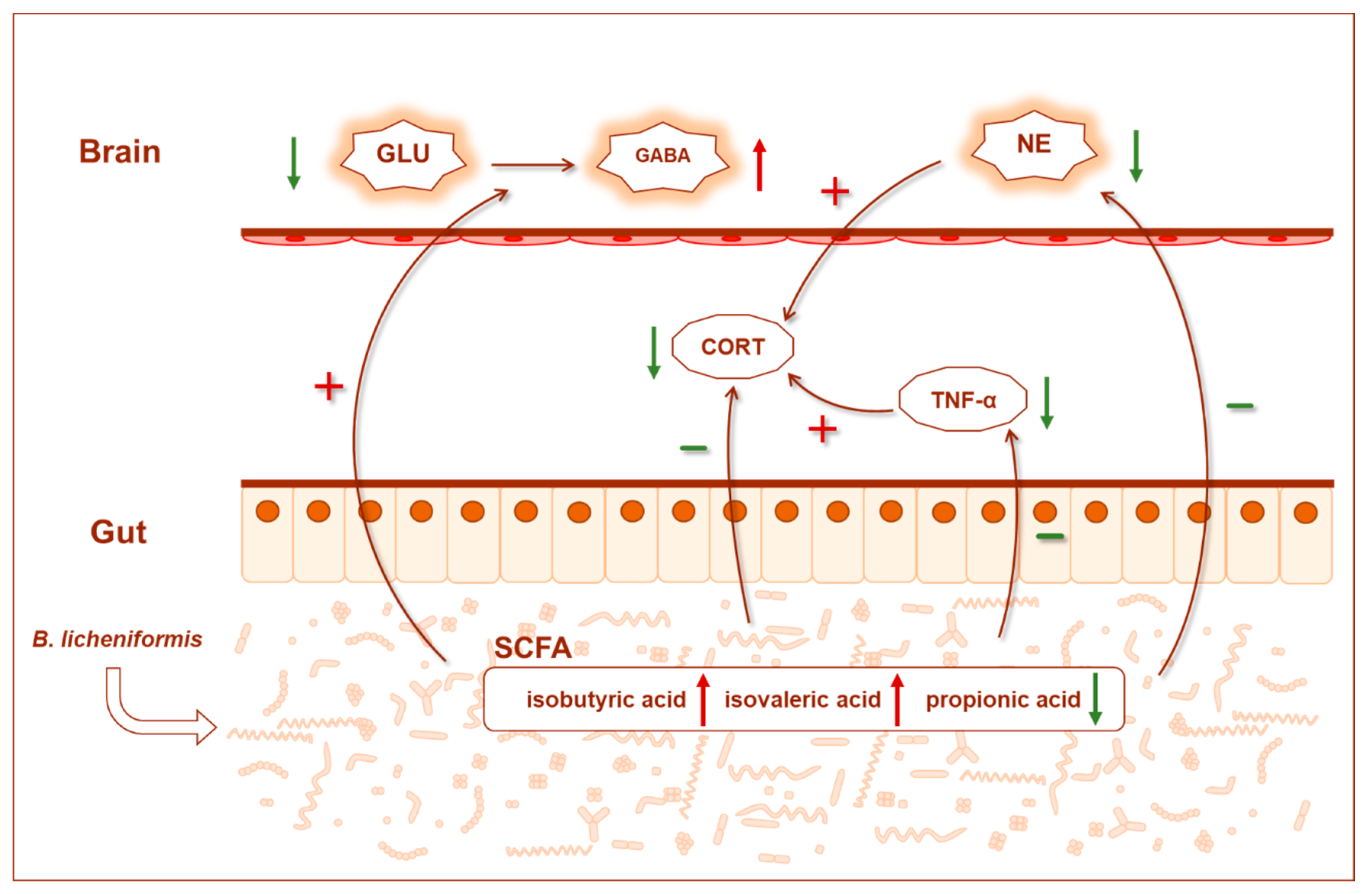
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, G.; Xie, F.; Yan, S.; Hu, X.; Jin, B.; Wang, J.; Wu, J.; Yin, D.; Xie, Q. Subhealth: Definition, Criteria for Diagnosis and Potential Prevalence in the Central Region of China. BMC Public Health 2013, 13, 446. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Wu, X.; Xing, J.; Li, L.; Zhao, Y.; Wang, J.; Zhang, Y.; Wang, Q.; Tang, Y.; Li, G.; et al. Xiaopiyishen Herbal Extract Granule Improves the Quality of Life among People with Fatigue-Predominant Subhealth and Liver-Qi Stagnation and Spleen-Qi Deficiency Syndrome. Evid.-Based Complement. Alter. Med. 2012, 2012, 509705. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.; Pedersen, O. The Human Intestinal Microbiome in Health and Disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The Human Microbiome Project. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef]
- Knox, N.C.; Forbes, J.D.; Peterson, C.; Van Domselaar, G.; Bernstein, C.N. The Gut Microbiome in Inflammatory Bowel Disease: Lessons Learned from Other Immune-Mediated Inflammatory Diseases. Am. J. Gastroenterol. 2019, 114, 1051. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of Short Chain Fatty Acids by the Gut Microbiota and their Impact on Human Metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Das, P.; Babaei, P.; Nielsen, J. Metagenomic Analysis of Microbe-Mediated Vitamin Metabolism in the Human Gut Microbiome. BMC Genom. 2019, 20, 208–211. [Google Scholar] [CrossRef]
- Cammarota, G.; Ianiro, G.; Ahern, A.; Carbone, C.; Temko, A.; Claesson, M.J.; Gasbarrini, A.; Tortora, G. Gut microbiome, Big Data and Machine Learning to Promote Precision Medicine for Cancer. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 635–648. [Google Scholar] [CrossRef]
- Yu, J.; Guo, H.; Xie, J.; Luo, J.; Li, Y.; Liu, L.; Ou, S.; Zhang, G.; Peng, X. The Alternate Consumption of Quercetin and Alliin in the Traditional Asian Diet Reshaped Microbiota and Altered Gene Expression of Colonic Epithelial Cells in Rats. J. Food Sci. 2019, 84, 678–686. [Google Scholar] [CrossRef]
- Wu, T. Biologic Characteristics of Bacillus lichniformis Strain 20386 and its Micro-ecologic Activities in vitro and in vivo. Chin. J. Microecol. 1990, 2, 1–12. [Google Scholar]
- Li, Y.; Liu, M.; Zhou, J.; Hou, B.; Su, X.; Liu, Z.; Yuan, J.; Li, M. Bacillus licheniformis Zhengchangsheng® Attenuates DSS-induced Colitis and Modulates the Gut Microbiota in Mice. Benef. Microbes 2019, 10, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.; Kim, S.K.; Park, K.S.; Jung, H.K.; Kwon, J.G.; Jang, B.I. A Double-Blind, Randomized, Active Drug Comparative, Parallel-Group, Multi-Center Clinical Study to Evaluate the Safety and Efficacy of Probiotics (Bacillus licheniformis, Zhengchangsheng® capsule) in Patients with Diarrhea. Intest. Res. 2014, 12, 236. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, B.Q.; Chen, X.F.; Yuan, Z.Q.; Fang, Q.F.; Yang, Z.; Sun, Z.F. Clinical Effect of Bacillus licheniformis Granules in Treating Infantile Hand-foot-and-mouth Disease(ordinary). Chin. J. Microecol. 2016, 28, 305–308. [Google Scholar]
