Cytokinins: Wide-Spread Signaling Hormones from Plants to Humans with High Medical Potential
Abstract
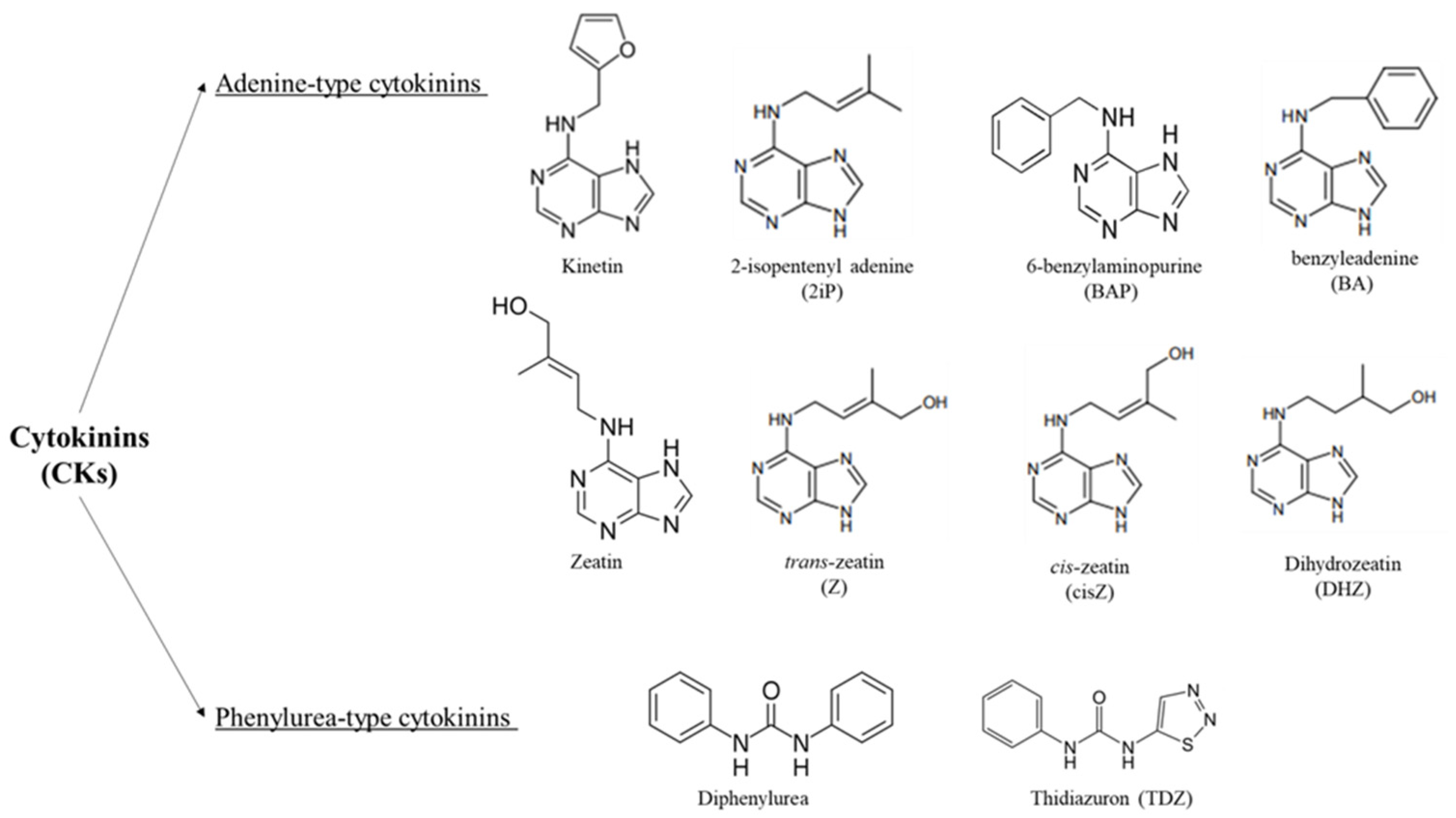
1. Cytokinins, Occurrence, Structure and Identification
2. Cytokinin Action in Plants
3. Cytokinins in Mammals
4. Antioxidant Activity of Cytokinins
5. Anti-Aging Activity of Cytokinins
6. Anticancer Activity of Cytokinins
7. Neuroprotective Activity of Cytokinins
8. Anti-Inflammatory and Immunomodulatory Effects of Cytokinins
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fathy, M.; Khalifa, E.M.; Fawzy, M.A. Modulation of inducible nitric oxide synthase pathway by eugenol and telmisartan in carbon tetrachloride-induced liver injury in rats. Life Sci. 2019, 216, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Fathy, M.; Nikaido, T. In vivo modulation of iNOS pathway in hepatocellular carcinoma by Nigella sativa. Environ. Health Prev. Med. 2013, 18, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Fathy, M.; Nikaido, T. In vivo attenuation of angiogenesis in hepatocellular carcinoma by Nigella sativa. Turk. J. Med. Sci. 2018, 48, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Fathy, M.; Okabe, M.; Othman, E.M.; Eldien, H.M.S.; Yoshida, T. Preconditioning of Adipose-Derived Mesenchymal Stem-Like Cells with Eugenol Potentiates Their Migration and Proliferation In Vitro and Therapeutic Abilities in Rat Hepatic Fibrosis. Molecules 2020, 25, 2020. [Google Scholar] [CrossRef]
- Fathy, M.; Okabe, M.; Eldien, H.M.S.; Yoshida, T. AT-MSCs Antifibrotic Activity is Improved by Eugenol through Modulation of TGF-β/Smad Signaling Pathway in Rats. Molecules 2020, 25, 348. [Google Scholar] [CrossRef]
- Fawzy, M.A.; Maher, S.A.; El-Rehany, M.A.; Welson, N.N.; Albezrah, N.K.A.; Batiha, G.E.-S.; Fathy, M. Vincamine Modulates the Effect of Pantoprazole in Renal Ischemia/Reperfusion Injury by Attenuating MAPK and Apoptosis Signaling Pathways. Molecules 2022, 27, 1383. [Google Scholar] [CrossRef]
- Cragg, G.M.; Pezzuto, J.M. Natural products as a vital source for the discovery of cancer chemotherapeutic and chemopreventive agents. Med. Princ. Pract. 2016, 25, 41–59. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 Years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef]
- Abdellatef, A.A.; Fathy, M.; Mohammed, A.E.-S.I.; Abu Bakr, M.S.; Ahmed, A.H.; Abbass, H.S.; El-Desoky, A.H.; Morita, H.; Nikaido, T.; Hayakawa, Y. Inhibition of cell-intrinsic NF-κB activity and metastatic abilities of breast cancer by aloe-emodin and emodic-acid isolated from Asphodelus microcarpus. J. Nat. Med. 2021, 75, 840–853. [Google Scholar] [CrossRef]
- Fathy, M.; Fawzy, M.A.; Hintzsche, H.; Nikaido, T.; Dandekar, T.; Othman, E.M. Eugenol exerts apoptotic effect and modulates the sensitivity of hela cells to cisplatin and radiation. Molecules 2019, 24, 3979. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.-M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.L.; Edrada-Ebel, R.; Quinn, R.J. The re-emergence of natural products for drug discovery in the genomics era. Nat. Rev. Drug Discov. 2015, 14, 111–129. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Alaaeldin, R.; Abdel-Rahman, I.A.M.; Hassan, H.A.; Youssef, N.; Allam, A.E.; Abdelwahab, S.F.; Zhao, Q.-L.; Fathy, M. Carpachromene Ameliorates Insulin Resistance in HepG2 Cells via Modulating IR/IRS1/PI3k/Akt/GSK3/FoxO1 Pathway. Molecules 2021, 26, 7629. [Google Scholar] [CrossRef] [PubMed]
