Abstract
Human milk contains <50% less protein (casein) than cow milk, but is equally effective in insulin secretion despite lower postingestion hyperaminoacidemia. Such potency of human milk might be modulated either by incretins (glucagon-like polypeptide-1,GLP-1); glucose-inhibitory-polypeptide, GIP), and/or by milk casein content. Healthy volunteers of both sexes were fed iso-lactose loads of two low-protein milks, i.e., human [Hum] (n = 8) and casein-deprived cow milk (Cow [↓Cas]) (n = 10), as well as loads of two high-protein milks, i.e., cow (n = 7), and casein-added human-milk (Hum [↑Cas]) (n = 7). Plasma glucose, insulin, C-peptide, incretins and amino acid concentrations were measured for 240′. All milks induced the same transient hyperglycemia. The early [20′–30′] insulin and C-peptide responses were comparable among all milk types apart from the low-protein (Cow [↓Cas]) milk, where they were reduced by <50% (p < 0.05 vs. others). When comparing the two high-protein milks, GLP-1 and GIP [5’–20’] responses with the (Hum [↑Cas]) milk were lower (by ≈2–3 fold, p < 0.007 and p < 0.03 respectively) than those with cow milk, whereas incretin secretion was substantially similar. Plasma amino acid increments largely reflected the milk protein content. Thus, neither casein milk content, nor incretin or amino acid concentrations, can account for the specific potency of human milk on insulin secretion, which remains as yet unresolved.
Keywords:
amino acids; casein; cow milk; dietary supplements; human milk; incretin; insulin; whey proteins 1. Introduction
Milk is a complex and complete nutritional substrate [1] endowed with many functional properties, largely due to a variety of substances and hormones, as well as of (oligo)peptides generated from the gut digestion of proteins [2,3,4,5]. Milk composition is species specific [1,6,7]. Compared to cow milk, human milk contains more lactose (≈5 vs. ≈7 g/100 mL, respectively), nearly the same amount of lipids and calories, but <50% less protein, depending on the lactation period [6,7,8,9]. Moreover, the protein fraction(s) are qualitatively and quantitatively different: the proteins are mainly in the soluble whey-fraction in human milk, whereas the (insoluble) casein(s) are predominant in cow milk [6,7,8,9]. Human milk is also rich in many hormones that regulate glucose homeostasis [10], and exhibits anti-inflammatory and antioxidant activities [11]. Although an infant’s growth is physiologically sustained by human milk, the latter can be replaced, and/or completed by, cow-derived formula-milk products [12,13] containing a reduced amount of protein (casein) as well as of salts. Indeed, an excessive intake of protein is not recommended in infants [14,15,16,17], because of the risks of abnormal weight gain in early childhood, and development of metabolic syndrome-related abnormalities later in life [15,16,17]. Mechanistically, these undesired effects were associated to greater insulin, insulin-like growth factor-1 (IGF-I) and amino acid concentrations following high-protein, cow-derived milk products, than those following human milk [17]. As a matter of fact, the role of anabolic hormones is crucial in orchestrating, along with the amino acids, infant growth.
Recently, Gunnerud et al. [18] first reported that the iso-lactose loads of cow and human milk stimulated insulin secretion to the same extent, despite a much lower protein concentration, and a less pronounced hyperaminoacidemia following human than cow milk. This finding appeared somehow unexpected, because amino acids are also potent stimulators of insulin secretion, in addition to, and synergistically with, glucose-derived lactose [19,20,21,22]. Therefore, a lower insulin response would have been expected following human milk.
As possible explanations for such a mismatch, at least two hypotheses could be forwarded: (1) Could the reported “equal” potency between human and cow milk on insulin stimulation be associated to a greater incretin response with the former, thus offsetting the (opposite) effects due to the lower protein content and the less marked postingestion hyperaminoacidemia? Incretins, among them the glucose-inhibitory-polypeptide (GIP) and the glucagon-like polypeptide-1 (GLP-1), are gastrointestinal hormones that are stimulated by nutrient ingestion and directly enhance insulin secretion [23]. Unfortunately, in the referenced study [18], the incretin response was incompletely reported, and the relatively short study duration (2 h) could have prevented the detection of possible late-occurring hormonal and metabolic changes. Alternatively: (2) Could the much lower casein content of human than cow milk have minimized any possible interference conveyed by casein itself on the whey protein-mediated insulin stimulation of human milk? Since casein coagulates in the acid pH of the stomach [24], the so-formed curd could impair whey protein intestinal digestion and amino acid absorption and, ultimately, compromise substrate-mediated signaling on insulin secretion.
Therefore, in this study we addressed the above hypotheses by testing, in young healthy volunteers, the effects of cow and human milk, as well as the manipulation of their casein content, on glucose, insulin, C-peptide, GLP-1, GIP and plasma amino acid concentrations. The insulin secretory capacity was tested using both ”natural” and experimentally-modified cow and human milk, the former prepared by decreasing casein concentration down to that of human milk, the latter by increasing it up to the cow-milk value.
2. Materials and Methods
2.1. Participants
Sixteen young healthy volunteers (7 males, 9 females; age: 23.1 ± 0.6 yrs.; BMI: 22.1 ± 0.8 kg/m2) were enrolled. The study was approved by the Ethical Committee of the Padova University and City Hospital (N° 2861P, on 8 July 2013, with a followed-on amendment in April, 2021) and was performed according to the guidelines of the 2013 Helsinki Declaration [25]. The protocol is registered at the ClinicalTrial.gov.site (ID: NCT04698889) (accessed on 4 February 2022). The studies were effectively started on April 2015, intermittently halted for lack of volunteers and/or support personnel, and finally completed (including all the analytical measurements and calculations) by 30 June 2021. The volunteers were sequentially recruited as they became available for the studies. Although the investigators’ intention-to-treat scheme was to apply a random allocation of the same number of volunteers to all the four arms of the protocol, some participants unpredictably refused to participate in some of the planned studies. Therefore, neither the number of participants of each test type, nor subject’s participation in all the planned tests, could be observed (See Appendix A, for the study planning and recruitment).
2.2. Type and Composition of Tested Milks
Four types of iso-lactose milk loads were administered in separate occasions: (1) natural cow whole milk (designated as: [Cow]) (to seven participants, 4 F, 3 M, BMI: 21.3 ± 1.3 Kg/m2); (2) natural human milk (designated as: [Hum]) (to eight participants, 3 F, 5 M, BMI: 23.8 ± 1.0 Kg/m2); (3) a cow-based whole milk, with a decreased total protein concentration (<1 g/100 mL) obtained by reducing its natural casein content, designated as: Cow [↓Cas], and with a target whey protein-to-casein ratio approaching that of human milk (between 60/40 and 70/30) on the first ≈1–6 months of lactation [9] (to ten participants, 5 F, 5 M, BMI: 22.4 ± 1.1 Kg/m2); and, finally, (4) a casein-added human milk (designated as: Hum [↑Cas]), matching the casein as well as the total protein content of natural cow milk, but with a casein-to-whey protein ratio (≈80/20) approaching that of cow whole milk [6] (to seven participants, 3 F, 4 M, BMI: 22.3 ± 1.1 Kg/m2). Therefore, a total of 32 test meal applications were performed. Six participants participated in three different studies, four in two different studies, whereas six participants were studied only once. Of the 32 studies, male participants contributed to a total of 17 studies, female participants to the remaining 15, both sexes being thus represented in a fairly well-balanced proportion in each experimental group. In repeated different tests performed on the same subject, at least three weeks were spaced between each of them. The analytical composition of the four milk types is reported in Table 1, whereas additional details about their preparation are reported in Appendix A.

Table 1.
Volumes and composition of the four milk types employed in the study.
2.3. Milk Provision and Preparation
2.3.1. Cow Milk
The cow milk was a commercial, pasteurized whole cow milk (Parmalat, Collecchio, Parma, Italy], with a label-reported concentration of 3.4% (g/vol) protein, 4.9% carbohydrate (lactose) and 3.5% fat. To this milk, lactose was added with the aim to raise its concentration to approximately 7 g/100 mL (Table 1). The milk was administered to each subject in volumes calculated to provide lactose at ≈0.35–0.36 g/kg body weight (corresponding to ≈25 g lactose in a reference 70 kg subject) (Table 1).
2.3.2. Human Milk
Human milk was provided by the “Bank of Donated Human Milk” of the Pediatric Dept. of the San Bortolo City Hospital (Vicenza, Italy), following a carefully-controlled procedure [27] approved by the Italian Ministry of Health (According to the guidelines of the Italian Ministry of Health (issued 5 December 2013). Published in the G.U. Serie Generale, n. 32, 8 February 2014). Milk batches were temporarily kept by the donating women in their home freezers, then collected, processed, stored and utilized following the protocol guidelines (See Appendix A for details). The milk batches utilized in the study were a mixture of milk samples collected by different donating women over the first ≈6 months of lactation. The volumes of administered human milk were calculated to provide the target lactose load [≈0.35–0.36 g/kg BW], to each study participants, by assuming a theoretical lactose concentration in human milk of ≈7 g/100 mL. As a matter of fact, the composition of human milk could not be directly measured before the studies, because we were committed not to thaw the milk batches days before the in vivo administration, for safety reasons. Therefore, such a time-restriction prevented a timely, pretest analysis by the laboratory. However, the effectively-administered lactose loads were re-calculated after the test following direct measurements of lactose concentrations in milk samples (Table 1) [See also Appendix A].
2.3.3. Low Casein Cow-Milk
The Cow [↓Cas] milk was an experimentally prepared, low-protein cow milk, with a ≈1% (w/vol) total protein concentration, but with a target WP/casein ratio approaching that of human milk in early-to-middle lactation (between 60/40 and 70/30) [9]. This milk was prepared by diluting a predetermined volume of cow whole milk with distilled water, in order to decrease the natural casein concentration to the desired value (i.e., that of natural human milk), followed by additions of calibrated amounts of (cow-derived) whey-proteins (Table 1) (See Appendix A for further details).
Lactose was added to this milk too, with the aim to raise its total concentration (resulting from the sum of the added and the natural lactose content of cow milk), to approximately 7 g/100 mL (Table 1). The administered milk volume was again calibrated to provide lactose in the individualized amount of ≈0.35–0.36 g/kg body weight (i.e., ≈25 g lactose in a reference 70 kg subject) (Table 1). Furthermore, fat (as milk cream) and salts (sodium, potassium, iron, calcium, phosphorus and magnesium, obtained from available hospital solutions) were also added to this milk, in amounts titrated to approximately match their (theoretical) concentrations to those of natural cow milk [28,29] (See Appendix A for additional, detailed specifications).