- Du, S.; Jia, Y.; Ren, S.; Gong, X.; Tang, H.; Wan-Shui, W.; Li-Ming, S. The Protective Effects of Bacillus licheniformis Preparation on Gastrointestinal Disorders and Inflammation Induced by Radiotherapy in Pediatric Patients with Central Nervous System tumor. Adv. Med. Sci.-Pol. 2018, 63, 134–139. [Google Scholar] [CrossRef]
- Liu, Q.; Zhiyong, L. Effect Observation on Bupi Yichang Pills Combined with Zhengchangsheng Capsules for Diarrhea-predominant Irritable Bowel Syndrome. J. New Chin. Med. 2018, 50, 91–93. [Google Scholar]
- Hao, Z.; Wang, W.; Guo, R.; Liu, H. Faecalibacterium prausnitzii (ATCC 27766) has Preventive and Therapeutic Effects on Chronic Unpredictable Mild Stress-induced Depression-like and Anxiety-like Behavior in Rats. Psychoneuroendocrino 2019, 104, 132–142. [Google Scholar] [CrossRef]
- Olson, C.A.; Vuong, H.E.; Yano, J.M.; Liang, Q.Y.; Nusbaum, D.J.; Hsiao, E.Y. The Gut Microbiota Mediates the Anti-Seizure Effects of the Ketogenic Diet. Cell 2018, 173, 1728–1741. [Google Scholar] [CrossRef]
- Porsolt, R.D.; Le Pichon, M.; Jalfre, M. Depression: A New Animal Model Sensitive to Antidepressant Treatments. Nature 1977, 266, 730–732. [Google Scholar] [CrossRef]
- Rodgers, R.J.; Dalvi, A. Anxiety, Defence and the Elevated Plus-maze. Neurosci. Biobehav. R. 1997, 21, 801–810. [Google Scholar] [CrossRef]
- den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.; Bakker, B.M. The Role of Short-chain Fatty Acids in the Interplay Between Diet, Gut Microbiota, and Host Energy Metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef]
- Oleskin, A.V.; Shenderov, B.A. Neuromodulatory Effects and Targets of the SCFAs and Gasotransmitters Produced by the Human Symbiotic Microbiota. Microb. Ecol. Health Dis. 2016, 27, 30971. [Google Scholar] [CrossRef] [PubMed]
- Spencer, R.L.; Deak, T. A Users Guide to HPA Axis Research. Physiol. Behav. 2017, 178, 43–65. [Google Scholar] [CrossRef] [PubMed]
- Esposito, G.; Capoccia, E.; Turco, F.; Palumbo, I.; Lu, J.; Steardo, A.; Cuomo, R.; Sarnelli, G.; Steardo, L. Palmitoylethanolamide Improves Colon Inflammation Through an Enteric Glia/toll like Receptor 4-dependent PPAR-α Activation. Gut 2014, 63, 1300–1312. [Google Scholar] [CrossRef] [PubMed]
- Lener, M.S.; Niciu, M.J.; Ballard, E.D.; Park, M.; Park, L.T.; Nugent, A.C.; Zarate, C.A. Glutamate and Gamma-Aminobutyric Acid Systems in the Pathophysiology of Major Depression and Antidepressant Response to Ketamine. Biol. Psychiat. 2017, 81, 886–897. [Google Scholar] [CrossRef] [PubMed]
- Lucki, I. The Spectrum of Behaviors Influenced by Serotonin. Biol. Psychiat. 1998, 44, 151–162. [Google Scholar] [CrossRef]