- Caplin, S.M.; Steward, F.C. Effect of Coconut Milk on the Growth of Explants from Carrot Root. Science 1948, 108, 655–657. [Google Scholar] [CrossRef]
- Kieber, J.J.; Schaller, G.E. The Perception of Cytokinin: A Story 50 Years in the Making: Figure 1. Plant Physiol. 2010, 154, 487–492. [Google Scholar] [CrossRef]
- Miller, C.O.; Skoog, F.; Von Saltza, M.H.; Strong, F.M. Kinetin, a cell division factor from deoxyribonucleic acid1. J. Am. Chem. Soc. 1955, 77, 1392. [Google Scholar] [CrossRef]
- Letham, D. Zeatin, a factor inducing cell division isolated from zea mays. Life Sci. 1963, 2, 569–573. [Google Scholar] [CrossRef]
- Jaworek, P.; Kopečný, D.; Zalabák, D.; Šebela, M.; Kouřil, S.; Hluska, T.; Končitíková, R.; Podlešáková, K.; Tarkowski, P. Occurrence and biosynthesis of cytokinins in poplar. Planta 2019, 250, 229–244. [Google Scholar] [CrossRef]
- Pokorná, E.; Hluska, T.; Galuszka, P.; Hallmark, H.T.; Dobrev, P.I.; Drábková, L.Z.; Filipi, T.; Holubová, K.; Plíhal, O.; Rashotte, A.M.; et al. Cytokinin N-glucosides: Occurrence, Metabolism and Biological Activities in Plants. Biomolecules 2020, 11, 24. [Google Scholar] [CrossRef]
- Crafts, C.B.; Miller, C.O. Detection and identification of cytokinins produced by mycorrhizal fungi. Plant Physiol. 1974, 54, 586–588. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.O.; Jameson, P.E.; Laloue, M.; Morris, J.W. Rapid identification of cytokinins by an immunological method. Plant Physiol. 1991, 95, 1156–1161. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Spinola, M.; Galvan, A.; Pignatiello, C.; Conti, B.; Pastorino, U.; Nicander, B.; Paroni, R.; Dragani, T.A. Identification and functional characterization of the candidate tumor suppressor gene TRIT1 in human lung cancer. Oncogene 2005, 24, 5502–5509. [Google Scholar] [CrossRef] [PubMed]
- Béres, T.; Zatloukal, M.; Voller, J.; Niemann, P.; Gahsche, M.C.; Tarkowski, P.; Novák, O.; Hanuš, J.; Strnad, M.; Doležal, K. Tandem mass spectrometry identification and LC–MS quantification of intact cytokinin nucleotides in K-562 human leukemia cells. Anal. Bioanal. Chem. 2010, 398, 2071–2080. [Google Scholar] [CrossRef]
- Seegobin, M.; Kisiala, A.; Noble, A.; Kaplan, D.; Brunetti, C.; Emery, R.J.N. Canis familiaris tissues are characterized by different profiles of cytokinins typical of the tRNA degradation pathway. FASEB J. 2018, 32, 6575–6581. [Google Scholar] [CrossRef]
- Barciszewski, J.; Mielcarek, M.; Stobiecki, M.; Siboska, G.; Clark, B.F.C. Identification of 6-furfuryladenine (kinetin) in human urine. Biochem. Biophys. Res. Commun. 2000, 279, 69–73. [Google Scholar] [CrossRef]