2.3.4. Casein-Added Human Milk
The Hum(↑Cas) milk] was prepared by adding cow casein (as calcium caseinate, Milk Protein 90, +Watt, Ponte San Nicolò, Padova, Italy), to natural human milk, in amounts calculated to attain a target total protein concentration similar to that of natural cow milk, but a WP-to-casein ratio similar to that of cow milk (≈20/80) [6]. Furthermore, in this milk type, the milk volumes to be administered were calculated to provide ≈0.35–0.36/kg BW lactose in each subject, under the assumption of an average lactose concentration in human milk of ≈7 g/100 mL (Table 1). The actual lactose loads administered to each subject with this milk too, were directly measured and recalculated after the studies (See Appendix A).
2.4. Experimental Procedures
The volunteers were admitted to the clinical study unit at ≈08:00 a.m. after >12 h. of overnight fast, and placed in a dedicated armchair. A 20 g cannula was inserted in an antecubital vein for blood withdrawal. After two baseline samples spaced by ≈10′, the milk load was administered and entirely consumed within 2′–5′. Starting from the end of milk ingestion (t = 0′), blood samples were collected at min 5′, 10′, 20′, 30′, 60′, 90′, 120′, 180′, and 240′, then immediately transferred to two series of plastic tubes, containing either Na-EDTA (6% w/vol), for glucose, amino acid, insulin and C-peptide determinations, or a protease inhibitor (EMD Millipore Corporation, Merck KGaA, Darmstadt, Germany), for GIP and GLP-1 assay. The tubes were kept on ice and delivered to the laboratory every 60′–90′. After centrifugation, aliquots of plasma were immediately frozen and kept at −80 °C until assayed. The participants rested in the armchair and did not eat anything over the entire duration of the test. Only drinking water was allowed.
2.5. Biochemical Analyses
The primary outcomes of the study were to measure plasma glucose, insulin, C-peptide and incretin concentration over the test. The secondary outcome was to measure plasma amino acids.
Plasma glucose concentration was determined by the glucose oxidase method, using a Yellow Spring glucose analyzer (Yellow Spring Inc., Yellow Springs, OH, USA). Insulin, C-peptide, GIP and GLP-1 plasma concentrations were determined by ELISA (Merck-Millipore Corporation, Merck KGaA, Darmstadt, Germany). The intra- and interassay variability (expressed as coefficient of variation, in %) were 2.2 and 4.5, respectively, for insulin, 6.12 and 9.21 for C-peptide, 7.4 and 8 for GLP-1, and 6.5 and 3.4 for GIP. The GLP-1 data of one subject out of the total ten of the Cow [↓Cas] milk group, as well as those of one subject out of eight participants of the Hum milk group, were excluded from calculation because they were considered outliers (See Appendix A). In one subject of the Hum [↓Cas] milk group, GIP was not determined for lack of plasma sample. Plasma amino acid concentrations were determined by gas chromatography–mass spectrometry (GCMS) using modifications of published methods [30,31,32] (See Appendix A for additional details and the CVs of plasma amino acid analysis). Lactose, fat and protein concentrations in the administered milks, were analyzed by standard laboratory methods.
2.6. Statistical Analysis
The data statistical analysis was performed either on absolute concentrations, or on their relative change (i.e., delta, Δ) in respect to baseline data, depending on the variability (expressed as coefficient of variation, CV) of the parameter’s baseline data among the groups’ means. Absolute concentration data were used where the CV was <2% (i.e., glucose), whereas Δ-changes were used when the CV was >2% (for the other parameters). Such a criterion was arbitrarily adopted to account for intertest (i.e., for intersubject group) variability. The incremental area(s) under the curve (iAUC) within specific time intervals (as detailed), were calculated using the trapezoidal approach, i.e., by calculating each area within two experimental times and adding them up. The one-way analysis of variance (ANOVA) was applied to analyze/compare one single set of data (i.e., fasting plasma glucose, insulin; the 240′ iAUC of any parameter, etc.) among the four experimental groups, followed by post hoc analysis using the Fisher’s LSD test. To analyze/compare multiple measurements of a single parameter over time between the experimental groups, the data were analyzed using the two-way ANOVA for repeated measurements, followed by the Fisher’s LSD test. The two tailed Student’s t test for unpaired data was used in some instances for head-to-head comparisons, as specifically indicated. The Statistica Software (version 10, TIBCO Sofware Inc., Palo Alto, CA, USA) was employed. A p value of <0.05 was considered statistically significant. Although the original study design was a cross-over one, it shifted to a partially-parallel one (i.e., one with independent groups), in the course of the protocol, because of the unavailability of some participants to undertake the planned study number, as reported above. Therefore, we adjusted power analysis to that applicable to four independent groups. Power analysis, sufficient to detect a ≈30% difference between mean pairs, with a RMSSE (root mean standardized square error) of <0.5, a power of 80%, and a two-sided level of significance of 0.05%, indicated a sample size of at least 29 test meal applications, therefore, below the total number of studies here executed.
3. Results
The study flow chart, participants’ enrollment and drop-outs before test initiation, their effective participation in the planned different milk tests, and other details, are reported in Appendix A. Each participant succeeded in completing the initiated test(s) for the planned 4 h duration. No participant reported any relevant side-effect except for minor abdominal discomfort in three of them, independent of the test type.
3.1. Test Milk Composition and Loads
The milks’ composition is visually depicted in Figure 1, and analytically reported in Table 1. The post hoc measured lactose concentrations in the administered milks were quite similar among the four milk types (Table 1). The actual lactose loads (in g/kg BW) resulted in being 0.347 with [Cow] milk, 0.343 with [Hum] milk, 0.337 with the Cow [↓Cas] milk, and 0.336 with the Hum [↑Cas] milk. The administered milk volumes were also quite similar across the four experimental groups, ranging from 4.82 to 5.47 mL/Kg body weight (Table 1). The measured total protein concentration in natural human milk was within the range of published milk data of the first six months of lactation (i.e., ≈1.0–1.4 g/100 mL) [9]. The calculated casein/WP ratio in the Cow [↓Cas] milk was [29/71] (The difference between the post hoc calculated [WP/Cas] ratio [≈70/30] of the Cow [↓Cas] milk (from Table 1), and the target value of Human milk [≈60/40] [9], as post hoc verified in our laboratory (see Appendix A and Figure A1), was due to the impossibility of measuring actual human milk protein composition before the test (see: Methods). Therefore, in the calculations applied to the preparation of the Cow [↓Cas] milk, we provisionally assumed a [WP/Cas] ratio [≈70/30], probably closer to that of the early two weeks of lactation [9]), than in the Hum [↑Cas] [83/17] (Table 1). Thus, casein deprivation and whey protein addition to Cow milk succeeded in the reversal of the casein/WP ratio of natural cow milk, to a value close to that of natural human milk in the early-to-middle lactation period(s) [9]. Conversely, casein addition to human milk succeeded in the reversal of the casein/WP ratio of natural human milk to the value found in natural cow milk [6].
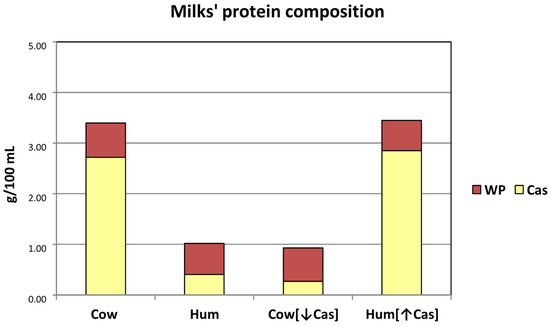
Figure 1.
Schematic protein composition of the milk types employed in the study. The Y axis reports the protein concentrations (g/100 mL) of each milk type. The (approximate) casein (Cas) and whey protein (WP) concentrations are reported as either yellow (lower) or brown (upper) part of the main bars. Abbreviations: Cas: casein; Hum: human; Cow [↓Cas]: low-casein cow milk; Hum [↑Cas]: high-casein human milk; WP: whey protein; WP/Cas: whey protein to casein ratio.
The fat content of cow milk was ≈50% greater than that of natural human milk, ≈60% greater than that of the Cow [↓Cas] milk, and ≈90% greater than that of the Hum [↑Cas] milk (Table 1). A strict statistical comparison of fat content among the four milk types (by one-way ANOVA) was prevented by the assumption of a (label-reported) 3.5% fat content in the commercial cow milk, not directly verified directly in our laboratory and therefore was not affected by analytical variations, as opposed to direct measurements of fat in the other milk types.
Glucose. Postabsorptive plasma glucose concentrations ranged between 90–92 mg/100 mL. Following the administration of each milk type, plasma glucose increased modestly (+10–20%) but significantly (p < 0.000001 by two-way ANOVA for repeated measurements, time effect) (Figure 2a), to peak values of 105–112 mg/100 mL, attained after 20′–30′ in all milk tests. No statistical difference in glucose concentrations was observed among the four milk types at any time point, selected intervals or incremental areas under the curve (data not reported) (iAUC) (p > 0.5 by ANOVA, either as group or interaction effect).
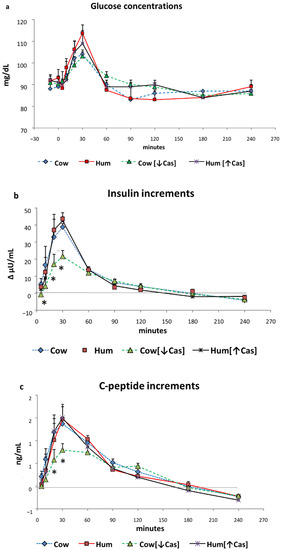
Figure 2.
Glucose, insulin, and C-peptide. Abbreviations: Hum: human; Cow [↓Cas]: low-casein cow milk; Hum [↑Cas]: high-casein human milk; WP: whey protein. (a): Plasma glucose concentrations (expressed as mg/dL) before and following milk administration over the 240 min study period. Data are mean ± SEM. No significant differences between the four milk types were detected at any time points. Sample size: Cow, n = 7; Hum, n = 8; Cow [↓Cas], n = 10; and Hum [↑Cas], n = 7. (b): Increments vs. basal values of plasma insulin concentrations (expressed as Δ μU/mL) following milk administration over the 240 min study period. Data are reported as mean ± SEM. The symbol (*) indicates the level(s) of the statistical differences (by 2-way ANOVA and the Fisher’s LSD test) between the Cow [↓Cas] milk and Cow milk at 10′ (p < 0.04), as well as between the Cow [↓Cas] milk and either Cow (p < 0.006 and p < 0.015), or the Hum (p < 0.05 and p < 0.025) milk at 20′ and 30′ respectively. Sample size: Cow, n = 7; Hum, n = 8; Cow [↓Cas], n = 10; and Hum [↑Cas], n = 7. (c): Increments vs. basal values of plasma C-peptide concentrations (expressed as Δ ng/mL) following milk administration over the 240 min study period. Data are reported as mean ± SEM. The symbol (*) indicates the level(s) of the statistical difference (by two-way ANOVA and the Fisher’s LSD test) at 20′ and 30′ between the Cow [↓Cas] milk and either the Cow (p < 0.012 and p < 0.016, respectively), the Hum (p = 0.067 and p < 0.005, respectively) or the Hum [↑Cas] milk (p < 0.01 and p < 0.004, respectively). Sample size: Cow, n = 7; Hum, n = 8; Cow [↓Cas], n = 10; and Hum [↑Cas], n = 7.