- McCall, J.G.; Al-Hasani, R.; Siuda, E.R.; Hong, D.Y.; Norris, A.J.; Ford, C.P.; Bruchas, M.R. CRH Engagement of the Locus Coeruleus Noradrenergic System Mediates Stress-Induced Anxiety. Neuron 2015, 87, 605–620. [Google Scholar] [CrossRef]
- Raskind, M.A.; Peterson, K.; Williams, T.; Hoff, D.J.; Hart, K.; Holmes, H.; Homas, D.; Hill, J.; Daniels, C.; Calohan, J.; et al. A Trial of Prazosin for Combat Trauma PTSD with Nightmares in Active-duty Soldiers Returned from Iraq and Afghanistan. Am. J. Psychiatry 2013, 170, 1003–1010. [Google Scholar] [CrossRef]
- Kakiyama, G.; Pandak, W.M.; Gillevet, P.M.; Hylemon, P.B.; Heuman, D.M.; Daita, K.; Takei, H.; Muto, A.; Nittono, H.; Ridlon, J.M.; et al. Modulation of the Fecal Bile Acid Profile by Gut Microbiota in Cirrhosis. J. Hepatol. 2013, 58, 949–955. [Google Scholar] [CrossRef]
- Sidebottom, A.M.; Chang, E.B. IBD Microbial Metabolome: The Good, Bad, and Unknown. Trends Endocrinol. Metab. 2020, 31, 807–809. [Google Scholar] [CrossRef]
- Sun, D.; Bai, R.; Zhou, W.; Yao, Z.; Liu, Y.; Tang, S.; Ge, X.; Luo, L.; Luo, C.; Hu, G.; et al. Angiogenin Maintains Gut Microbe Homeostasis by Balancing α-Proteobacteria and Lachnospiraceae. Gut 2021, 70, 666–676. [Google Scholar] [CrossRef]
- Wilson, B.A.; Ho, M. Pasteurella multocida: From Zoonosis to Cellular Microbiology. Clin. Microbiol. Rev. 2013, 26, 631–655. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Zeng, B.; Zhou, C.; Liu, M.; Fang, Z.; Xu, X.; Zeng, L.; Chen, J.; Fan, S.; Du, X.; et al. Gut Microbiome Remodeling induces Depressive-like Behaviors through a Pathway Mediated by the Host’s Metabolism. Mol. Psychiatry 2016, 21, 786–796. [Google Scholar] [CrossRef] [PubMed]
- Shariat, P.; Mitchell, W.J.; Boyd, A.; Priest, F.G. Anaerobic Metabolism in Bacillus licheniformis NCIB 6346. Microbiology 1995, 141, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Machiels, K.; Joossens, M.; Sabino, J.; De Preter, V.; Arijs, I.; Eeckhaut, V.; Ballet, V.; Claes, K.; Van Immerseel, F.; Verbeke, K.; et al. A Decrease of the Butyrate-producing Species Roseburia hominis and Faecalibacterium prausnitzii Defines Dysbiosis in Patients with Ulcerative Colitis. Gut 2014, 63, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Berni, C.R.; Sangwan, N.; Stefka, A.T.; Nocerino, R.; Paparo, L.; Aitoro, R.; Calignano, A.; Khan, A.A.; Gilbert, J.A.; Nagler, C.R. Lactobacillus rhamnosus GG-supplemented Formula Expands Butyrate-producing Bacterial Strains in Food Allergic Infants. ISME J. 2016, 10, 742–750. [Google Scholar] [CrossRef]
- Ohkawara, S.; Furuya, H.; Nagashima, K.; Asanuma, N.; Hino, T. Oral Administration of Butyrivibrio Fibrisolvens, a Butyrate-producing Bacterium, Decreases the Formation of Aberrant Crypt Foci in the Colon and Rectum of Mice. J. Nutr. 2005, 135, 2878–2883. [Google Scholar] [CrossRef]