- Barciszewski, J.; Siboska, G.E.; Pedersen, B.O.; Clark, B.F.; Rattan, S.I. Evidence for the presence of kinetin in DNA and cell extracts. FEBS Lett. 1996, 393, 197–200. [Google Scholar] [CrossRef]
- Samanovic, M.I.; Tu, S.; Novak, O.; Iyer, L.M.; McAllister, F.E.; Aravind, L.; Gygi, S.P.; Hubbard, S.R.; Strnad, M.; Darwin, K.H. Proteasomal control of cytokinin synthesis protects mycobacterium tuberculosis against nitric oxide. Mol. Cell 2015, 57, 984–994. [Google Scholar] [CrossRef]
- Naseem, M.; Sarukhanyan, E.; Dandekar, T. LONELY-GUY knocks every door: Crosskingdom microbial pathogenesis. Trends Plant Sci. 2015, 20, 781–783. [Google Scholar] [CrossRef]
- Großkinsky, D.K.; Naseem, M.; Abdelmohsen, U.R.; Plickert, N.; Engelke, T.; Griebel, T.; Zeier, J.; Novak, O.; Strnad, M.; Pfeifhofer, H.; et al. Cytokinins mediate resistance against pseudomonas syringae in tobacco through increased antimicrobial phytoalexin synthesis independent of salicylic acid signaling. Plant Physiol. 2011, 157, 815–830. [Google Scholar] [CrossRef]
- Horgan, R.; Hewett, E.; Horgan, J.; Purse, J.; Wareing, P. A new cytokinin from Populus x robusta. Phytochemistry 1975, 14, 1005–1008. [Google Scholar] [CrossRef]
- Strnad, M. The aromatic cytokinins. Physiol. Plant. 1997, 101, 674–688. [Google Scholar] [CrossRef]
- Kieber, J.J. Tribute to Folke Skoog: Recent Advances in our Understanding of Cytokinin Biology. J. Plant Growth Regul. 2002, 21, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Liu, L.; Kong, Z.; Li, S.; Lu, L.; Kabir, N.; Chen, G.; Zhang, J.; Qanmber, G.; Liu, Z. Identification of GhLOG gene family revealed that GhLOG3 is involved in regulating salinity tolerance in cotton (Gossypium hirsutum L.). Plant Physiol. Biochem. 2021, 166, 328–340. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Jameson, G.B.; Guo, Y.; Song, J.; Jameson, P.E. The LONELY GUY gene family: From mosses to wheat, the key to the formation of active cytokinins in plants. Plant Biotechnol. J. 2022. [Google Scholar] [CrossRef] [PubMed]
- Nayar, S. Exploring the Role of a Cytokinin-Activating Enzyme LONELY GUY in Unicellular Microalga Chlorella variabilis. Front. Plant Sci. 2021, 11, 611871. [Google Scholar] [CrossRef]
- Moramarco, F.; Pezzicoli, A.; Salvini, L.; Leuzzi, R.; Pansegrau, W.; Balducci, E. A LONELY GUY protein of Bordetella pertussis with unique features is related to oxidative stress. Sci. Rep. 2019, 9, 17016. [Google Scholar] [CrossRef]
- Naseem, M.; Bencurova, E.; Dandekar, T. The Cytokinin-Activating LOG-Family Proteins Are Not Lysine Decarboxylases. Trends Biochem. Sci. 2018, 43, 232–236. [Google Scholar] [CrossRef]
- Barciszewski, J.; Rattan, S.I.; Siboska, G.; Clark, B.F. Kinetin—45 years on. Plant Sci. 1999, 148, 37–45. [Google Scholar] [CrossRef]
- Barciszewski, J.; Siboska, G.E.; Pedersen, B.O.; Clark, B.F.; Rattan, S.I. A mechanism for the in vivo formation of N6-furfuryladenine, kinetin, as a secondary oxidative damage product of DNA. FEBS Lett. 1997, 414, 457–460. [Google Scholar] [CrossRef]
- Barciszewski, J.; Siboska, G.E.; Pedersen, B.O.; Clark, B.F.; Rattan, S.I. Furfural, a precursor of the cytokinin hormone kinetin, and base propenals are formed by hydroxyl radical damage of DNA. Biochem. Biophys. Res. Commun. 1997, 238, 317–319. [Google Scholar] [CrossRef] [PubMed]
- Wawrzyniak, D.; Rolle, K.; Barciszewski, J. Aktywność biologiczna N6-furfuryloadenozyny [Biological activity of N6-furfuryladenosine]. Postepy Biochem. 2019, 65, 109–117. (In Polish) [Google Scholar] [CrossRef]
- Hertz, N.T.; Berthet, A.; Sos, M.L.; Thorn, K.S.; Burlingame, A.L.; Nakamura, K.; Shokat, K.M. A neo-substrate that amplifies cat-alytic activity of parkinson’s-disease-related kinase PINK1. Cell 2013, 154, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Rolly, N.; Mun, B.-G.; Yun, B.-W. Insights into the Transcriptional Regulation of Branching Hormonal Signaling Pathways Genes under Drought Stress in Arabidopsis. Genes 2021, 12, 298. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.K.; Gouda, G.; Donde, R.; Vadde, R.; Behera, L. In silico characterization of the impact of mutation (LEU112PRO) on the structure and function of carotenoid cleavage dioxygenase 8 in Oryza sativa. Phytochemistry 2020, 175, 112365. [Google Scholar] [CrossRef] [PubMed]
- Chefdor, F.; Héricourt, F.; Koudounas, K.; Carqueijeiro, I.; Courdavault, V.; Mascagni, F.; Bertheau, L.; Larcher, M.; Depierreux, C.; Lamblin, F.; et al. Highlighting type A RRs as potential regulators of the dkHK1 multi-step phosphorelay pathway in Populus. Plant Sci. 2018, 277, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Savelieva, E.M.; Oslovsky, V.E.; Karlov, D.S.; Kurochkin, N.N.; Getman, I.A.; Lomin, S.N.; Sidorov, G.V.; Mikhailov, S.N.; Osolodkin, D.I.; Romanov, G.A. Cytokinin activity of N6-benzyladenine derivatives assayed by interaction with the receptors in planta, in vitro, and in silico. Phytochemistry 2018, 149, 161–177. [Google Scholar] [CrossRef]
- Li, P.; Lei, K.; Li, Y.; He, X.; Wang, S.; Liu, R.; Ji, L.; Hou, B. Identification and characterization of the first cytokinin glycosyltransferase from rice: Glucosyl Zeatin and Glucosyl Ribosylzeatin from Vinca rosea Crown Gall. Rice 2019, 12, 19. [Google Scholar] [CrossRef]
- Morris, R.O. Mass Spectroscopic Identification of Cytokinins: Glucosyl Zeatin and Glucosyl Ribosylzeatin from Vinca rosea Crown Gall. Plant Physiol. 1977, 59, 1029–1033. [Google Scholar] [CrossRef]
- Goindi, S.; Guleria, A.; Aggarwal, N. Development and evaluation of solid lipid nanoparticles of N-6-furfuryl adenine for preven-tion of photoaging. J. Biomed. Nanotechnol. 2015, 11, 1734–1746. [Google Scholar] [CrossRef]
- Maia Campos, P.M.B.G.; de Camargo Júnior, F.B.; de Andrade, J.P.; Gaspar, L.R. Efficacy of cosmetic formulations containing dis-persion of liposome with magnesium ascorbyl phosphate, alpha-lipoic acid and kinetin. Photochem. Photobiol. 2012, 88, 748–752. [Google Scholar] [CrossRef] [PubMed]
