Retinoic Acid: Sexually Dimorphic, Anti-Insulin and Concentration-Dependent Effects on Energy
Abstract
1. Introduction
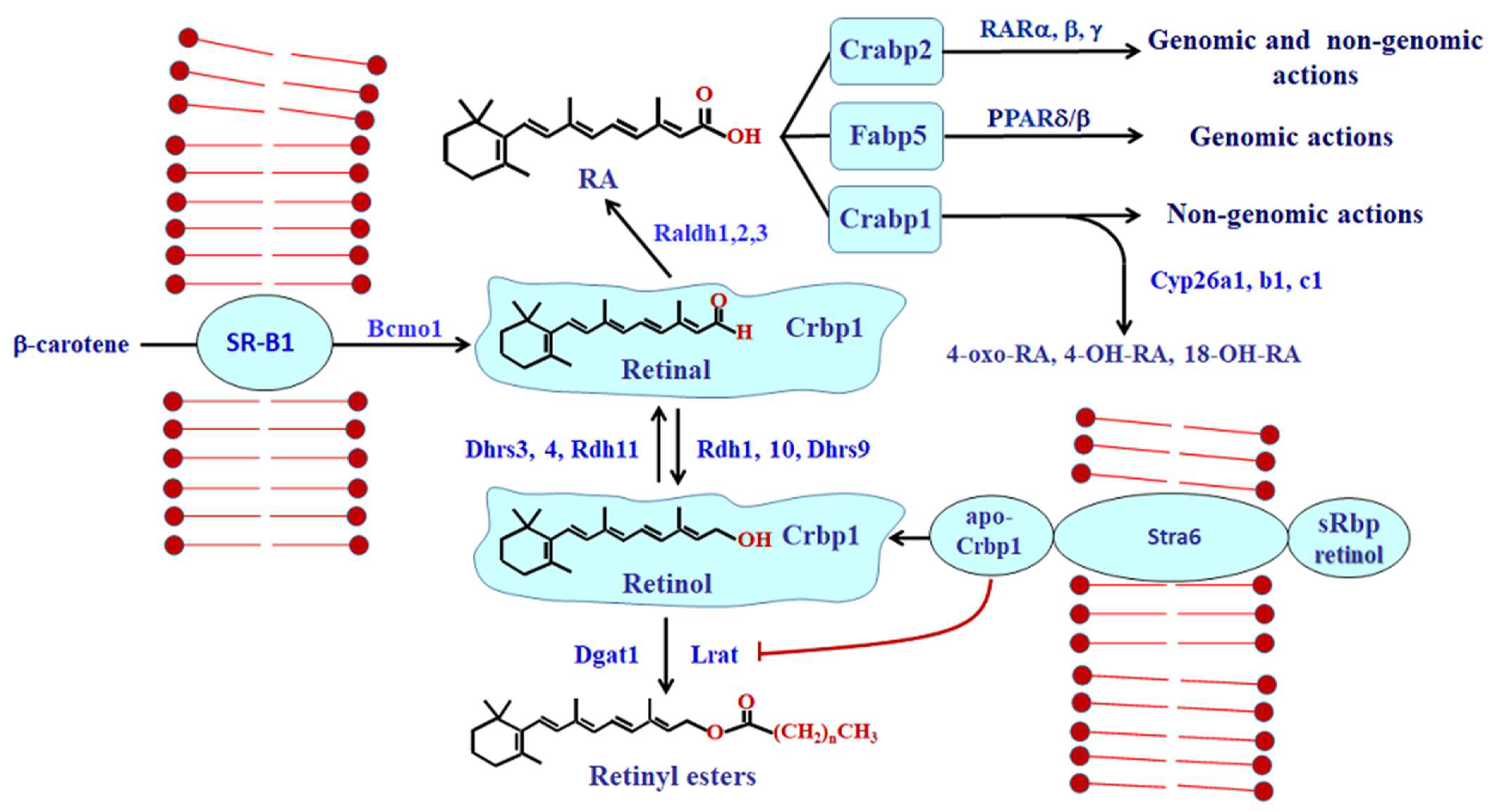
2. The Retinoid Metabolon
2.1. RA Homeostasis
2.2. Complexity of Retinoid-Related Enzyme Actions
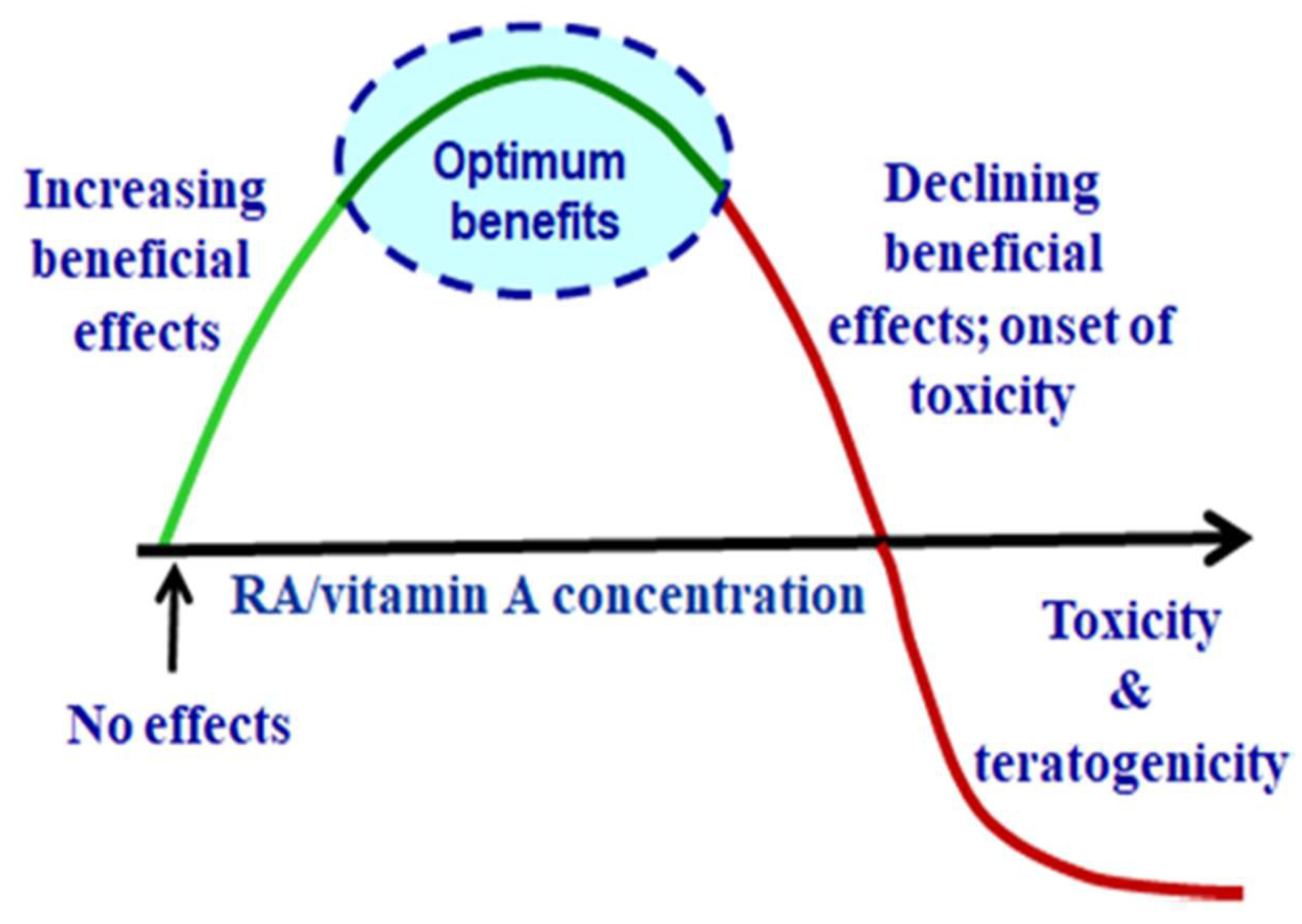
3. Vitamin A/Retinoic Acid Hormesis
3.1. RA Hormesis
3.2. Contrary RA Effects
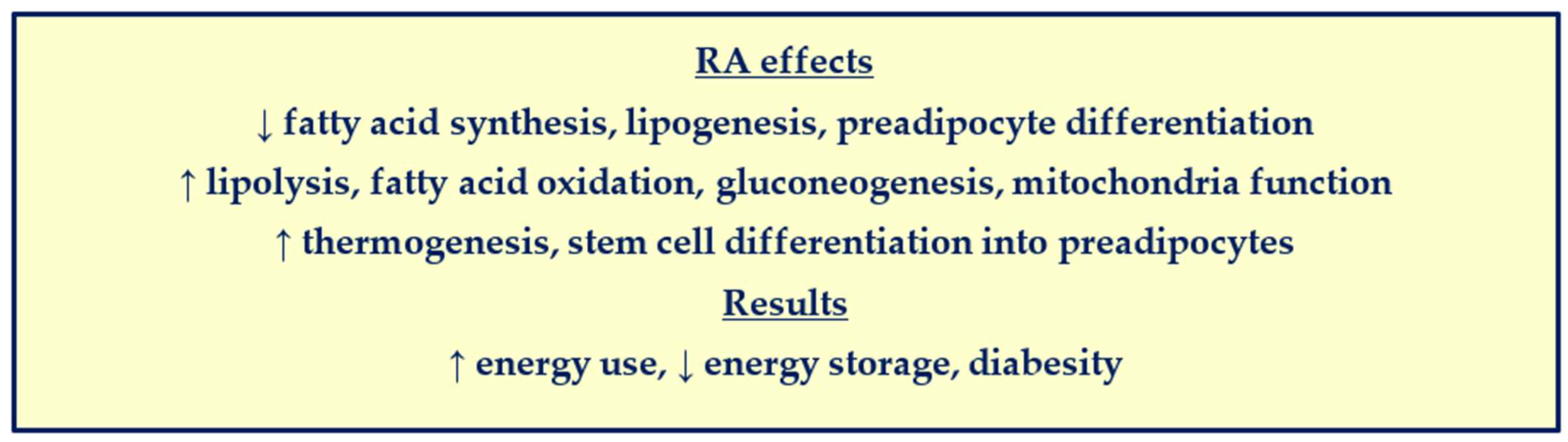
4. RA Control of Preadipocyte Differentiation and Obesity
5. Retinoids Direct Pancreatic Islet Development in Mammals
5.1. Emergence of Pancreatic Buds
5.2. Vitamin A Induces Pancreas Development
5.3. RA Induces Pancreagenesis
5.4. Requirement of Crbp1 for Normal Pancreagenesis
5.5. Retinoid Receptors and Pancreagenesis
5.6. RA and Human Pancreas Development
5.7. Summary RA and Pancreagenesis
6. Retinoids Maintain Pancreatic Islet Function in Post-Natal Mammals
6.1. Vitamin A Protects against Type 1 Diabetes
6.2. RA Inhibits Glucagon Secretion
6.3. The Advantage of Avoiding Diets Copious in Vitamin A Content
6.4. Pancreas Function in the Lrat-Null Mouse
6.5. 9-cis-Retinoic Acid Reduces Glucose-Stimulated Insulin Secretion
6.6. Summary of RA Effects on Post-Natal Pancreas Function
7. The Serum Retinol-Binding Protein (Rbp4) and Insulin Resistance
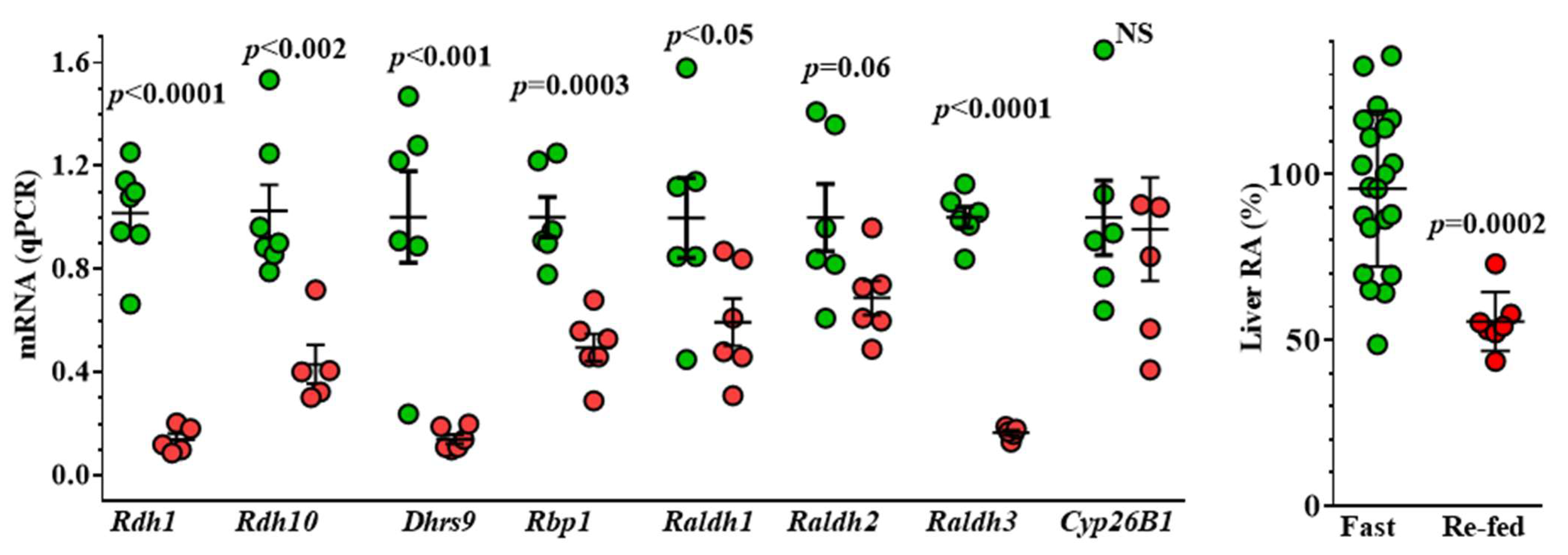
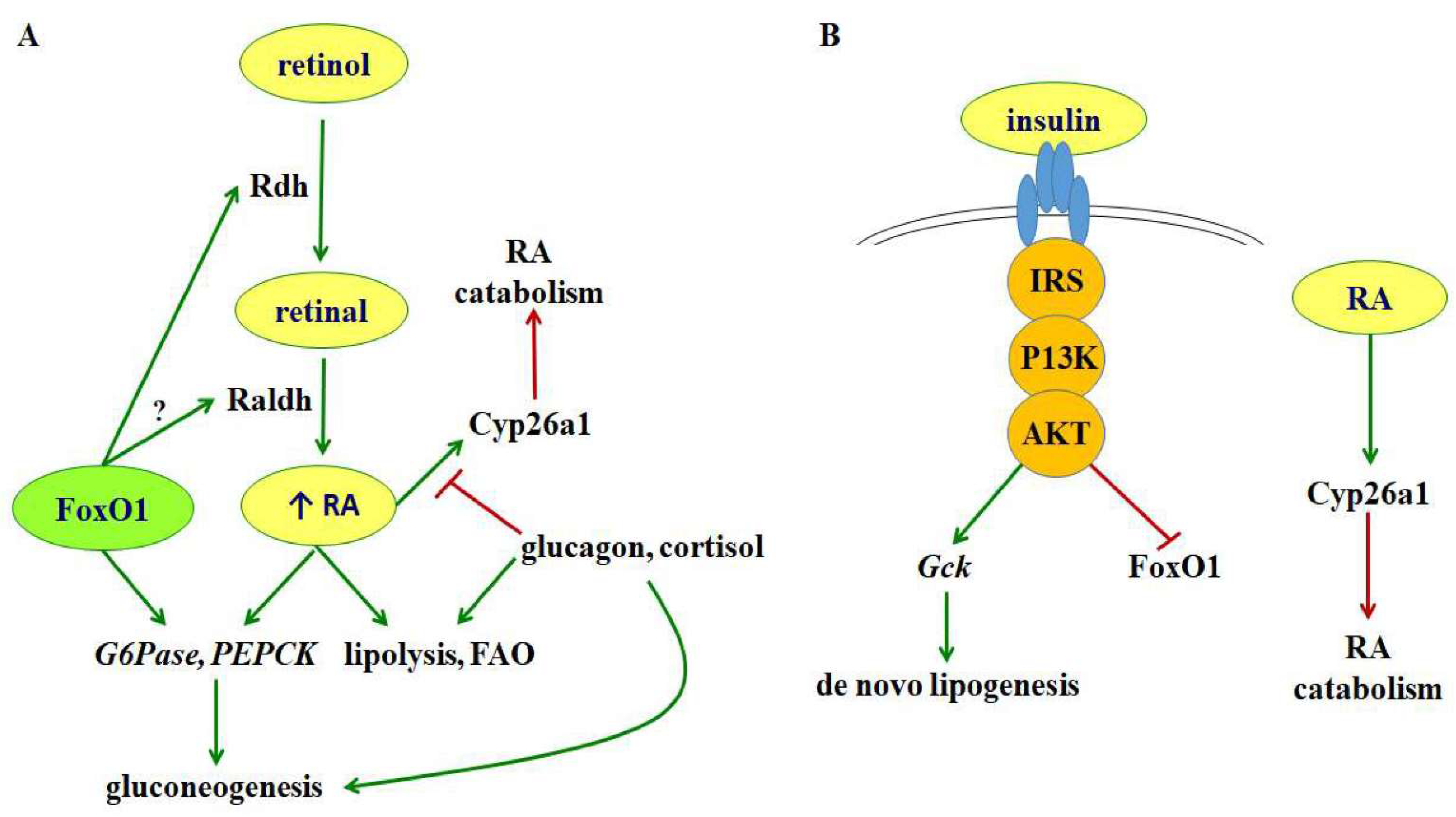
8. Insulin Suppresses Liver RA Concentrations
9. Glucagon and Cortisol Diminish RA Induction of Liver Cyp26a1
10. RA Exerts Tissue-Specific, Sexually Dimorphic Actions over Energy Use
11. Sex Hormones Effect RA Concentrations and Action
12. Conclusions
- Experimental protocols should avoid chow diets with their higher (copious) amounts of vitamin A, which rescued several knockouts of the retinoid metabolon;
- RA dosing should heed hormesis effects;
- RA quantification should rely on fasted vs. re-fed states, and avoid ad lib fed animals;
- The assessment of RA-regulated gene expression, should consider fasting vs. re-feeding;
- Knockouts of retinoid metabolon genes to provide reproducible manipulation of tissue RA concentrations should be considered;
- Phenotypic evaluation should include both males and females;
- Experiments with model animals should require sufficient numbers to achieve reliable results.
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dollé, P. Developmental Expression of Retinoic Acid Receptors (RARs). Nucl. Recept Signal. 2009, 7, e006. [Google Scholar] [CrossRef] [PubMed]
- Pino-Lagos, K.; Guo, Y.; Noelle, R.J. Retinoic Acid: A Key Player in Immunity. Biofactors 2010, 36, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Cione, E.; Caroleo, M.C.; Cannataro, R.; Perri, M.; Pingitore, A.; Genchi, G. Vitamin A and Diabesity: New Insight for Drug Discovery. Mini Rev. Med. Chem. 2016, 16, 738–742. [Google Scholar] [CrossRef] [PubMed]
- Pawlikowski, B.; Wragge, J.; Siegenthaler, J.A. Retinoic Acid Signaling in Vascular Development. Genesis 2019, 57, e23287. [Google Scholar] [CrossRef]
- Gewiss, R.; Topping, T.; Griswold, M.D. Cycles, Waves, and Pulses: Retinoic Acid and the Organization of Spermatogenesis. Andrology 2020, 8, 892–897. [Google Scholar] [CrossRef]
- Sirbu, I.O.; Chiş, A.R.; Moise, A.R. Role of Carotenoids and Retinoids during Heart Development. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2020, 1865, 158636. [Google Scholar] [CrossRef]
- Obrochta, K.M.; Krois, C.R.; Campos, B.; Napoli, J.L. Insulin Regulates Retinol Dehydrogenase Expression and All-Trans-Retinoic Acid Biosynthesis through FoxO1. J. Biol. Chem. 2015, 290, 7259–7268. [Google Scholar] [CrossRef]
- Preston, J.D.; Reynolds, L.J.; Pearson, K.J. Developmental Origins of Health Span and Life Span: A Mini-Review. Gerontology 2018, 64, 237–245. [Google Scholar] [CrossRef]
- Napoli, J.L. Physiological Insights into All-Trans-Retinoic Acid Biosynthesis. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2012, 1821, 152–167. [Google Scholar] [CrossRef]
- Kedishvili, N.Y. Retinoic Acid Synthesis and Degradation. Subcell. Biochem. 2016, 81, 127–161. [Google Scholar] [CrossRef]
- Napoli, J.L. Cellular Retinoid Binding-Proteins, CRBP, CRABP, FABP5: Effects on Retinoid Metabolism, Function and Related Diseases. Pharmacol. Ther. 2017, 173, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yu, J.; Kane, M.A.; Moise, A.R. Modulation of Retinoid Signaling: Therapeutic Opportunities in Organ Fibrosis and Repair. Pharmacol. Ther. 2020, 205, 107415. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Kane, M.A.; Napoli, J.L. Multiple Retinol and Retinal Dehydrogenases Catalyze All-Trans-Retinoic Acid Biosynthesis in Astrocytes. J. Biol. Chem. 2011, 286, 6542–6553. [Google Scholar] [CrossRef]
- Lei, Z.; Chen, W.; Zhang, M.; Napoli, J.L. Reduction of All-Trans-Retinal in the Mouse Liver Peroxisome Fraction by the Short-Chain Dehydrogenase/Reductase RRD: Induction by the PPAR Alpha Ligand Clofibrate. Biochemistry 2003, 42, 4190–4196. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.; Ruuska, S.E.; Levinthal, D.J.; Noy, N. Distinct Roles for Cellular Retinoic Acid-Binding Proteins I and II in Regulating Signaling by Retinoic Acid. J. Biol. Chem. 1999, 274, 23695–23698. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.-N. Cellular Retinoic Acid Binding Proteins: Genomic and Non-Genomic Functions and Their Regulation. Subcell. Biochem. 2016, 81, 163–178. [Google Scholar] [CrossRef]
- Fiorella, P.D.; Napoli, J.L. Expression of Cellular Retinoic Acid Binding Protein (CRABP) in Escherichia coli. Characterization and Evidence That Holo-CRABP Is a Substrate in Retinoic Acid Metabolism. J. Biol. Chem. 1991, 266, 16572–16579. [Google Scholar] [CrossRef]
- Fiorella, P.D.; Napoli, J.L. Microsomal Retinoic Acid Metabolism. Effects of Cellular Retinoic Acid-Binding Protein (Type I) and C18-Hydroxylation as an Initial Step. J. Biol. Chem. 1994, 269, 10538–10544. [Google Scholar] [CrossRef]
- Nelson, C.H.; Peng, C.-C.; Lutz, J.D.; Yeung, C.K.; Zelter, A.; Isoherranen, N. Direct Protein-Protein Interactions and Substrate Channeling between Cellular Retinoic Acid Binding Proteins and CYP26B1. FEBS Lett. 2016, 590, 2527–2535. [Google Scholar] [CrossRef]
- Zhong, G.; Ortiz, D.; Zelter, A.; Nath, A.; Isoherranen, N. CYP26C1 Is a Hydroxylase of Multiple Active Retinoids and Interacts with Cellular Retinoic Acid Binding Proteins. Mol. Pharmacol. 2018, 93, 489–503. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, P.; Krois, C.R.; Kane, M.A.; Napoli, J.L. Altered Vitamin A Homeostasis and Increased Size and Adiposity in the Rdh1-Null Mouse. FASEB J. 2007, 21, 2886–2896. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Vuckovic, M.; Yoo, H.S.; Fox, N.; Rodriguez, A.; McKessy, K.; Napoli, J.L. Retinoic Acid Exerts Sexually Dimorphic Effects on Muscle Energy Metabolism and Function. J. Biol. Chem. 2021, 297, 101101. [Google Scholar] [CrossRef] [PubMed]
- Krois, C.R.; Vuckovic, M.G.; Huang, P.; Zaversnik, C.; Liu, C.S.; Gibson, C.E.; Wheeler, M.R.; Obrochta, K.M.; Min, J.H.; Herber, C.B.; et al. RDH1 Suppresses Adiposity by Promoting Brown Adipose Adaptation to Fasting and Re-Feeding. Cell. Mol. Life Sci. 2019, 76, 2425–2447. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Vuckovic, M.G.; Smullin, C.P.; Kim, M.; Lo, C.P.-S.; Devericks, E.; Yoo, H.S.; Tintcheva, M.; Deng, Y.; Napoli, J.L. Modest Decreases in Endogenous All-Trans-Retinoic Acid Produced by a Mouse Rdh10 Heterozygote Provoke Major Abnormalities in Adipogenesis and Lipid Metabolism. Diabetes 2018, 67, 662–673. [Google Scholar] [CrossRef] [PubMed]
- Niederreither, K.; Vermot, J.; Fraulob, V.; Chambon, P.; Dolle, P. Retinaldehyde Dehydrogenase 2 (RALDH2)-Independent Patterns of Retinoic Acid Synthesis in the Mouse Embryo. Proc. Natl. Acad. Sci. USA 2002, 99, 16111–16116. [Google Scholar] [CrossRef] [PubMed]
- Dupé, V.; Matt, N.; Garnier, J.-M.; Chambon, P.; Mark, M.; Ghyselinck, N.B. A Newborn Lethal Defect Due to Inactivation of Retinaldehyde Dehydrogenase Type 3 Is Prevented by Maternal Retinoic Acid Treatment. Proc. Natl. Acad. Sci. USA 2003, 100, 14036–14041. [Google Scholar] [CrossRef]
- Ross, A.C.; Zolfaghari, R. Cytochrome P450s in the Regulation of Cellular Retinoic Acid Metabolism. Annu. Rev. Nutr. 2011, 31, 65–87. [Google Scholar] [CrossRef]
- Mcilroy, G.D.; Delibegovic, M.; Owen, C.; Stoney, P.N.; Shearer, K.D.; McCaffery, P.J.; Mody, N. Fenretinide Treatment Prevents Diet-Induced Obesity in Association with Major Alterations in Retinoid Homeostatic Gene Expression in Adipose, Liver, and Hypothalamus. Diabetes 2013, 62, 825–836. [Google Scholar] [CrossRef]
- Isoherranen, N.; Zhong, G. Biochemical and Physiological Importance of the CYP26 Retinoic Acid Hydroxylases. Pharmacol. Ther. 2019, 204, 107400. [Google Scholar] [CrossRef]
- Posch, K.C.; Burns, R.D.; Napoli, J.L. Biosynthesis of All-Trans-Retinoic Acid from Retinal. Recognition of Retinal Bound to Cellular Retinol Binding Protein (Type I) as Substrate by a Purified Cytosolic Dehydrogenase. J. Biol. Chem. 1992, 267, 19676–19682. [Google Scholar] [CrossRef]
- Yang, D.; Krois, C.R.; Huang, P.; Wang, J.; Min, J.; Yoo, H.S.; Deng, Y.; Napoli, J.L. Raldh1 Promotes Adiposity during Adolescence Independently of Retinal Signaling. PLoS ONE 2017, 12, e0187669. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-I.; Ganesan, S.; Luo, S.X.; Wu, Y.-W.; Park, E.; Huang, E.J.; Chen, L.; Ding, J.B. Aldehyde Dehydrogenase 1a1 Mediates a GABA Synthesis Pathway in Midbrain Dopaminergic Neurons. Science 2015, 350, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Calabrese, E.J.; Lin, Z.; Lian, B.; Zhang, X. Similarities between the Yin/Yang Doctrine and Hormesis in Toxicology and Pharmacology. Trends Pharmacol. Sci. 2020, 41, 544–556. [Google Scholar] [CrossRef] [PubMed]
- Nau, H. Teratogenicity of Isotretinoin Revisited: Species Variation and the Role of All-Trans-Retinoic Acid. J. Am. Acad. Dermatol. 2001, 45, S183–S187. [Google Scholar] [CrossRef]
- Eckhoff, C.; Bailey, J.R.; Collins, M.D.; Slikker, W.; Nau, H. Influence of Dose and Pharmaceutical Formulation of Vitamin A on Plasma Levels of Retinyl Esters and Retinol and Metabolic Generation of Retinoic Acid Compounds and Beta-Glucuronides in the Cynomolgus Monkey. Toxicol. Appl. Pharmacol. 1991, 111, 116–127. [Google Scholar] [CrossRef]
- Tzimas, G.; Thiel, R.; Chahoud, I.; Nau, H. The Area under the Concentration-Time Curve of All-Trans-Retinoic Acid Is the Most Suitable Pharmacokinetic Correlate to the Embryotoxicity of This Retinoid in the Rat. Toxicol. Appl. Pharmacol. 1997, 143, 436–444. [Google Scholar] [CrossRef]
- Biesalski, H.K. Comparative Assessment of the Toxicology of Vitamin A and Retinoids in Man. Toxicology 1989, 57, 117–161. [Google Scholar] [CrossRef]
- Lind, T.; Lind, P.M.; Hu, L.; Melhus, H. Studies of Indirect and Direct Effects of Hypervitaminosis A on Rat Bone by Comparing Free Access to Food and Pair-Feeding. Upsala J. Med. Sci 2018, 123, 82–85. [Google Scholar] [CrossRef]
- Amengual, J.; Ribot, J.; Bonet, M.L.; Palou, A. Retinoic Acid Treatment Enhances Lipid Oxidation and Inhibits Lipid Biosynthesis Capacities in the Liver of Mice. Cell. Physiol. Biochem. 2010, 25, 657–666. [Google Scholar] [CrossRef]
- Sato, M.; Hiragun, A.; Mitsui, H. Preadipocytes Possess Cellular Retinoid Binding Proteins and Their Differentiation Is Inhibited by Retinoids. Biochem. Biophys. Res. Commun. 1980, 95, 1839–1845. [Google Scholar] [CrossRef]
- Wu, A.-M.; Huang, C.-Q.; Lin, Z.-K.; Tian, N.-F.; Ni, W.-F.; Wang, X.-Y.; Xu, H.-Z.; Chi, Y.-L. The Relationship between Vitamin A and Risk of Fracture: Meta-Analysis of Prospective Studies. J. Bone Miner. Res. 2014, 29, 2032–2039. [Google Scholar] [CrossRef] [PubMed]
- Chertow, B.S.; Baker, G.R. The Effects of Vitamin A on Insulin Release and Glucose Oxidation in Isolated Rat Islets. Endocrinology 1978, 103, 1562–1572. [Google Scholar] [CrossRef]
- Noy, N. The One-Two Punch: Retinoic Acid Suppresses Obesity Both by Promoting Energy Expenditure and by Inhibiting Adipogenesis. Adipocyte 2013, 2, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.C.; Noy, N. All-Trans-Retinoic Acid Represses Obesity and Insulin Resistance by Activating Both Peroxisome Proliferation-Activated Receptor Beta/Delta and Retinoic Acid Receptor. Mol. Cell. Biol. 2009, 29, 3286–3296. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.C.; DeSantis, D.; Soltanian, H.; Croniger, C.M.; Noy, N. Retinoic Acid Upregulates Preadipocyte Genes to Block Adipogenesis and Suppress Diet-Induced Obesity. Diabetes 2012, 61, 1112–1121. [Google Scholar] [CrossRef]
- Hudak, C.S.; Gulyaeva, O.; Wang, Y.; Park, S.-M.; Lee, L.; Kang, C.; Sul, H.S. Pref-1 Marks Very Early Mesenchymal Precursors Required for Adipose Tissue Development and Expansion. Cell Rep. 2014, 8, 678–687. [Google Scholar] [CrossRef]
- Wang, B.; Yang, Q.; Harris, C.L.; Nelson, M.L.; Busboom, J.R.; Zhu, M.-J.; Du, M. Nutrigenomic Regulation of Adipose Tissue Development—Role of Retinoic Acid: A Review. Meat Sci. 2016, 120, 100–106. [Google Scholar] [CrossRef]
- Wang, B.; Fu, X.; Liang, X.; Deavila, J.M.; Wang, Z.; Zhao, L.; Tian, Q.; Zhao, J.; Gomez, N.A.; Trombetta, S.C.; et al. Retinoic Acid Induces White Adipose Tissue Browning by Increasing Adipose Vascularity and Inducing Beige Adipogenesis of PDGFRα+ Adipose Progenitors. Cell Discov. 2017, 3, 17036. [Google Scholar] [CrossRef]
- Ziouzenkova, O.; Orasanu, G.; Sharlach, M.; Akiyama, T.E.; Berger, J.P.; Viereck, J.; Hamilton, J.A.; Tang, G.; Dolnikowski, G.G.; Vogel, S.; et al. Retinaldehyde Represses Adipogenesis and Diet-Induced Obesity. Nat. Med. 2007, 13, 695–702. [Google Scholar] [CrossRef]
- Reichert, B.; Yasmeen, R.; Jeyakumar, S.M.; Yang, F.; Thomou, T.; Alder, H.; Duester, G.; Maiseyeu, A.; Mihai, G.; Harrison, E.H.; et al. Concerted Action of Aldehyde Dehydrogenases Influences Depot-Specific Fat Formation. Mol. Endocrinol. 2011, 25, 799–809. [Google Scholar] [CrossRef]
- Muenzner, M.; Tuvia, N.; Deutschmann, C.; Witte, N.; Tolkachov, A.; Valai, A.; Henze, A.; Sander, L.E.; Raila, J.; Schupp, M. Retinol-Binding Protein 4 and Its Membrane Receptor STRA6 Control Adipogenesis by Regulating Cellular Retinoid Homeostasis and Retinoic Acid Receptor α Activity. Mol. Cell. Biol. 2013, 33, 4068–4082. [Google Scholar] [CrossRef] [PubMed]
- Pictet, R.L.; Clark, W.R.; Williams, R.H.; Rutter, W.J. An Ultrastructural Analysis of the Developing Embryonic Pancreas. Dev. Biol. 1972, 29, 436–467. [Google Scholar] [CrossRef]
- Jørgensen, M.C.; Ahnfelt-Rønne, J.; Hald, J.; Madsen, O.D.; Serup, P.; Hecksher-Sørensen, J. An Illustrated Review of Early Pancreas Development in the Mouse. Endocr. Rev. 2007, 28, 685–705. [Google Scholar] [CrossRef]
- Herrera, P.L.; Huarte, J.; Sanvito, F.; Meda, P.; Orci, L.; Vassalli, J.D. Embryogenesis of the Murine Endocrine Pancreas; Early Expression of Pancreatic Polypeptide Gene. Development 1991, 113, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Chertow, B.S.; Blaner, W.S.; Baranetsky, N.G.; Sivitz, W.I.; Cordle, M.B.; Thompson, D.; Meda, P. Effects of Vitamin A Deficiency and Repletion on Rat Insulin Secretion in Vivo and in Vitro from Isolated Islets. J. Clin. Investig. 1987, 79, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Matthews, K.A.; Rhoten, W.B.; Driscoll, H.K.; Chertow, B.S. Vitamin A Deficiency Impairs Fetal Islet Development and Causes Subsequent Glucose Intolerance in Adult Rats. J. Nutr. 2004, 134, 1958–1963. [Google Scholar] [CrossRef] [PubMed]
- Arregi, I.; Climent, M.; Iliev, D.; Strasser, J.; Gouignard, N.; Johansson, J.K.; Singh, T.; Mazur, M.; Semb, H.; Artner, I.; et al. Retinol Dehydrogenase-10 Regulates Pancreas Organogenesis and Endocrine Cell Differentiation via Paracrine Retinoic Acid Signaling. Endocrinology 2016, 157, 4615–4631. [Google Scholar] [CrossRef]
- Martín, M.; Gallego-Llamas, J.; Ribes, V.; Kedinger, M.; Niederreither, K.; Chambon, P.; Dollé, P.; Gradwohl, G. Dorsal Pancreas Agenesis in Retinoic Acid-Deficient Raldh2 Mutant Mice. Dev. Biol. 2005, 284, 399–411. [Google Scholar] [CrossRef]
- Oström, M.; Loffler, K.A.; Edfalk, S.; Selander, L.; Dahl, U.; Ricordi, C.; Jeon, J.; Correa-Medina, M.; Diez, J.; Edlund, H. Retinoic Acid Promotes the Generation of Pancreatic Endocrine Progenitor Cells and Their Further Differentiation into Beta-Cells. PLoS ONE 2008, 3, e2841. [Google Scholar] [CrossRef]
- Fan, X.; Molotkov, A.; Manabe, S.-I.; Donmoyer, C.M.; Deltour, L.; Foglio, M.H.; Cuenca, A.E.; Blaner, W.S.; Lipton, S.A.; Duester, G. Targeted Disruption of Aldh1a1 (Raldh1) Provides Evidence for a Complex Mechanism of Retinoic Acid Synthesis in the Developing Retina. Mol. Cell. Biol. 2003, 23, 4637–4648. [Google Scholar] [CrossRef]
- Kane, M.A.; Folias, A.E.; Pingitore, A.; Perri, M.; Krois, C.R.; Ryu, J.Y.; Cione, E.; Napoli, J.L. CrbpI Modulates Glucose Homeostasis and Pancreas 9-Cis-Retinoic Acid Concentrations. Mol. Cell. Biol. 2011, 31, 3277–3285. [Google Scholar] [CrossRef] [PubMed]
- Trasino, S.E.; Benoit, Y.D.; Gudas, L.J. Vitamin A Deficiency Causes Hyperglycemia and Loss of Pancreatic β-Cell Mass. J. Biol. Chem. 2015, 290, 1456–1473. [Google Scholar] [CrossRef] [PubMed]
- Lorberbaum, D.S.; Kishore, S.; Rosselot, C.; Sarbaugh, D.; Brooks, E.P.; Aragon, E.; Xuan, S.; Simon, O.; Ghosh, D.; Mendelsohn, C.; et al. Retinoic Acid Signaling within Pancreatic Endocrine Progenitors Regulates Mouse and Human β Cell Specification. Development 2020, 147, dev189977. [Google Scholar] [CrossRef] [PubMed]
- Tiyaboonchai, A.; Cardenas-Diaz, F.L.; Ying, L.; Maguire, J.A.; Sim, X.; Jobaliya, C.; Gagne, A.L.; Kishore, S.; Stanescu, D.E.; Hughes, N.; et al. GATA6 Plays an Important Role in the Induction of Human Definitive Endoderm, Development of the Pancreas, and Functionality of Pancreatic β Cells. Stem Cell Rep. 2017, 8, 589–604. [Google Scholar] [CrossRef]
- Pagliuca, F.W.; Millman, J.R.; Gürtler, M.; Segel, M.; Van Dervort, A.; Ryu, J.H.; Peterson, Q.P.; Greiner, D.; Melton, D.A. Generation of Functional Human Pancreatic β Cells in Vitro. Cell 2014, 159, 428–439. [Google Scholar] [CrossRef]
- Chertow, B.S.; Webb, M.D.; Leidy, J.W.; Cordle, M.B. Protective Effects of Retinyl Palmitate on Streptozotocin- and Alloxan-Induced Beta Cell Toxicity and Diabetes in the Rat. Res. Commun. Chem. Pathol. Pharmacol. 1989, 63, 27–44. [Google Scholar]
- Eltony, S.A.; Elmottaleb, N.A.; Gomaa, A.M.; Anwar, M.M.; El-Metwally, T.H. Effect of All-Trans Retinoic Acid on the Pancreas of Streptozotocin-Induced Diabetic Rat. Anat. Rec. 2016, 299, 334–351. [Google Scholar] [CrossRef]
- Koprivica, I.; Gajic, D.; Saksida, T.; Cavalli, E.; Auci, D.; Despotovic, S.; Pejnovic, N.; Stosic-Grujicic, S.; Nicoletti, F.; Stojanovic, I. Orally Delivered All-Trans-Retinoic Acid- and Transforming Growth Factor-β-Loaded Microparticles Ameliorate Type 1 Diabetes in Mice. Eur. J. Pharmacol. 2019, 864, 172721. [Google Scholar] [CrossRef]
- Driscoll, H.K.; Chertow, B.S.; Jelic, T.M.; Baltaro, R.J.; Chandor, S.B.; Walker, E.M.; Dadgari, J.M.; Pofahl, A.B. Vitamin A Status Affects the Development of Diabetes and Insulitis in BB Rats. Metabolism 1996, 45, 248–253. [Google Scholar] [CrossRef]
- Basu, T.K.; Basualdo, C. Vitamin A Homeostasis and Diabetes Mellitus. Nutrition 1997, 13, 804–806. [Google Scholar] [CrossRef]
- Pearson, J.A.; Wong, F.S.; Wen, L. The Importance of the Non Obese Diabetic (NOD) Mouse Model in Autoimmune Diabetes. J. Autoimmun. 2016, 66, 76–88. [Google Scholar] [CrossRef]
- Reeves, P.G. Components of the AIN-93 Diets as Improvements in the AIN-76A Diet. J. Nutr. 1997, 127, 838S–841S. [Google Scholar] [CrossRef]
- Zunino, S.J.; Storms, D.H.; Stephensen, C.B. Diets Rich in Polyphenols and Vitamin A Inhibit the Development of Type I Autoimmune Diabetes in Nonobese Diabetic Mice. J. Nutr. 2007, 137, 1216–1221. [Google Scholar] [CrossRef]
- Van, Y.-H.; Lee, W.-H.; Ortiz, S.; Lee, M.-H.; Qin, H.-J.; Liu, C.-P. All-Trans Retinoic Acid Inhibits Type 1 Diabetes by T Regulatory (Treg)-Dependent Suppression of Interferon-Gamma-Producing T-Cells without Affecting Th17 Cells. Diabetes 2009, 58, 146–155. [Google Scholar] [CrossRef]
- Stosić-Grujicić, S.; Cvjetićanin, T.; Stojanović, I. Retinoids Differentially Regulate the Progression of Autoimmune Diabetes in Three Preclinical Models in Mice. Mol. Immunol. 2009, 47, 79–86. [Google Scholar] [CrossRef]
- Phillips, B.E.; Garciafigueroa, Y.; Engman, C.; Liu, W.; Wang, Y.; Lakomy, R.J.; Meng, W.S.; Trucco, M.; Giannoukakis, N. Arrest in the Progression of Type 1 Diabetes at the Mid-Stage of Insulitic Autoimmunity Using an Autoantigen-Decorated All-Trans Retinoic Acid and Transforming Growth Factor Beta-1 Single Microparticle Formulation. Front. Immunol. 2021, 12, 586220. [Google Scholar] [CrossRef]
- Chertow, B.S.; Driscoll, H.K.; Primerano, D.A.; Cordle, M.B.; Matthews, K.A. Retinoic Acid Receptor Transcripts and Effects of Retinol and Retinoic Acid on Glucagon Secretion from Rat Islets and Glucagon-Secreting Cell Lines. Metabolism 1996, 45, 300–305. [Google Scholar] [CrossRef]
- De Luca, L.M.; Shores, R.L.; Spangler, E.F.; Wenk, M.L. Inhibition of Initiator-Promoter-Induced Skin Tumorigenesis in Female SENCAR Mice Fed a Vitamin A-Deficient Diet and Reappearance of Tumors in Mice Fed a Diet Adequate in Retinoid or Beta-Carotene. Cancer Res. 1989, 49, 5400–5406. [Google Scholar]
- McCARTHY, P.T.; Cerecedo, L.R. Vitamin A Deficiency in the Mouse. J. Nutr. 1952, 46, 361–376. [Google Scholar] [CrossRef]
- Williams, R.J.; Pelton, R.B. Individuality in Nutrition: Effects of Vitamin A-Deficient and Other Deficient Diets on Experimental Animals. Proc. Natl. Acad. Sci. USA 1966, 55, 126–134. [Google Scholar] [CrossRef]
- Obrochta, K.M.; Kane, M.A.; Napoli, J.L. Effects of Diet and Strain on Mouse Serum and Tissue Retinoid Concentrations. PLoS ONE 2014, 9, e99435. [Google Scholar] [CrossRef]
- Quadro, L.; Blaner, W.S.; Salchow, D.J.; Vogel, S.; Piantedosi, R.; Gouras, P.; Freeman, S.; Cosma, M.P.; Colantuoni, V.; Gottesman, M.E. Impaired Retinal Function and Vitamin A Availability in Mice Lacking Retinol-Binding Protein. EMBO J. 1999, 18, 4633–4644. [Google Scholar] [CrossRef]
- E, X.; Zhang, L.; Lu, J.; Tso, P.; Blaner, W.S.; Levin, M.S.; Li, E. Increased Neonatal Mortality in Mice Lacking Cellular Retinol-Binding Protein II. J. Biol. Chem. 2002, 277, 36617–36623. [Google Scholar] [CrossRef]
- Batten, M.L.; Imanishi, Y.; Maeda, T.; Tu, D.C.; Moise, A.R.; Bronson, D.; Possin, D.; Van Gelder, R.N.; Baehr, W.; Palczewski, K. Lecithin-Retinol Acyltransferase Is Essential for Accumulation of All-Trans-Retinyl Esters in the Eye and in the Liver. J. Biol. Chem. 2004, 279, 10422–10432. [Google Scholar] [CrossRef]
- Liu, L.; Gudas, L.J. Disruption of the Lecithin:Retinol Acyltransferase Gene Makes Mice More Susceptible to Vitamin A Deficiency. J. Biol. Chem. 2005, 280, 40226–40234. [Google Scholar] [CrossRef]
- Ruiz, A.; Ghyselinck, N.B.; Mata, N.; Nusinowitz, S.; Lloyd, M.; Dennefeld, C.; Chambon, P.; Bok, D. Somatic Ablation of the Lrat Gene in the Mouse Retinal Pigment Epithelium Drastically Reduces Its Retinoid Storage. Investig. Opthalmol. Vis. Sci. 2007, 48, 5377–5387. [Google Scholar] [CrossRef]
- Yen, C.-L.E.; Monetti, M.; Burri, B.J.; Farese, R.V. The Triacylglycerol Synthesis Enzyme DGAT1 Also Catalyzes the Synthesis of Diacylglycerols, Waxes, and Retinyl Esters. J. Lipid Res. 2005, 46, 1502–1511. [Google Scholar] [CrossRef]
- Graham, K.L.; Werner, B.J.; Moyer, K.M.; Patton, A.K.; Krois, C.R.; Yoo, H.S.; Tverskoy, M.; LaJevic, M.; Napoli, J.L.; Sobel, R.A.; et al. DGAT1 Inhibits Retinol-Dependent Regulatory T Cell Formation and Mediates Autoimmune Encephalomyelitis. Proc. Natl. Acad. Sci. USA 2019, 116, 3126–3135. [Google Scholar] [CrossRef]
- Brun, P.-J.; Grijalva, A.; Rausch, R.; Watson, E.; Yuen, J.J.; Das, B.C.; Shudo, K.; Kagechika, H.; Leibel, R.L.; Blaner, W.S. Retinoic Acid Receptor Signaling Is Required to Maintain Glucose-Stimulated Insulin Secretion and β-Cell Mass. FASEB J. 2015, 29, 671–683. [Google Scholar] [CrossRef]
- Trasino, S.E.; Tang, X.-H.; Jessurun, J.; Gudas, L.J. Retinoic Acid Receptor B2 Agonists Restore Glycaemic Control in Diabetes and Reduce Steatosis. Diabetes Obes. Metab. 2016, 18, 142–151. [Google Scholar] [CrossRef]
- Morgenstern, J.; Fleming, T.; Kliemank, E.; Brune, M.; Nawroth, P.; Fischer, A. Quantification of All-Trans Retinoic Acid by Liquid Chromatography-Tandem Mass Spectrometry and Association with Lipid Profile in Patients with Type 2 Diabetes. Metabolites 2021, 11, 60. [Google Scholar] [CrossRef] [PubMed]
- Kane, M.A.; Folias, A.E.; Pingitore, A.; Perri, M.; Obrochta, K.M.; Krois, C.R.; Cione, E.; Ryu, J.Y.; Napoli, J.L. Identification of 9-Cis-Retinoic Acid as a Pancreas-Specific Autacoid That Attenuates Glucose-Stimulated Insulin Secretion. Proc. Natl. Acad. Sci. USA 2010, 107, 21884–21889. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.S.; Napoli, J.L. Regulation of Rdh5 in Pancreas Beta Cells. 2022; manuscript to be sumitted. [Google Scholar]
- Olsen, T.; Blomhoff, R. Retinol, Retinoic Acid, and Retinol-Binding Protein 4 Are Differentially Associated with Cardiovascular Disease, Type 2 Diabetes, and Obesity: An Overview of Human Studies. Adv. Nutr. 2020, 11, 644–666. [Google Scholar] [CrossRef]
- Kawaguchi, R.; Yu, J.; Honda, J.; Hu, J.; Whitelegge, J.; Ping, P.; Wiita, P.; Bok, D.; Sun, H. A Membrane Receptor for Retinol Binding Protein Mediates Cellular Uptake of Vitamin A. Science 2007, 315, 820–825. [Google Scholar] [CrossRef]
- Kawaguchi, R.; Yu, J.; Ter-Stepanian, M.; Zhong, M.; Cheng, G.; Yuan, Q.; Jin, M.; Travis, G.H.; Ong, D.; Sun, H. Receptor-Mediated Cellular Uptake Mechanism That Couples to Intracellular Storage. ACS Chem. Biol. 2011, 6, 1041–1051. [Google Scholar] [CrossRef]
- Berry, D.C.; O’Byrne, S.M.; Vreeland, A.C.; Blaner, W.S.; Noy, N. Cross Talk between Signaling and Vitamin A Transport by the Retinol-Binding Protein Receptor STRA6. Mol. Cell. Biol. 2012, 32, 3164–3175. [Google Scholar] [CrossRef]
- Yang, Q.; Graham, T.E.; Mody, N.; Preitner, F.; Peroni, O.D.; Zabolotny, J.M.; Kotani, K.; Quadro, L.; Kahn, B.B. Serum Retinol Binding Protein 4 Contributes to Insulin Resistance in Obesity and Type 2 Diabetes. Nature 2005, 436, 356–362. [Google Scholar] [CrossRef]
- Mody, N.; Graham, T.E.; Tsuji, Y.; Yang, Q.; Kahn, B.B. Decreased Clearance of Serum Retinol-Binding Protein and Elevated Levels of Transthyretin in Insulin-Resistant Ob/Ob Mice. Am. J. Physiol.-Endocrinol. Metab. 2008, 294, E785–E793. [Google Scholar] [CrossRef][Green Version]
- Moraes-Vieira, P.M.; Yore, M.M.; Dwyer, P.M.; Syed, I.; Aryal, P.; Kahn, B.B. RBP4 Activates Antigen-Presenting Cells, Leading to Adipose Tissue Inflammation and Systemic Insulin Resistance. Cell Metab. 2014, 19, 512–526. [Google Scholar] [CrossRef]
- Noy, N. Vitamin A in Regulation of Insulin Responsiveness: Mini Review. Proc. Nutr. Soc. 2016, 75, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-W.; Park, S.W.; Lin, Y.-L.; Burton, F.H.; Wei, L.-N. Cellular Retinoic Acid Binding Protein 1 Protects Mice from High-Fat Diet-Induced Obesity by Decreasing Adipocyte Hypertrophy. Int. J. Obes. 2020, 44, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Bonet, M.L.; Ribot, J.; Galmés, S.; Serra, F.; Palou, A. Carotenoids and Carotenoid Conversion Products in Adipose Tissue Biology and Obesity: Pre-Clinical and Human Studies. Biochim. Biophys Acta-Mol. Cell Biol. Lipids 2020, 1865, 158676. [Google Scholar] [CrossRef] [PubMed]
- Bento, C.; Matos, A.C.; Cordeiro, A.; Ramalho, A. Vitamin A Deficiency Is Associated with Body Mass Index and Body Adiposity in Women with Recommended Intake of Vitamin A. Nutr. Hosp. 2018, 35, 1072–1078. [Google Scholar] [CrossRef]
- Galmés, S.; Palou, A.; Serra, F. Increased Risk of High Body Fat and Altered Lipid Metabolism Associated to Suboptimal Consumption of Vitamin A Is Modulated by Genetic Variants Rs5888 (SCARB1), Rs1800629 (UCP1) and Rs659366 (UCP2). Nutrients 2020, 12, 2588. [Google Scholar] [CrossRef]
- Moore, M.C.; Coate, K.C.; Winnick, J.J.; An, Z.; Cherrington, A.D. Regulation of Hepatic Glucose Uptake and Storage in Vivo. Adv. Nutr. 2012, 3, 286–294. [Google Scholar] [CrossRef]
- McCommis, K.S.; Butler, A.A. The Importance of Keeping Time in the Liver. Endocrinology 2021, 162, bqaa230. [Google Scholar] [CrossRef]
- Myers, S.R.; Biggers, D.W.; Neal, D.W.; Cherrington, A.D. Intraportal Glucose Delivery Enhances the Effects of Hepatic Glucose Load on Net Hepatic Glucose Uptake in Vivo. J. Clin. Investig. 1991, 88, 158–167. [Google Scholar] [CrossRef][Green Version]
- Klyuyeva, A.V.; Belyaeva, O.V.; Goggans, K.R.; Krezel, W.; Popov, K.M.; Kedishvili, N.Y. Changes in Retinoid Metabolism and Signaling Associated with Metabolic Remodeling during Fasting and in Type I Diabetes. J. Biol. Chem. 2021, 296, 100323. [Google Scholar] [CrossRef]
- Kitada, M.; Ogura, Y.; Koya, D. Rodent Models of Diabetic Nephropathy: Their Utility and Limitations. Int. J. Nephrol. Renov. Dis. 2016, 9, 279–290. [Google Scholar] [CrossRef]
- Rui, L. Energy Metabolism in the Liver. Compr. Physiol. 2014, 4, 177–197. [Google Scholar] [CrossRef] [PubMed]
- Thatcher, J.E.; Isoherranen, N. The Role of CYP26 Enzymes in Retinoic Acid Clearance. Expert Opin. Drug Metab. Toxicol. 2009, 5, 875–886. [Google Scholar] [CrossRef] [PubMed]
- White, J.A.; Guo, Y.D.; Baetz, K.; Beckett-Jones, B.; Bonasoro, J.; Hsu, K.E.; Dilworth, F.J.; Jones, G.; Petkovich, M. Identification of the Retinoic Acid-Inducible All-Trans-Retinoic Acid 4-Hydroxylase. J. Biol. Chem. 1996, 271, 29922–29927. [Google Scholar] [CrossRef] [PubMed]
- Loudig, O.; Babichuk, C.; White, J.; Abu-Abed, S.; Mueller, C.; Petkovich, M. Cytochrome P450RAI(CYP26) Promoter: A Distinct Composite Retinoic Acid Response Element Underlies the Complex Regulation of Retinoic Acid Metabolism. Mol. Endocrinol. 2000, 14, 1483–1497. [Google Scholar] [CrossRef]
- Yoo, H.S.; Napoli, J.L. The glucocorticoid receptor represses whereas C/EBPβ can enhance or repress CYP26A1 transcription. 2022; manuscript submitted. [Google Scholar]
- Tuteja, S.; Qu, L.; Vujkovic, M.; Dunbar, R.L.; Chen, J.; DerOhannessian, S.; Rader, D.J. Genetic Variants Associated with Plasma Lipids Are Associated with the Lipid Response to Niacin. J. Am. Heart Assoc. 2018, 7, e03488. [Google Scholar] [CrossRef]
- Teslovich, T.M.; Musunuru, K.; Smith, A.V.; Edmondson, A.C.; Stylianou, I.M.; Koseki, M.; Pirruccello, J.P.; Ripatti, S.; Chasman, D.I.; Willer, C.J.; et al. Biological, Clinical and Population Relevance of 95 Loci for Blood Lipids. Nature 2010, 466, 707–713. [Google Scholar] [CrossRef]
- Partridge, E.C.; Chhetri, S.B.; Prokop, J.W.; Ramaker, R.C.; Jansen, C.S.; Goh, S.-T.; Mackiewicz, M.; Newberry, K.M.; Brandsmeier, L.A.; Meadows, S.K.; et al. Occupancy Maps of 208 Chromatin-Associated Proteins in One Human Cell Type. Nature 2020, 583, 720–728. [Google Scholar] [CrossRef]
- Boyle, A.P.; Hong, E.L.; Hariharan, M.; Cheng, Y.; Schaub, M.A.; Kasowski, M.; Karczewski, K.J.; Park, J.; Hitz, B.C.; Weng, S.; et al. Annotation of Functional Variation in Personal Genomes Using RegulomeDB. Genome Res. 2012, 22, 1790–1797. [Google Scholar] [CrossRef]
- Bonet, M.L.; Ribot, J.; Palou, A. Lipid Metabolism in Mammalian Tissues and Its Control by Retinoic Acid. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2012, 1821, 177–189. [Google Scholar] [CrossRef]
- Rhinn, M.; Schuhbaur, B.; Niederreither, K.; Dollé, P. Involvement of Retinol Dehydrogenase 10 in Embryonic Patterning and Rescue of Its Loss of Function by Maternal Retinaldehyde Treatment. Proc. Natl. Acad. Sci. USA 2011, 108, 16687–16692. [Google Scholar] [CrossRef] [PubMed]
- Ashique, A.M.; May, S.R.; Kane, M.A.; Folias, A.E.; Phamluong, K.; Choe, Y.; Napoli, J.L.; Peterson, A.S. Morphological Defects in a Novel Rdh10 Mutant That Has Reduced Retinoic Acid Biosynthesis and Signaling. Genesis 2012, 50, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Siegenthaler, J.A.; Ashique, A.M.; Zarbalis, K.; Patterson, K.P.; Hecht, J.H.; Kane, M.A.; Folias, A.E.; Choe, Y.; May, S.R.; Kume, T.; et al. Retinoic Acid from the Meninges Regulates Cortical Neuron Generation. Cell 2009, 139, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Tong, M.-H.; Yang, Q.-E.; Davis, J.C.; Griswold, M.D. Retinol Dehydrogenase 10 Is Indispensible for Spermatogenesis in Juvenile Males. Proc. Natl. Acad. Sci. USA 2013, 110, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Haushalter, C.; Asselin, L.; Fraulob, V.; Dollé, P.; Rhinn, M. Retinoic Acid Controls Early Neurogenesis in the Developing Mouse Cerebral Cortex. Dev. Biol. 2017, 430, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-H.; Kakkad, B.; Ong, D.E. Estrogen Directly Induces Expression of Retinoic Acid Biosynthetic Enzymes, Compartmentalized between the Epithelium and Underlying Stromal Cells in Rat Uterus. Endocrinology 2004, 145, 4756–4762. [Google Scholar] [CrossRef]
- Flynn, J.M.; Meadows, E.; Fiorotto, M.; Klein, W.H. Myogenin Regulates Exercise Capacity and Skeletal Muscle Metabolism in the Adult Mouse. PLoS ONE 2010, 5, e13535. [Google Scholar] [CrossRef]
- Rousseau, C.; Nichol, J.N.; Pettersson, F.; Couture, M.-C.; Miller, W.H. ERbeta Sensitizes Breast Cancer Cells to Retinoic Acid: Evidence of Transcriptional Crosstalk. Mol. Cancer Res. 2004, 2, 523–531. [Google Scholar]
- Hevener, A.L.; Zhou, Z.; Moore, T.M.; Drew, B.G.; Ribas, V. The Impact of ERα Action on Muscle Metabolism and Insulin Sensitivity—Strong Enough for a Man, Made for a Woman. Mol. Metab. 2018, 15, 20–34. [Google Scholar] [CrossRef]
- De Paoli, M.; Zakharia, A.; Werstuck, G.H. The Role of Estrogen in Insulin Resistance: A Review of Clinical and Preclinical Data. Am. J. Pathol. 2021, 191, 1490–1498. [Google Scholar] [CrossRef]
- Ombra, M.N.; Di Santi, A.; Abbondanza, C.; Migliaccio, A.; Avvedimento, E.V.; Perillo, B. Retinoic Acid Impairs Estrogen Signaling in Breast Cancer Cells by Interfering with Activation of LSD1 via PKA. Biochim. Biophys. Acta-Gene Regul. Mech. 2013, 1829, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Miro Estruch, I.; de Haan, L.H.J.; Melchers, D.; Houtman, R.; Louisse, J.; Groten, J.P.; Rietjens, I.M.C.M. The Effects of All-Trans Retinoic Acid on Estrogen Receptor Signaling in the Estrogen-Sensitive MCF/BUS Subline. J. Recept. Signal. Transduct. Res. 2018, 38, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.F.; Li, M.T.; Von Hagen, S.; Zhang, Y.F.; Irwin, R.J. Androgen Modulation of the Messenger Ribonucleic Acid of Retinoic Acid Receptors in the Prostate, Seminal Vesicles, and Kidney in the Rat. Endocrinology 1997, 138, 553–559. [Google Scholar] [CrossRef][Green Version]
- Udhane, S.S.; Pandey, A.V.; Hofer, G.; Mullis, P.E.; Flück, C.E. Retinoic Acid Receptor Beta and Angiopoietin-like Protein 1 Are Involved in the Regulation of Human Androgen Biosynthesis. Sci. Rep. 2015, 5, 10132. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Napoli, J.L. Retinoic Acid: Sexually Dimorphic, Anti-Insulin and Concentration-Dependent Effects on Energy. Nutrients 2022, 14, 1553. https://doi.org/10.3390/nu14081553
Napoli JL. Retinoic Acid: Sexually Dimorphic, Anti-Insulin and Concentration-Dependent Effects on Energy. Nutrients. 2022; 14(8):1553. https://doi.org/10.3390/nu14081553
Chicago/Turabian StyleNapoli, Joseph L. 2022. "Retinoic Acid: Sexually Dimorphic, Anti-Insulin and Concentration-Dependent Effects on Energy" Nutrients 14, no. 8: 1553. https://doi.org/10.3390/nu14081553
APA StyleNapoli, J. L. (2022). Retinoic Acid: Sexually Dimorphic, Anti-Insulin and Concentration-Dependent Effects on Energy. Nutrients, 14(8), 1553. https://doi.org/10.3390/nu14081553





