Abstract
Familial hypercholesterolemia (FH) is a genetic disease characterized by high low-density lipoprotein (LDL) cholesterol (LDL-c) concentrations that increase cardiovascular risk and cause premature death. The most frequent cause of the disease is a mutation in the LDL receptor (LDLR) gene. Diabetes is also associated with an increased risk of cardiovascular disease and mortality. People with FH seem to be protected from developing diabetes, whereas cholesterol-lowering treatments such as statins are associated with an increased risk of the disease. One of the hypotheses to explain this is based on the toxicity of LDL particles on insulin-secreting pancreatic β-cells, and their uptake by the latter, mediated by the LDLR. A healthy lifestyle and a relatively low body mass index in people with FH have also been proposed as explanations. Its association with superimposed diabetes modifies the phenotype of FH, both regarding the lipid profile and cardiovascular risk. However, findings regarding the association and interplay between these two diseases are conflicting. The present review summarizes the existing evidence and discusses knowledge gaps on the matter.
1. Introduction
1.1. Familial Hypercholesterolemia
Familial hypercholesterolemia (FH) is a genetic disease characterized by high low-density lipoprotein (LDL) cholesterol (LDL-c) concentrations that increase cardiovascular risk and cause premature death [1]. The most frequent mutations are found in the LDL receptor gene (-LDLR- responsible for LDL uptake), though other genes involved in LDL metabolism can also cause the disease, such as apolipoprotein B 100 (APOB), apolipoprotein E (APOE) or proprotein convertase subtilisin/Kexin-type 9 (PCSK9) [2,3]. Heterozygous FH (HeFH) (one affected allele) is the usual presentation form, with a prevalence of 1/250 [4], higher in isolated regions [5,6,7]. LDL-c concentrations in people with HeFH are often twice those of the general population [8]. Homozygous FH (HoFH) is infrequent (1/160,000–1/300,000) but more severe, with LDL-c concentrations exceeding 500 mg/dL from birth. Without treatment, subjects with HoFH develop atherosclerosis before the age of 20 and die before 30 [9]. The diagnosis of FH is usually made based on LDL-c concentrations, family history, and the presence of corneal arcus, xanthomas, or xanthelasmas [8]. Although affected individuals have a higher cardiovascular risk than the general population [10], subjects with the same mutation show enormous phenotype variability. These differences might be explained by other factors such as the type of mutation [11], age [12], gender [10,13], or the existence of other concomitant diseases [14].
1.2. Diabetes Mellitus
Diabetes mellitus (DM) is a group of metabolic disorders defined by increased blood glucose concentrations. The most frequent types of DM are type 1 diabetes (-T1DM- mediated by autoimmune destruction of pancreatic ß cells and absolute insulin deficiency), type 2 diabetes (-T2DM- caused by progressive loss of insulin secretion in the context of insulin resistance) and gestational DM (first diagnosed during pregnancy), but there are also other, less frequent forms of the disease, such as monogenic DM or drug-induced DM [15]. A correct classification of DM is important since both treatment and follow-up depend on it. The prevalence of DM has doubled since the 1990s [16]; nowadays, there are about 537 million subjects with DM around the world (mostly T2DM), and this is expected to continue increasing in the near future [17]. Its complex physiopathology involves modifiable factors such as weight, diet, or physical activity [18], and non-modifiable factors such as genetics, age, or gender [19]. Patients have an increased all-cause mortality [20], but about 50% die because of cardiovascular complications [21], especially women [22], and people with long-standing disease [23,24]. This cardiovascular risk is enhanced in the presence of other risk factors such as smoking, hypertension, or dyslipidemia that contribute to endothelial damage and the progression of atherosclerosis [25].
The prevalence of DM is generally lower in people with FH than in the general population [26], suggesting a relationship between glucose and lipid metabolism. The aim of this paper is to summarize the existing evidence and contribute to the understanding of the complex underlying mechanisms that relate DM and HF.
2. Familial Hypercholesterolemia and Diabetes: Molecular Causes
2.1. Genetics of FH
FH is the most common monogenic disorder. It has high penetrance (90%) and autosomal dominant inheritance [1] and is caused by mutations in genes related to LDL metabolism.
HeFH is mainly caused by loss-of-function mutations in LDLR (85–90%) or APOB (5%), or gain-of-function mutations in PCSK9 (1–3%) [27]. Mutations have also been identified in APOE [3] and in the adaptor protein type 1 gene (LDLRAP1), the latter with autosomal recessive inheritance [28]. However, 10–40% of patients with a clinical phenotype of FH have negative genetic tests, probably representing severe polygenic forms of hypercholesterolemia [29].
HoFH is a more severe form that involves two mutations in the aforementioned genes. According to the combination of mutations, HoFH is classified into the following: true homozygotes (two equal mutations in both alleles of the same gene, mostly in LDLR); compound heterozygotes (a different mutation in each allele of the same gene); double heterozygotes (two different mutations in different genes); autosomal recessive hypercholesterolemia (mutations in LDLRAP1) [9]. The phenotype of HFHo will depend on the degree of residual LDLR activity, which is defined by the genetic defect. Indeed, in some cases, the LDLR protein is almost absent (less than 2%), leading to the most extreme phenotypes [30].
LDLR is the most frequently affected gene in HF and more than 3000 mutations have been described so far, most of them disease-causing or pathogenic [2]. Traditionally, mutations were classified into classes I to V, with class I mutations being the most severe, where no protein synthesis is present (large rearrangements, insertions, nonsense frameshifts, or splicing mutations). Classes II-IV include alterations in LDLR transport, LDLR binding, internalization, or recycling of LDLR, corresponding to in-frame, missense mutations, or small deletions [27]. Currently, there is a tendency to simplify this classification into class 1 and non-class 1 mutations [31], which would correspond to null or defective alleles, respectively, and this correlates with the severity of the individual phenotype. Null LDLR allele carriers present with very high LDL-c concentrations, premature coronary heart disease and poor response to treatment [32]. However, LDL-c concentrations have been shown to improve cardiovascular risk prediction more than the genetic defect per se. A cohort study in 12,245 FH LDLR mutation carriers showed that the classification of pathogenic LDLR variants according to LDL-c concentration percentile was indeed more accurate than class 1 vs. non-class 1. The relative risk of major cardiovascular events ranged from 2.2 in subjects with an LDL-c concentration below the 75th percentile to 13 when the LDL-c concentration was above the 98th percentile of the cohort [33].
APOB was the second gene identified to be associated with FH, also called familial defective APOB [34]. It is less frequent than FH caused by LDLR mutations, and there are currently about 35 pathogenic mutations described, generally located in the LDLR-binding domain of apolipoprotein B (apoB) [27]. The most common is the R3500Q mutation, which accounts for 5–10% of FH cases in northern Europe [35]. Patients with this form of FH present with less severe phenotypes than LDLR mutation carriers and have lower LDL-c concentrations and less cardiovascular events [36].
FH type 3 is caused by gain of function mutations in PCSK9 [37], and there are about 30 pathogenic variants reported [27]. The phenotype is variable, with variants such as p. (Asp374Tyr), which causes an extreme FH phenotype with very high LDL-c concentrations and premature coronary heart disease [38], and other mutations affecting distinct domains of the protein, leading to milder phenotypes and better response to treatment [39].
In patients with an FH phenotype but no mutation identified, a polygenic mechanism should be considered, caused by the aggregation of common LDL-c-raising genetic variants or single nucleotide polymorphisms (SNPs), which can be studied using validated polygenic risk scores [40,41].
There are other genes that are no longer considered to cause FH, such as STAP1, which seemed to be associated with the disease, but subsequent in vitro and family segregation studies have shown that it does not cause FH [42,43].
2.2. Genetics of Type 2 Diabetes
Regarding the genetics of DM, there are both monogenic forms, including neonatal diabetes mellitus and maturity-onset diabetes of the young (MODY), and the following polygenic forms: T1DM or T2DM [44]. Neonatal diabetes is caused mainly by paternally inherited duplications in chromosome 6q24 that cause overexpression of paternally imprinted genes, mutations in KATP channels, potassium inwardly rectifying channels, subfamily J, member 11 (KCNJ11) or ATP Binding Cassette Subfamily C Member 8 (ABCC8) genes, among others [45]. Mutations in the hepatocyte nuclear factor 1-α (HNF1A), 4-α (HNF4A), 1-ß (HNF1B/TCF2) and glucokinase (GCK) genes are responsible for most of the cases of MODY [46].
The development of T2DM depends on both environmental [47] and genetic causes. The genetics of T2DM are very complex, and genome-wide association studies and whole-genome sequencing have shown more than seventy genes related to the pathogenesis of the disease [48,49]. A large number of SNPs have been described in more than 400 distinct genomic regions [50]. The heritability of T2DM ranges from 20 to 80% [51], the highest concordance corresponding to monozygotic twins [52]. Despite the huge number of risk SNPs identified, each one accounts only for a small effect on the risk of T2DM, around 10–20% increase per risk allele [44]. Because of this, various genetic risk scores have been developed to evaluate the cumulative effect of multiple SNPs and to identify individuals with a high genetic risk of T2DM [53,54].
The genes with the most reported risk variants are KCNJ11, peroxisome proliferator-activated receptor gamma (PPARG), HNF1B/TCF2 and wolfram syndrome 1 (wolframin) (WFS1), confirmed by genome-wide association studies [55]. Other genes related to T2DM are insulin receptor substrate 1 gene (IRS1) and IRS-2, ABCC8, Phosphatase and Tensin Homolog (PTEN), Zinc Transporter-8 Gene (SLC30A8), GATA Binding Protein 6 (GATA6), ISL LIM Homeobox 1 (ISL-1), Transcription Factor 7-like 2 (TCF7L2), Insulin-like Growth Factor 2 mRNA-Binding Protein 2 (IGF2BP2), among many others [48,50,56].
The effects of variants in these genes can lead to impaired insulin response, decreasing insulin sensitivity, loss of the ß cell morphology, generate oxidative stress in the pancreas, destruction of pancreatic β-cells altering insulin biosynthesis, causing insulin receptor dysfunction, etc. [48,56]. Due to the polygenic feature, many genes and their SNPs contribute to an enhanced risk of T2DM, which together with environmental triggers, like obesity, leads to the development of the disease [51].
2.3. Genetic Studies Assessing the Link between Hyperlipidemia and Type 2 Diabetes
Mendelian randomization studies suggest that there is an overlap between the risks of DM and hyperlipidemia. Indeed, after combining and analysing existing information provided by three large consortia, Fall et al. report a significant association between gene variants determining higher LDL-c and a lower risk of T2DM, whereas the association with variants determining HDL-c and triglycerides was less clear [57,58]. When constructing the risk scores, the authors excluded SNPs associated with adiposity, which they considered a possible confounder. White et al. used a modified approach in a dataset combining several genome-wide association studies, including 188,577 individuals with measured blood lipids and 34,840 with T2DM. A 130 SNP score was developed for LDL-c (explaining 7.9% of its variance), and 140 SNP scores, for HDL-c and triglycerides. For each SD (38 mg/dL) estimated increase in LDL-c, the risk of T2DM was reduced by 21% (R 0.79 (0.71–0.88)). For triglycerides, every 89 mg/dL estimated increase was also associated with a reduction in T2DM (OR 0.83 (0.72–0.95)), as was the case for every 16 mg/dL estimated increase in HDL-c (OR 0.83 (0.76–0.90)) [59]. Although the protective effect of triglycerides seems somewhat unexpected, other studies in different ethnic groups agree with this finding [60,61].
3. Familial Hypercholesterolemia and Glucose Metabolism: Risk of Diabetes
3.1. Epidemiological Studies
In 2019, the worldwide prevalence of DM was 9.3%, higher in men (9.6 vs. 9%) and in high-income countries (10.4 vs. 4%) [17]. Most epidemiological studies in FH subjects have shown a lower DM prevalence than in the general population (see Table 1). In a Dutch cohort with more than 14,000 FH subjects, only 2.8% had DM [62], whereas a British cohort showed an even lower prevalence (0.8%) [63], and intermediate results were described in 263 French-Canadian patients with FH [64]. Recently, a Spanish study with more than 1700 subjects with FH found a T2DM prevalence close to 6%, around one third of the national average [65]. However, another recently published Spanish study, performed on the island of Gran Canaria, showed an unexpectedly high prevalence of DM in HeFH LDLR mutation carriers (25%) [66]. Other studies show a high prevalence of DM too, above 20%, but in patients with only clinical diagnosis of FH without genetic confirmation [67,68].

Table 1.
Prevalence of diabetes in representative populations with FH.
Regarding the relationship between FH mutations and DM, the results are not consistent. Patients with mutations in APOB, with a less severe phenotype, had a higher prevalence of T2DM (1.91%) than LDLR mutation carriers, and amongst these, the most severe phenotype (receptor-negative) had the lowest prevalence of DM (1.12%) [26]. In accordance with these findings, PCSK9 InsLEU mutation carriers had a higher prevalence of DM and a lower incidence of coronary heart disease. However, other studies have not found an association between mutation type and DM [74,75].