- Chiu, P.C.; Chan, C.C.; Lin, H.M.; Chiu, H.C. The clinical anti-aging effects of topical kinetin and niacinamide in Asians: A ran-domized, double-blind, placebo-controlled, split-face comparative trial. J. Cosmet. Dermatol. 2007, 6, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, S.S.; Mekureyaw, M.F.; Pandey, C.; Roitsch, T. Role of Cytokinins for Interactions of Plants with Microbial Pathogens and Pest Insects. Front. Plant Sci. 2019, 10, 1777. [Google Scholar] [CrossRef]
- Naseem, M.; Wölfling, M.; Dandekar, T. Cytokinins for immunity beyond growth, galls and green islands. Trends Plant Sci. 2014, 19, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Zeng, D.; Que, C.; da Silva, J.A.T.; Xu, S.; Li, D. Comparative Transcriptomic and Metabolic Analyses Reveal the Molecular Mechanism of Ovule Development in the Orchid, Cymbidium sinense. Front. Plant Sci. 2021, 12, 814275. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Guan, P.; Gu, J.; Yang, X.; Wang, F.; Qi, M.; Li, T.; Liu, Y. Exogenous DA-6 Improves the Low Night Temperature Tolerance of Tomato Through Regulating Cytokinin. Front. Plant Sci. 2020, 11, 2290. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin as a regulatory hub of plant hormone levels and action in stress situations. Plant Biol. 2021, 23, 7–19. [Google Scholar] [CrossRef]
- Antoniadi, I.; Novák, O.; Gelová, Z.; Johnson, A.; Plíhal, O.; Simerský, R.; Mik, V.; Vain, T.; Mateo-Bonmatí, E.; Karady, M.; et al. Cell-surface receptors enable perception of extracellular cytokinins. Nat. Commun. 2020, 11, 4284. [Google Scholar] [CrossRef]
- Prerostova, S.; Jarosova, J.; Dobrev, P.I.; Hluskova, L.; Motyka, V.; Filepova, R.; Knirsch, V.; Gaudinova, A.; Kieber, J.; Vankova, R. Heat Stress Targeting Individual Organs Reveals the Central Role of Roots and Crowns in Rice Stress Responses. Front. Plant Sci. 2022, 12, 799249. [Google Scholar] [CrossRef]
- Ferreira, F.J.; Kieber, J.J. Cytokinin signaling. Curr. Opin. Plant Biol. 2005, 8, 518–525. [Google Scholar] [CrossRef]
- Choi, J.; Choi, D.; Lee, S.; Ryu, C.M.; Hwang, I. Cytokinins and plant immunity: Old foes or new friends? Trends Plant Sci. 2011, 16, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.; Sheen, J.; Müller, B. Cytokinin signaling networks. Ann. Rev. Plant Biol. 2012, 63, 353–380. [Google Scholar] [CrossRef] [PubMed]
- Skoog, F.; Miller, C.O. Chemical regulation of growth and organ formation in plant tissues cultured in vitro. Symp. Soc. Exp. Biol. 1957, 11, 118–130. [Google Scholar] [PubMed]
- Veselova, S.V.; Nuzhnaya, T.V.; Burkhanova, G.F.; Rumyantsev, S.D.; Khusnutdinova, E.K.; Maksimov, I.V. Ethylene-Cytokinin Interaction Determines Early Defense Response of Wheat against Stagonospora nodorum Berk. Biomolecules 2021, 11, 174. [Google Scholar] [CrossRef]
- Gupta, R.; Pizarro, L.; Leibman-Markus, M.; Marash, I.; Bar, M. Cytokinin response induces immunity and fungal pathogen resistance, and modulates trafficking of the PRR LeEIX2 in tomato. Mol. Plant Pathol. 2020, 21, 1287–1306. [Google Scholar] [CrossRef]