Insulin. Postabsorptive plasma insulin concentrations ranged between ≈10 and ≈16 μU/mL, without differences between the four studies. Despite the small increments of plasma glucose, plasma insulin increased in all studies by ≈3.5–4 fold vs. baseline (p < 0.00001 by two-way ANOVA for repeated measurements, time effect), to peak values at 30′ with all test milks (Figure 2b). The Cow, Hum and Hum [↑Cas] milk determined the same insulin increments (i.e., not different among the groups, p > 0.05 at any time point, by two-way ANOVA for repeated measurements, group effect). In contrast, at 10′, 20′ and 30′ the insulin increments with the Cow [↓Cas] milk were between ≈1 and ≈4-fold lower than those with Cow, Hum and Hum [↑Cas] (p < 0.05–<0.006, by two-way ANOVA for repeated measurements, group effect, followed by the Fisher’s LSD test) milks (Figure 2b).
3.2. Glucose
Postabsorptive plasma glucose concentrations ranged between 90–92 mg/dL. Following the administration of each milk type, plasma glucose increased modestly (+10–20%) but significantly (p < 0.001 by ANOVA for repeated measurements, time effect) (Figure 2a), to peak values of 105–112 mg/dL, attained after 20′–30′ in all studies. No statistical difference in glucose concentrations was observed among the four milk types at any time point, selected intervals or incremental areas under the curve (iAUC) (data not reported) (p > 0.5 by the two-way ANOVA for repeated measurements, group effect).
3.3. Insulin
Postabsorptive plasma insulin concentrations ranged between ≈10 and ≈16 μU/mL, without differences between the four studies. Despite the small increments of plasma glucose, plasma insulin increased in all studies by ≈3.5–4 fold vs. baseline (p < 0.0001 by the ANOVA for repeated measurements, time effect), to peak values at 30′ with all test milks (Figure 2b). The Cow, Hum and Hum [↑Cas] milks determined the same insulin increments (i.e., not different among the groups, p > 0.05 at any time point, by two-way ANOVA for repeated measurements, group effect). In contrast, at 10′, 20′ and 30′ the insulin increments with the Cow [↓Cas] milk were between ≈1 and ≈4-fold lower than those with Cow, Hum and Hum [↑Cas] (p < 0.05; < 0.006, by two-way ANOVA for repeated measurements, group effect, followed by the Fisher’s LSD test) milks (Figure 2b).
3.4. C-Peptide
Postabsorptive plasma C-peptide concentrations ranged between 1.0 to 1.2 ng/mL, without differences among the four studies, and increased significantly paralleling the insulin increase (Figure 2c). The C-peptide increments with Cow [↓Cas] milk at 20′ and 30′ were lower those following either the Cow (p = 0.011 and p = 0.016), Hum (p = 0.067 and p < 0.005), or Hum [↑Cas] (p < 0.01 and p < 0.004) milk (Figure 2c).
3.5. GLP-1 and GIP
Postabsorptive plasma GLP-1 concentrations ranged between 2.5 to 4.0 pM, without differences between the four studies. Following Cow milk ingestion, GLP-1 concentrations increased to a variable extent over the 4 h study (p < 0.00001 by two-way ANOVA for repeated measurements, time effect, and p = 0.01, interaction effect). In all groups, the pattern of GLP-1 increments (including, to a less extent, that following Hum [↑Cas] milk), was approximately biphasic, with an early secretion peak at 20′–30′, followed by a second one at 60′–90′ (Figure 3a). With Hum milk, GLP-1 peaked at 30′ as well, but the increment was modest and slowly decreased thereafter. With the Cow [↓Cas] milk, there was a marked and progressive GLP-1 increase, peaking between 60′–90′ and slowly decreasing thereafter. The GLP-1 increments with Cow milk at 10′ were greater than those observed with either Hum (p = 0.04) or Hum [↑Cas] milk (p < 0.04); greater at 20′ than those with either Hum (p < 0.035) or Cow [↓Cas] milk (p < 0.025); and greater at 60′ than that with Cow [↓Cas] milk (p < 0.045) (by ANOVA and the Fisher’s LSD test). At 90′, the increment with Cow milk tended to be greater, albeit insignificantly (p = 0.06) than that with Hum milk.
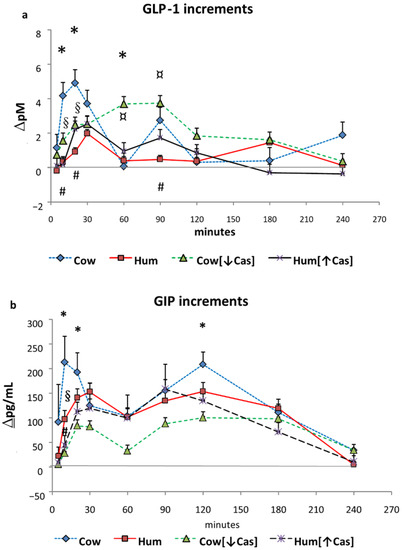
Figure 3.
GLP-1 and GIP. Abbreviations: GIP: glucose-inhibitory-polypeptide; GLP-1: glucagon-like polypeptide-1; Hum: human; Cow [↓Cas]: low-casein cow milk; Hum [↑Cas]: high-casein human milk. (a): Increments vs. baseline values of plasma GLP-1 concentrations (expressed as Δ pM/mL) following milk administration over the 240 min study period. Data are means ± SEM. The symbols indicate the statistical difference (by two-way ANOVA the Fisher’s LSD test): (*) at 10′, 20′ and at 60′ between Cow [n = 7] and Cow [↓Cas] milk (n = 9) (p = 0.025, p < 0.04, and p < 0.002, respectively); [#] at 10′, 20′ and 90′ between Cow [n = 7], and Hum [n = 7] milk (p = 0.002, p < 0.0015, p = 0.066); [§] at 10′ and 20′ between Cow [n = 7] and Hum [↑Cas] [n = 6] milk (p < 0.002 and p < 0.04, respectively); and finally [¤] between Hum [n = 7] and Cow [↓Cas] milk [n = 9] at 60′ and 90′ (p < 0.005 at both time points) (by two-way ANOVA the Fisher’s LSD test). (b): Increments vs. baseline values of plasma GIP concentrations (expressed as Δ pM/mL) following milk administration over the 240 min study period. Data are means ± SEM. The symbols indicate the levels of statistical difference (by two-way ANOVA the Fisher’s LSD test): (*) at 10′, 20′ and 120′ between Cow [n = 7] and the Cow [↓Cas] milk [n = 9] (p < 0.0001, p = 0.015 and p < 0.02 respectively); at 10′ between the Cow [n = 7] and either [§] the Hum [n = 7] (p < 0.02) or the [#] Hum [↑Cas] [n = 6] (p = 0.001) milk.
Due to the biphasic mode of GLP-1 response(s) (Figure 3a), we arbitrarily calculated their iAUCs separately, i.e., both within the [5′–20′] and the [60′–240′] time intervals. With Cow milk, the [5′–20′] GLP-1 iAUC was greater than that with either Hum (by ≈7-fold, p < 0.002), Cow [↓Cas] (by ≈2-fold, p < 0.035) or Hum [↑Cas] (by ≈5-fold, p < 0.007) milk (Figure 4a). In contrast, the [60′–240′] GLP-1 iAUC was not significantly different among the groups, although it was ≈1-fold to ≈5-fold greater with Cow [↓Cas] milk than with Cow (p = 0.094), Hum (p = 0.056) and Hum [↑Cas] (p = 0.087) milk types (Figure 4c).
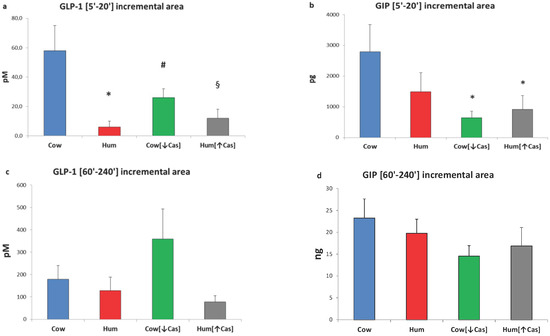
Figure 4.
GLP-1 and GIP incremental areas. Abbreviations: GIP: glucose-inhibitory-polypeptide; GLP-1: glucagon-like polypeptide-1; Hum: human; Cow [↓Cas]: low-casein cow milk; Hum [↑Cas]: high-casein human milk. (a): Integrated increments of GLP-1 vs. baseline over the [5′–20′] period. The symbols indicate significant differences between Cow milk [n = 7] and either [*] Hum (n = 7) (p < 0.002), [#] Cow [↓Cas] [n = 9] (p < 0.035) or [§] Hum [↑Cas] milk [n = 6] (p < 0.007) (by one-way ANOVA and the Fisher’s LSD test). (b): Integrated increments of GIP vs. baseline over the [5′–20′] period. The symbol (*) indicates a significant difference between Cow [n = 7] and either the Cow [↓Cas] milk [n = 9] (p < 0.012) or the Hum [↑Cas] milk [n = 6] (p = 0.03) (by one-way ANOVA and the Fisher’s LSD test). (c): Integrated increments of GLP-1 vs. baseline over the [60′–240′] period. There was no significant difference among the four milk types. (d): Integrated increments of GIP vs. baseline over the [60′–240′] period. There was no significant difference among the four milk types.
Postabsorptive GIP concentrations ranged between 1.7 to 1.9 pg/L, without differences among the four studies. Following milk administration, GIP concentrations increased to a variable extent over the 4 h study (p < 0.000001 by two-way ANOVA for repeated measurements, time effect). GIP response was biphasic, with the first peak occurring between 10′ and 30′, and the second one between 90′ and 120′ (Figure 3b). The GIP increment with Cow milk at 10′ was greater than that with the Cow [↓Cas] milk (p < 0.03) and not statistically different from those of other milk types at any other time point. However, with Cow milk the [5′–20′] GIP iAUC was ≈4-fold greater than that with the Cow [↓Cas] milk (p < 0.012), as well as 3-fold greater than that with the Hum [↑Cas] milk (p < 0.03) (Figure 4b) (by one-way ANOVA, followed by the Fisher’s LSD test). Within the [60′–240′] interval, the GIP iAUC was not significantly different among the groups (Figure 4d).
3.6. Plasma Amino Acids
Total plasma amino acids increased with all milk loads (p < 0.0001 by two-way ANOVA for repeated measurements, time effect) (Figure 5a), to an extent grossly reflecting their content in the administered milks, peaking at ≈10′–30′ with Cow, Hum and Cow [↓Cas] milks, and at ≈60′ with the Hum [↑Cas] milk. At 60′, the increment of total amino acids with the Hum [↑Cas] was greater than that following both the Hum (p < 0.003) and the Cow [↓Cas] (p < 0.009) (by one-way ANOVA and the Fisher’s LSD test). Plasma BCAAs increased, although with variable extent, with all milk types too (p < 0.0001 by two-way ANOVA for repeated measurements, time effect) (Figure 5b), peaking at 20′–30′ with Cow, Hum and Cow [↓Cas] milks, and at 90′ with the Hum [↑Cas] milk. At 20′, 60′ and 90, the increments of BCAA concentrations with the Hum [↑Cas] milk were greater than those with other milk types (Figure 5b). The sum phenylalanine (Phe) and tyrosine (Tyr) (Figure 5c) increased, although with a variable extent, also with all milk types (p < 0.002 by two-way ANOVA for repeated measurements, interaction effect), again peaking at 20′–30′ with Cow, Hum and Cow [↓Cas] milks, and at 90′ with the Hum [↑Cas] milk. There was no significant difference in the sum of Phe and Tyr increments among the groups at any time point.
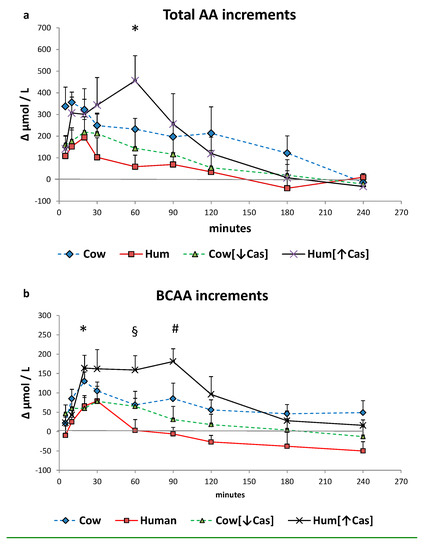
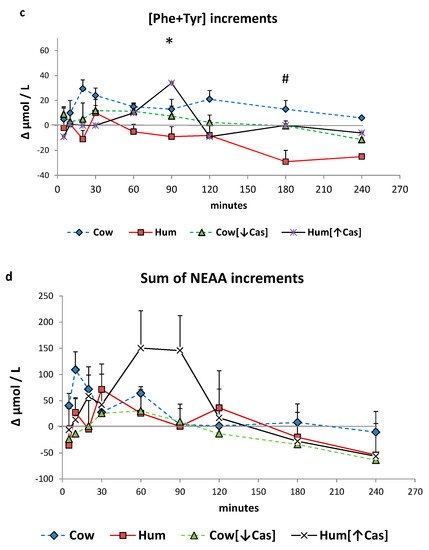
Figure 5.
Plasma amino acid increments. Abbreviations: AA: amino acids; BCAA: branched-chain AA; Phe: phenylalanine; Tyr: tyrosine; Hum: human; Cow [↓Cas]: low-casein cow milk; Hum [↑Cas]: high-casein human milk. (a): Increments vs. baseline of the sum of total amino acid [AA] concentration over the 4-h study period following milk ingestion. The symbol (*) at 5′ indicates a significant difference (by the two-way ANOVA and the Fisher’s LSD test) between Cow milk [n = 7] and either Hum [n = 7] [p < 0.02], Cow [↓Cas] [n = 10] (p < 0.025) or Hum [↑Cas] [n = 7] (p < 0.04) milk. The symbol (#) at 10′ indicates a significant different between Cow and either Hum (p < 0.025) or the Cow [↓Cas] (p < 0.03) milk (by one-way ANOVA and the Fisher’s LSD test). The symbol (§) at 60′ indicates a significant difference between the Hum [↑Cas] milk, and either Hum (p = 0.0025) or Cow [↓Cas] milk (p = 0.008), whereas that with Cow milk was of borderline significance (p = 0.075) (by one-way ANOVA and the Fisher’s LSD test). (b): Increments vs. baseline of the sum of branched-chain amino acids (BCAA) concentration over the 4 h study period following milk ingestion. The symbol (*) at 20′ indicates a significant difference (by one-way ANOVA and the Fisher’s LSD test) between the Hum [↑Cas] [n = 7] milk and either Hum [n = 7] (p < 0.035) or Cow [↓Cas] [n = 10] (p < 0.016) milks. The symbol (#) at 60′ indicates a significant difference between the Hum [↑Cas] milk and either Hum [p < 0.005] or Cow [↓Cas] (p = 0.05) milks. The symbol (§) at 90′ indicates a significant difference between the Hum [↑Cas] milk and either Hum (p < 0.001) or Cow [↓Cas] [p < 0.003] milk (by one-way ANOVA and the Fisher’s LSD test). (c): Sum of concentration increments vs. baseline of phenylalanine [Phe] and tyrosine [Tyr] over the 4 hours after milk ingestion. The symbol [*] at 90’ indicates a significant difference between the Hum [↑Cas] [n = 7] milk and either the Cow [↓Cas] [n = 10] (p < 0.001) or the Cow [n = 7] milk (p < 0.02). The symbol [#] at 180’ indicates a significant difference between Hum milk [n = 7] and either the Cow [=7] (p < 0.001), the Cow [↓Cas] [n = 10] (p < 0.015) or the Hum [↑Cas] milk (p = 0.02). (d): Sum of concentration increments vs. baseline of non-essential amino acids [NEAA] (alanine, glycine, proline, OH-proline, serine, cysteine, glutamic acid, tyrosine) over the 4 h after milk ingestion. There was no significant difference between the groups at any time point.
The sum of NEAA increased, although to a variable extent, with all milk types too (p < 0.002 by 2-WAY ANOVA for repeated measurements, interaction effect), again peaking at 20′–30′ with Cow, Hum and Cow [↓Cas] milks, and at 90′ with the Hum [↑Cas] milk. There was no significant difference in the sum of NEAA increments between the groups at any time point.
The iAUC of the sum of all amino acids, the BCAAs, Phe and Tyr, as well as of the NEAAs, within either the first or the final two hours following milk ingestion, are shown in Figure 6. The [0′–120′] iAUCs were greater than those of the [120′–240′] interval (p < 0.001 or less in any amino acid group, The [120′–240′] areas were lower (p = 0.001) than [0′–120′] ones (by the paired t test within each AA group) in each experimental milk group, except for the that of NEAAs in the Cow milk group, showing a fast amino acid absorption. The iAUCs of the sum of all amino acids, as either the [0′–120′] or the [120′–240′] iAUC (Figure 6a), were not significantly different among the four milk types, possibly because of large intragroup variations. The iAUC of the BCAA with Hum milk was lower than that with Cow milk (p < 0.03) in the [120′–240′] interval, as well as lower than that with Hum [↑Cas] in the [0′–120′] interval (p < 0.03). Conversely, with Hum [↑Cas] milk the iAUC of the BCAA was greater than that with the Cow [↓Cas] milk in the [0′–120′] interval, as well as lower than that with Hum milk in the [120′–240′] interval.
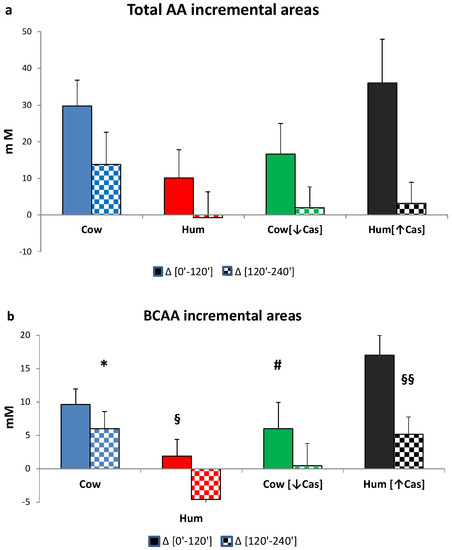
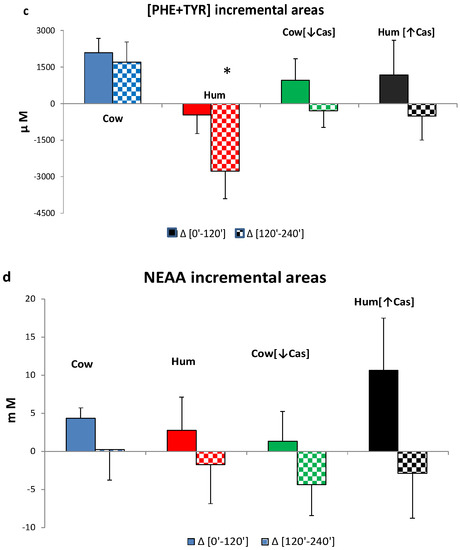
Figure 6.
Incremental areas of amino acids. Abbreviations: AA: amino acids; BCAA: branchedchain AA; Phe: phenylalanine; Tyr: tyrosine; Hum: human; Cow [↓Cas]: low-casein cow milk; Hum [↑Cas]: high-casein human milk. (a): Integrated incremental areas under the curve (iAUC) over baseline, of total AA concentrations, in the [0′–120′] (left full bars) and the [120′–240′] iAUC (right checkered bars) time intervals, respectively. There was no difference, among the four milk types, in either the [0′–120′] or the [120′–240′] iAUC. The [120′–240′] areas were lower (p < 0.0001) than the [0′–120′] ones (by the paired t test) in all milk groups. (b): Integrated incremental areas under the curve (iAUC) over baseline, of the BCAA concentrations, in the [0′–120′] (left full bars) and the [120′–240′] iAUC (right checkered bars) time intervals, respectively. The symbol (*) indicates a significant difference (by one-way ANOVA and the Fisher’s LSD test) between Cow and Hum milk (p < 0.03) in the [120′–240′] iAUC. The symbol (§) indicates a significant difference (p < 0.03) between Hum and Hum [↑Cas] in the [0′–120′] iAUC. The symbol (#) indicates a significant difference (p < 0.02) Cow [↓Cas] and Hum [↑Cas] in the [0′–120′] iAUC. The symbol (§§) indicates a significant difference (p < 0.04) between Hum and Hum [↑Cas] milk the [120′–240′] iAUC. The [120′–240′] areas were significantly lower (p < 0.0001) than the [0′–120′] ones (by paired t test). (c): Integrated incremental areas over baseline, of the sum of phenylalanine (Phe) and tyrosine (Tyr) concentrations, in the [0′–120′] (left bars, full colors) and the [120′–240′] (right bars, checkered-pattern colors) time intervals, respectively. The symbol (*) indicates a significant difference between Hum and Cow milk in the [120′–240′] (p < 0.003), as well as borderline-significant difference between the Hum and the Cow [-Cas] milk (p = 0.057). The [120′–240′] areas were significantly lower [p < 0.0001] than the [0′–120′] ones (by paired t test). (d): Integrated incremental areas over baseline, of the sum of non-essential amino acids (NEAA) (alanine, glycine, proline, serine, hydroxyproline, cysteine, glutamate, tyrosine), in the [0′–120′] (left bars, full colors) and the [120′–240′] (right bars, checkered-pattern colors) time intervals, respectively. There were no differences in the iAUC in any study group. The [120′–240′] areas were lower (p = 0.001) than [0′–120′] ones (by the paired t test) in each experimental group except for the Cow milk group.
The (Phe+Tyr) iAUC in the [0′–120′] iAUC was not different between the four milk types, whereas in the [120′–240′] interval the iAUC with Hum milk was significantly lower than that with Cow milk (p < 0.003), and nearly significantly lower than that Cow [↓Cas] milk (p = 0.054) (Figure 6c).
There was no significant difference between the four groups in the NEAA iAUC in either the [0′–120′] or the [120′–240′] time intervals (Figure 6d).
No significant correlation was found between the increase of plasma amino acid concentrations (both as total and as the BCAA only) and the increase of either insulin, C-peptide or incretin concentrations within the selected time intervals (i.e., in the 5′–20′, 10′–30′, and 60′–120′ integrated areas under the curve, iAUC).
4. Discussion
In this study, we show that the early insulin and C-peptide responses to the iso-lactose loads of natural human and cow milk in young healthy volunteers are similar, despite the much lower protein content of human milk. This observation is relevant because the early (i.e., within the first ≈10′–30′) insulin secretion represents the key homeostatic factor to controlling postprandial plasma glucose concentration following nutrient ingestion [33]. Notably, both glucose and amino acids synergistically cooperate to stimulate the early insulin secretion [19,21]. Such a specific potency of human milk appeared somehow intriguing and deserved further investigation. Our study, while confirming the seminal observation by Gunnerud et al. [18], was designed to provide additional insight into the possible mechanisms for such a “specific” potency of human milk. We focused our study on the role on incretins and plasma amino acids, by also exploring the effects of casein in experimentally modified human and cow milk, the former casein-added, the latter casein-deprived.
Under the iso-lactose conditions of this study, the response of incretins, i.e., the gastrointestinal hormones that cooperate with glucose and amino acids in the enhancement of insulin secretion [23], do not appear to be responsible for the equal potency of human and cow milk in the insulin stimulation, despite the ≈70% lower protein concentration of the former (Table 1). As a matter of fact, the incretin concentrations in the early (i.e., between [5′–20′]) phase following human milk administration are not greater, rather lower, than those following cow milk, and no greater either, thereafter (Figure 3 and Figure 4). In the referenced study [18], the incretin data were incompletely reported, therefore preventing a full comparison between human and cow milk. Secondly, we show that the experimental increase of casein (henceforth, of total protein) concentration in human milk, to a value close to that of natural cow milk, does not impair its capacity to enhance the insulin and incretin responses. This finding excludes a possible interference by casein itself, added to human milk, on the insulin-stimulatory effect conveyed by whey proteins and/or glucose. Conversely, the experimental reduction of casein in natural cow milk, to a value close to that of human milk, impairs its early insulin and incretin responses. Third, neither plasma amino acids are apparently involved in the equally potent insulin and C-peptide stimulation of human and cow milk, since plasma aminoacidemia following human milk is not greater, but rather lower, than that following cow milk. Our study, while confirming the seminal results of Gunnerud et al. [18], adds new information useful in the understanding of the possible mechanisms explaining the specific potency of human milk.
Casein deprivation in cow milk reduced the early insulin and C-peptide response (by ≈50% at the 30′ peak), in respect to that of both cow and human natural milks (Figure 2b,c), and despite post-milk plasma amino acid increments similar or even slightly greater following the low-protein cow than human natural milk (Figure 5 and Figure 6). Thus, plasma amino acids were not apparently involved in the reduced insulin early response to the low-protein cow milk administration. However, the lower early [5′–20′] GLP-1 and GIP responses following the low-protein rather than the natural cow milk (Figure 4a,b) suggest a role of casein in sustaining the response of incretins, and possibly, also of insulin, to natural cow milk.
Despite a more marked postingestion hyperaminoacidemia (either as total amino acids or of the BCAA only), within the [5′–90′] interval following the casein-added human milk, the insulin response was not altered, i.e., it was neither increased nor decreased in respect to that of natural human milk. The interpretation of such a finding is difficult and possibly double-faceted. On the one side, while the more marked hyperaminoacidemia after the casein-added human milk should have stimulated insulin secretion further, such a potential, additional effect could have been blunted by some interference at the intestinal level, between the casein itself and the WP-mediated stimulation of insulin secretion (Figure 5b). It is well established that WP is more potent than casein (or cheese) on insulin secretion [21,34], since it behaves as a “fast” protein, more rapidly digested and absorbed than the “slow” protein casein, and leading to a brisker and more marked postingestion hyperaminoacidemia [34,35,36]. It is then possible that the main actor in the stimulation of insulin secretion is the concentration of the whey proteins themselves. As a matter of fact, from the data of Table 1, the calculated WP total concentrations did not differ much among the four milk types. However, such a conclusion would not apply to the low-protein cow milk, that, despite an estimated WP concentration comparable to that of the other milk types, exhibited a lower early insulin response (Figure 2).
The failure to detect significant correlations between the increase of plasma amino acid concentrations (both as total AA and as the BCAA only) and the increase of either insulin, C-peptide or incretin concentrations at selected time intervals, confirms the complexity of the mechanism(s) controlling hormone response following the ingestion of mixed nutrients. In addition, analytical variation(s) in the amino acid analysis, greater than that for hormones (see also Appendix A), could have prevented the detection of significant differences among the experimental groups.
A species-specific effect of the milk proteins on insulin secretion could not be excluded either. In other words, the whey protein fraction of human milk may be more efficient for insulin stimulation in humans, than those from another species, i.e., cow. A species specificity in the protein structure and amino acid sequences does actually exist in human and cow milk proteins [37]. Clearly, this hypothesis requires further studies to be confirmed.
Although casein and whey proteins might modify gastric motility, the existing literature is quite contrasting about their effects [38,39,40]. Unfortunately, in our study we did not measure either gastric motility, curd formation in the stomach, or intestinal amino acid absorption. Nevertheless, amino acid absorption apparently was not impaired by either casein removal or addition, as indirectly judged by the measured plasma amino acid increments following the low-casein or the high-casein cow, and human milk loads (Figure 5 and Figure 6).
An objective limitation of our study is that it has been conducted in young adults, not in infants. A study like ours would not, however, be unfeasible in infants, for ethical and practical limitations. Rather, the present study could offer some elements in the interpretation of the mechanisms of action of human and cow milk on insulin secretion.
The experimental manipulation of cow and human milks could have altered the milks’ physiological assembly. Milk structure is very complex, i.e., lipids are emulsified in membrane-coated globules, whereas the proteins are in colloidal dispersions as micelles [41,42,43]. It cannot be excluded that the milk qualitative composition was altered by the dilution/addition procedures here used.
We extended our study to four hours, to be able to detect possible late-occurring differences among the tested milks, whereas in previous studies the duration was limited to 2 h [18,21]. The longer duration of our study allowed us to better describe the time-dependent pattern of incretin response to milk ingestion. GIP is produced by K-cells of the proximal part of the small intestine, whereas GLP-1 is produced by L-cells located in more distal intestinal tracts [23]. The GIP response to milk ingestion was more clearly biphasic than that of GLP-1 (Figure 3), and may reflects the intestinal location of the incretin-producing cells, as well as the different rates of casein and whey protein digestion.
The lysozyme content of human milk is ≈0.8–1 g/L, accounting for ≈8% of total whey proteins [8], whereas lysozyme is almost undetectable (≈1.5 μg/L) in cow milk [44]. Although it cannot be excluded that the higher lysozyme concentration of natural human rather than cow milk played a specific role in insulin and C-peptide stimulation, to our knowledge no experimental data exist on the effect of lysozyme on insulin secretion in vivo. This hypothesis should, however, be directly tested.
Human milk contains ≈20–25% of total nitrogen-containing substances as non-protein nitrogen [7,8]. In our study, we did not consider nor measure this fraction. The possible role of nonprotein nitrogen on hormone secretion might worth exploring. We also did not measure nor consider the oligosaccharides of milk, particularly those of human milk [7]. Their possible effects on either the glycemic responses or on any interference on substrate intestinal absorption would require specific studies.
The fat content of cow milk was apparently greater than that of natural human milk, of Cow [↓Cas] milk, and of the Hum [↑Cas] milk (Table 1). Whether fat per se stimulates insulin secretion in humans is controversial [45,46]. Nevertheless, one target of the study was to test the cow and human milks head-to-head, i.e., as they were, without any manipulation except for the lactose content. Although we acknowledge that the difference in fat content between these two milk types could theoretically have affected the results, the insulin and C peptide responses were not different between these two milk types, nor between the natural Cow and the Hum [↑Cas] milk. If anything, the greater fat content of cow milk should have resulted in a greater insulin and C-peptide response than that observed with both human milk-containing oral loads, which was not the case. Conversely, there was no difference in fat content between the two low-protein milks (i.e., the Hum and the Cow [↓Cas] milks), another head-to-head comparison of our study.
Oral fat (particularly monounsaturated fatty acids) can stimulate incretin secretion [47,48,49,50]. Therefore, the greater GLP-1 and GIP responses observed at some time points following the cow rather than the other milk types (Figure 3a,b) could well be due to its greater fat content, however with apparently little or no impact on insulin secretion, as discussed above.
5. Conclusions
Our study provides novel data on the characterization of the effects of human milk, compared to those of cow milk, on insulin and incretin responses, as well as on the possible role of milk proteins and plasma amino acids on these effects. This information may be helpful to better understand the physiology of hormone secretion following milk ingestion in humans.
Author Contributions
P.T.: designed research (project conception, research plan, and study overview); analyzed data; performed statistical analysis; wrote the paper; had primary responsibility for final content. A.T.: hands-on conduct of the experiments, results discussion. M.V. (Monica Vettore): hands-on conduct of the experiments, sample and data analysis. E.I.: provided essential reagents and materials, and analyzed data. A.L.: participated in and discussed the study design and the results, and provided essential reagents. E.F.: contributed to study design and provided essential materials. E.A.R.: conducted research (hands-on conduct of the experiments) and discussed the results. M.V. (Monica Vedovato): contributed to participant recruitment, study execution and data analysis and interpretation. G.V.: contributed to project conception, provided essential materials and discussed the results. M.B.: contributed to study design, provided essential materials and discussed the results. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by an institutional grant (ex 60%, N. 60A07-0391/14 and DOR2291115/22) of the University of Padova, Italy.
Institutional Review Board Statement
The study was approved by the Ethical Committee of the Padova University and City Hospital (No. 2861P, on 8 July 2013, with a follow-on amendment on 22 April 2021) and was performed according to the guidelines of the 2013 Helsinki Declaration [18]. The protocol is registered at the ClinicalTrial.gov.site (ID: NCT04698889).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data of this study can be found upon request to corresponding author.
Acknowledgments
The young volunteers of the CUAMM University College (Padova, Italy) are warmly appreciated for their contribution to the in vivo tests. We thank the +Watt Company (Ponte San Nicolò, Padova, Italy) for kindly furnishing us with the Milk Protein 90 product.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
Appendix A.1. Participants
Young healthy volunteers of both sexes were recruited predominantly among the students of the CUAMM University College of Padova, Padova (Italy). All subjects were healthy, and none referred to either a personal or a familial history of diabetes, impaired glucose tolerance, or any metabolic, hormonal, hepatic, renal, and cardiovascular disease. Their hemoglobin Hb1c values were normal (5.1 ± 0.2%, mean ± SD).
Appendix A.2. Participants’ Flow Chart
Appendix A.3. Steps of Recruitment
- Advertisements/invitation to participate in the study were placed at Students’ university college(s).
- Personal data and accurate familiar and personal medical history from potential candidates of both sexes, were collected by the investigators. An in-depth enquiry about present or past pathologies and/or other exclusion features (lifestyle, addictions, alcohol use or abuse, smoking, pharmacological treatments) was performed.
- A detailed description of the aims of the study, and of potential adverse reactions, including an accurate overview analysis of the consent form to be signed prior to the in vivo test, were submitted to each candidate.
- Of the twenty subjects initially selected, two refused to participate in the study for unforeseen subsequent commitments. One enrolled male subject participated in two studies, but then his data were not included in the experimental group(s), because of post hoc blood glucose values resulting in the range of impaired fasting glucose (IFG) criteria (i.e., 112–116 mg/dL), and also because the investigators had reasonable doubts about his observance of the required fasting period (>12 h overnight) as well as of alcohol abstinence prior to studies.
- Seventeen subjects were finally enrolled (n = 17, 8 males, 9 females, age: 23–28 yrs.). They were sequentially and randomly assigned to either one or more of the four experimental groups. Although the investigators made them the proposal to participate in all the four test types, none of them agreed. Therefore, six participants completed three studies, four two studies, while the remainder six participants underwent one study only. Thus, a total of 32 studies were performed. Repeated studies in the same volunteer were spaced by at least two weeks. Since the volunteers were sequentially enrolled over 2–3 yrs., no randomization could be planned at the start of the protocol, rather it was sequentially applied, as far as possible, when the volunteers became available.
- Following the analyses of plasma samples after the studies, the GLP-1 data of one subject, out of the total ten of the Cow [↓Cas] milk group, as well as those of one subject out of eight subjects of the Hum milk group, were excluded from calculation, because of outlier behavior (values >4-fold greater than the mean ± 1 SD of the group). In one subject of the Hum [↓Cas] milk group, GIP was not determined for lack of plasma sample.
Appendix A.4. Milk Sources and Preparation
The four types of milk tested were: (1) natural cow whole milk (designated as: Cow); (2) natural human milk (designated as Hum); (3) a cow-based whole-milk, prepared experimentally by diluting cow milk with water and subsequently adding calibrated amounts of whey proteins (WP), with the aim to attain both a total protein concentration, and a casein/WP ratio, approximating those expected in human milk in the early-to-middle lactation period (designated as: Cow [↓Cas]); and, finally, (4) a cow-based human milk with added (cow) casein, with the aim to match the casein and the total protein content, as well as the casein/WP ratio, of cow milk (designated as: Hum [↑Cas]).
Appendix A.5. Milk Protein Composition and Analysis
We relied on literature-derived values of casein/WP ratios for both cow (i.e., 80/20) and human (≈40/60) natural milk (see Refs. [1,4,5,6,7] of the main MS), the latter as the average of 15 days to 3–12 months of lactation (7). Since the collection periods of the human milk used in this study were between 15 days and 6 mo. of lactation (see below), literature-retrieved average data fell well within the expected casein/WP ratio of the human milk employed in this study.
Nevertheless, in preliminary analyses we directly determined in our laboratory the protein composition of human and cow whole milk samples, by combining centrifugation and polyacrylamide gel electrophoresis (Table A1). Twenty mL of milk were centrifuged for 1 h at 5000× g at 4 °C, to remove fat. Then, the clean, fat-deprived layer was transferred into ultracentrifuge tubes (UltraPlus Centrifuge Ware-Nalgene) and centrifuged for 4 h at 100,000× g at 4 °C (Optima Max-XP Ultracentrifuge-Beckman Coulter), to separate the casein (bottom pellet) and the soluble whey protein (top layer, defined as “serum”). Protein concentrations in the whole milk samples and the serum fraction were determined by the Lowry method) [Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem.1951; 193:265–275], that of casein was calculated as the difference between the two.

Table A1.
Protein concentration of human and cow milk samples as determined in our laboratory.
Table A1.
Protein concentration of human and cow milk samples as determined in our laboratory.
| Cow Milk | Human Milk | |
|---|---|---|
| Concentrations (g/100 mL) | ||
| Total protein | 3.40 | 1.02 |
| Serum protein | 0.65 | 0.58 |
| Calculated casein | 2.75 | 0.44 |
| Casein/WP ratio | 81/19 | 43/57 |
The data of Table A1 show a good correspondence between the directly determined and the literature data, of the concentrations of cow and human milk protein, as well as of the casein and WP protein fractions (Refs. [1,4,5,6,7]). However, since we could not determine the casein and WP concentrations in all the human milk samples utilized in the study, we used the literature-derived data as average values for the calculations.
Appendix A.6. SDS PAGE Electrophoresis: Methodology
The milk proteins were then separated by polyacrylamide gel electrophoresis (12%) under denaturing conditions (SDS-PAGE). The cow milk samples of casein and serum were processed separately, because of the high casein content of cow milk, whereas human milk was analyzed as a whole, because of its low casein content. Fixed amounts of each sample (20 µg of protein) were diluted in a loading buffer (20 mM Tris-HCl pH 6.8, glycerol 10% vol/vol, β-mercaptoethanol 1% vol/vol, SDS 1% w/vol, 0.2% bromophenol blue, deionized H2O).
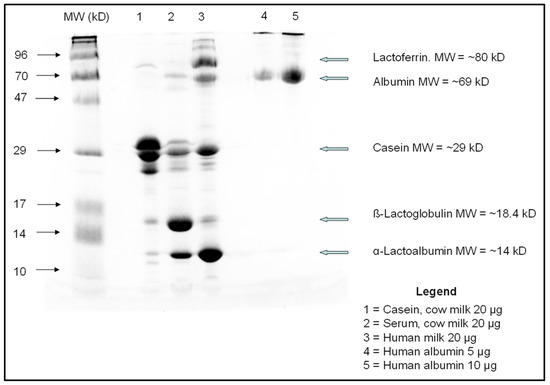
Figure A1.
Polyacrylamide gel electrophoresis of human and cow milk samples. The quantities reported in the Legend are those of the protein amount loaded. The MW values reported on the first lane (from the left) are those of the reference MW standards used, those on the left the MW of the listed proteins. Abbreviations used: MW = molecular weight; kD = kiloDalton.
The samples were loaded into gel dipped in running buffer (glycine 18.8 g/L, Tris 3 g/L, SDS 1 g/L, and deionized H2O, pH 8.3), using the Hoefer MiniVE electrophoretic unit (Amersham Pharmacia Biotech, Sunnyvale, CA, USA). Ten and five µL of human albumin (Sigma Aldrich, St. Louis, MO, USA) were used as a loading control. The gel was run at 150 V for 2 h at 4 °C. After the electrophoresis, the gel was stained by a Coomassie blue R-250 (Sigma Aldrich, Carlsbad, CA, USA) solution for 1 h at room temperature. Thereafter, the gel was incubated overnight at 4° with a destain solution (methanol 45% vol/vol, deionized H2O 45% vol/vol, Acetic Acid 10% vol/vol) to remove excess stain. Blue bands were visible after overnight incubation. The intensity of the signals was quantified by VersaDoc Imaging System (BioRad Laboratories, Hercules, CA, USA).
As shown in the Figure above, the casein content of cow milk far exceeded that of human milk, whereas the gross whey protein fraction content was typical of each milk type: β-lacto globulin was the most abundant whey protein in cow milk, whereas α-lacto albumin was the most abundant in human milk, as expected (Refs. [1,4,5,6,7]).
Appendix A.7. Milk Sources and Preparation
Appendix A.7.1. Cow Milk
The cow milk was a commercially available, pasteurized whole cow milk (Parmalat, Collecchio, Parma, Italy), with reported 3.4% (g/vol) protein, 4.9% lactose and 3.5% fat concentrations. It was administered to 7 subjects (3 M, 4 F).
Appendix A.7.2. Human Milk
Human milk was kindly provided by the “Donated Human Milk Bank” of the Pediatric Dept. at the San Bortolo City Hospital (Vicenza, Italy). Milk batches from donating women between the first 15 days to ≈6 months of lactation, were collected into 200 mL plastic containers, immediately stored in a home freezer and kept at −20 °C under temperature control. The frozen milk batches were regularly collected from the donors’ homes through a dedicated service, and safely delivered to the chemical laboratory of the “Centrale del Latte” of Vicenza, Italy. After thawing at 4 °C, milk samples from different donors were mixed, pasteurized at 62.5 °C for 30 min according to the Holder method (Ref. [19] of the main MS), then refrozen at –20 °C.
According to the approved guidelines, these milk batches had to be utilized in the neonates within 3 months from pasteurization. Since the availability of human milk occasionally exceeded the amounts required for premature infant nutrition at the Pediatric Dept, batches of human milk close to the established postcollection deadline (<24 h) but not yet needed for infants, were made available for other uses, such as for experimental purposes as those of this protocol.
Each single pasteurized milk batch usually contained 100–120 mL milk, whereas milk volumes to be administered to the volunteers ranged between 250–350 mL (according to subject’s body weight, BW, see below). Therefore, two to three different batches of milk were thawed the night before the study day for each study, mixed and kept stirring overnight at 4 °C before administration to each volunteer.
Human milk was administered to 8 subjects (5 M, 3 F). Its analytical composition is reported on Table 1. Since we were committed to thaw the milk batches just the night before the day of the in vivo testing, the composition of human milk could not be directly measured in the laboratory before the studies. Therefore, we had to provisionally assume a “standard” protein composition of human milk, as well as a “standard” lactose concentration, from data of published studies (Refs. [1,4,5,6,7] of main MS). Such a provisional composition was used as gross reference to calculate the amounts of lactose, and consequently, of the milk volumes, to be administered on a pro-kilo basis to the participants.
Appendix A.7.3. Low Casein Cow-Milk
The Cow [↓Cas] milk was a low-protein experimental milk, at a target ≈1% (w/vol) total protein concentration, but with a reversed casein-to-WP ratio, approaching that of human milk of early-to-middle lactation (i.e., 40/60) (Ref. [7] of the main MS). This milk was prepared by diluting commercial cow milk with distilled water in order to decrease the casein concentration to a value close to that of early-lactation human milk. As outlined above, we calculated such dilution by assuming from literature data (Ref. [4] of the main MS) a [80/20] casein-to-WP ratio of commercial cow milk.
Thereafter, whey proteins (lactoferrin, α-lacto albumin and lysozyme, all purchased from ACEF, Fiorenzuola d’Arda, Piacenza, Italy), as well as salts and fat, were added and accurately mixed with the diluted cow milk, with the purpose to approximately match their content between the experimental Cow [↓Cas] milk and “average” Hum milk, on the basis of published data (Refs. [1,4,5,6,7] of the main MS). The whey protein(s) were added with the purpose of comparing at best the two low casein (i.e., the Cow [↓Cas] and the Hum) milks, in respect to the WP sub-fractions.
The added whey proteins were titrated to each volunteer’s body weight. Lactoferrin, that is almost absent in cow milk (Ref. [4] of the main MS), was added in 0.015 g/Kg BW. Lacto albumin was added in 0.0129 g/Kg BW, that, together with its estimated residual amount in cow milk (≈0.00055 g/Kg BW), ended up as a total of 0.0135 g/Kg BW. Finally, a small amount of lysozyme (0.0025 g/Kg BW) was added too, in order to match its content between the Cow [↓Cas] and Hum milk. Lysozyme is contained only in trace amounts in cow milk (Ref. [39] of main MS), but is more abundant in human milk (i.e., ≈0.05 g/mL) (Ref. [6] of main MS). As a result, the total amount of administered whey proteins with the Cow [↓Cas] milk was ≈0.03004 g/Kg BW, with an estimated WP/Casein ratio of ≈70/30. Fat (as milk cream) and salts (sodium, potassium, iron, calcium, phosphorus and magnesium, obtained from available hospital solutions) were also added (in g/Kg BW) to this milk, in amounts titrated to match their (theoretical) concentrations to that of natural cow milk (Refs. [21,22] of the main MS). The resulting test milk suspension was gently but accurately shaken by hand for at least 10′ to ensure a homogeneous, complete mixing of the components. The achievement of extensive mixing was verified and confirmed by substrate concentration analysis in multiple, random samples from the final milk volume (data not shown). This milk was administered to 10 participants (5 M, 5 F).
Appendix A.7.4. Casein-Added Human Milk
The casein-added human milk (labelled as: Hum [↑Cas] milk) was prepared by adding ≈0.1 g/kg BW of a micellar caseinate product (Milk Protein 90, kindly furnished by +Watt, Ponte San Nicolò, Padova, Italy), to natural human milk, assuming a natural human milk casein concentration of ≈0.25 g/100 mL, to match the final total protein concentration to that of natural cow milk (i.e., 3.4%, g/100 mL) (Ref. [4] of MS) (Figure 1). We provisionally assumed a ≈1 g/100 mL total protein concentration of Hum milk, that is within the range reported in the first six months of the lactation period (Ref. [7] of the main MS), of which the WP fraction represents ≈60% of total protein. Indeed, the target total protein concentration was successfully achieved, as determined on post hoc analyses (Table 1, main MS). No attempt was made to match the natural human milk lactose, lipid and electrolyte concentrations, with those of other milk types. This milk was administered to 7 subjects (4 M, 3 F).
Appendix A.8. Lactose Addition to Cow-Based Milk(s)
To both cow-based milk types, lactose (purchased from VWR International, Haasrode Research Park Zone 3, Geldenaaksebaan 464 B-3001 Leuven, Belgium), was added in titrated amounts, to attain a final lactose concentration similar to that expected in Hum milk (≈7 g/100 mL) (Ref. [4] of the main MS). The actual lactose concentration in the human milk-containing test milks, effectively administered to the volunteers, resulting from post hoc laboratory analysis, are reported in Table 1 (see main MS). As reported above, no lactose was added to human-based milk types. The target total lactose concentration was successfully achieved in all test milks, with a minor exception of the lactose-added cow milk.
In our calculation, we did not take into account nor measure, either oligosaccharides (likely present at very low concentrations in cow milk) (Ref. [4] of the main MS), or non-protein nitrogen that accounts for ≈25% of total nitrogen in human milk (Refs. [4,6] of the main MS).
Except for commercial cow milk, the other milk types were kept stirring for at least 1 h the night before the study day, maintained overnight at 4 °C, and again vigorously shaken at room temperature on the morning of the study day.
Appendix A.9. Administered Milk Volumes
The milk volumes to be administered to each volunteer were then calculated to deliver between 0.33–0.36 g lactose per Kg body weight (i.e., ≈25 g in a standard 70 Kg subject). The actual volumes of administered milk (on a pro-kilo basis) resulted in being fairly well matched among the four milk types (Table 1 of the main MS).
Appendix A.10. Plasma Amino Acid Analysis by Gas Chromatography-Mass Spectrometry (GCMS)
Plasma amino acid concentrations were determined by GCMS using modifications of published methods (Refs. [23,24,25] the main MS). Five-hundred μL of plasma were thawed and dispensed in 2 mL cryotubes. One hundred μL of 300 μM norleucine solution was added as internal standard (SI). A calibrated standard curve was prepared and run in the same assay. Plasma aliquots were deproteinized with 100 μL 6N HCl and loaded onto SPE (solid phase extraction) AG 50W-X 2 columns, pre-activated with 6 mL distilled water and 6 mL 1M HCl. The columns were washed with 6 mL 0.5 M HCl. Elution of the amino acid-containing fraction was accomplished with 6 mL of a 4M NH4OH solution. Following overnight stay at room temperature to evaporate excess NH3, the samples were frozen at 80 °C and lyophilized overnight. Dried samples were then derivatized with 140 μL of a MTBSTFA solution/acetonitrile, at a 1/1 ratio, for 1 h at 110 °C, and injected by an auto sampler into a 5973N Agilent Gas Chromatography–Mass Spectrometer (GCMS) (Agilent Technologies, Palo Alto, Ca, USA), using a column flow rate of 1.3 mL/min and electron impact ionization. About ≈60% of all amino acid analyses were performed as single assay, the remaining ≈40% in either duplicates or more. In each assay, repeated injections were performed to control for analytical variations. The injection reproducibility, expressed as coefficient of variation (CV), applied to sum of all the analyzed (n = 18) amino acids, was 18.6 ± 4.3 (±1 SD), whereas that applied to the sum of the branched chain amino acids was 15.4 ± 1.8. Estimates of intra-assay, interassay and intrasubject variation, resulting from repeated analyses of the two baseline samples in three subjects, who underwent three different milk load tests, was 26.5 ± 3.7 (±1 SD) for the sum of all the amino acids analyzed, and 25.8 ± 8.4 for the sum of the branched-chain amino acids.
References
- Pereira, P.C. Milk nutritional composition and its role in human health. Nutrition 2014, 30, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, R.; Meisel, H. Food-derived peptides with biological activity: From research to food applications. Curr. Opin. Biotechnol. 2007, 18, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Madureira, A.; Tavares, T.; Gomes, A.; Pintado, M.; Malcata, F. Invited review: Physiological properties of bioactive peptides obtained from whey proteins. J. Dairy Sci. 2010, 93, 437–455. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fan, Y.; Shaikh, A.S.; Wang, Z.; Wang, D.; Tan, H. Dezhou donkey (Equus asinus) milk a potential treatment strategy for type 2 diabetes. J. Ethnopharmacol. 2019, 246, 112221. [Google Scholar] [CrossRef] [PubMed]
- Lionetti, L.; Cavaliere, G.; Bergamo, P.; Trinchese, G.; De Filippo, C.; Gifuni, G.; Gaita, M.; Pignalosa, A.; Donizzetti, I.; Putti, R.; et al. Diet supplementation with donkey milk upregulates liver mitochondrial uncoupling, reduces energy efficiency and improves antioxidant and antiinflammatory defences in rats. Mol. Nutr. Food Res. 2012, 56, 1596–1600. [Google Scholar] [CrossRef] [PubMed]
- Haug, A.; Høstmark, A.T.; Harstad, O.M. Bovine milk in human nutrition—A review. Lipids Health Dis. 2007, 6, 25. [Google Scholar] [CrossRef]
- Andreas, N.J.; Kampmann, B.; Le-Doare, K.M. Human breast milk: A review on its composition and bioactivity. Early Hum. Dev. 2015, 91, 629–635. [Google Scholar] [CrossRef]
- Lönnerdal, B. Nutritional and physiologic significance of human milk proteins. Am. J. Clin. Nutr. 2003, 77, 1537S–1543S. [Google Scholar] [CrossRef]
- Lönnerdal, B.; Erdmann, P.; Thakkar, S.K.; Sauser, J.; Destaillats, F. Longitudinal evolution of true protein, amino acids and bioactive proteins in breast milk: A developmental perspective. J. Nutr. Biochem. 2017, 41, 1–11. [Google Scholar] [CrossRef]
- Savino, F.; Liguori, S.A.; Sorrenti, M.; Fissore, M.F.; Oggero, R. Breast Milk Hormones and Regulation of Glucose Homeostasis. Int. J. Pediatr. 2011, 2011, 1–11. [Google Scholar] [CrossRef]
- Trinchese, G.; Cavaliere, G.; De Filippo, C.; Aceto, S.; Prisco, M.; Chun, J.T.; Penna, E.; Negri, R.; Muredda, L.; Demurtas, A.; et al. Human Milk and Donkey Milk, Compared to Cow Milk, Reduce Inflammatory Mediators and Modulate Glucose and Lipid Metabolism, Acting on Mitochondrial Function and Oleylethanolamide Levels in Rat Skeletal Muscle. Front. Physiol. 2018, 9, 32. [Google Scholar] [CrossRef] [PubMed]
- Hernell, O. Human milk vs. cow’s milk and the evolution of infant formulas. Nestle Nutr. Workshop Ser. Pediatr. Program. 2011, 67, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Castilho, S.D.; Filho, A.D.A.B. The history of infant nutrition. J. Pediatr. 2010, 86, 179–188. [Google Scholar] [CrossRef] [PubMed]
- European Childhood Obesity Trial Study Group; Koletzko, B.; Von Kries, R.; Closa, R.; Escribano, J.; Scaglioni, S.; Giovannini, M.; Beyer, J.; Demmelmair, H.; Gruszfeld, D.; et al. Lower protein in infant formula is associated with lower weight up to age 2 y: A randomized clinical trial. Am. J. Clin. Nutr. 2009, 89, 1836–1845. [Google Scholar] [CrossRef]
- Oddy, W.H. Infant feeding and obesity risk in the child. Breastfeed. Rev. 2012, 20, 7–12. [Google Scholar]
- Liotto, N. Protein content of infant formula for the healthy full-term infant. Am. J. Clin. Nutr. 2020, 111, 946–947. [Google Scholar] [CrossRef]
- European Childhood Obesity Trial Study Group; Socha, P.; Grote, V.; Gruszfeld, D.; Janas, R.; Demmelmair, H.; Closa-Monasterolo, R.; Subías, J.E.; Scaglioni, S.; Verduci, E.; et al. Milk protein intake, the metabolic-endocrine response, and growth in infancy: Data from a randomized clinical trial. Am. J. Clin. Nutr. 2011, 94, 1776S–1784S. [Google Scholar] [CrossRef]
- Gunnerud, U.; Holst, J.J.; Östman, E.; Björck, I. The glycemic, insulinemic and plasma amino acid responses to equi-carbohydrate milk meals, a pilot- study of bovine and human milk. Nutr. J. 2012, 11, 83. [Google Scholar] [CrossRef]
- Floyd, J.C.; Fajans, S.S.; Pek, S.; A Thiffault, C.; Knopf, R.F.; Conn, J.W. Synergistic Effect of Essential Amino Acids and Glucose upon Insulin Secretion in Man. Diabetes 1970, 19, 109–115. [Google Scholar] [CrossRef]
- Schmid, R.; Schulte-Frohlinde, E.; Schusdziarra, V.; Neubauer, J.; Stegmann, M.; Maier, V.; Classen, M. Contribution of Postprandial Amino Acid Levels to Stimulation of Insulin, Glucagon, and Pancreatic Polypeptide in Humans. Pancreas 1992, 7, 698–704. [Google Scholar] [CrossRef]
- Nilsson, M.; Stenberg, M.; Frid, A.H.; Holst, J.J.; Björck, I.M. Glycemia and insulinemia in healthy participants after lactose-equivalent meals of milk and other food proteins: The role of plasma amino acids and incretins. Am. J. Clin. Nutr. 2004, 80, 1246–1253. [Google Scholar] [CrossRef] [PubMed]
- Van Loon, L.J.; Kruijshoop, M.; Menheere, P.P.; Wagenmakers, A.J.; Saris, W.H.; Keizer, H.A. Amino acid ingestion strongly enhances insulin secretion in patients with long-term type 2 diabetes. Diabetes Care 2003, 26, 625–630. [Google Scholar] [CrossRef]
- Nauck, M.A.; Meier, J.J. Incretin hormones: Their role in health and disease. Diabetes Obes. Metab. 2018, 20 (Suppl. 1), 5–21. [Google Scholar] [CrossRef] [PubMed]
- Egger, L.; Ménard, O.; Baumann, C.; Duerr, D.; Schlegel, P.; Stoll, P.; Vergères, G.; Dupont, D.; Portmann, R. Digestion of milk proteins: Comparing static and dynamic in vitro digestion systems with in vivo data. Food Res. Int. 2019, 118, 32–39. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Livesey, G. A perspective on food energy standards for nutrition labelling. Br. J. Nutr. 2001, 85, 271–287. [Google Scholar] [CrossRef] [PubMed]
- Arslanoglu, S.; Bertino, E.; Tonetto, P.; De Nisi, G.; Ambruzzi, A.M.; Biasini, A.; Profeti, C.; Spreghini, M.R.; Moro, G.E. Guidelines for the establishment and operation of a donor human milk bank. J. Matern. Neonatal Med. 2010, 23, 1–20. [Google Scholar] [CrossRef]
- Strata, A. Milk and derivatives. Functional properties. Progr. Nutr. 2013, 15, 3–32. (In Italian) [Google Scholar]
- Bijl, E.; van Valenberg, H.J.F.; Huppertz, T.; van Hooijdonk, A.C.M. Protein, casein, and micellar salts in milk: Current content and historical perspectives. J. Dairy Sci. 2013, 96, 5455–5464. [Google Scholar] [CrossRef]
- Adams, R.F. Determination of amino acid profiles in biological samples by gas chromatography. J. Chromatogr. A 1974, 95, 189–212. [Google Scholar] [CrossRef]
- Schwenk, W.F.; Berg, P.J.; Beaufrere, B.; Miles, J.M.; Haymond, M.W. Use of t-butyldimethylsilylation in GC/MS analysis of physiologic compounds in plasma using electron impact ionization. Anal. Biochem. 1982, 141, 101–109. [Google Scholar] [CrossRef]
- Csapó, J.; Albert, C.S.; Lók, K.; Csapó-Kiss, Z.S. Separation and determination of the amino acids by ion exchange column chromatography applying post-column derivatization. Acta Univ. Sapientiae Aliment. 2008, 5, 29. [Google Scholar]
- Garber, A. The importance of early insulin secretion and its impact on glycaemic regulation. Int. J. Obes. 2000, 24, S32–S37. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hall, W.L.; Millward, D.J.; Long, S.J.; Morgan, L.M. Casein and whey exert different effects on plasma amino acid profiles, gastrointestinal hormone secretion and appetite. Br. J. Nutr. 2003, 89, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Boirie, Y.; Dangin, M.; Gachon, P.; Vasson, M.-P.; Maubois, J.-L.; Beaufrère, B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc. Natl. Acad. Sci. USA 1997, 94, 14930–14935. [Google Scholar] [CrossRef]
- Salehi, A.; Gunnerud, U.; Muhammed, S.J.; Östman, E.; Holst, J.J.; Björck, I.; Rorsman, P. The insulinogenic effect of whey protein is partially mediated by a direct effect of amino acids and GIP on β-cells. Nutr. Metab. 2012, 9, 48. [Google Scholar] [CrossRef]
- Magnuson, S.J.; Henry, J.F.; Yip, T.T.; Hutchens, W.T. Structural Homology of Human, Bovine, and Porcine Milk Lactoferrins: Evidence for Shared Antigenic Determinants. Pediatr. Res. 1990, 28, 176–181. [Google Scholar] [CrossRef]
- Calbet, J.A.L.; Holst, J.J. Gastric emptying, gastric secretion and enterogastrone response after administration of milk proteins or their peptide hydrolysates in humans. Eur. J. Nutr. 2004, 43, 127–139. [Google Scholar] [CrossRef]
- Stanstrup, J.; Schou, S.S.; Holmer-Jensen, J.; Hermansen, K.; Dragsted, L.O. Whey Protein Delays Gastric Emptying and Suppresses Plasma Fatty Acids and Their Metabolites Compared to Casein, Gluten, and Fish Protein. J. Proteome Res. 2014, 13, 2396–2408. [Google Scholar] [CrossRef]
- Ma, J.; Stevens, J.E.; Cukier, K.; Maddox, A.F.; Wishart, J.M.; Jones, K.L.; Clifton, P.M.; Horowitz, M.; Rayner, C.K. Effects of a Protein Preload on Gastric Emptying, Glycemia, and Gut Hormones After a Carbohydrate Meal in Diet-Controlled Type 2 Diabetes. Diabetes Care 2009, 32, 1600–1602. [Google Scholar] [CrossRef]
- Carver, J.A.; Holt, C. Functional and dysfunctional folding, association and aggregation of caseins. Adv. Protein Chem. Struct. Biol. 2019, 118, 163–216. [Google Scholar] [CrossRef] [PubMed]
- Permyakov, E.A.; Permyakov, S.E.; Breydo, L.; Redwan, E.M.; Almehdar, H.A.; Uversky, V.N. Disorder in Milk Proteins: Lactalbumin. Part A. Structural Properties and Conformational Behavior. Curr. Protein Pept. Sci. 2016, 17, 352–367. [Google Scholar] [CrossRef] [PubMed]
- Bourlieu, C.; Michalski, M.-C. Structure–function relationship of the milk fat globule. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Kuczyńska, B.; Puppel, K.; Gołȩbiewski, M.; Metera, E.; Sakowski, T.; Słoniewski, K.; Gołębiewski, M. Differences in whey protein content between cow’s milk collected in late pasture and early indoor feeding season from conventional and organic farms in Poland. J. Sci. Food Agric. 2012, 92, 2899–2904. [Google Scholar] [CrossRef] [PubMed]
- Böttger, I.; Dobbs, R.; Faloona, G.R.; Unger, R.H. The Effects of Triglyceride Absorption upon Glucagon, Insulin, and Gut Glucagon-Like Immunoreactivity. J. Clin. Investig. 1973, 52, 2532–2541. [Google Scholar] [CrossRef]
- Imamura, F.; Micha, R.; Wu, J.H.; de Oliveira Otto, M.C.; Otite, F.O.; Abioye, A.I.; Mozaffarian, D. Effects of Saturated Fat, Polyunsaturated Fat, Monounsaturated Fat, and Carbohydrate on Glucose-Insulin Homeostasis: A Systematic Review and Meta-analysis of Randomised Controlled Feeding Trials. PLoS Med. 2016, 13, e1002087. [Google Scholar] [CrossRef] [PubMed]
- Baggio, L.L.; Drucker, D.J. Biology of incretins: GLP-1 and GIP. Gastroenterology 2007, 132, 2131–2157. [Google Scholar] [CrossRef]
- Krarup, T.; Holst, J.J.; Larsen, K.L. Responses and molecular heterogeneity of IR-GIP after intraduodenal glucose and fat. Am. J. Physiol. Metab. 1985, 249, E195–E200. [Google Scholar] [CrossRef]
- Beysen, C.; Karpe, F.; Fielding, B.A.; Clark, A.; Levy, J.C.; Frayn, K.N. Interaction between specific fatty acids, GLP-1 and insulin secretion in humans. Diabetologia 2002, 45, 1533–1541. [Google Scholar] [CrossRef]
- Rocca, A.S.; LaGreca, J.; Kalitsky, J.; Brubaker, P.L. Monounsaturated Fatty Acid Diets Improve Glycemic Tolerance through Increased Secretion of Glucagon-Like Peptide-1*. Endocrinology 2001, 142, 1148–1155. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).