3.2. Lipid-Lowering Treatment and Risk of Diabetes
In recent years, many drugs have been developed to treat hypercholesterolemia, and several studies have shown that they could alter glucose tolerance, highlighting the link between cholesterol and glucose metabolism (see Table 2).

Table 2.
Studies assessing the association between lipid-lowering drugs and disorders of glucose metabolism.
3.2.1. Statins
Statins are the treatment of choice for hypercholesterolemia, both in primary and secondary prevention [93,94]. They inhibit the 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMG-CoA reductase), increase LDLR expression, and reduce plasma LDL-c concentration by over 50% [95]. New-onset DM (NODM) has a prevalence of 9–12% and is one of most recognized side effects of statins [76,96]. Risk increases with age in women [97], and in people with more than two risk factors for DM (impaired fasting plasma glucose, hypertriglyceridemia, hypertension, obesity, or the metabolic syndrome) [77,78]. The risk of DM seems to be independent of LDL-c concentrations [76,79] and varies according to statin type and dose, as well as exposure time [80,98]. Nevertheless, this association with NODM should not discourage health professionals from prescribing these drugs, given their proven cardiovascular benefit, especially in high-risk individuals [99,100]. Simvastatin, atorvastatin, and rosuvastatin have shown more glucose impairment, while pitavastatin has a lower risk of NODM compared with atorvastatin and rosuvastatin [81,96,101]. Pravastatin has also shown favourable results, probably related to its lower liposolubility and limited potency [82]. However, FH subjects seem to be protected against these diabetogenic effects [70].
3.2.2. Ezetimibe
Ezetimibe inhibits intestinal absorption of cholesterol by blocking the Niemann-Pick C1 like1 (NPC1L1) transporter [102], and is frequently used as a concomitant treatment to statins. Its relationship with glucose metabolism is controversial. Several studies have shown that fasting plasma glucose, glycosylated haemoglobin (HbA1c) and insulin sensitivity improve with ezetimibe treatment, both in DM and non-DM individuals [103,104]. This drug also improves inflammation markers and obesity and reduces waist circumference [83]. Based on these positive results, a possible compensatory effect on the diabetogenic effects of statins has been studied. Dragi et al. found that the combination of low-dose-pravastatin plus ezetimibe improved insulin resistance and inflammation compared with high-dose-pravastatin alone [84]. In 2018, a meta-analysis concluded that patients who used low-dose-statins plus ezetimibe for more than 3 months had lower fasting plasma glucose compared with those treated with high-dose statins [105]. Nevertheless, no differences in the HOMA-IR index were found when two statins in monotherapy were compared with a combination of low-dose-statin plus ezetimibe [85]. No significant differences were found either, in a recent study that compared statins alone versus their combination with ezetimibe in glucose intolerant patients followed for 7 years [106]. Other studies have found neutral [107] or deleterious effects on glycemic metabolism with ezetimibe, with an increase in HbA1c and hepatic long-chain fatty acids in patients with non-alcoholic fatty liver disease [86].
The discrepancies in the results could be explained by the small number of participants in some studies, insufficient follow-up, or the presence of other lipid-lowering drugs that could act as confounders.
3.2.3. PCSK9 Inhibitors (PCSK9-i)
Inhibition of the PCSK9 enzyme prevents LDLR degradation after cellular internalization, reducing LDL-c by about 60%. Approved in 2015, monoclonal antibodies against PCSK9 (alirocumab and evolocumab) have shown a favourable safety profile with few side effects [108], but the consequences on glucose metabolism are still not clear. Despite the fact that most clinical studies have not found an association between PCSK9-i and NODM or worsening of pre-existing DM [87,109].
A large study including more than 96,000 individuals followed for 1.5 years found a small but significant increase in plasma glucose and HbA1c but not a higher incidence of NODM in those treated with PCSK9-i [88]. In 2020, a meta-analysis found that alirocumab was associated with a reduction in the risk of DM and, when compared with ezetimibe in monotherapy, evolocumab was also associated with this risk reduction. However, when used in combination with statins, an increased risk of NODM was found in the PCSK9-i group, even though the use of statins was equivalent between the experimental and active comparator arms [89]. It seems that the combination with other lipid-lowering drugs (especially statins) could change the studies’ results due to the discrepancies in background treatment between groups. Furthermore, mendelian randomization studies must be interpreted carefully. As is the case for other lipid-lowering drugs, follow-up is often limited and could be insufficient to see an effect on glucose metabolism [110].
3.2.4. Bempedoic Acid
Bempedoic acid is a newly developed drug that inhibits adenosine triphosphate citrate lyase, increasing LDLR expression and reducing LDL-c [90]. In the phase 3 “CLEAR” studies, bempedoic acid was associated with a reduced incidence of DM and an improvement in fasting blood glucose and HbA1c in week 12 in pre-DM or DM subjects, without increasing NODM risk for 1 year [90,91]. A recent meta-analysis found a reduction of 34% in NODM risk [91].
3.2.5. Other Cholesterol-Lowering Drugs
Nicotinic acid (B3 vitamin) reduces triglyceride and LDL-c concentrations and raises high-density lipoprotein cholesterol (HDL-c) by up to 35% [111]. It is associated with an increased risk of NODM and higher fasting plasma glucose and HbA1c, especially in predisposed individuals, with a dose-dependent effect [112]. Niacin has other side effects, such as flushing, and does not reduce cardiovascular events in secondary prevention [113], so its use is currently limited.
Bile acid sequestrants (resins) reduce bile acid reabsorption and increase hepatic LDLR, lowering LDL-c by 15–25%. They improve the glucose profile but do not cause hypoglycemia in T2DM subjects. Similar results have been found with different resins and in both pre-DM and healthy individuals [92,112,114]. Although they have a moderate lipid-lowering effect, they could be useful in subjects with DM because of their dual effects on lipid and glucose metabolism.
3.3. Genetics and Metabolism
The cause of the lower prevalence of DM in FH subjects found in most studies is not clearly known yet. In vitro, long exposure to fatty acids has been associated to β-cells dysfunction and reduced insulin secretion, especially when coexisting with hyperglycemia [115,116]. Moreover, in vitro studies have shown that intracellular cholesterol accumulation also induces apoptosis of pancreatic β-cells [117]. LDL particle uptake causes β-cell death in a dose-dependent manner, and this toxicity can be counteracted by HDL, very LDL (VLDL) particles, or antioxidants [118]. Supporting these findings, polymorphisms in ATP-binding cassette transporter 1 gene (ABCA1), involved in cholesterol efflux and HDL synthesis, have been associated to obesity, the metabolic syndrome, and DM [119,120]. On the β-cell, HDL particles have an anti-inflammatory effect and participate in cholesterol efflux [121]. Higher HDL-c levels are associated with less hyperglycemia and HDL particle size is inversely correlated to T2DM risk in the general population [122].
A large meta-analysis of genetic association studies assessing the effects of cholesterol-lowering variants in or near NPC1L1, HMGCR, PCSK9, ABCG5/G8 and LDLR showed an overall increased risk of DM with an odds ratio of 1.19–2.42 for every 1 mmol/L (38.6 mg/dL) reduction in LDLc [110]. However, there was rather high heterogeneity in the meta-analysis, suggesting gene-specific associations with DM. Indeed, the highest risk of T2DM was associated with variants in or near NPC1L1, whereas the HMGCR locus was associated with body mass index and waist-to-hip ratio, and PCSK9, with higher fasting and two-hour glucose concentrations [110].
The lipotoxicity hypothesis could, at least partially, explain how statins increase NODM and how FH reduces the risk of DM. The rise in LDLR increases LDL particle uptake by pancreatic β-cells, thereby promoting dysfunction and apoptosis, especially in those with baseline glucose disturbances. On the other hand, genetic mutations that prevent cholesterol input, like FH, could be protective and explain the inverse relationship between mutation severity and DM prevalence [123]. However, clinical studies do not clearly reflect this theory. No differences in insulin, C peptide, or fasting plasma glucose concentrations have been found comparing FH with non-FH subjects, regardless of their insulin sensitivity [124,125,126]. Indeed, in some studies, FH has even been associated with an increased risk of impaired glucose metabolism [7,127].
In vivo studies show controversial results. When comparing prediabetic wildtype vs. LDLR knock-out (KO) mice, no differences were observed in glucose levels, although less insulin secretion and more β-cell apoptosis were seen in LDLR KO mice [128].
In a study in PCSK9 KO and PCSK9/LDLR double knock-out mice, the former showed reduced insulin secretion and glucose intolerance, as well as cholesteryl ester accumulation in β-cells compared with WT mice. In the double knock-out mice, these alterations were restored, supporting the hypothesis that LDLR, the target of PCSK9, is responsible for the phenotype [129]. However, a later study with PCSK9 KO and PCSK9 ß-cell specific KO mice does not show any alteration on glucose homeostasis nor in β-cell function [130].
Thus, other molecular or environmental factors are probably involved in DM risk. For example, plasma lipoprotein(a) (Lp(a)) has been shown to be higher in HeFH compared with the general population [131], and an inverse association has been described between Lp(a) concentrations and the risk of T2DM [132]. However, this effect has to be confirmed, and a mechanism explaining it is still to be found.
Regarding environmental factors, a study comparing a cohort of 2185 HeFH subjects from the Spanish Dyslipidaemia Registry with a representative sample of the background population showed more favorable cardiovascular risk profiles in the former. Indeed, HeFH subjects without cardiovascular disease showed a lower body mass index and a lower prevalence of smoking than the background populations, suggesting that the lower prevalence of T2DM could, at least partially, be explained by a healthier lifestyle in patients with FH [133].
4. Coexistence of Diabetes and Familial Hypercholesterolemia: Clinical Consequences
4.1. Effects on the Lipoproteins
Cardiovascular disease is the leading cause of death in people with DM. Traditionally, DM has been considered to increase the risk of ischemic heart disease, stroke, and peripheral arterial disease by 2–4 times [134]. Although recent studies show that contemporary treatment for cardiovascular risk has reduced the excess mortality associated with the disease, DM remains a very strong independent risk factor for cardiovascular morbidity and mortality [135]. Therefore, since FH is associated with an elevated risk of premature atherosclerosis, it is conceptually reasonable to assume that the coexistence of both DM and FH has a strong impact on cardiovascular disease risk.
While decreased clearance of LDL particles and accumulation of LDL-c is the main determinant for increased cardiovascular disease in FH, multiple interconnected mechanisms have been involved in vascular damage caused by DM, including hyperglycemia-induced overproduction of reactive oxygen species, accumulation of advanced glycation products, activation of protein kinase C and chronic inflammation [136]. In addition, DM is also responsible for a characteristic cluster of lipid disorders with high atherogenic potential, known as diabetic dyslipidemia. Although diabetic dyslipidemia and FH share hyperbetalipoproteinemia as the fundamental mechanism for atherogenesis, the mechanisms behind them and their biochemical expression are different.
The hallmarks of diabetic dyslipidemia are hypertriglyceridemia and decreased HDL-c, whereas LDL-c concentrations are normal or only slightly increased. Although the mechanisms of diabetic dyslipidemia are not completely understood, it is accepted that insulin resistance is its main underlying element [137]. Under physiological conditions, insulin inhibits lipolysis in adipose tissue and activates lipoprotein lipase, an enzyme involved in the plasma clearance of triglycerides from VLDL and chylomicrons. In a state of insulin resistance, lipolysis is not inhibited, and increased circulating free fatty acids are readily taken up by the liver and used as substrates for synthesis and subsequent release of VLDL. Hypertriglyceridemia stimulates the enzymatic activity of cholesteryl ester transfer protein and, during their passage through the circulation, VLDL particles transfer their triglycerides to HDL and LDL in exchange for cholesteryl esters [137]. Triglyceride-enriched HDL undergoes lysis by hepatic lipase, a mechanism by which they are converted into small, dense particles with reduced antioxidant, anti-inflammatory, and anti-atherogenic capacity compared to normal HDL. The smaller HDLs, in turn, are cleared more rapidly from the circulation, resulting in a decrease in HDL-c and apolipoprotein A-1 (apoA-1) concentrations [137]. In a similar manner, LDL particles also become smaller and denser due to a higher ratio of protein to lipid (LDL phenotype B). These LDL particles are resistant to receptor binding, pass more readily through the arterial wall, bind to proteoglycans and are more susceptible to oxidation [138]. On the whole, although LDL-c is not characteristically increased, diabetic dyslipidemia is characterised by an increase in the total number of apoB-containing particles (VLDL, IDL, and LDL).