- Tiwari, S.; Verma, N.; Prasad, S.M.; Singh, V.P. Cytokinin alleviates cypermethrin toxicity in Nostoc muscorum by involving nitric oxide: Regulation of exopolysaccharides secretion, PS II photochemistry and reactive oxygen species homeostasis. Chemosphere 2020, 259, 127356. [Google Scholar] [CrossRef]
- Wu, L.-Y.; Shang, G.-D.; Wang, F.-X.; Gao, J.; Wan, M.-C.; Xu, Z.-G.; Wang, J.-W. Dynamic chromatin state profiling reveals regulatory roles of auxin and cytokinin in shoot regeneration. Dev. Cell 2022, 57, 526–542.e7. [Google Scholar] [CrossRef]
- Werner, S.; Bartrina, I.; Schmülling, T. Cytokinin regulates vegetative phase change in Arabidopsis thaliana through the miR172/TOE1-TOE2 module. Nat. Commun. 2021, 12, 5816. [Google Scholar] [CrossRef]
- Nguyen, H.N.; Nguyen, T.Q.; Kisiala, A.B.; Emery, R.J.N. Beyond transport: Cytokinin ribosides are translocated and active in regulating the development and environmental responses of plants. Planta 2021, 254, 45. [Google Scholar] [CrossRef]
- Kerr, S.C.; Patil, S.B.; Germain, A.D.S.; Pillot, J.; Saffar, J.; Ligerot, Y.; Aubert, G.; Citerne, S.; Bellec, Y.; Dun, E.A.; et al. Integration of the SMXL/D53 strigolactone signalling repressors in the model of shoot branching regulation in Pisum sativum. Plant J. 2021, 107, 1756–1770. [Google Scholar] [CrossRef]
- Ravanfar, S.A.; Karimi, E.; Mehrabanjoubani, P.; Ebrahimi, M. Enhancement of phenolic and flavonoids compounds, antioxidant and cytotoxic effects in regenerated red cabbage by application of Zeatin. Nat. Prod. Res. 2018, 34, 898–902. [Google Scholar] [CrossRef] [PubMed]
- Sameena, P.P.; Kalaji, H.M.; Żuk-Gołaszewska, K.; Horaczek, T.; Sierka, E.; Puthur, J.T. 6-Benzylaminopurine Alleviates the Impact of Cu2+ Toxicity on Photosynthetic Performance of Ricinus communis L. Seedlings. Int. J. Mol. Sci. 2021, 22, 13349. [Google Scholar] [CrossRef] [PubMed]
- Jha, S.; Chaudhuri, K.N.; Ghosh, B. The root: A potential new source of competent cells for high-frequency regeneration in Tylophora indica. Plant Cell Rep. 2004, 22, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Pramita, A.D.; Kristanti, A.N.; Sugiharto; Utami, E.S.W.; Manuhara, Y.S.W. Production of biomass and flavonoid of Gynura procumbens (Lour.) Merr shoots culture in temporary immersion system. J. Genet. Eng. Biotechnol. 2018, 16, 639–643. [Google Scholar] [CrossRef]
- Chen, B.; Yang, H. 6-Benzylaminopurine alleviates chilling injury of postharvest cucumber fruit through modulating antioxidant system and energy status. J. Sci. Food Agric. 2012, 93, 1915–1921. [Google Scholar] [CrossRef]
- Mlejnek, P.; Procházka, S. Activation of caspase-like proteases and induction of apoptosis by isopentenyladenosine in tobacco BY-2 cells. Planta 2002, 215, 158–166. [Google Scholar] [CrossRef]
- Mlejnek, P.; Doležel, P. Apoptosis induced by N6-substituted derivatives of adenosine is related to intracellular accumulation of corresponding mononucleotides in HL-60 cells. Toxicol. Vitr. 2005, 19, 985–990. [Google Scholar] [CrossRef]
- Wu, J.J.; Weinstein, G.D.; Kricorian, G.J.; Kormeili, T.; McCullough, J.L. