Analysis of Serum Th2 Cytokines in Infants with Non-IgE Mediated Food Allergy Compared to Healthy Infants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.1.1. Infants
- Group 1: patients with mild to moderate non-IgE-mediated CMA. Patients within 4 months of life, fed with adapted formula milk, with gastrointestinal (abdominal discomfort, colic and fussing, food refusal, vomiting, diarrhea, constipation, hematochezia) and/or cutaneous symptoms (skin rash, itching, erythema, atopic dermatitis), and negative serum IgE to cow milk.
- Group 2: healthy infants of comparable age and not following any special diet, enrolled during outpatient routine health checks.
2.1.2. Serology
2.1.3. Fecal Calprotectin
2.2. Total RNA Extraction
2.3. Reverse Transcription
2.4. Transcription Levels of IL4, IL5, IL10, and IL13 by Real-Time PCR Assays
2.5. Statistical Analysis
3. Results
3.1. Subjects Characteristic
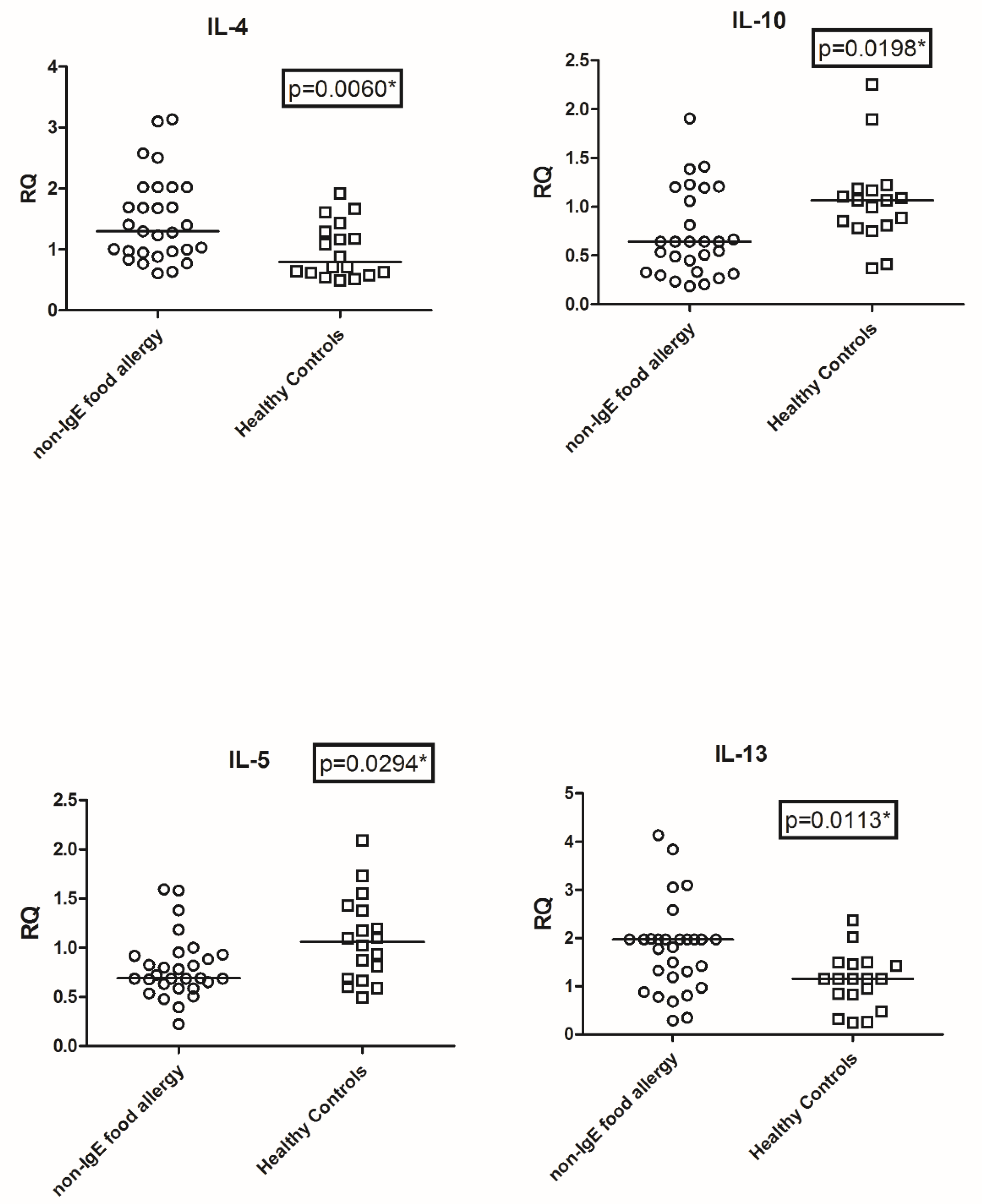
3.2. Transcriptional Level of IL4, IL5, IL10, and IL13
4. Discussion
Strengths and Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IL | Interleukin |
| Th | T helper cell |
| Me | Median |
| TaqMan RT-PCR | TaqMan real-time reverse-transcription |
| CMA | Cow milk allergy |
| RQ | Relative quantification |
| GAPDH | Glyceraldhyde tryphosphate dehydrogenase |
| eHF | estensive hydrolyzed formula |
References
- Cianferoni, A. Non-IgE Mediated Food Allergy. Curr. Pediatr. Rev. 2020, 16, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Fiocchi, A.; Bognanni, A.; Brożek, J.; Ebisawa, M.; Schünemann, H.; Ansotegui, I.J.; WAO DRACMA Guideline Group. World Allergy Organization (WAO) Diagnosis and Rationale for Action against Cow’s Milk Allergy (DRACMA) Guidelines update—I–Plan and definitions. World Allergy Organ. J. 2022, 15, 100609. [Google Scholar] [CrossRef] [PubMed]
- Vitaliti, G.; Cimino, C.; Coco, A.; Praticò, A.D.; Lionetti, E. The immunopathogenesis of cow’s milk protein allergy (CMPA). Ital. J. Pediatr. 2012, 38, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fulkerson, P.C.; Rothenberg, M.E. Targeting eosinophils in allergy, inflammation and beyond. Nat. Rev. Drug Discov. 2013, 12, 117–129. [Google Scholar] [CrossRef] [Green Version]
- Qurashi, T.A.; Bhat, G.A.; Khan, M.S.; Rasool, R.; Sameen, F.; Hassan, I.; Mudassar, S. Interleukin 4 and Interleukin 4 receptor alpha gene variants and risk of atopy—A case control study based assessment. Clin. Immunol. 2021, 229, 108783. [Google Scholar] [CrossRef]
- Venter, C.; Brown, T.; Meyer, R.; Walsh, J.; Shah, N.; Nowak-Węgrzyn, A.; Chen, T.-X.; Fleischer, D.M.; Heine, R.G.; Levin, M.; et al. Better recognition, diagnosis and management of non-IgE-mediated cow’s milk allergy in infancy: iMAP—an international interpretation of the MAP (Milk Allergy in Primary Care) guideline. Clin. Transl. Allergy 2017, 7, 26, Erratum in Clin. Transl. Allergy 2018, 8, 4. [Google Scholar] [CrossRef]
- Koletzko, S.; Niggemann, B.; Arato, A.; Dias, J.A.; Heuschkel, R.; Husby, S.; Mearin, M.L.; Papadopoulou, A.; Ruemmele, F.M.; Staiano, A.; et al. Diagnostic Approach and Management of Cow’s-Milk Protein Allergy in Infants and Children: Espghan gi committee practical guidelines. J. Pediatric Gastroenterol. Nutr. 2012, 55, 221–229. [Google Scholar] [CrossRef]
- Vandenplas, Y. Prevention and Management of Cow’s Milk Allergy in Non-Exclusively Breastfed Infants. Nutrients 2017, 9, 731. [Google Scholar] [CrossRef]
- Meyer, R.; Groetch, M.; Venter, C. When Should Infants with Cow’s Milk Protein Allergy Use an Amino Acid Formula? A Practical Guide. J. Allergy Clin. Immunol. Pr. 2018, 6, 383–399. [Google Scholar] [CrossRef]
- Beşer, Ö.F.; Sancak, S.; Erkan, T.; Kutlu, T.; Cokugras, H.C.; Çokuğraş, F.Ç. Can Fecal Calprotectin Level Be Used as a Markers of Inflammation in the Diagnosis and Follow-Up of Cow’s Milk Protein Allergy? Allergy Asthma Immunol. Res. 2014, 6, 33–38. [Google Scholar] [CrossRef] [Green Version]
- Orivuori, L.; Mustonen, K.; de Goffau, M.C.; Hakala, S.; Paasela, M.; Roduit, C.; Dalphin, J.C.; Genuneit, J.; Lauener, R.; Riedler, J.; et al. High level of fecal calprotectin at age 2 months as a marker of intestinal inflammation predicts atopic dermatitis and asthma by age 6. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2015, 45, 928–939. [Google Scholar] [CrossRef] [PubMed]
- Tovo, P.-A.; Rabbone, I.; Tinti, D.; Galliano, I.; Trada, M.; Daprà, V.; Cerutti, F.; Bergallo, M. Enhanced expression of human endogenous retroviruses in new-onset type 1 diabetes: Potential pathogenetic and therapeutic implications. Autoimmunity 2020, 53, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, T.; Itabashi, K.; Imai, T. Retrospective Multicenter Survey on Food-Related Symptoms Suggestive of Cow’s Milk Allergy in NICU Neonates. Allergol. Int. 2013, 62, 85–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sicherer, S.H.; Sampson, H.A. Food allergy: Epidemiology, pathogenesis, diagnosis, and treatment. J. Allergy Clin. Immunol. 2014, 133, 291–307.e5. [Google Scholar] [CrossRef] [PubMed]
- Georgountzou, A.; Kokkinou, D.; Taka, S.; Maggina, P.; Lakoumentas, J.; Papaevangelou, V.; Tsolia, M.; Xepapadaki, P.; Andreakos, E.; Papadopoulos, N.G. Differential maturation trajectories of innate antiviral immunity in health and atopy. Pediatr. Allergy Immunol. 2021, 32, 1843–1856. [Google Scholar] [CrossRef] [PubMed]
- Prokešová, L.; Zizka, J.; Kocourková, I.; Novotná, O.; Petrásková, P.; Šterzl, I.; Lodinová-Žádníková, R. Cytokine levels in healthy and allergic mothers and their children during the first year of life. Pediatr. Allergy Immunol. 2006, 17, 175–183. [Google Scholar] [CrossRef]
- Lappalainen, M.; Roponen, M.; Pekkanen, J.; Huttunen, K.; Hirvonen, M.-R. Maturation of cytokine-producing capacity from birth to 1 yr of age. Pediatr. Allergy Immunol. 2009, 20, 714–725. [Google Scholar] [CrossRef]
- Schultz, C.; Strunk, T.; Temming, P.; Matzke, N.; Härtel, C. Reduced IL-10 production and -receptor expression in neonatal T lymphocytes. Acta Paediatr. 2007, 96, 1122–1125. [Google Scholar] [CrossRef]
- Harris, V.R.; Cooper, A.J. Atopic dermatitis: The new frontier. Med J. Aust. 2017, 207, 351–356. [Google Scholar] [CrossRef]
- Wang, R.; Deng, Y.; Zhang, Y.; Li, X.; Sun, L.; Deng, Q.; Liu, Y.; Gooneratne, R.; Li, J. Modulation of Intestinal Barrier, Inflammatory Response, and Gut Microbiota by Pediococcus pentosaceus zy-B Alleviates Vibrio parahaemolyticus Infection in C57BL/6J Mice. J. Agric. Food Chem. 2022, 70, 1865–1877. [Google Scholar] [CrossRef]
- Koninckx, C.R.; Donat, E.; Benninga, M.A.; Broekaert, I.J.; Gottrand, F.; Kolho, K.-L.; Lionetti, P.; Miele, E.; Orel, R.; Papadopoulou, A.; et al. The Use of Fecal Calprotectin Testing in Paediatric Disorders: A Position Paper of the European Society for Paediatric Gastroenterology and Nutrition Gastroenterology Committee. J. Pediatr. Gastroenterol. Nutr. 2021, 72, 617–640. [Google Scholar] [CrossRef] [PubMed]
- D’Auria, E.; Salvatore, S.; Acunzo, M.; Peroni, D.; Pendezza, E.; Di Profio, E.; Fiore, G.; Zuccotti, G.V.; Verduci, E. Hydrolysed Formulas in the Management of Cow’s Milk Allergy: New Insights, Pitfalls and Tips. Nutrients 2021, 13, 2762. [Google Scholar] [CrossRef] [PubMed]
| Target | Sequence 5′→3′ |
|---|---|
| GAPDHF | -CCAAGGTCATCCATGACAAC- |
| GAPDHR | -GTGGCAGTGATGGCATGGAC- |
| GAPDH-6FAM | -6FAM-TGGAGAAGGCTGGGGCTCAT-TAMRA |
| IL4F | -ACTTTGAACAGCCTCACAGAG- |
| IL4R | -TTGGAGGCAGCAAAGATGTC- |
| IL4P-6FAM | 6FAM-CTGTGCACCGAGTTGACCGTA-TAMRA |
| IL5F | -GCTCTTGGAGCTGCCTACGT- |
| IL5R | -CAAGGTCTCTTTCACCAATGCA- |
| IL5P-6FAM | -6FAM-ATGCCATCCCCACAGAAATTCCCAC-TAMRA |
| IL10F | -ATGAAGGATCAGCTGGACAACTT- |
| IL10R | -CCTTGATGTCTGGGTCTTGGT- |
| IL10P-6FAM | -6FAM-ACCTGGGTTGCCAAGCCTTGTCTG-TAMRA |
| IL13F | -CTCATTGAGGAGCTGGTCAACA- |
| IL13R | -TCCATACCATGCTGCCATTG- |
| IL13P | 6FAM-CACCCAGAACCAGAAGGCTCCGC-TAMRA |
| Non-IgE Food Allergy (n = 34) | Healthy Controls (n = 19) | p-Value | |
|---|---|---|---|
| Age at enrollment | |||
| Weeks (median, range) | 10 (4–6) | 8 (2–16) | 0.248 * |
| Gender | |||
| Female, n (%) | 16 (47.0) | 10 (52.6) | 1 # |
| Male, n (%) | 18 (53.0) | 9 (47.4) | 1 # |
| Birth weight | |||
| Grams (median, range) | 3180 (2960–3390) | 3070 (2895–3235) | 0.307 * |
| Gestational age | |||
| Weeks (median, range) | 36 (35–37) | 37 (35–39) | 0.250 * |
| Nationality | |||
| Italian, n (%) | 25 (73.5) | 14 (73.7) | 0.69 # |
| Foreign, n (%) | 9 (26.5) | 5 (26.3) | 0.693 * |
| Dietary treatment | |||
| Amino acid formulas, n (%) | 5 (14.7) | - | - |
| Hydrolyzed casein formulas, n (%) | 29 (85.3) | - | - |
| Breastmilk, n (%) | 12 (63) | ||
| Standard formula, n (%) | 7 (37) |
| Non-Ig E Food Allergy (n = 34) | Healthy Controls (n = 19) | p Value | |
|---|---|---|---|
| Fecal calprotectin | |||
| µg/g (median, range) | 2176 (366–4210) | 368 (120–840) | 0.015 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savino, F.; Giuliani, F.; Giraudi, S.; Galliano, I.; Montanari, P.; Daprà, V.; Bergallo, M. Analysis of Serum Th2 Cytokines in Infants with Non-IgE Mediated Food Allergy Compared to Healthy Infants. Nutrients 2022, 14, 1565. https://doi.org/10.3390/nu14081565
Savino F, Giuliani F, Giraudi S, Galliano I, Montanari P, Daprà V, Bergallo M. Analysis of Serum Th2 Cytokines in Infants with Non-IgE Mediated Food Allergy Compared to Healthy Infants. Nutrients. 2022; 14(8):1565. https://doi.org/10.3390/nu14081565
Chicago/Turabian StyleSavino, Francesco, Francesca Giuliani, Stefano Giraudi, Ilaria Galliano, Paola Montanari, Valentina Daprà, and Massimiliano Bergallo. 2022. "Analysis of Serum Th2 Cytokines in Infants with Non-IgE Mediated Food Allergy Compared to Healthy Infants" Nutrients 14, no. 8: 1565. https://doi.org/10.3390/nu14081565
APA StyleSavino, F., Giuliani, F., Giraudi, S., Galliano, I., Montanari, P., Daprà, V., & Bergallo, M. (2022). Analysis of Serum Th2 Cytokines in Infants with Non-IgE Mediated Food Allergy Compared to Healthy Infants. Nutrients, 14(8), 1565. https://doi.org/10.3390/nu14081565