Several studies have assessed the presence of phenotypic features of diabetic dyslipidemia in non-diabetic subjects with FH. LDL particles from both HoFH and HeFH patients appear to be larger, more buoyant, and more resistant to oxidation than those from healthy controls [139]. Thus, the qualitative properties of LDL do not seem to play a significant role in the development of atherosclerosis in people with FH. Furthermore, patients with FH usually have normal triglyceride concentrations. However, experimental studies have suggested that defective LDLR promotes liver uptake of chylomicrons and remnants and increases VLDL secretion [140,141]. In fact, disturbed triglyceride-rich lipoprotein metabolism and, particularly, postprandial dyslipoproteinemia have been proposed as a putative modulator of cardiovascular risk in HeFH [142]. The possible role of lipoprotein lipase in postprandial hyperlipemia among subjects with HeFH has not been specifically studied. However, individuals with HeFH who carry an LPL gene variant that reduces lipoprotein lipase activity, show higher triglyceride levels and lower HDL-c levels than non-carriers of this mutation [143]. This suggests that a decreased lipoprotein lipase activity, as occurs in insulin resistance, could condition the phenotype of HeFH. Finally, results have been discordant regarding serum concentrations of HDL-c in subjects with FH [141]. This is probably related to the fact that, in subjects with FH, there is an increase in both synthesis and catabolism of HDL particles, but there may be an imbalance between both processes that varies depending on population-specific genetic or environmental factors. Increased apoA-1 catabolism due to increased cholesteryl ester transfer protein activity favours the generation of small HDL particles rich in triglycerides and apolipoprotein E [144,145]. Moreover, HDL particles in subjects with FH may show different functional abnormalities not detectable by measuring HDL-c alone. This may include a defective ability to reverse cholesterol transport from macrophages and impaired anti-inflammatory and antioxidant capacity [144,145].
As mentioned above and depicted in Figure 1, it is reasonable to think that subjects with FH who develop DM may have alterations in lipid metabolism resulting from the additive effect of both diseases. A few studies have compared the clinical characteristics and lipid profiles of HeFH subjects with and without T2DM [68,74,146]. Patients with DM were older, had a higher prevalence of hypertension, and had a higher body mass index than patients without DM. As expected, they also had a lipid profile more characteristic of diabetic dyslipidemia, including higher triglyceride and lower HDL-c and apoA-1 concentrations [68,74,146], as well as higher concentrations of markers of subclinical systemic inflammation, such as C-reactive protein and neutrophil count [68], typical of individuals with insulin resistance.
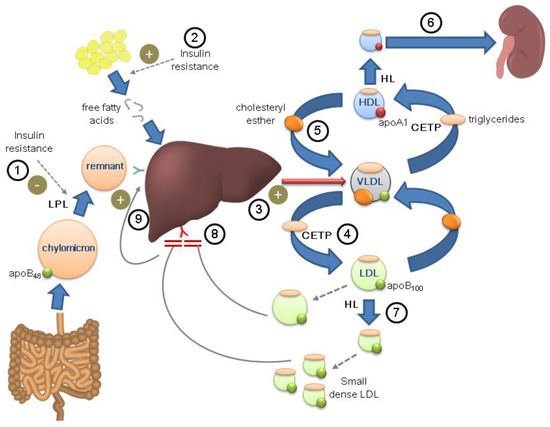
Figure 1.
Potential combination of the physiopathological mechanisms of diabetes and familial hypercholesterolemia in the same individual. Diabetic dyslipidemia. Insulin resistance reduces lipoprotein lipase activity (LPL) ①, decreasing plasma triglyceride clearance, and promotes the release of free fatty acids ②, which are taken up by the liver and used for the synthesis and release of VLDL ③. VLDL exchange triglycerides and cholesterol esters with LDL ④ and HDL ⑤ through the action of cholesteryl ester transfer protein (CETP). Triglyceride-rich HDL particles, through the action of hepatic lipase (HL), are converted into smaller particles, with less anti-atherogenic properties, which are cleared more rapidly in the kidney ⑥. LDL particles also become smaller and denser (LDL phenotype B), more pro-atherogenic ⑦. Familial hypercholesterolemia. The genetic defect in LDL receptor prevents its uptake and metabolism in the liver, favoring the accumulation of LDL particles ⑧. This generates an increase in the uptake of chylomicrons and remnants in the liver ⑨, in turn boosting the synthesis of VLDL.
4.2. Effects on Chronic Arterial Wall Inflammation and Endothelial Dysfunction
In recent decades, abundant scientific evidence has highlighted the preponderant role of immunological and inflammatory mechanisms in the development and progression of atherosclerosis. As mentioned above, inflammatory mechanisms may be particularly important in the development of cardiovascular disease in individuals with T2DM. Epidemiological studies have shown that insulin resistance is associated with high concentrations of uric acid and a wide set of acute phase reactants and markers of endothelial dysfunction [147,148]. In addition, obesity, commonly present among people with T2DM, perpetuates the maintenance of a state of chronic inflammation as adipose tissue secretes a variety of proinflammatory adipocytokines such as tumour necrosis factor α, interleukins 1, 6, and 8, resistin, adiponectin, leptin, and adipsin [149].
Increased blood concentrations of different biomarkers of systemic inflammation, endothelial activation, and oxidative stress [150,151] have also been reported in FH subjects, and some authors have postulated their possible role as tools for cardiovascular risk stratification in HeFH [152]. In any case, these studies reveal that DM and FH could share a greater predisposition to the activation of pathways leading to arterial wall inflammation and endothelial activation, promoting early mechanisms of atherosclerosis induction.
4.3. Effects on the Cardiovascular Risk
Contrary to theoretical assumptions and evidence from the general population, in which the role of DM as a cardiovascular risk factor is incontrovertible, studies that have evaluated the association between DM and cardiovascular disease in HeFH have offered contradictory results. Over the past two decades, a considerable number of studies have assessed the role of classical cardiovascular risk factors in patients with HeFH. A multi-centre retrospective cohort study performed in the Netherlands on 2400 patients (112,943 person-years) [153] found that, along with male gender, smoking, hypertension, low HDL-c and Lp(a), DM was independently associated with the presence of at least one cardiovascular event (RR 2.19; 95% CI: 1.36–3.54). Very recently, another methodologically similar study, which evaluated 1050 Japanese patients with HeFH over 19 years, also demonstrated that DM was an independent risk factor for a composite of major adverse cardiovascular events (HR 1.81; 95% CI: 1.12–2.25) [154]. However, the results of cross-sectional studies were mixed (see Table 3), and in many of them, DM was no longer significantly associated with the presence of cardiovascular disease after adjustment for other covariates. In many of the studies that found no association, either the population size was small or the prevalence of DM was very low, possibly limiting the statistical power to detect the association between DM and cardiovascular disease. In fact, a meta-analysis of 27 studies, published in 2018, aimed at assessing the association between cardiovascular disease and several classical risk factors, adding up to 41,831 subjects and 6629 cardiovascular events, found that DM was indeed an independent risk factor in HeFH (OR 1.95; 95% CI: 1.33–2.57), along with age, male sex, hypertension, body mass index, smoking, increased Lp(a), low HDL-c and a family history of cardiovascular disease [14].
In recent years, mainly due to the wide variation in established cardiovascular disease rates, even among individuals who share the same mutation and belong to the same family, there has been a growing interest in finding tools for cardiovascular risk stratification in subjects with HeFH. To this end, predictive models specifically designed for HeFH have been developed, and, strikingly, DM was not a factor to be taken into account in any of them. The first one, the Montreal-FH-SCORE, was calculated on the basis of retrospective data from a sample of 670 patients carrying a known FH-causing mutation in the LDLR gene, and it combines five predictor variables (age, gender, smoking, hypertension, and untreated HDL-c levels) [155]. In light of these findings, the authors conducted a specific study to investigate the impact of DM on cardiovascular disease in FH, using data from 1412 patients (73 with DM) from the FH Canada Registry. Although patients with DM had a higher prevalence of established cardiovascular disease, their results confirmed that including DM did not improve risk prediction with respect to the Montreal-FH-SCORE [146]. Subsequently, two mathematical models for cardiovascular risk prediction have been developed, but, unlike the Montreal-FH-SCORE, which had the limitation of being based on retrospective data, these were generated using prospective data from registries that collected incident cardiovascular events. The SAFEHEART Risk Equation was estimated using data from 2404 Spanish patients (104 with DM) with HeFH. Age, male sex, history of previous atherosclerotic cardiovascular disease, high blood pressure, increased body mass index, active smoking, and LDL-c and Lp(a) concentrations, but not DM, were independent predictors of incident cardiovascular events [156]. The FH-Risk SCORE was developed from a multinational prospective cohort of 3881 adults (152 with DM) with HeFH and no prior history of atherosclerotic cardiovascular disease. DM was not among the selected variables for the FH-Risk SCORE equation either, which incorporates sex, age, HDL-c, LDL-c, hypertension, smoking, and Lp(a) concentration as independent risk factors for 10-year atherosclerotic cardiovascular disease [157]. It should be noted that, until the publication of these two large studies, only a few long-term prospective studies had been carried out to assess the occurrence of new cardiovascular events in subjects with FH and, again, DM was not a significant risk factor in any of them [36,158,159].
Overall, the information available to date suggests that the role of DM as a cardiovascular risk factor in the FH population is smaller than in the general population. However, as their authors themselves acknowledge, due to the low prevalence among the FH population, even the highest quality prospective studies included small numbers of patients with DM and may not have had sufficient statistical power to determine the true effect of the disease [156,157]. Therefore, as has already been cautioned before [160], it is probably premature to underestimate the role of DM, and clinical judgement should be applied to establish the individual risk of a person with both FH and DM, considering other specific variables related to the disease, such as type of DM, time since diagnosis, or target organ damage, as recommended in clinical practice guidelines [161].

Table 3.
Cross-sectional studies that have assessed the association between diabetes and cardiovascular disease in subjects with heterozygous familial hypercholesterolemia.
Table 3.
Cross-sectional studies that have assessed the association between diabetes and cardiovascular disease in subjects with heterozygous familial hypercholesterolemia.
| Author, Year | Study Type * | Country | FH Diagnostic Criteria ** | N | Diabetes (%) | Univariate Association OR (95% CI) | Multivariate Association OR (95% CI) | Adjusting Covariates |
|---|---|---|---|---|---|---|---|---|
| Hopkins, 2001 [162] | RR | USA | MEDPED criteria | 262 | 3.0 | NS | NS | Age, sex, BMI, smoking, waist to hip ratio, hypertension, HDL-c, triglycerides, small LDL, Lp(a), homocysteine, insulin, white cell count, C-reactive protein, xanthomas, intima-medial thickness, angiotensin-converting enzyme I/D polymorphism |
| De Sauvage, 2003 [163] | MC | Netherlands | Genetic test or definite DLCN criteria | 526 | 2.1 | 17.61 (2.25–137.8) | NS | Age, sex, BMI, smoking, total-c, LDL-c, HDL-c, triglycerides, Lp(a), apo A1, apo B, homocysteine |
| Allard, 2014 [164] | SC | Canada | Definite DLCN criteria | 409 | 6.4 | 3.2 (1.9–5.6) | 3.6 (2.0–6.5) | Sex, BMI, smoking, family history of premature CVD, hypertension, LDL-c, HDL-c, triglycerides, Lp(a) |
| Alonso, 2014 [165] | MC | Spain | Genetic test | 1960 | 3.9 | Non reported | NS | Sex, BMI, smoking, hypertension, HDL-c, triglycerides, Lp(a), type of mutation, xanthomas |
| Besseling, 2014 [62] | NR | Netherlands | Genetic test | 14,283 | 2.8 | 6.40 (5.21–7.86) | 1.37 (1.03–1.82) | Age, sex, BMI, smoking, hypertension, lipid profile |
| Pereira, 2014 [166] | SC | Brazil | Definite or probable DLCN criteria | 202 | 17.3 | 2.23 (1.05–4.75) | NS | Age, sex, BMI, smoking, hypertension, sedentary lifestyle, LDL-c, HDL-c, triglycerides, glucose, creatinine, xanthomas, corneal arcus, ankle-brachial index, claudication |
| Chan, 2015 [167] | SC | Australia | Genetic test | 390 | 1.3 | 2.74 (1.06–7.08) | NS | Obesity, smoking, hypertension, CKD, LDL-c, HDL-c, triglycerides, Lp(a) |
| De Goma, 2016 [168] | NR | USA | Genetic test or any set of clinical criteria | 1295 | 13 | 3.08 (2.04–4.64) | 1.74 (1.08–2.82) | Age, smoking, hypertension, total-c, low HDL-c |
| Paquette, 2016 [155] | SC | Canada | Genetic test | 670 | 3.3 | 3.5 (1.45–8.47) | NS | Age, sex, BMI, smoking, hypertension, prior statin use, total-c, LDL-c, HDL-c, triglycerides, VLDL-c, non-HDL-c, Lp(a), apoB |
| Paquette, 2017 [169] | MC | Canada | Genetic test | 1388 | 4.5 | 3.28 (1.92–5.619 | NS | Age, sex, BMI, smoking, hypertension, prior statin use, total-c, LDL-c, HDL-c, triglycerides, VLDL-c, non-HDL-c, Lp(a), apo B |
| Galema Boers, 2017 [170] | SC | Netherlands | Genetic test or definite or probable DLCN criteria | 821 | 4 | 4.39 (2.15–8.97) | NS | Age, sex, BMI, smoking, hypertension, family history of CVD, previous cardiovascular disease, triglycerides, high LDL-c, low HDL-c. |
| Paquette, 2019 [146] | MC | Canada | Definite, probable or possible DLCN criteria | 1412 | 5.2 | 2.9 (1.8–4.7) | NS | Montreal-FH-SCORE |
| Pérez-Calahorra, 2019 [171] | NR | Spain | Genetic test or definite or probable DLCN criteria | 1958 | 6.5 | 4.99 (3.43–7.26) | NS | |
| Michikura, 2022 [172] | SC | Japan | Genetic test | 176 | 12 | Non reported | NS | Age, sex, BMI, smoking, hypertension, LDL-c, HDL-c, triglycerides, Achilles tendon elasticity index |
* Type of study. SC: single-centre; MC: multicentre; RR: regional registry; NR: national registry. ** Diagnostic criteria. MEDPED: Make Early Diagnosis to Prevent Early Deaths System; DLCN: Dutch Lipid Clinic Network; NS: Not significant; BMI: body mass index; CVD: cardiovascular disease; c: cholesterol.
5. Knowledge Gaps and Further Research
The previous sections have highlighted the interplay between lipid and glucose metabolism, but also the controversy in this area. The inverse correlation between LDL-c concentrations and the risk of DM is supported by the low risk of DM in most populations with HF, by mendelian randomization studies, and by the increased risk of DM associated with some cholesterol-lowering agents, especially statins. However, results are inconsistent, and robust mechanistic studies are sparse. Furthermore, healthy behavior in people with FH could be associated with lower body mass index and a lower risk of T2DM.
There are several approaches that could fill in some of the existing knowledge gaps.
- In FH populations where DM is more frequent than in the general population, family co-segregation studies could be performed, comparing the prevalence of DM and pre-DM in FH-causing mutation carriers and non-carriers in the same families;
- Studies focused on glucose tolerance, insulin secretion, and insulin resistance in whole-body and β-cell specific LDLR (or other FH-related genes) knock-out animal models, as performed already for PCSK9 [129,130];
- FH-causing-mutation-specific studies in β-cells and islets, assessing their viability and function;
- Larger and longer prospective studies assessing the incidence of DM in FH and non-FH populations, as well as the cardiovascular risk of the combination of FH and DM.
6. Conclusions
Both DM and FH are associated with an increased risk of cardiovascular disease. Many studies suggest that FH is protective against the development of DM and that cholesterol-lowering treatments, especially statins, increase the risk of DM. Indeed, the LDLR is hypothesized to play a role in the toxicity of (or protection from) cholesterol on the β-cells. Their reduced amount or function in HF would protect the cells against LDL particle entry, whereas their increase would promote it and, thus, damage the β-cells. Nevertheless, this hypothesis is still to be proven. Indeed, a healthy lifestyle associated with a relatively low body mass index in people with FH could also account for some of the protection against DM. On the other hand, there are also studies showing an increased prevalence of DM in people with FH, and not all cholesterol-lowering drugs are associated with an increased risk of DM. The combination of FH and DM would be expected to be associated with an especially high risk of cardiovascular disease. However, existing evidence suggests that other classical cardiovascular risk factors modulate cardiovascular risk in FH, but DM does not play a highly relevant role. Short follow-up and small numbers of people with DM advise that this conclusion should be drawn with caution. Much research is still needed to fully understand the interplay between glucose and lipid metabolism in FH and DM.
Author Contributions
Conceptualization, A.M.W., R.M.S.-H. and M.B.; writing—original draft preparation, A.M.G.-L., R.M.S.-H. and M.B.; writing—review and editing, R.M.S.-H. and A.M.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Instituto de Salud Carlos III (ISCIII), Spain with grants CM 19/00116 (AMGL), INT20/00032 (RMSH) and PI 20/0084, partially funded with European Union Regional Development Funds, “A way of doing Europe”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to thank Yeray Brito-Casillas for assistance with reference management.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Goldstein, J.L.; Schrott, H.G.; Hazzard, W.R.; Bierman, E.L.; Motulsky, A.G. Hyperlipidemia in coronary heart disease. II. Genetic analysis of lipid levels in 176 families and delineation of a new inherited disorder, combined hyperlipidemia. J. Clin. Investig. 1973, 52, 1544–1568. [Google Scholar] [CrossRef] [PubMed]
- Landrum, M.J.; Lee, J.M.; Benson, M.; Brown, G.; Chao, C.; Chitipiralla, S.; Gu, B.; Hart, J.; Hoffman, D.; Hoover, J.; et al. ClinVar: Public archive of interpretations of clinically relevant variants. Nucleic Acids Res. 2016, 44, D862–D868. [Google Scholar] [CrossRef] [PubMed]
- Cenarro, A.; Etxebarria, A.; De Castro-Orós, I.; Stef, M.; Bea, A.M.; Palacios, L.; Mateo-Gallego, R.; Benito-Vicente, A.; Ostolaza, H.; Tejedor, T.; et al. The p.Leu167del Mutation in APOE Gene Causes Autosomal Dominant Hypercholesterolemia by Down-regulation of LDL Receptor Expression in Hepatocytes. J. Clin. Endocrinol. Metab. 2016, 101, 2113–2121. [Google Scholar] [CrossRef]
- De Ferranti, S.D.; Rodday, A.M.; Mendelson, M.M.; Wong, J.B.; Leslie, L.K.; Sheldrick, R.C. Prevalence of familial hypercholesterolemia in the 1999 to 2012 United States national health and nutrition examination surveys (NHANES). Circulation 2016, 133, 1067–1072. [Google Scholar] [CrossRef]
- Steyn, K.; Goldberg, Y.P.; Kotze, M.J.; Steyn, M.; Swanepoel, A.S.P.; Fourie, J.M.; Coetzee, G.A.; Van Der Westhuyzen, D.R. Estimation of the prevalence of familial hypercholesterolaemia in a rural Afrikaner community by direct screening for three Afrikaner founder low density lipoprotein receptor gene mutations. Hum. Genet. 1996, 98, 479–484. [Google Scholar] [CrossRef]
- Couture, P.; Morissette, J.; Gaudet, D.; Vohl, M.C.; Gagné, C.; Bergeron, J.; Després, J.P.; Simard, J. Fine mapping of low-density lipoprotein receptor gene by genetic linkage on chromosome 19p13.1-p13.3 and study of the founder effect of four French Canadian low-density lipoprotein receptor gene mutations. Atherosclerosis 1999, 143, 145–151. [Google Scholar] [CrossRef]
- Sánchez-Hernández, R.M.; Tugores, A.; Nóvoa, F.J.; Brito-Casillas, Y.; Expósito-Montesdeoca, A.B.; Garay, P.; Bea, A.M.; Riaño, M.; Pocovi, M.; Civeira, F.; et al. The island of Gran Canaria: A genetic isolate for familial hypercholesterolemia. J. Clin. Lipidol. 2019, 13, 618–626. [Google Scholar] [CrossRef]
- Benito-Vicente, A.; Uribe, K.B.; Jebari, S.; Galicia-Garcia, U.; Ostolaza, H.; Martin, C. Familial Hypercholesterolemia: The Most Frequent Cholesterol Metabolism Disorder Caused Disease. Int. J. Mol. Sci. 2018, 19, 3426. [Google Scholar] [CrossRef]
- Cuchel, M.; Bruckert, E.; Ginsberg, H.N.; Raal, F.J.; Santos, R.D.; Hegele, R.A.; Kuivenhoven, J.A.; Nordestgaard, B.G.; Descamps, O.S.; Steinhagen-Thiessen, E.; et al. Homozygous familial hypercholesterolaemia: New insights and guidance for clinicians to improve detection and clinical management. A position paper from the Consensus Panel on Familial Hypercholesterolaemia of the European Atherosclerosis Society. Eur. Heart J. 2014, 35, 2146–2157. [Google Scholar] [CrossRef]
- Perak, A.M.; Ning, H.; De Ferranti, S.D.; Gooding, H.C.; Wilkins, J.T.; Lloyd-Jones, D.M. Long-Term Risk of Atherosclerotic Cardiovascular Disease in US Adults with the Familial Hypercholesterolemia Phenotype. Circulation 2016, 134, 9–19. [Google Scholar] [CrossRef]
- Di Taranto, M.D.; Giacobbe, C.; Fortunato, G. Familial hypercholesterolemia: A complex genetic disease with variable phenotypes. Eur. J. Med. Genet. 2020, 63, 103831. [Google Scholar] [CrossRef] [PubMed]
- Masana, L.; Zamora, A.; Plana, N.; Comas-Cufí, M.; Garcia-Gil, M.; Martí-Lluch, R.; Ponjoan, A.; Alves-Cabratosa, L.; Elosua, R.; Marrugat, J.; et al. Incidence of Cardiovascular Disease in Patients with Familial Hypercholesterolemia Phenotype: Analysis of 5 Years Follow-Up of Real-World Data from More than 1.5 Million Patients. J. Clin. Med. 2019, 8, 1080. [Google Scholar] [CrossRef] [PubMed]
- Alonso, R.; Mata, N.; Castillo, S.; Fuentes, F.; Saenz, P.; Muñiz, O.; Galiana, J.; Figueras, R.; Diaz, J.L.; Gomez-Enterría, P.; et al. Cardiovascular disease in familial hypercholesterolaemia: Influence of low-density lipoprotein receptor mutation type and classic risk factors. Atherosclerosis 2008, 200, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Akioyamen, L.E.; Genest, J.; Chu, A.; Inibhunu, H.; Ko, D.T.; Tu, J.V. Risk factors for cardiovascular disease in heterozygous familial hypercholesterolemia: A systematic review and meta-analysis. J. Clin. Lipidol. 2019, 13, 15–30. [Google Scholar] [CrossRef]
- Committee, A.D.A.P.P. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022, 45, S17–S38. [Google Scholar] [CrossRef]
- Liu, J.; Ren, Z.H.; Qiang, H.; Wu, J.; Shen, M.; Zhang, L.; Lyu, J. Trends in the incidence of diabetes mellitus: Results from the Global Burden of Disease Study 2017 and implications for diabetes mellitus prevention. BMC Public Health 2020, 20, 1415. [Google Scholar] [CrossRef]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef]
- Kolb, H.; Martin, S. Environmental/lifestyle factors in the pathogenesis and prevention of type 2 diabetes. BMC Med. 2017, 15, 131. [Google Scholar] [CrossRef]
- Boles, A.; Kandimalla, R.; Reddy, P.H. Dynamics of diabetes and obesity: Epidemiological perspective. Biochim. Biophys. Acta. Mol. Basis Dis. 2017, 1863, 1026–1036. [Google Scholar] [CrossRef]
- Htay, T.; Soe, K.; Lopez-Perez, A.; Doan, A.H.A.; Romagosa, M.A.; Aung, K.K. Mortality and Cardiovascular Disease in Type 1 and Type 2 Diabetes. Curr. Cardiol. Rep. 2019, 21, 45. [Google Scholar] [CrossRef]
- Morrish, N.J.; Wang, S.L.; Stevens, L.K.; Fuller, J.H.; Keen, H. Mortality and causes of death in the WHO Multinational Study of Vascular Disease in Diabetes. Diabetologia 2001, 44 (Suppl. 2), S14–S21. [Google Scholar] [CrossRef] [PubMed]
- Glovaci, D.; Fan, W.; Wong, N.D. Epidemiology of Diabetes Mellitus and Cardiovascular Disease. Curr. Cardiol. Rep. 2019, 21, 21. [Google Scholar] [CrossRef]
- Rawshani, A.; Sattar, N.; Franzén, S.; Rawshani, A.; Hattersley, A.T.; Svensson, A.M.; Eliasson, B.; Gudbjörnsdottir, S. Excess mortality and cardiovascular disease in young adults with type 1 diabetes in relation to age at onset: A nationwide, register-based cohort study. Lancet 2018, 392, 477–486. [Google Scholar] [CrossRef]
- Gregg, E.W.; Cheng, Y.J.; Srinivasan, M.; Lin, J.; Geiss, L.S.; Albright, A.L.; Imperatore, G. Trends in cause-specific mortality among adults with and without diagnosed diabetes in the USA: An epidemiological analysis of linked national survey and vital statistics data. Lancet 2018, 391, 2430–2440. [Google Scholar] [CrossRef]
- Dokken, B.B. The pathophysiology of cardiovascular disease and diabetes: Beyond blood pressure and lipids. Diabetes Spectr. 2008, 21, 160–165. [Google Scholar] [CrossRef]
- Besseling, J.; Kastelein, J.J.P.; Defesche, J.C.; Hutten, B.A.; Hovingh, G.K. Association between familial hypercholesterolemia and prevalence of type 2 diabetes mellitus. JAMA 2015, 313, 1029–1036. [Google Scholar] [CrossRef]
- Bruikman, C.S.; Hovingh, G.K.; Kastelein, J.J.P. Molecular basis of familial hypercholesterolemia. Curr. Opin. Cardiol. 2017, 32, 262–266. [Google Scholar] [CrossRef]
- Soutar, A.K.; Naoumova, R.P.; Traub, L.M. Genetics, clinical phenotype, and molecular cell biology of autosomal recessive hypercholesterolemia. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1963–1970. [Google Scholar] [CrossRef]
- Civeira, F.F.; De Castro-Orós, I.; Pocoví, M. The genetic basis of familial hypercholesterolemia: Inheritance, linkage, and mutations. Appl. Clin. Genet. 2010, 3, 53. [Google Scholar] [CrossRef]
- Sánchez-Hernández, R.M.; Civeira, F.; Stef, M.; Perez-Calahorra, S.; Almagro, F.; Plana, N.; Novoa, F.J.; Sáenz-Aranzubía, P.; Mosquera, D.; Soler, C.; et al. Homozygous Familial Hypercholesterolemia in Spain: Prevalence and Phenotype-Genotype Relationship. Circ. Cardiovasc. Genet. 2016, 9, 504–510. [Google Scholar] [CrossRef]
- Goldberg, A.C.; Hopkins, P.N.; Toth, P.P.; Ballantyne, C.M.; Rader, D.J.; Robinson, J.G.; Daniels, S.R.; Gidding, S.S.; de Ferranti, S.D.; Ito, M.K.; et al. Familial hypercholesterolemia: Screening, diagnosis and management of pediatric and adult patients: Clinical guidance from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J. Clin. Lipidol. 2011, 5, S1–S8. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.D.; Gidding, S.S.; Hegele, R.A.; Cuchel, M.A.; Barter, P.J.; Watts, G.F.; Baum, S.J.; Catapano, A.L.; Chapman, M.J.; Defesche, J.C.; et al. Defining severe familial hypercholesterolaemia and the implications for clinical management: A consensus statement from the International Atherosclerosis Society Severe Familial Hypercholesterolemia Panel. Lancet Diabetes Endocrinol. 2016, 4, 850–861. [Google Scholar] [CrossRef]
- Hartgers, M.L.; Hovingh, G.K.; Kastelein, J.J.; Huijgen, R. Familial Hypercholesterolemia: Classification of Mutation Severity According to Percentile Low-Density Lipoprotein Cholesterol Useful for Predicting Coronary Artery Disease Risk. Circulation 2016, 134, A19939. [Google Scholar]
- Soria, L.F.; Ludwig, E.H.; Clarke, H.R.G.; Vega, G.L.; Grundy, S.M.; McCarthy, B.J. Association between a specific apolipoprotein B mutation and familial defective apolipoprotein B-100. Proc. Natl. Acad. Sci. USA 1989, 86, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Tybjærg-Hansen, A.; Humphries, S.E. Familial defective apolipoprotein B-100: A single mutation that causes hypercholesterolemia and premature coronary artery disease. Atherosclerosis 1992, 96, 91–107. [Google Scholar] [CrossRef]
- Futema, M.; Taylor-Beadling, A.; Williams, M.; Humphries, S.E. Genetic testing for familial hypercholesterolemia-past, present, and future. J. Lipid Res. 2021, 62, 100139. [Google Scholar] [CrossRef]
- Abifadel, M.; Varret, M.; Rabès, J.P.; Allard, D.; Ouguerram, K.; Devillers, M.; Cruaud, C.; Benjannet, S.; Wickham, L.; Erlich, D.; et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat. Genet. 2003, 34, 154–156. [Google Scholar] [CrossRef]
- Naoumova, R.P.; Tosi, I.; Patel, D.; Neuwirth, C.; Horswell, S.D.; Marais, A.D.; Van Heyningen, C.; Soutar, A.K. Severe hypercholesterolemia in four British families with the D374Y mutation in the PCSK9 gene: Long-term follow-up and treatment response. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2654–2660. [Google Scholar] [CrossRef]
- Sánchez-Hernández, R.M.; Di Taranto, M.D.; Benito-Vicente, A.; Uribe, K.B.; Lamiquiz-Moneo, I.; Larrea-Sebal, A.; Jebari, S.; Galicia-Garcia, U.; Nóvoa, F.J.; Boronat, M.; et al. The Arg499His gain-of-function mutation in the C-terminal domain of PCSK9. Atherosclerosis 2019, 289, 162–172. [Google Scholar] [CrossRef]
- Talmud, P.J.; Shah, S.; Whittall, R.; Futema, M.; Howard, P.; Cooper, J.A.; Harrison, S.C.; Li, K.; Drenos, F.; Karpe, F.; et al. Use of low-density lipoprotein cholesterol gene score to distinguish patients with polygenic and monogenic familial hypercholesterolaemia: A case-control study. Lancet 2013, 381, 1293–1301. [Google Scholar] [CrossRef]
- Khera, A.V.; Chaffin, M.; Aragam, K.G.; Haas, M.E.; Roselli, C.; Choi, S.H.; Natarajan, P.; Lander, E.S.; Lubitz, S.A.; Ellinor, P.T.; et al. Genome-wide polygenic scores for common diseases identify individuals with risk equivalent to monogenic mutations. Nat. Genet. 2018, 50, 1219–1224. [Google Scholar] [CrossRef] [PubMed]
- Loaiza, N.; Hartgers, M.L.; Reeskamp, L.F.; Balder, J.W.; Rimbert, A.; Bazioti, V.; Wolters, J.C.; Winkelmeijer, M.; Jansen, H.P.G.; Dallinga-Thie, G.M.; et al. Taking One Step Back in Familial Hypercholesterolemia: STAP1 Does Not Alter Plasma LDL (Low-Density Lipoprotein) Cholesterol in Mice and Humans. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 973–985. [Google Scholar] [CrossRef] [PubMed]
- Lamiquiz-Moneo, I.; Restrepo-Córdoba, M.A.; Mateo-Gallego, R.; Bea, A.M.; del Pino Alberiche-Ruano, M.; García-Pavía, P.; Cenarro, A.; Martín, C.; Civeira, F.; Sánchez-Hernández, R.M. Predicted pathogenic mutations in STAP1 are not associated with clinically defined familial hypercholesterolemia. Atherosclerosis 2020, 292, 143–151. [Google Scholar] [CrossRef]
- Wang, X.; Strizich, G.; Hu, Y.; Wang, T.; Kaplan, R.C.; Qi, Q. Genetic markers of type 2 diabetes: Progress in genome-wide association studies and clinical application for risk prediction. J. Diabetes 2016, 8, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Colclough, K.; Gloyn, A.L.; Pollin, T.I. Monogenic diabetes: A gateway to precision medicine in diabetes. J. Clin. Investig. 2021, 131, e142244. [Google Scholar] [CrossRef] [PubMed]
- Weinreich, S.S.; Bosma, A.; Henneman, L.; Rigter, T.; Spruijt, C.M.J.; Grimbergen, A.J.E.M.A.; Breuning, M.H.; De Koning, E.J.P.; Losekoot, M.; Cornel, M.C. A decade of molecular genetic testing for MODY: A retrospective study of utilization in The Netherlands. Eur. J. Hum. Genet. 2015, 23, 29–33. [Google Scholar] [CrossRef]
- Dong, G.; Qu, L.; Gong, X.; Pang, B.; Yan, W.; Wei, J. Effect of Social Factors and the Natural Environment on the Etiology and Pathogenesis of Diabetes Mellitus. Int. J. Endocrinol. 2019, 2019, 8749291. [Google Scholar] [CrossRef]
- Yahaya, T.O.; Salisu, T.F. A Review of Type 2 Diabetes Mellitus Predisposing Genes. Curr. Diabetes Rev. 2019, 16, 52–61. [Google Scholar] [CrossRef]
- Mambiya, M.; Shang, M.; Wang, Y.; Li, Q.; Liu, S.; Yang, L.; Zhang, Q.; Zhang, K.; Liu, M.; Nie, F.; et al. The Play of Genes and Non-genetic Factors on Type 2 Diabetes. Front. Public Health 2019, 7, 349. [Google Scholar] [CrossRef]
- Sirdah, M.M.; Reading, N.S. Genetic predisposition in type 2 diabetes: A promising approach toward a personalized management of diabetes. Clin. Genet. 2020, 98, 525–547. [Google Scholar] [CrossRef]
- Prasad, R.B.; Groop, L. Genetics of type 2 diabetes-pitfalls and possibilities. Genes 2015, 6, 87–123. [Google Scholar] [CrossRef] [PubMed]
- Medici, F.; Hawa, M.; Ianari, A.; Pyke, D.A.; Leslie, R.D.G. Concordance rate for type II diabetes mellitus in monozygotic twins: Actuarial analysis. Diabetologia 1999, 42, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Imamura, M.; Shigemizu, D.; Tsunoda, T.; Iwata, M.; Maegawa, H.; Watada, H.; Hirose, H.; Tanaka, Y.; Tobe, K.; Kaku, K.; et al. Assessing the clinical utility of a genetic risk score constructed using 49 susceptibility alleles for type 2 diabetes in a Japanese population. J. Clin. Endocrinol. Metab. 2013, 98, E1667–E1673. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, C.; Si, S.; Li, Y.; Li, W.; Yuan, T.; Xue, F. Genomic risk score provides predictive performance for type 2 diabetes in the UK biobank. Acta Diabetol. 2021, 58, 467–474. [Google Scholar] [CrossRef]
- Hertel, J.K.; Johansson, S.; Midthjell, K.; Nygård, O.; Njølstad, P.R.; Molven, A. Type 2 diabetes genes—Present status and data from Norwegian studies. Nor. Epidemiol. 2013, 23, 9–22. [Google Scholar] [CrossRef]
- Szabo, M.; Máté, B.; Csép, K.; Benedek, T. Genetic Approaches to the Study of Gene Variants and Their Impact on the Pathophysiology of Type 2 Diabetes. Biochem. Genet. 2018, 56, 22–55. [Google Scholar] [CrossRef]
- Fall, T.; Xie, W.; Poon, W.; Yaghootkar, H.; Magi, R.; Knowles, J.W.; Lyssenko, V.; Weedon, M.; Frayling, T.M.; Ingelsson, E. Using genetic variants to assess the relationship between circulating lipids and type 2 diabetes. Diabetes 2015, 64, 2676–2684. [Google Scholar] [CrossRef]
- Swerdlow, D.I.; Sattar, N. Blood Lipids and Type 2 Diabetes Risk: Can Genetics Help Untangle the Web? Diabetes 2015, 64, 2344–2345. [Google Scholar] [CrossRef][Green Version]
- White, J.; Swerdlow, D.I.; Preiss, D.; Fairhurst-Hunter, Z.; Keating, B.J.; Asselbergs, F.W.; Sattar, N.; Humphries, S.E.; Hingorani, A.D.; Holmes, M.V. Association of Lipid Fractions with Risks for Coronary Artery Disease and Diabetes. JAMA Cardiol. 2016, 1, 692–699. [Google Scholar] [CrossRef]
- Klimentidis, Y.C.; Wineinger, N.E.; Vazquez, A.I.; De Los Campos, G. Multiple metabolic genetic risk scores and type 2 diabetes risk in three racial/ethnic groups. J. Clin. Endocrinol. Metab. 2014, 99, E1814–E1818. [Google Scholar] [CrossRef]
- Klimentidis, Y.C.; Chougule, A.; Arora, A.; Frazier-Wood, A.C.; Hsu, C.H. Triglyceride-Increasing Alleles Associated with Protection against Type-2 Diabetes. PLoS Genet. 2015, 11, e1005204. [Google Scholar] [CrossRef] [PubMed]
- Besseling, J.; Kindt, I.; Hof, M.; Kastelein, J.J.P.; Hutten, B.A.; Hovingh, G.K. Severe heterozygous familial hypercholesterolemia and risk for cardiovascular disease: A study of a cohort of 14,000 mutation carriers. Atherosclerosis 2014, 233, 219–223. [Google Scholar] [CrossRef]
- Neil, H.A.W.; Betteridge, D.J.; Broome, K.; Durrington, P.N.; Hawkins, M.M.; Humphries, S.E.; Mann, J.I.; Miller, J.P.; Thompson, G.R.; Thorogood, M.; et al. Mortality in treated heterozygous familial hypercholesterolaemia: Implications for clinical management. Atherosclerosis 1999, 142, 105–112. [Google Scholar] [CrossRef]
- Ferriéres, J.; Lambert, J.; Lussier-Cacan, S.; Davignon, J. Coronary artery disease in heterozygous familial hypercholesterolemia patients with the same LDL receptor gene mutation. Circulation 1995, 92, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Climent, E.; Pérez-Calahorra, S.; Marco-Benedí, V.; Plana, N.; Sánchez, R.; Ros, E.; Ascaso, J.F.; Puzo, J.; Almagro, F.; Lahoz, C.; et al. Effect of LDL cholesterol, statins and presence of mutations on the prevalence of type 2 diabetes in heterozygous familial hypercholesterolemia. Sci. Rep. 2017, 7, 5596. [Google Scholar] [CrossRef]
- Sánchez-Hernández, R.M.; González-Lleó, A.M.; Tugores, A.; Brito-Casillas, Y.; Civeira, F.; Boronat, M.; Wägner, A. Familial hypercholesterolemia in Gran Canaria: Founder mutation effect and high frequency of diabetes. Clin. E Investig. En Arterioscler. 2021, 33, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Lalić, K.; Rajković, N.; Popović, L.; Lukač, S.S.; Stošić, L.; Rasulić, I.; Lalić, N.M. The effects of 3-year statin therapy and the achievement of LDL cholesterol target values in familial hypercholesterolemia patients: An experience from Serbia. Atherosclerosis 2018, 277, 298–303. [Google Scholar] [CrossRef]
- Sun, D.; Cao, Y.X.; You, X.D.; Zhou, B.Y.; Li, S.; Guo, Y.L.; Zhang, Y.; Wu, N.Q.; Zhu, C.G.; Gao, Y.; et al. Clinical and genetic characteristics of familial hypercholesterolemia patients with type 2 diabetes. J. Endocrinol. Investig. 2019, 42, 591–598. [Google Scholar] [CrossRef]
- Vuorio, A.F.; Turtola, H.; Piilahti, K.-M.; Repo, P.; Kanninen, T.; Kontula, K. Familial Hypercholesterolemia in the Finnish North Karelia. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 3127–3138. [Google Scholar] [CrossRef]
- Fuentes, F.; Alcala-Diaz, J.F.; Watts, G.F.; Alonso, R.; Muñiz, O.; Díaz-Díaz, J.L.; Mata, N.; Sanchez Muñoz-Torrero, J.F.; Brea, Á.; Galiana, J.; et al. Statins do not increase the risk of developing type 2 diabetes in familial hypercholesterolemia: The SAFEHEART study. Int. J. Cardiol. 2015, 201, 79–84. [Google Scholar] [CrossRef]
- Saavedra, Y.G.L.; Dufour, R.; Baass, A. Familial hypercholesterolemia: PCSK9 InsLEU genetic variant and prediabetes/diabetes risk. J. Clin. Lipidol. 2015, 9, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Skoumas, I.; Ioakeimidis, N.; Vlachopoulos, C.; Chrysohoou, C.; Michalakeas, C.; Georgakopoulos, C.; Katsi, V.; Panagiotakos, D.; Tousoulis, D. Statin Therapy and Risk of Diabetes Mellitus in Aging Patients with Heterozygous Familial Hypercholesterolemia or Familial Combined Hyperlipidemia: A 10-Year Follow-Up. Angiology 2018, 69, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.; Martagon, A.J.; Galan Ramirez, G.A.; Antonio-Villa, N.E.; Vargas-Vázquez, A.; Elias-Lopez, D.; Gonzalez-Retana, G.; Rodríguez-Encinas, B.; Ceballos-Macías, J.J.; Romero-Zazueta, A.; et al. Familial hypercholesterolemia in Mexico: Initial insights from the national registry. J. Clin. Lipidol. 2021, 15, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Climent, E.; Pérez-Calahorra, S.; Benaiges, D.; Pintó, X.; Suárez-Tembra, M.; Plana, N.; Sánchez-Hernández, R.M.; Valdivielso, P.; Ascaso, J.F.; Pedro-Botet, J. Clinical and genetic differences between heterozygous familial hypercholesterolemia patients with and without type 2 diabetes. Rev. Esp. Cardiol. 2020, 73, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Ryan, K.A.; Jaworek, T.J.; Southam, L.; Reid, J.G.; Overton, J.D.; Baras, A.; Puurunen, M.K.; Zeggini, E.; Taylor, S.I.; et al. Familial Hypercholesterolemia and Type 2 Diabetes in the Old Order Amish. Diabetes 2017, 66, 2054–2058. [Google Scholar] [CrossRef] [PubMed]
- Sattar, N.; Preiss, D.; Murray, H.M.; Welsh, P.; Buckley, B.M.; de Craen, A.J.; Seshasai, S.R.K.; McMurray, J.J.; Freeman, D.J.; Jukema, J.W.; et al. Statins and risk of incident diabetes: A collaborative meta-analysis of randomised statin trials. Lancet 2010, 375, 735–742. [Google Scholar] [CrossRef]
- Waters, D.D.; Ho, J.E.; Boekholdt, S.M.; Demicco, D.A.; Kastelein, J.J.P.; Messig, M.; Breazna, A.; Pedersen, T.R. Cardiovascular event reduction versus new-onset diabetes during atorvastatin therapy: Effect of baseline risk factors for diabetes. J. Am. Coll. Cardiol. 2013, 61, 148–152. [Google Scholar] [CrossRef]
- Cederberg, H.; Stančáková, A.; Yaluri, N.; Modi, S.; Kuusisto, J.; Laakso, M. Increased risk of diabetes with statin treatment is associated with impaired insulin sensitivity and insulin secretion: A 6 year follow-up study of the METSIM cohort. Diabetologia 2015, 58, 1109–1117. [Google Scholar] [CrossRef]
- Khan, S.U.; Rahman, H.; Okunrintemi, V.; Riaz, H.; Khan, M.S.; Sattur, S.; Kaluski, E.; Lincoff, A.M.; Martin, S.S.; Blaha, M.J. Association of Lowering Low-Density Lipoprotein Cholesterol with Contemporary Lipid-Lowering Therapies and Risk of Diabetes Mellitus: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2019, 8, e011581. [Google Scholar] [CrossRef]
- Ko, M.J.; Jo, A.J.; Kim, Y.J.; Kang, S.H.; Cho, S.; Jo, S.H.; Park, C.Y.; Yun, S.C.; Lee, W.J.; Park, D.W. Time- and Dose-Dependent Association of Statin Use with Risk of Clinically Relevant New-Onset Diabetes Mellitus in Primary Prevention: A Nationwide Observational Cohort Study. J. Am. Heart Assoc. 2019, 8, e011320. [Google Scholar] [CrossRef]
- Choi, J.Y.; Choi, C.U.; Hwang, S.Y.; Choi, B.G.; Jang, W.Y.; Kim, D.Y.; Kim, W.; Park, E.J.; Lee, S.; Na, J.O.; et al. Effect of Pitavastatin Compared with Atorvastatin andRosuvastatin on New-Onset Diabetes Mellitus in PatientsWith Acute Myocardial Infarction. Am. J. Cardiol. 2018, 122, 922–928. [Google Scholar] [CrossRef] [PubMed]
- Freeman, D.J.; Norrie, J.; Sattar, N.; Neely, R.D.G.; Cobbe, S.M.; Ford, I.; Isles, C.; Lorimer, A.R.; Macfarlane, P.W.; McKillop, J.H.; et al. Pravastatin and the development of diabetes mellitus: Evidence for a protective treatment effect in the West of Scotland Coronary Prevention Study. Circulation 2001, 103, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Hiramitsu, S.; Ishiguro, Y.; Matsuyama, H.; Yamada, K.; Kato, K.; Noba, M.; Uemura, A.; Yoshida, S.; Matsubara, Y.; Kani, A.; et al. The effects of ezetimibe on surrogate markers of cholesterol absorption and synthesis in Japanese patients with dyslipidemia. J. Atheroscler. Thromb. 2010, 17, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Dagli, N.; Yavuzkir, M.; Karaca, I. The effects of high dose pravastatin and low dose pravastatin and ezetimibe combination therapy on lipid, glucose metabolism and inflammation. Inflammation 2007, 30, 230–235. [Google Scholar] [CrossRef]
- Her, A.Y.; Kim, J.Y.; Kang, S.M.; Choi, D.; Jang, Y.; Chung, N.; Manabe, I.; Lee, S.H. Effects of atorvastatin 20 mg, rosuvastatin 10 mg, and atorvastatin/ezetimibe 5 mg/5 mg on lipoproteins and glucose metabolism. J. Cardiovasc. Pharmacol. Ther. 2010, 15, 167–174. [Google Scholar] [CrossRef]
- Takeshita, Y.; Takamura, T.; Honda, M.; Kita, Y.; Zen, Y.; Kato, K.I.; Misu, H.; Ota, T.; Nakamura, M.; Yamada, K.; et al. The effects of ezetimibe on non-alcoholic fatty liver disease and glucose metabolism: A randomised controlled trial. Diabetologia 2014, 57, 878–890. [Google Scholar] [CrossRef]
- Sabatine, M.S.; Leiter, L.A.; Wiviott, S.D.; Giugliano, R.P.; Deedwania, P.; De Ferrari, G.M.; Murphy, S.A.; Kuder, J.F.; Gouni-Berthold, I.; Lewis, B.S.; et al. Cardiovascular safety and efficacy of the PCSK9 inhibitor evolocumab in patients with and without diabetes and the effect of evolocumab on glycaemia and risk of new-onset diabetes: A prespecified analysis of the FOURIER randomised controlled trial. Lancet Diabetes Endocrinol. 2017, 5, 941–950. [Google Scholar] [CrossRef]
- De Carvalho, L.S.F.; Campos, A.M.; Sposito, A.C. Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) Inhibitors and Incident Type 2 Diabetes: A Systematic Review and Meta-analysis With Over 96,000 Patient-Years. Diabetes Care 2018, 41, 364–367. [Google Scholar] [CrossRef]
- Chen, Q.; Wu, G.; Li, C.; Qin, X.; Liu, R.; Zhang, M. Safety of Proprotein Convertase Subtilisin/Kexin Type 9 Monoclonal Antibodies in Regard to Diabetes Mellitus: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Am. J. Cardiovasc. Drugs 2020, 20, 343–353. [Google Scholar] [CrossRef]
- Leiter, L.A.; Banach, M.; Catapano, A.L.; Duell, P.B.; Gotto, A.M.; Laufs, U.; Mancini, G.B.J.; Ray, K.K.; Hanselman, J.C.; Ye, Z.; et al. Bempedoic acid in patients with type 2 diabetes mellitus, prediabetes, and normoglycaemia: A post hoc analysis of efficacy and glycaemic control using pooled data from phase 3 clinical trials. Diabetes. Obes. Metab. 2022. [Google Scholar] [CrossRef]
- Masson, W.; Lobo, M.; Lavalle-Cobo, A.; Masson, G.; Molinero, G. Effect of bempedoic acid on new onset or worsening diabetes: A meta-analysis. Diabetes Res. Clin. Pract. 2020, 168, 108369. [Google Scholar] [CrossRef] [PubMed]
- Handelsman, Y.; Goldberg, R.B.; Garvey, W.T.; Fonseca, V.A.; Rosenstock, J.; Jones, M.R.; Lai, Y.L.; Jin, X.; Misir, S.; Nagendran, S.; et al. Colesevelam hydrochloride to treat hypercholesterolemia and improve glycemia in prediabetes: A randomized, prospective study. Endocr. Pract. 2010, 16, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Kazi, D.S.; Penko, J.M.; Bibbins-Domingo, K. Statins for Primary Prevention of Cardiovascular Disease: Review of Evidence and Recommendations for Clinical Practice. Med. Clin. N. Am. 2017, 101, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Tramacere, I.; Boncoraglio, G.B.; Banzi, R.; Del Giovane, C.; Kwag, K.H.; Squizzato, A.; Moja, L. Comparison of statins for secondary prevention in patients with ischemic stroke or transient ischemic attack: A systematic review and network meta-analysis. BMC Med. 2019, 17, 67. [Google Scholar] [CrossRef] [PubMed]
- Karlson, B.W.; Palmer, M.K.; Nicholls, S.J.; Lundman, P.; Barter, P.J. Doses of rosuvastatin, atorvastatin and simvastatin that induce equal reductions in LDL-C and non-HDL-C: Results from the VOYAGER meta-analysis. Eur. J. Prev. Cardiol. 2016, 23, 744–747. [Google Scholar] [CrossRef] [PubMed]
- Adhyaru, B.B.; Jacobson, T.A. Safety and efficacy of statin therapy. Nat. Rev. Cardiol. 2018, 15, 757–769. [Google Scholar] [CrossRef] [PubMed]
- Goodarzi, M.O.; Li, X.; Krauss, R.M.; Rotter, J.I.; Chen, Y.D.I. Relationship of sex to diabetes risk in statin trials. Diabetes Care 2013, 36, e100–e101. [Google Scholar] [CrossRef]
- Preiss, D.; Seshasai, S.R.K.; Welsh, P.; Murphy, S.A.; Ho, J.E.; Waters, D.D.; DeMicco, D.A.; Barter, P.; Cannon, C.P.; Sabatine, M.S.; et al. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: A meta-analysis. JAMA 2011, 305, 2556–2564. [Google Scholar] [CrossRef]
- Cholesterol Treatment Trialists’ (CTT) Collaborators Efficacy of cholesterol-lowering therapy in 18 686 people with diabetes in 14 randomised trials of statins: A meta-analysis. Lancet 2008, 371, 117–125. [CrossRef]
- Mach, F.; Ray, K.K.; Wiklund, O.; Corsini, A.; Catapano, A.L.; Bruckert, E.; De Backer, G.; Hegele, R.A.; Hovingh, G.K.; Jacobson, T.A.; et al. Adverse effects of statin therapy: Perception vs. the evidence—Focus on glucose homeostasis, cognitive, renal and hepatic function, haemorrhagic stroke and cataract. Eur. Heart J. 2018, 39, 2526–2539. [Google Scholar] [CrossRef]
- Sasaki, J.; Iwashita, M.; Kono, S. Statins: Beneficial or adverse for glucose metabolism. J. Atheroscler. Thromb. 2006, 13, 123–129. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Garcia-Calvo, M.; Lisnock, J.M.; Bull, H.G.; Hawes, B.E.; Burnett, D.A.; Braun, M.P.; Crona, J.H.; Davis, H.R.; Dean, D.C.; Detmers, P.A.; et al. The target of ezetimibe is Niemann-Pick C1-Like 1 (NPC1L1). Proc. Natl. Acad. Sci. USA 2005, 102, 8132–8137. [Google Scholar] [CrossRef] [PubMed]
- Tsunoda, T.; Nozue, T.; Yamada, M.; Mizuguchi, I.; Sasaki, M.; Michishita, I. Effects of ezetimibe on atherogenic lipoproteins and glucose metabolism in patients with diabetes and glucose intolerance. Diabetes Res. Clin. Pract. 2013, 100, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Hiramitsu, S.; Miyagishima, K.; Ishii, J.; Matsui, S.; Naruse, H.; Shiino, K.; Kitagawa, F.; Ozaki, Y. Effect of ezetimibe on lipid and glucose metabolism after a fat and glucose load. J. Cardiol. 2012, 60, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Shang, H.; Wu, J. Effect of ezetimibe on glycemic control: A systematic review and meta-analysis of randomized controlled trials. Endocrine 2018, 60, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-B.; Kim, B.; Han, K.; Kim, J.A.; Roh, E.; Hong, S.H.; Choi, K.M.; Baik, S.H.; Yoo, H.J. Combination of Statin and Ezetimibe versus Statin Monotherapy on Cardiovascular Disease and Type 2 Diabetes Incidence among Adults with Impaired Fasting Glucose: A Propensity-Matched Nationwide Cohort Study. J. Lipid Atheroscler. 2021, 10, 303–312. [Google Scholar] [CrossRef]
- Kikuchi, K.; Nezu, U.; Inazumi, K.; Miyazaki, T.; Ono, K.; Orime, K.; Shirakawa, J.; Sato, K.; Koike, H.; Wakasugi, T.; et al. Double-blind randomized clinical trial of the effects of ezetimibe on postprandial hyperlipidaemia and hyperglycaemia. J. Atheroscler. Thromb. 2012, 19, 1093–1101. [Google Scholar] [CrossRef][Green Version]
- Ascaso, J.F. Inhibition of proprotein convertase subtilisin/kexin type 9 in the treatment of hypercholesterolemia. Endocrinol. Nutr. 2016, 63, 255–257. [Google Scholar] [CrossRef]
- Ganda, O.P.; Plutzky, J.; Sanganalmath, S.K.; Bujas-Bobanovic, M.; Koren, A.; Mandel, J.; Letierce, A.; Leiter, L.A. Efficacy and safety of alirocumab among individuals with diabetes mellitus and atherosclerotic cardiovascular disease in the ODYSSEY phase 3 trials. Diabetes. Obes. Metab. 2018, 20, 2389–2398. [Google Scholar] [CrossRef]
- Lotta, L.A.; Sharp, S.J.; Burgess, S.; Perry, J.R.B.; Stewart, I.D.; Willems, S.M.; Luan, J.; Ardanaz, E.; Arriola, L.; Balkau, B.; et al. Association Between Low-Density Lipoprotein Cholesterol-Lowering Genetic Variants and Risk of Type 2 Diabetes: A Meta-analysis Supplemental content. JAMA 2016, 316, 1383–1391. [Google Scholar] [CrossRef]
- Malik, S.; Kashyap, M.L. Niacin, lipids, and heart disease. Curr. Cardiol. Rep. 2003, 5, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Zafrir, B.; Jain, M. Lipid-lowering therapies, glucose control and incident diabetes: Evidence, mechanisms and clinical implications. Cardiovasc. Drugs Ther. 2014, 28, 361–377. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Sharma, A.; Krishnamoorthy, P.; Garg, J.; Virmani, D.; Sharma, T.; Stefanini, G.; Kostis, J.B.; Mukherjee, D.; Sikorskaya, E. Role of Niacin in Current Clinical Practice: A Systematic Review. Am. J. Med. 2017, 130, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Vega, G.L.; Dunn, F.L.; Grundy, S.M. Effect of colesevelam hydrochloride on glycemia and insulin sensitivity in men with the metabolic syndrome. Am. J. Cardiol. 2011, 108, 1129–1135. [Google Scholar] [CrossRef] [PubMed]
- Ritz-Laser, B.; Meda, P.; Constant, I.; Klages, N.; Charollais, A.; Morales, A.; Magnan, C.; Ktorza, A.; Philippe, J. Glucose-induced preproinsulin gene expression is inhibited by the free fatty acid palmitate. Endocrinology 1999, 140, 4005–4014. [Google Scholar] [CrossRef] [PubMed]
- Briaud, I.; Harmon, J.S.; Kelpe, C.L.; Segu, V.B.G.; Poitout, V. Lipotoxicity of the pancreatic beta-cell is associated with glucose-dependent esterification of fatty acids into neutral lipids. Diabetes 2001, 50, 315–321. [Google Scholar] [CrossRef]
- Lu, X.; Liu, J.; Hou, F.; Liu, Z.; Cao, X.; Seo, H.; Gao, B. Cholesterol induces pancreatic β cell apoptosis through oxidative stress pathway. Cell Stress Chaperones 2011, 16, 539–548. [Google Scholar] [CrossRef]
- Cnop, M.; Hannaert, J.C.; Grupping, A.Y.; Pipeleers, D.G. Low density lipoprotein can cause death of islet β-cells by its cellular uptake and oxidative modification. Endocrinology 2002, 143, 3449–3453. [Google Scholar] [CrossRef]
- Villarreal-Molina, M.T.; Aguilar-Salinas, C.A.; Rodríguez-Cruz, M.; Riaño, D.; Villalobos-Comparan, M.; Coral-Vazquez, R.; Menjivar, M.; Yescas-Gomez, P.; Königsoerg-Fainstein, M.; Romero-Hidalgo, S.; et al. The ATP-binding cassette transporter A1 R230C variant affects HDL cholesterol levels and BMI in the Mexican population: Association with obesity and obesity-related comorbidities. Diabetes 2007, 56, 1881–1887. [Google Scholar] [CrossRef]
- Haghvirdizadeh, P.; Ramachandran, V.; Etemad, A.; Heidari, F.; Ghodsian, N.; Bin Ismail, N.; Ismail, P. Association of ATP-Binding Cassette Transporter A1 Gene Polymorphisms in Type 2 Diabetes Mellitus among Malaysians. J. Diabetes Res. 2015, 2015, 289846. [Google Scholar] [CrossRef]
- Dullaart, R.P.F.; Annema, W.; de Boer, J.F.; Tietge, U.J.F. Pancreatic β-cell function relates positively to HDL functionality in well-controlled Type 2 diabetes mellitus. Atherosclerosis 2012, 222, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Sokooti, S.; Flores-Guerrero, J.L.; Kieneker, L.M.; Heerspink, H.J.L.; Connelly, M.A.; Bakker, S.J.L.; Dullaart, R.P.F. HDL Particle Subspecies and Their Association with Incident Type 2 Diabetes: The PREVEND Study. J. Clin. Endocrinol. Metab. 2021, 106, 1761–1772. [Google Scholar] [CrossRef] [PubMed]
- Perego, C.; Da Dalt, L.; Pirillo, A.; Galli, A.; Catapano, A.L.; Norata, G.D. Cholesterol metabolism, pancreatic β-cell function and diabetes. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 2149–2156. [Google Scholar] [CrossRef] [PubMed]
- Paolisso, G.; Ferrannini, E.; D’Amore, A.; Volpe, C.; Varricchio, M.; D’Onofrio, F. Effects of physiological plasma insulin levels on glucose turnover parameters in familial hypercholesterolemia. Atherosclerosis 1993, 101, 111–115. [Google Scholar] [CrossRef]
- Galvan, A.Q.; Santoro, D.; Natali, A.; Sampietro, T.; Boni, C.; Masoni, A.; Buzzigoli, G.; Ferrannini, E. Insulin sensitivity in familial hypercholesterolemia. Metabolism 1993, 42, 1359–1364. [Google Scholar] [CrossRef]
- Karhapää, P.; Voutilainen, E.; Kovanen, P.T.; Laakso, M. Insulin resistance in familial and nonfamilial hypercholesterolemia. Arterioscler. Thromb. Vasc. Biol. 1993, 13, 41–47. [Google Scholar] [CrossRef]
- Koks, N.; de Vries, M.A.; Birnie, E.; Alipour, A.; Castro Cabezas, M. Glucose-dependent leucocyte activation in familial hypercholesterolemia. Eur. J. Clin. Investig. 2017, 47, 839–846. [Google Scholar] [CrossRef]
- Oliveira, R.B.d.; Carvalho, C.P.d.F.; Polo, C.C.; Dorighello, G.d.G.; Boschero, A.C.; Oliveira, H.C.F.d.; Collares-Buzato, C.B. Impaired compensatory beta-cell function and growth in response to high-fat diet in LDL receptor knockout mice. Int. J. Exp. Pathol. 2014, 95, 296–308. [Google Scholar] [CrossRef]
- Da Dalt, L.; Ruscica, M.; Bonacina, F.; Balzarotti, G.; Dhyani, A.; Di Cairano, E.; Baragetti, A.; Arnaboldi, L.; De Metrio, S.; Pellegatta, F.; et al. PCSK9 deficiency reduces insulin secretion and promotes glucose intolerance: The role of the low-density lipoprotein receptor. Eur. Heart J. 2019, 40, 357–368. [Google Scholar] [CrossRef]
- Peyot, M.L.; Roubtsova, A.; Lussier, R.; Chamberland, A.; Essalmani, R.; Murthy Madiraju, S.R.; Seidah, N.G.; Prentki, M.; Prat, A. Substantial PCSK9 inactivation in β-cells does not modify glucose homeostasis or insulin secretion in mice. Biochim. Biophys. Acta. Mol. Cell Biol. Lipids 2021, 1866, 158968. [Google Scholar] [CrossRef]
- Tada, H.; Kawashiri, M.A.; Yoshida, T.; Teramoto, R.; Nohara, A.; Konno, T.; Inazu, A.; Mabuchi, H.; Yamagishi, M.; Hayashi, K. Lipoprotein(A) in familial hypercholesterolemia with proprotein convertase subtilisin/kexin type 9 (PCSK9) gain-of-function mutations. Circ. J. 2016, 80, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Paige, E.; Masconi, K.L.; Tsimikas, S.; Kronenberg, F.; Santer, P.; Weger, S.; Willeit, J.; Kiechl, S.; Willeit, P. Lipoprotein(a) and incident type-2 diabetes: Results from the prospective Bruneck study and a meta-analysis of published literature. Cardiovasc. Diabetol. 2017, 16, 38. [Google Scholar] [CrossRef] [PubMed]
- Perez-Calahorra, S.; Civeira, F.; Guallar-Castillón, P.; Pinto, X.; Banegas, J.R.; Pedro-Botet, J.; Suarez-Tembra, M.; Mauri, M.; Soler, C.; Rodriguez-Artalejo, F.; et al. Behavioural cardiovascular risk factors and prevalence of diabetes in subjects with familial hypercholesterolaemia. Eur. J. Prev. Cardiol. 2020, 27, 1649–1660. [Google Scholar] [CrossRef] [PubMed]
- Fox, C.S.; Coady, S.; Sorlie, P.D.; D’agostino, R.B.; Pencina, M.J.; Vasan, R.S.; Meigs, J.B.; Levy, D.; Savage, P.J. Increasing Cardiovascular Disease Burden Due to Diabetes Mellitus the Framingham Heart Study. Circulation 2007, 115, 1544–1550. [Google Scholar] [CrossRef]
- Raghavan, S.; Vassy, J.L.; Ho, Y.L.; Song, R.J.; Gagnon, D.R.; Cho, K.; Wilson, P.W.F.; Phillips, L.S. Diabetes Mellitus-Related All-Cause and Cardiovascular Mortality in a National Cohort of Adults. J. Am. Heart Assoc. 2019, 8, e011295. [Google Scholar] [CrossRef]
- Katakami, N. Mechanism of Development of Atherosclerosis and Cardiovascular Disease in Diabetes Mellitus. J. Atheroscler. Thromb. 2018, 25, 27–39. [Google Scholar] [CrossRef]
- Wu, L.; Parhofer, K.G. Diabetic dyslipidemia. Metabolism. 2014, 63, 1469–1479. [Google Scholar] [CrossRef]
- Tribble, D.L.; Rizzo, M.; Chait, A.; Lewis, D.M.; Blanche, P.J.; Krauss, R.M. Enhanced oxidative susceptibility and reduced antioxidant content of metabolic precursors of small, dense low-density lipoproteins. Am. J. Med. 2001, 110, 103–110. [Google Scholar] [CrossRef]
- Paiker, J.E.; Raal, F.J.; Waisberg, R.; Buthelezi, E.P. Quantity versus quality of LDL cholesterol in patients with familial hypercholesterolemia-which is more important? Clin. Chim. Acta 2001, 314, 167–173. [Google Scholar] [CrossRef]
- Kolovou, G.D.; Kostakou, P.M.; Anagnostopoulou, K.K. Familial hypercholesterolemia and triglyceride metabolism. Int. J. Cardiol. 2011, 147, 349–358. [Google Scholar] [CrossRef]
- Chemello, K.V.; García-Nafría, J.; Gallo, A.; Martín, C.; Lambert, G.; Blom, D. Lipoprotein metabolism in familial hypercholesterolemia. J. Lipid Res. 2021, 62, 100062. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.C.; Watts, G.F. Postprandial lipoprotein metabolism in familial hypercholesterolemia: Thinking outside the box. Metabolism 2012, 61, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Wittekoek, M.E.; Pimstone, S.N.; Reymer, P.W.; Feuth, L.; Botma, G.J.; Defesche, J.C.; Prins, M.; Hayden, M.R.; Kastelein, J.J. A common mutation in the lipoprotein lipase gene (N291S) alters the lipoprotein phenotype and risk for cardiovascular disease in patients with familial hypercholesterolemia. Circulation. 1998, 97, 729–735. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ganjali, S.; Momtazi, A.A.; Banach, M.; Kovanen, P.T.; Stein, E.A.; Sahebkar, A. HDL abnormalities in familial hypercholesterolemia: Focus on biological functions. Prog. Lipid Res. 2017, 67, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Pedro-Botet, J.; Climent, E.; Benaiges, D. Familial Hypercholesterolemia: Do HDL Play a Role? Biomedicines 2021, 9, 810. [Google Scholar] [CrossRef] [PubMed]
- Paquette, M.; Bernard, S.; Ruel, I.; Blank, D.W.; Genest, J.; Baass, A. Diabetes is associated with an increased risk of cardiovascular disease in patients with familial hypercholesterolemia. J. Clin. Lipidol. 2019, 13, 123–128. [Google Scholar] [CrossRef]
- Spiga, R.; Marini, M.A.; Mancuso, E.; Di Fatta, C.; Fuoco, A.; Perticone, F.; Andreozzi, F.; Mannino, G.C.; Sesti, G. Uric Acid Is Associated with Inflammatory Biomarkers and Induces Inflammation via Activating the NF-κB Signaling Pathway in HepG2 Cells. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1241–1249. [Google Scholar] [CrossRef]
- Mirza, S.; Hossain, M.; Mathews, C.; Martinez, P.; Pino, P.; Gay, J.L.; Rentfro, A.; McCormick, J.B.; Fisher-Hoch, S.P. Type 2-diabetes is associated with elevated levels of TNF-alpha, IL-6 and adiponectin and low levels of leptin in a population of Mexican Americans: A cross-sectional study. Cytokine 2012, 57, 136–142. [Google Scholar] [CrossRef]
- Derosa, G.; Fogari, E.; D’Angelo, A.; Bianchi, L.; Bonaventura, A.; Romano, D.; Maffioli, P. Adipocytokine levels in obese and non-obese subjects: An observational study. Inflammation 2013, 36, 914–920. [Google Scholar] [CrossRef]
- Rahman, T.; Hamzan, N.S.; Mokhsin, A.; Rahmat, R.; Ibrahim, Z.O.; Razali, R.; Thevarajah, M.; Nawawi, H. Enhanced status of inflammation and endothelial activation in subjects with familial hypercholesterolaemia and their related unaffected family members: A case control study. Lipids Health Dis. 2017, 16, 81. [Google Scholar] [CrossRef]
- Ganjali, S.; Keshavarz, R.; Hosseini, S.; Mansouri, A.; Mannarino, M.R.; Pirro, M.; Jamialahmadi, T.; Sahebkar, A. Evaluation of Oxidative Stress Status in Familial Hypercholesterolemia. J. Clin. Med. 2021, 10, 5867. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, A.; Liberale, L.; Reiner, Ž.; Carbone, F.; Montecucco, F.; Sahebkar, A. Inflammatory Biomarkers for Cardiovascular Risk Stratification in Familial Hypercholesterolemia. Rev. Physiol. Biochem. Pharmacol. 2020, 177, 25–52. [Google Scholar] [CrossRef] [PubMed]
- Jansen, A.C.M.; Van Aalst-Cohen, E.S.; Tanck, M.W.; Trip, M.D.; Lansberg, P.J.; Liem, A.H.; Roeters Van Lennep, H.W.O.; Sijbrands, E.J.G.; Kastelein, J.J.P. The contribution of classical risk factors to cardiovascular disease in familial hypercholesterolaemia: Data in 2400 patients. J. Intern. Med. 2004, 256, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Tada, H.; Okada, H.; Nohara, A.; Yamagishi, M.; Takamura, M.; Kawashiri, M.A. Effect of Cumulative Exposure to Low-Density Lipoprotein-Cholesterol on Cardiovascular Events in Patients with Familial Hypercholesterolemia. Circ. J. 2021, 85, 2073–2078. [Google Scholar] [CrossRef] [PubMed]
- Paquette, M.; Dufour, R.; Baass, A. The Montreal-FH-SCORE: A new score to predict cardiovascular events in familial hypercholesterolemia. J. Clin. Lipidol. 2017, 11, 80–86. [Google Scholar] [CrossRef]
- Pérez De Isla, L.; Alonso, R.; Mata, N.; Fernández-Pérez, C.; Muñiz, O.; Díaz-Díaz, J.L.; Saltijeral, A.; Fuentes-Jiménez, F.; De Andrés, R.; Zambón, D.; et al. Predicting Cardiovascular Events in Familial Hypercholesterolemia: The SAFEHEART Registry (Spanish Familial Hypercholesterolemia Cohort Study). Circulation 2017, 135, 2133–2144. [Google Scholar] [CrossRef]
- Paquette, M.; Bernard, S.; Cariou, B.; Hegele, R.A.; Genest, J.; Trinder, M.; Brunham, L.R.; Béliard, S.; Baass, A. Familial Hypercholesterolemia-Risk-Score: A New Score Predicting Cardiovascular Events and Cardiovascular Mortality in Familial Hypercholesterolemia. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 2632–2640. [Google Scholar] [CrossRef]
- Panagiotakos, D.B.; Pitsavos, C.; Skoumas, J.; Chrysohoou, C.; Toutouza, M.; Stefanadis, C.I.; Toutouzas, P.K. Importance of LDL/HDL cholesterol ratio as a predictor for coronary heart disease events in patients with heterozygous familial hypercholesterolaemia: A 15-year follow-up (1987–2002). Curr. Med. Res. Opin. 2003, 19, 89–94. [Google Scholar]
- Humphries, S.E.; Cooper, J.A.; Capps, N.; Durrington, P.N.; Jones, B.; McDowell, I.F.W.; Soran, H.; Neil, A.H.W. Coronary heart disease mortality in severe vs. non-severe familial hypercholesterolaemia in the Simon Broome Register. Atherosclerosis 2019, 281, 207. [Google Scholar] [CrossRef]
- Gidding, S.S. Diabetes and familial hypercholesterolemia: An unhealthy marriage. Rev. Española Cardiol. 2020, 73, 705–706. [Google Scholar] [CrossRef]
- Cosentino, F.; Grant, P.J.; Aboyans, V.; Bailey, C.J.; Ceriello, A.; Delgado, V.; Federici, M.; Filippatos, G.; Grobbee, D.E.; Hansen, T.B.; et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur. Heart J. 2020, 41, 255–323. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, P.N.; Stephenson, S.; Wu, L.L.; Riley, W.A.; Xin, Y.; Hunt, S.C. Evaluation of coronary risk factors in patients with heterozygous familial hypercholesterolemia. Am. J. Cardiol. 2001, 87, 547–553. [Google Scholar] [CrossRef]
- De Sauvage Nolting, P.R.W.; Defesche, J.C.; Buirma, R.J.A.; Hutten, B.A.; Lansberg, P.J.; Kastelein, J.J.P. Prevalence and significance of cardiovascular risk factors in a large cohort of patients with familial hypercholesterolaemia. J. Intern. Med. 2003, 253, 161–168. [Google Scholar] [CrossRef]
- Allard, M.D.; Saeedi, R.; Yousefi, M.; Frohlich, J. Risk stratification of patients with familial hypercholesterolemia in a multi-ethnic cohort. Lipids Health Dis. 2014, 13, 65. [Google Scholar] [CrossRef] [PubMed]
- Alonso, R.; Andres, E.; Mata, N.; Fuentes-Jiménez, F.; Badimón, L.; López-Miranda, J.; Padró, T.; Muñiz, O.; Díaz-Díaz, J.L.; Mauri, M.; et al. Lipoprotein(a) levels in familial hypercholesterolemia: An important predictor of cardiovascular disease independent of the type of LDL receptor mutation. J. Am. Coll. Cardiol. 2014, 63, 1982–1989. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.; Miname, M.; Makdisse, M.; Filho, R.K.; Santos, R.D. Association of Peripheral Arterial and Cardiovascular Diseases inFamilial Hypercholesterolemia. Arq. Bras. Cardiol. 2014, 103, 118. [Google Scholar] [CrossRef]
- Chan, D.C.; Pang, J.; Hooper, A.J.; Burnett, J.R.; Bell, D.A.; Bates, T.R.; Van Bockxmeer, F.M.; Watts, G.F. Elevated lipoprotein(a), hypertension and renal insufficiency as predictors of coronary artery disease in patients with genetically confirmed heterozygous familial hypercholesterolemia. Int. J. Cardiol. 2015, 201, 633–638. [Google Scholar] [CrossRef]
- Degoma, E.M.; Ahmad, Z.S.; O’Brien, E.C.; Kindt, I.; Shrader, P.; Newman, C.B.; Pokharel, Y.; Baum, S.J.; Hemphill, L.C.; Hudgins, L.C.; et al. Treatment gaps in adults with heterozygous familial hypercholesterolemia in the United States. Circ. Cardiovasc. Genet. 2016, 9, 240–249. [Google Scholar] [CrossRef]
- Paquette, M.; Brisson, D.; Dufour, R.; Khoury, É.; Gaudet, D.; Baass, A. Cardiovascular disease in familial hypercholesterolemia: Validation and refinement of the Montreal-FH-SCORE. J. Clin. Lipidol. 2017, 11, 1161–1167. [Google Scholar] [CrossRef]
- Galema-Boers, A.M.; Lenzen, M.J.; Engelkes, S.R.; Sijbrands, E.J.; Roeters van Lennep, J.E. Cardiovascular risk in patients with familial hypercholesterolemia using optimal lipid-lowering therapy. J. Clin. Lipidol. 2018, 12, 409–416. [Google Scholar] [CrossRef]
- Perez-Calahorra, S.; Laclaustra, M.; Marco-Benedí, V.; Lamiquiz-Moneo, I.; Pedro-Botet, J.; Plana, N.; Sanchez-Hernandez, R.M.; Amor, A.J.; Almagro, F.; Fuentes, F.; et al. Effect of lipid-lowering treatment in cardiovascular disease prevalence in familial hypercholesterolemia. Atherosclerosis 2019, 284, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Michikura, M.; Ogura, M.; Hori, M.; Matsuki, K.; Makino, H.; Hosoda, K.; Harada-Shiba, M. Association between Achilles Tendon Softness and Atherosclerotic Cardiovascular Disease in Patients with Familial Hypercholesterolemia. J. Atheroscler. Thromb. 2022, 63151. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).