Daily Inclusion of Resistant Starch-Containing Potatoes in a Dietary Guidelines for Americans Dietary Pattern Does Not Adversely Affect Cardiometabolic Risk or Intestinal Permeability in Adults with Metabolic Syndrome: A Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.3. Dietary Control, Assessment, and Compliance
2.4. Biospecimen Collection and Processing
2.5. Clinical Chemistries, Metabolic Hormones, and Endotoxemia
2.6. Vascular Function
2.7. Arginine Metabolism and Nitric Oxide Metabolites
2.8. Lipid Peroxidation and Plasma Antioxidants
2.9. Gastrointestinal Permeability Test
2.10. Gut Microbiome and Fecal Short-Chain Fatty Acids
2.11. Statistical Analyses
3. Results
3.1. Participants, Compliance, & Dietary Intakes
3.2. Changes in Cardiometabolic Health Parameters following the 2-week Intervention
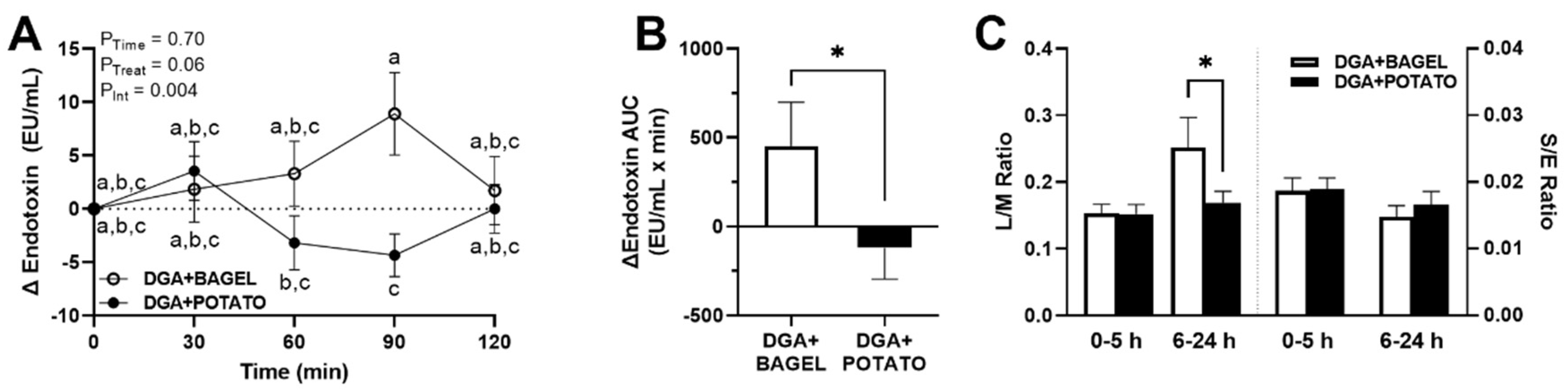
3.3. Intervention Effects on Fasting Endotoxemia and Vascular Health
3.4. Intervention Effects on Postprandial Glycemia, Cholecystokinin, and Lipid Peroxidation
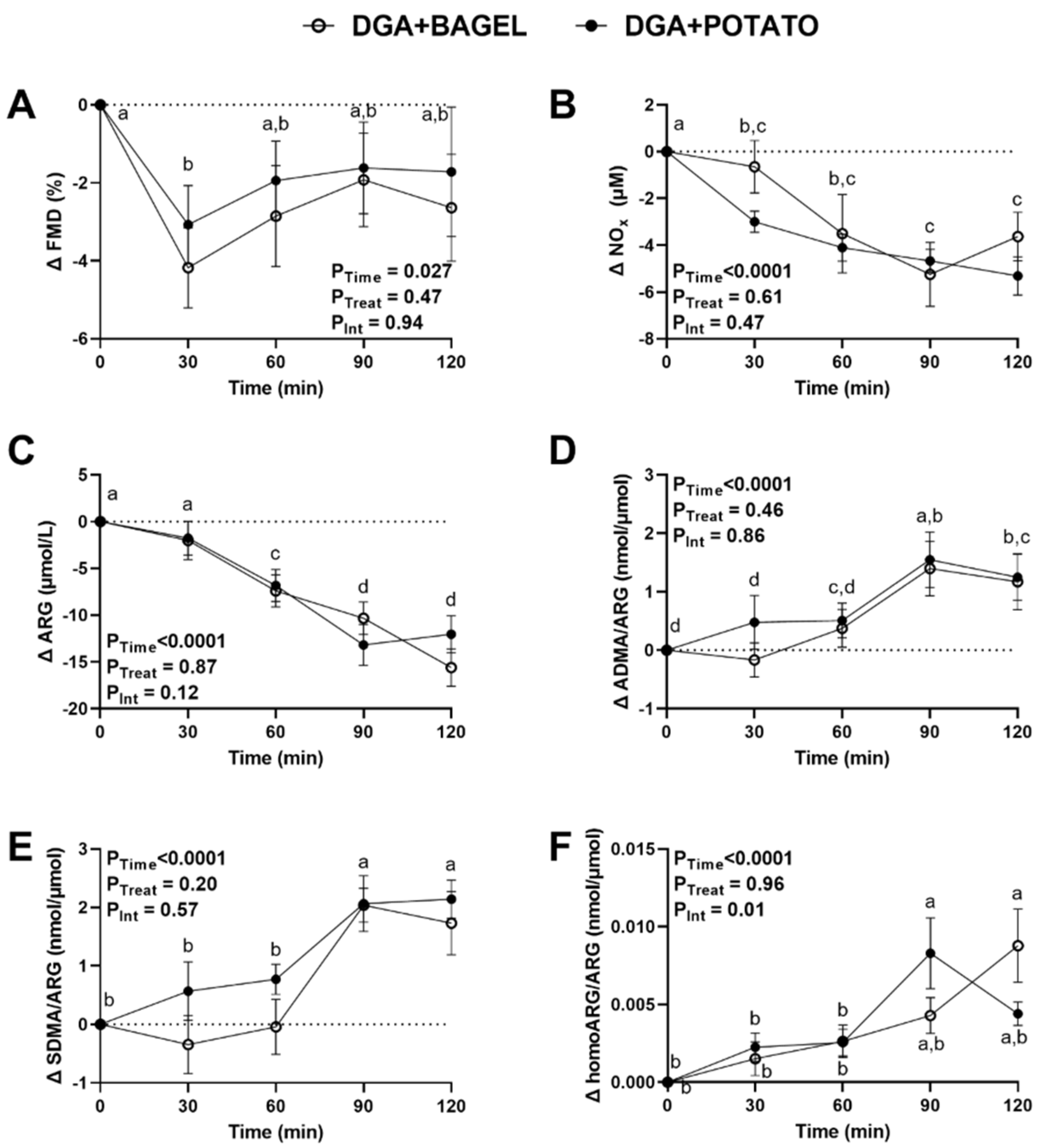
3.5. Intervention Effects on Postprandial Vascular Health and Nitric Oxide Homeostasis
3.6. Intervention Effects on Postprandial Endotoxemia and Gut Permeability
3.7. Intervention Effects on Microbiome Composition and Function
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kochanek, K.; Xu, J.; Arias, E. Mortality in the United States, 2019. In NCHS Data Brief; National Center for Health Statistics: Hyattsville, MD, USA, 2020; Volume 395, pp. 1–8. [Google Scholar]
- Jacome-Sosa, M.; Parks, E.J.; Bruno, R.S.; Tasali, E.; Lewis, G.F.; Schneeman, B.O.; Rains, T.M. Postprandial metabolism of macronutrients and cardiometabolic risk: Recent developments, emerging concepts, and future directions. Adv. Nutr. 2016, 7, 364–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mah, E.; Bruno, R.S. Postprandial hyperglycemia on vascular endothelial function: Mechanisms and consequences. Nutr. Res. 2012, 32, 727–740. [Google Scholar] [CrossRef] [PubMed]
- DECODE Study Group, the European Diabetes Epidemiology Group. Glucose tolerance and cardiovascular mortality: Comparison of fasting and 2-hour diagnostic criteria. Arch. Intern. Med. 2001, 161, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Mah, E.; Noh, S.K.; Ballard, K.D.; Matos, M.E.; Volek, J.S.; Bruno, R.S. Postprandial hyperglycemia impairs vascular endothelial function in healthy men by inducing lipid peroxidation and increasing asymmetric dimethylarginine:arginine. J. Nutr. 2011, 141, 1961–1968. [Google Scholar] [CrossRef]
- Cani, P.D.; Delzenne, N.M. Interplay between obesity and associated metabolic disorders: New insights into the gut microbiota. Curr. Opin. Pharmacol. 2009, 9, 737–743. [Google Scholar] [CrossRef]
- Cani, P.D.; Osto, M.; Geurts, L.; Everard, A. Involvement of gut microbiota in the development of low-grade inflammation and type 2 diabetes associated with obesity. Gut Microbes 2012, 3, 279–288. [Google Scholar] [CrossRef] [Green Version]
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart disease and stroke statistics-2021 update: A report from the American Heart Association. Circulation 2021, 143, e254–e743. [Google Scholar] [CrossRef]
- US Department of Agriculture; US Department of Health and Human Services. Dietary Guidelines for Americans, 2020–2025, 9th ed.; United States Department of Agriculture: Washington, DC, USA, 2020. Available online: http://dietaryguidelines.gov (accessed on 12 January 2022).
- Mozaffarian, D.; Hao, T.; Rimm, E.B.; Willett, W.C.; Hu, F.B. Changes in diet and lifestyle and long-term weight gain in women and men. N. Engl. J. Med. 2011, 364, 2392–2404. [Google Scholar] [CrossRef] [Green Version]
- Borgi, L.; Rimm, E.B.; Willett, W.C.; Forman, J.P. Potato intake and incidence of hypertension: Results from three prospective US cohort studies. BMJ 2016, 353, i2351. [Google Scholar] [CrossRef] [Green Version]
- Muraki, I.; Rimm, E.B.; Willett, W.C.; Manson, J.E.; Hu, F.B.; Sun, Q. Potato consumption and risk of type 2 diabetes: Results from three prospective cohort studies. Diabetes Care 2016, 39, 376–384. [Google Scholar] [CrossRef] [Green Version]
- Camire, M.E.; Kubow, S.; Donnelly, D.J. Potatoes and human health. Crit. Rev. Food Sci. Nutr. 2009, 49, 823–840. [Google Scholar] [CrossRef] [PubMed]
- Borch, D.; Juul-Hindsgaul, N.; Veller, M.; Astrup, A.; Jaskolowski, J.; Raben, A. Potatoes and risk of obesity, type 2 diabetes, and cardiovascular disease in apparently healthy adults: A systematic review of clinical intervention and observational studies. Am. J. Clin. Nutr. 2016, 104, 489–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larsson, S.C.; Wolk, A. Potato consumption and risk of cardiovascular disease: 2 prospective cohort studies. Am. J. Clin. Nutr. 2016, 104, 1245–1252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liese, A.D.; Weis, K.E.; Schulz, M.; Tooze, J.A. Food intake patterns associated with incident type 2 diabetes: The Insulin Resistance Atherosclerosis Study. Diabetes Care 2009, 32, 263–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGill, C.R.; Kurilich, A.C.; Davignon, J. The role of potatoes and potato components in cardiometabolic health: A review. Ann. Med. 2013, 45, 467–473. [Google Scholar] [CrossRef]
- Atkinson, F.S.; Foster-Powell, K.; Brand-Miller, J.C. International tables of glycemic index and glycemic load values: 2008. Diabetes Care 2008, 31, 2281–2283. [Google Scholar] [CrossRef] [Green Version]
- Patterson, M.A.; Maiya, M.; Stewart, M.L. Resistant starch content in foods commonly consumed in the United States: A narrative review. J. Acad. Nutr. Diet. 2020, 120, 230–244. [Google Scholar] [CrossRef]
- Maier, T.V.; Lucio, M.; Lee, L.H.; VerBerkmoes, N.C.; Brislawn, C.J.; Bernhardt, J.; Lamendella, R.; McDermott, J.E.; Bergeron, N.; Heinzmann, S.S.; et al. Impact of dietary resistant starch on the human gut microbiome, metaproteome, and metabolome. MBio 2017, 8, e01343-17. [Google Scholar] [CrossRef] [Green Version]
- Venkataraman, A.; Sieber, J.R.; Schmidt, A.W.; Waldron, C.; Theis, K.R.; Schmidt, T.M. Variable responses of human microbiomes to dietary supplementation with resistant starch. Microbiome 2016, 4, 33. [Google Scholar] [CrossRef] [Green Version]
- Ballard, K.D.; Mah, E.; Guo, Y.; Pei, R.; Volek, J.S.; Bruno, R.S. Low-fat milk ingestion prevents postprandial hyperglycemia-mediated impairments in vascular endothelial function in obese individuals with metabolic syndrome. J. Nutr. 2013, 143, 1602–1610. [Google Scholar] [CrossRef]
- Li, X.; Watanabe, K.; Kimura, I. Gut microbiota dysbiosis drives and implies novel therapeutic strategies for diabetes mellitus and related metabolic diseases. Front. Immunol. 2017, 8, 1882. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, R.A.; Nishiyama, S.K.; Wray, D.W.; Richardson, R.S. Ultrasound assessment of flow-mediated dilation. Hypertension 2010, 55, 1075–1085. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.A.; Benedict, F.G. A biometric study of human basal metabolism. Proc. Natl. Acad. Sci. USA 1918, 4, 370–373. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, E.T.; Ohls, J.; Carlson, S.; Fleming, K. The Healthy Eating Index: Design and applications. J. Am. Diet. Assoc. 1995, 95, 1103–1108. [Google Scholar] [CrossRef]
- US Department of Agriculture. Agricultural Research Services. FoodData Central. Available online: https://fdc.nal.usda.gov (accessed on 4 January 2022).
- Murphy, M.M.; Douglass, J.S.; Birkett, A. Resistant starch intakes in the United States. J. Am. Diet. Assoc. 2008, 108, 67–78. [Google Scholar] [CrossRef]
- Lewis, S.J.; Heaton, K.W. Stool form scale as a useful guide to intestinal transit time. Scand. J. Gastroenterol. 1997, 32, 920–924. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Sapper, T.N.; Mah, E.; Moller, M.V.; Kim, J.B.; Chitchumroonchokchai, C.; McDonald, J.D.; Bruno, R.S. Green tea extract treatment reduces NFkappaB activation in mice with diet-induced nonalcoholic steatohepatitis by lowering TNFR1 and TLR4 expression and ligand availability. J. Nutr. Biochem. 2017, 41, 34–41. [Google Scholar] [CrossRef]
- Stein, J.H.; Korcarz, C.E.; Hurst, R.T.; Lonn, E.; Kendall, C.B.; Mohler, E.R.; Najjar, S.S.; Rembold, C.M.; Post, W.S. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: A consensus statement from the american society of echocardiography carotid intima-media thickness task force endorsed by the society for vascular medicine. J. Am. Soc. Echocardiogr. 2008, 21, 93–111. [Google Scholar] [CrossRef]
- McDonald, J.D.; Chitchumroonchokchai, C.; Li, J.; Mah, E.; Labyk, A.N.; Reverri, E.J.; Ballard, K.D.; Volek, J.S.; Bruno, R.S. Replacing carbohydrate during a glucose challenge with the egg white portion or whole eggs protects against postprandial impairments in vascular endothelial function in prediabetic men by limiting increases in glycaemia and lipid peroxidation. Br J. Nutr. 2018, 119, 259–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodges, J.K.; Zhu, J.; Yu, Z.; Vodovotz, Y.; Brock, G.; Sasaki, G.Y.; Dey, P.; Bruno, R.S. Intestinal-level anti-inflammatory bioactivities of catechin-rich green tea: Rationale, design, and methods of a double-blind, randomized, placebo-controlled crossover trial in metabolic syndrome and healthy adults. Contemp. Clin. Trials Commun. 2020, 17, 100495. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M.; Nadeau, A.; Lamsam, J.; Nord, S.L.; Ryks, M.; Burton, D.; Sweetser, S.; Zinsmeister, A.R.; Singh, R. Understanding measurements of intestinal permeability in healthy humans with urine lactulose and mannitol excretion. Neurogastroenterol. Motil. 2010, 22, e15–e26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Valle-Pinero, A.Y.; Van Deventer, H.E.; Fourie, N.H.; Martino, A.C.; Patel, N.S.; Remaley, A.T.; Henderson, W.A. Gastrointestinal permeability in patients with irritable bowel syndrome assessed using a four probe permeability solution. Clin. Chim. Acta 2013, 418, 97–101. [Google Scholar] [CrossRef] [Green Version]
- Yu, Z.; Morrison, M. Improved extraction of PCR-quality community DNA from digesta and fecal samples. Biotechniques 2004, 36, 808–812. [Google Scholar] [CrossRef]
- Dey, P.; Sasaki, G.Y.; Wei, P.; Li, J.; Wang, L.; Zhu, J.; McTigue, D.; Yu, Z.; Bruno, R.S. Green tea extract prevents obesity in male mice by alleviating gut dysbiosis in association with improved intestinal barrier function that limits endotoxin translocation and adipose inflammation. J. Nutr. Biochem. 2019, 67, 78–89. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [Green Version]
- Han, J.; Lin, K.; Sequeira, C.; Borchers, C.H. An isotope-labeled chemical derivatization method for the quantitation of short-chain fatty acids in human feces by liquid chromatography-tandem mass spectrometry. Anal. Chim. Acta 2015, 854, 86–94. [Google Scholar] [CrossRef]
- Traber, M.G.; Buettner, G.R.; Bruno, R.S. The relationship between vitamin C status, the gut-liver axis, and metabolic syndrome. Redox Biol. 2019, 21, 101091. [Google Scholar] [CrossRef]
- Van Buuren, S.; Groothuis-Oudshoorn, K. mice: Multivariate imputation by chained equations in R. J. Stat. Softw. 2010, 45, 1–67. [Google Scholar] [CrossRef] [Green Version]
- Rubin, D.B. Multiple Imputation for Nonresponse in Surveys; John Wiley & Sons: Hoboken, NJ, USA, 2004; Volume 81. [Google Scholar]
- Kaul, A.; Mandal, S.; Davidov, O.; Peddada, S.D. Analysis of microbiome data in the presence of excess zeros. Front. Microbiol. 2017, 8, 2114. [Google Scholar] [CrossRef] [PubMed]
- Nearing, J.T.; Douglas, G.M.; Hayes, M.G.; MacDonald, J.; Desai, D.K.; Allward, N.; Jones, C.M.A.; Wright, R.J.; Dhanani, A.S.; Comeau, A.M.; et al. Microbiome differential abundance methods produce different results across 38 datasets. Nat. Commun. 2022, 13, 342. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Peddada, S.D. Analysis of microbial compositions: A review of normalization and differential abundance analysis. NPJ Biofilms Microbiomes 2020, 6, 60. [Google Scholar] [CrossRef] [PubMed]
- Kratz, A.; Ferraro, M.; Sluss, P.M.; Lewandrowski, K.B. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Laboratory reference values. N. Engl. J. Med. 2004, 351, 1548–1563. [Google Scholar] [CrossRef] [PubMed]
- So, J.; Avendano, E.E.; Raman, G.; Johnson, E.J. Potato consumption and risk of cardio-metabolic diseases: Evidence mapping of observational studies. Syst. Rev. 2020, 9, 274. [Google Scholar] [CrossRef] [PubMed]
- Raigond, P.; Ezekiel, R.; Raigond, B. Resistant starch in food: A review. J. Sci. Food Agric. 2015, 95, 1968–1978. [Google Scholar] [CrossRef]
- Miketinas, D.C.; Shankar, K.; Maiya, M.; Patterson, M.A. Usual dietary intake of resistant starch in US adults from NHANES 2015–2016. J. Nutr. 2020, 150, 2738–2747. [Google Scholar] [CrossRef]
- Krishnan, S.; Adams, S.H.; Allen, L.H.; Laugero, K.D.; Newman, J.W.; Stephensen, C.B.; Burnett, D.J.; Witbracht, M.; Welch, L.C.; Que, E.S.; et al. A randomized controlled-feeding trial based on the Dietary Guidelines for Americans on cardiometabolic health indexes. Am. J. Clin. Nutr. 2018, 108, 266–278. [Google Scholar] [CrossRef] [Green Version]
- King, D.E.; Mainous, A.G., 3rd; Lambourne, C.A. Trends in dietary fiber intake in the United States, 1999–2008. J. Acad. Nutr. Diet. 2012, 112, 642–648. [Google Scholar] [CrossRef]
- McDonald, J.D.; Mah, E.; Chitchumroonchokchai, C.; Dey, P.; Labyk, A.N.; Villamena, F.A.; Volek, J.S.; Bruno, R.S. Dairy milk proteins attenuate hyperglycemia-induced impairments in vascular endothelial function in adults with prediabetes by limiting increases in glycemia and oxidative stress that reduce nitric oxide bioavailability. J. Nutr. Biochem. 2019, 63, 165–176. [Google Scholar] [CrossRef] [PubMed]
- McDonald, J.D.; Mah, E.; Dey, P.; Olmstead, B.D.; Sasaki, G.Y.; Villamena, F.A.; Bruno, R.S. Dairy milk, regardless of fat content, protects against postprandial hyperglycemia-mediated impairments in vascular endothelial function in adults with prediabetes by limiting oxidative stress responses that reduce nitric oxide bioavailability. J. Nutr. Biochem. 2019, 63, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.; Gertz, E.R.; Adams, S.H.; Newman, J.W.; Pedersen, T.L.; Keim, N.L.; Bennett, B.J. Effects of a diet based on the Dietary Guidelines on vascular health and TMAO in women with cardiometabolic risk factors. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thaiss, C.A.; Levy, M.; Grosheva, I.; Zheng, D.; Soffer, E.; Blacher, E.; Braverman, S.; Tengeler, A.C.; Barak, O.; Elazar, M.; et al. Hyperglycemia drives intestinal barrier dysfunction and risk for enteric infection. Science 2018, 359, 1376–1383. [Google Scholar] [CrossRef] [Green Version]
- Blaak, E.E.; Canfora, E.E.; Theis, S.; Frost, G.; Groen, A.K.; Mithieux, G.; Nauta, A.; Scott, K.; Stahl, B.; van Harsselaar, J.; et al. Short chain fatty acids in human gut and metabolic health. Benef. Microbes 2020, 11, 411–455. [Google Scholar] [CrossRef]
- Park, J.-h.; Kotani, T.; Konno, T.; Setiawan, J.; Kitamura, Y.; Imada, S.; Usui, Y.; Hatano, N.; Shinohara, M.; Saito, Y.; et al. Promotion of intestinal epithelial cell turnover by commensal bacteria: Role of short-chain fatty acids. PLoS ONE 2016, 11, e0156334. [Google Scholar] [CrossRef]
- Fukuda, S.; Toh, H.; Hase, K.; Oshima, K.; Nakanishi, Y.; Yoshimura, K.; Tobe, T.; Clarke, J.M.; Topping, D.L.; Suzuki, T.; et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 2011, 469, 543–547. [Google Scholar] [CrossRef]
- Klingbeil, E.A.; Cawthon, C.; Kirkland, R.; de La Serre, C.B. Potato-resistant starch supplementation improves microbiota dysbiosis, inflammation, and gut-brain signaling in high fat-fed rats. Nutrients 2019, 11, 2710. [Google Scholar] [CrossRef] [Green Version]
- Liang, D.; Zhang, L.; Chen, H.; Zhang, H.; Hu, H.; Dai, X. Potato resistant starch inhibits diet-induced obesity by modifying the composition of intestinal microbiota and their metabolites in obese mice. Int. J. Biol. Macromol. 2021, 180, 458–469. [Google Scholar] [CrossRef]
- DeMartino, P.; Johnston, E.A.; Petersen, K.S.; Kris-Etherton, P.M.; Cockburn, D.W. Additional resistant starch from one potato side dish per day alters the gut microbiota but not fecal short-chain fatty acid concentrations. Nutrients 2022, 14, 721. [Google Scholar] [CrossRef] [PubMed]
- Nishiwaki, H.; Hamaguchi, T.; Ito, M.; Ishida, T.; Maeda, T.; Kashihara, K.; Tsuboi, Y.; Ueyama, J.; Shimamura, T.; Mori, H.; et al. Short-chain fatty acid-producing gut microbiota is decreased in Parkinson’s disease but not in rapid-eye-movement sleep behavior disorder. MSystems 2020, 5, e00797-20. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Li, L. Modulation of short-chain fatty acids as potential therapy method for type 2 diabetes mellitus. Can. J. Infect. Dis. Med. Microbiol. 2021, 2021, 6632266. [Google Scholar] [CrossRef] [PubMed]



| Nutrient | Bagel (100 g, Plain) | Potato (350 g, Raw) |
|---|---|---|
| Energy (kcal) 1 | 275 | 275 |
| Total Fat (g) | 1.6 | 0.3 |
| Protein (g) | 10.5 | 7.47 |
| Carbohydrate (g) | 53.4 | 63.2 |
| Total Dietary Fiber (g) | 2.3 | 4.5 |
| Resistant Starch (g) 2 | 0 | 17.5 |
| Sodium (mg) | 534 | 17 |
| Potassium (mg) | 101 | 1455 |
| All (n = 27) | Male (n = 13) | Female (n = 14) | p | |
|---|---|---|---|---|
| Age (year) 1 | 32.5 ± 1.3 | 31.9 ± 1.5 | 33.1 ± 2.1 | 0.67 |
| BMI (kg/m2) | 35.0 ± 1.0 | 34.9 ± 1.0 | 35.1 ± 1.7 | 0.91 |
| Waist Circumference (cm) | 109.8 ± 2.4 | 114.5 ± 2.1 | 105.4 ± 3.8 | 0.051 |
| SBP (mmHg) | 120.5 ± 1.6 | 125.2 ± 2.3 | 116.1 ± 1.6 | 0.004 |
| DBP (mmHg) | 82.9 ± 1.1 | 84.5 ± 1.3 | 81.4 ± 1.7 | 0.15 |
| Left cIMT (mm) | 0.61 ± 0.02 | 0.58 ± 0.03 | 0.64 ± 0.03 | 0.24 |
| Right cIMT (mm) | 0.60 ± 0.02 | 0.57 ± 0.03 | 0.63 ± 0.03 | 0.18 |
| Plasma Triglyceride (mg/dL) | 127.6 ± 15.5 | 171.2 ± 25.5 | 87.0 ± 10.3 | 0.008 |
| Plasma HDL-C (mg/dL) | 36.7 ± 1.4 | 34.7 ± 2.3 | 38.5 ± 1.6 | 0.19 |
| Plasma Glucose (mg/dL) | 103.7 ± 1.6 | 100.9 ± 2.7 | 106.4 ± 1.5 | 0.09 |
| Plasma Insulin (µIU/mL) | 19.9 ± 4.4 | 13.3 ± 1.1 | 26.5 ± 8.4 | 0.15 |
| HOMA-IR | 5.2 ± 1.2 | 3.3 ± 0.3 | 7.1 ± 2.4 | 0.14 |
| Plasma Ascorbic Acid (µmol/L) | 40.9 ± 4.2 | 41.7 ± 6.2 | 40.2 ± 5.9 | 0.87 |
| Plasma Uric Acid (µmol/L) | 355.2 ± 16.7 | 419.4 ± 13.2 | 300.2 ± 19.0 | <0.001 |
| MetS Criteria 2,3 | ||||
| 3 Risk Factors (%) | 67 | 62 | 71 | 0.70 |
| 4 Risk Factors (%) | 22 | 23 | 21 | >0.99 |
| 5 Risk Factors (%) | 11 | 15 | 7 | 0.60 |
| Waist Circumference (%) | 96 | 92 | 100 | 0.48 |
| HDL-C (%) | 89 | 85 | 93 | 0.60 |
| Glucose (%) | 85 | 69 | 100 | 0.04 |
| Blood Pressure (%) | 44 | 54 | 36 | 0.45 |
| Triglyceride (%) | 37 | 62 | 14 | 0.02 |
| DGA + BAGEL | DGA + POTATO | ||||||
|---|---|---|---|---|---|---|---|
| Parameter | Day 0 | Day 14 | Day 0 | Day 14 | PTime | PTreatment | PInteraction |
| BMI (kg/m2) | 35.6 ± 1.2 | 35.1 ± 1.1 | 35.7 ± 1.2 | 35.2 ± 1.1 | 0.24 | 0.53 | 0.36 |
| Waist Circumference (cm) | 108.5 ± 2.2 | 107.8 ± 2.4 | 108.8 ± 2.2 | 107.5 ± 2.4 | 0.20 | 0.83 | 0.86 |
| SBP (mmHg) | 123.1 ± 1.9 | 113.6 ± 1.9 | 119.1 ± 2.2 | 114.6 ± 1.8 | 0.0001 | 0.18 | 0.06 |
| DBP (mmHg) | 81.5 ± 1.3 | 75.9 ± 1.7 | 81.9 ± 1.5 | 77.7 ± 1.4 | 0.001 | 0.20 | 0.40 |
| Glucose (mg/dL) | 104.4 ± 2.6 | 101.9 ± 2.4 | 109.5 ± 2.8 | 102.3 ± 2.6 | 0.04 | 0.14 | 0.16 |
| Insulin (μIU/mL) | 21.1 ± 5.5 | 14.2 ± 2.0 | 17.0 ± 2.0 | 14.9 ± 2.4 | 0.03 | 0.39 | 0.23 |
| HOMA-IR | 5.5 ± 1.5 | 3.6 ± 0.5 | 4.7 ± 0.6 | 3.8 ± 0.6 | 0.02 | 0.48 | 0.38 |
| Cholecystokinin (pmol/L) | 15.9 ± 6.0 | 14.3 ± 3.1 | 17.5 ± 5.1 | 14.2 ± 3.4 | 0.40 | 0.90 | 0.75 |
| Malondialdehyde (μmol/L) | 1.87 ± 0.1 | 1.78 ± 0.1 | 1.86 ± 0.1 | 1.93 ± 0.1 | 0.81 | 0.13 | 0.11 |
| Endotoxin (EU/mL) | 26.6 ± 2.0 | 21.2 ± 2.9 | 22.3 ± 2.1 | 23.3 ± 2.7 | 0.35 | 0.55 | 0.14 |
| NOx (μmol/L) | 28.6 ± 5.3 | 29.8 ± 3.7 | 23.9 ± 1.8 | 30.1 ± 2.3 | 0.17 | 0.62 | 0.36 |
| Arginine (μmol/L) | 62.0 ± 2.6 | 66.2 ± 2.8 | 66.4 ± 3.3 | 65.7 ± 2.8 | 0.51 | 0.39 | 0.18 |
| ADMA (nmol/L) | 577.2 ± 22.0 | 570.5 ± 17.9 | 592.6 ± 22.7 | 567.7 ± 23.1 | 0.29 | 0.58 | 0.37 |
| ADMA/Arginine (nmol/μmol) | 9.60 ± 0.45 | 8.97 ± 0.43 | 9.33 ± 0.49 | 8.95 ± 0.47 | 0.12 | 0.66 | 0.64 |
| SDMA (nmol/L) | 529.6 ± 16.8 | 581.7 ± 28.8 | 567.4 ± 21.3 | 586.9 ± 26.4 | 0.04 | 0.21 | 0.33 |
| SDMA/Arginine (nmol/μmol) | 8.91 ± 0.45 | 9.22 ± 0.71 | 9.02 ± 0.52 | 9.36 ± 0.63 | 0.49 | 0.76 | 0.97 |
| Homoarginine (μmol/L) | 1.88 ± 0.1 | 2.08 ± 0.2 | 2.04 ± 0.2 | 2.11 ± 0.2 | 0.07 | 0.10 | 0.24 |
| Homoarginine/Arginine (nmol/μmol) | 30.7 ± 2.0 | 31.7 ± 2.1 | 32.2 ± 2.3 | 32.6 ± 2.4 | 0.34 | 0.51 | 0.78 |
| Parameter | DGA + BAGEL | DGA + POTATO | p 1 |
|---|---|---|---|
| Glucose (mg/dL × min) | 2649 ± 499 | 2808 ± 565 | 0.73 |
| Insulin (μIU/mL × min) | 5940 ± 670 | 7086 ± 1372 | 0.29 |
| Cholecystokinin (pmol/L × min) | −196 ± 266 | −67 ± 344 | 0.80 |
| Malondialdehyde (μmol/L × min) | 57.2 ± 6.8 | 45.4 ± 7.2 | 0.08 |
| FMD (% × min) | −259 ± 75 | −198 ± 71 | 0.48 |
| NOx (μmol/L × min) | −240 ± 88 | −433 ± 48 | 0.11 |
| ARG (μmol/L × min) | −826 ± 187 | −833 ± 182 | 0.98 |
| ADMA/ARG (nmol/μmol × min) | 66 ± 35 | 95 ± 29 | 0.47 |
| SDMA/ARG (nmol/μmol × min) | 76 ± 37 | 134 ± 34 | 0.25 |
| homoARG/ARG (nmol/μmol × min) | 0.39 ± 0.18 | 0.45 ± 0.12 | 0.78 |
| DGA + BAGEL | DGA + POTATO | ||
|---|---|---|---|
| μmol/g Feces | p-Value | ||
| Acetate | 52.3 ± 4.7 | 46.6 ± 3.9 | 0.19 |
| Propionate | 23.1 ± 2.2 | 19.3 ± 2.1 | 0.03 |
| Isobutyrate | 2.7 ± 0.2 | 2.3 ± 0.2 | 0.13 |
| Butyrate | 21.3 ± 2.7 | 17.9 ± 2.6 | 0.16 |
| 2-Methylbutryrate | 1.6 ± 0.2 | 1.4 ± 0.1 | 0.23 |
| Isovalerate | 1.8 ± 0.2 | 1.6 ± 0.2 | 0.16 |
| Valerate | 3.1 ± 0.3 | 2.4 ± 0.3 | 0.02 |
| 4-Methylvalerate | 0.04 ± 0.01 | 0.03 ± 0.01 | 0.23 |
| Hexanoate | 1.0 ± 0.3 | 0.7 ± 0.2 | 0.38 |
| Total SCFA | 106.9 ± 9.5 | 92.2 ± 8.5 | 0.09 |
| mol % of total SCFA | |||
| Acetate | 49.3 ± 1.0 | 51.6 ± 1.0 | 0.03 |
| Propionate | 21.9 ± 0.8 | 20.7 ± 0.8 | 0.03 |
| Isobutyrate | 2.9 ± 0.2 | 2.8 ± 0.2 | 0.82 |
| Butyrate | 18.4 ± 1.0 | 17.5 ± 1.1 | 0.39 |
| 2-Methylbutryrate | 1.7 ± 0.2 | 1.7 ± 0.2 | 0.99 |
| Isovalerate | 2.0 ± 0.2 | 2.0 ± 0.2 | 0.84 |
| Valerate | 3.0 ± 0.2 | 2.8 ± 0.2 | 0.02 |
| 4-Methylvalerate | 0.03 ± 0.0 | 0.03 ± 0.0 | 0.54 |
| Hexanoate | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.74 |
| mol % of straight SCFA | |||
| Acetate | 52.8 ± 1.0 | 55.3 ± 1.1 | 0.02 |
| Propionate | 23.5 ± 0.8 | 22.1 ± 0.8 | 0.04 |
| Butyrate | 19.6 ± 1.1 | 18.7 ± 1.1 | 0.36 |
| Valerate | 3.3 ± 0.3 | 3.0 ± 0.2 | 0.04 |
| Hexanoate | 0.9 ± 0.2 | 0.9 ± 0.2 | 0.76 |
| mol % of branched SCFA | |||
| Isobutyrate | 44.3 ± 0.6 | 44.3 ± 0.6 | 0.99 |
| 2-Methylbutryrate | 25.1 ± 0.4 | 25.4 ± 0.5 | 0.55 |
| Isovalerate | 29.8 ± 0.4 | 29.7 ± 0.4 | 0.86 |
| 4-Methylvalerate | 0.8 ± 0.3 | 0.6 ± 0.2 | 0.39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, S.; Shaw, E.L.; Quarles, W.R.; Sasaki, G.Y.; Dey, P.; Hodges, J.K.; Pokala, A.; Zeng, M.; Bruno, R.S. Daily Inclusion of Resistant Starch-Containing Potatoes in a Dietary Guidelines for Americans Dietary Pattern Does Not Adversely Affect Cardiometabolic Risk or Intestinal Permeability in Adults with Metabolic Syndrome: A Randomized Controlled Trial. Nutrients 2022, 14, 1545. https://doi.org/10.3390/nu14081545
Cao S, Shaw EL, Quarles WR, Sasaki GY, Dey P, Hodges JK, Pokala A, Zeng M, Bruno RS. Daily Inclusion of Resistant Starch-Containing Potatoes in a Dietary Guidelines for Americans Dietary Pattern Does Not Adversely Affect Cardiometabolic Risk or Intestinal Permeability in Adults with Metabolic Syndrome: A Randomized Controlled Trial. Nutrients. 2022; 14(8):1545. https://doi.org/10.3390/nu14081545
Chicago/Turabian StyleCao, Sisi, Emily L. Shaw, William R. Quarles, Geoffrey Y. Sasaki, Priyankar Dey, Joanna K. Hodges, Avinash Pokala, Min Zeng, and Richard S. Bruno. 2022. "Daily Inclusion of Resistant Starch-Containing Potatoes in a Dietary Guidelines for Americans Dietary Pattern Does Not Adversely Affect Cardiometabolic Risk or Intestinal Permeability in Adults with Metabolic Syndrome: A Randomized Controlled Trial" Nutrients 14, no. 8: 1545. https://doi.org/10.3390/nu14081545
APA StyleCao, S., Shaw, E. L., Quarles, W. R., Sasaki, G. Y., Dey, P., Hodges, J. K., Pokala, A., Zeng, M., & Bruno, R. S. (2022). Daily Inclusion of Resistant Starch-Containing Potatoes in a Dietary Guidelines for Americans Dietary Pattern Does Not Adversely Affect Cardiometabolic Risk or Intestinal Permeability in Adults with Metabolic Syndrome: A Randomized Controlled Trial. Nutrients, 14(8), 1545. https://doi.org/10.3390/nu14081545