- Wu, W.; Sun, Y.; Luo, N.; Cheng, C.; Jiang, C.; Yu, Q.; Cheng, S.; Ge, J. Integrated 16S rRNA Gene Sequencing and LC-MS Analysis Revealed the Interplay Between Gut Microbiota and Plasma Metabolites in Rats with Ischemic Stroke. J. Mol. Neurosci. 2021, 71, 2095–2106. [Google Scholar] [CrossRef]
- Stilling, R.M.; van de Wouw, M.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. The Neuropharmacology of Butyrate: The Bread and Butter of the Microbiota-gut-brain axis? Neurochem. Int. 2016, 99, 110–132. [Google Scholar] [CrossRef]
- Lobel, L.; Garrett, W. Butyrate Makes Macrophages “Go Nuclear” against Bacterial Pathogens. Immunity 2019, 50, 275–278. [Google Scholar] [CrossRef]
- Donohoe, D.R.; Holley, D.; Collins, L.B.; Montgomery, S.A.; Whitmore, A.C.; Hillhouse, A.; Curry, K.P.; Renner, S.W.; Greenwalt, A.; Ryan, E.P.; et al. A Gnotobiotic Mouse Model Demonstrates that Dietary Fiber Protects Against Colorectal Tumorigenesis in A Microbiota- and Butyrate-dependent Manner. Cancer Discov. 2014, 4, 1387–1397. [Google Scholar] [CrossRef]
- Tyagi, A.; Yu, M.; Darby, T.; Vaccaro, C.; Li, J.; Owens, J.; Hsu, E.; Adams, J.; Weitzmann, M.; Jones, R.; et al. The Microbial Metabolite Butyrate Stimulates Bone Formation via T Regulatory Cell-Mediated Regulation of WNT10B Expression. Immunity 2018, 49, 1116–1131. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Harrington, J.M.; Millar, S.R.; Perry, I.J.; O’Toole, P.W.; Phillips, C.M. Gut Microbiota Associations with Metabolic Health and Obesity Status in Older Adults. Nutrients 2020, 12, 2364. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Choi, J.; Lee, H. Flavonoid-Rich Orange Juice Intake and Altered Gut Microbiome in Young Adults with Depressive Symptom: A Randomized Controlled Study. Nutrients 2020, 12, 1815. [Google Scholar] [CrossRef]
- Heimann, E.; Nyman, M.; Pålbrink, A.; Lindkvist-Petersson, K.; Degerman, E. Branched Short-chain Fatty Acids Modulate Glucose and Lipid Metabolism in Primary Adipocytes. Adipocyte 2016, 5, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.; Rao, A.V.; Wolever, T.M. Different Substrates and Methane Producing Status Affect Short-chain Fatty Acid Profiles Produced by In Vitro Fermentation of Human Feces. J. Nutr. 2000, 130, 1932–1936. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Wang, Z.; Wang, D.; Lawrence, P.; Wang, X.; Kothapalli, K.S.D.; Greenwald, J.; Liu, R.; Park, H.G.; Brenna, J.T. BCFA-enriched Vernix-monoacylglycerol Reduces LPS-induced Inflammatory Markers in Human Enterocytes in vitro. Pediatr. Res. 2018, 83, 874–879. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, Z.; Greenwald, J.; Kothapalli, K.S.D.; Park, H.G.; Liu, R.; Mendralla, E.; Lawrence, P.; Wang, X.; Brenna, J.T. BCFA Suppresses LPS Induced IL-8 mRNA Expression in Human Intestinal Epithelial Cells. Prostaglandins Leukot. Essent. Fat. Acids 2017, 116, 27–31. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Q.; Zhang, Y.L.; Pei, C.X.; Zhang, S.L.; Wang, Y.X.; Yang, W.Z.; Bai, Y.S.; Shi, Z.G.; Liu, X.N. Effects of Isobutyrate Supplementation on Ruminal Microflora, Rumen Enzyme Activities and Methane Emissions in Simmental Steers. J. Anim. Physiol. Anim. Nutr. 2015, 99, 123–131. [Google Scholar] [CrossRef]
- Cho, K.M.; Kim, Y.S.; Lee, M.; Lee, H.Y.; Bae, Y.S. Isovaleric Acid Ameliorates Ovariectomy-induced Osteoporosis by Inhibiting Osteoclast Differentiation. J. Cell. Mol. Med. 2021, 25, 4287–4297. [Google Scholar] [CrossRef]
- Blakeney, B.A.; Crowe, M.S.; Mahavadi, S.; Murthy, K.S.; Grider, J.R. Branched Short-Chain Fatty Acid Isovaleric Acid Causes Colonic Smooth Muscle Relaxation via cAMP/PKA Pathway. Dig. Dis. Sci. 2019, 64, 1171–1181. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Wang, Y.; Tao, Y.; Lu, S.; Zhu, X.; Li, D. Improvement of N-caproic Acid Production with Ruminococcaceae bacterium CPB6: Selection of Electron Acceptors and Carbon Sources and Optimization of the Culture Medium. Microb. Cell Factories 2018, 17, 99. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.H.; Raison, C.L. The role of Inflammation in Depression: From Evolutionary Imperative to Modern Treatment Target. Nat. Rev. Immunol. 2016, 16, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Saresella, M.; Marventano, I.; Barone, M.; La Rosa, F.; Piancone, F.; Mendozzi, L.; D’Arma, A.; Rossi, V.; Pugnetti, L.; Roda, G.; et al. Alterations in Circulating Fatty Acid Are Associated with Gut Microbiota Dysbiosis and Inflammation in Multiple Sclerosis. Front. Immunol. 2020, 11, 1390. [Google Scholar] [CrossRef] [PubMed]
- Orange, J.S.; Salazar-Mather, T.P.; Opal, S.M.; Spencer, R.L.; Miller, A.H.; McEwen, B.S.; Biron, C.A. Mechanism of Interleukin 12-mediated Toxicities during Experimental Viral Infections: Role of Tumor Necrosis Factor and Glucocorticoids. J. Exp. Med. 1995, 181, 901–914. [Google Scholar] [CrossRef]
- Swain, M.G.; Appleyard, C.B.; Wallace, J.L.; Maric, M. TNF-alpha Facilitates Inflammation-induced Glucocorticoid Secretion in Rats with Biliary Obstruction. J. Hepatol. 1997, 26, 361–368. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, S.; Meng, C.; Hao, Z.; Liu, H. Bacillus licheniformis Reshapes the Gut Microbiota to Alleviate the Subhealth. Nutrients 2022, 14, 1642. https://doi.org/10.3390/nu14081642
Feng S, Meng C, Hao Z, Liu H. Bacillus licheniformis Reshapes the Gut Microbiota to Alleviate the Subhealth. Nutrients. 2022; 14(8):1642. https://doi.org/10.3390/nu14081642
Chicago/Turabian StyleFeng, Siyuan, Chen Meng, Zikai Hao, and Hong Liu. 2022. "Bacillus licheniformis Reshapes the Gut Microbiota to Alleviate the Subhealth" Nutrients 14, no. 8: 1642. https://doi.org/10.3390/nu14081642
APA StyleFeng, S., Meng, C., Hao, Z., & Liu, H. (2022). Bacillus licheniformis Reshapes the Gut Microbiota to Alleviate the Subhealth. Nutrients, 14(8), 1642. https://doi.org/10.3390/nu14081642





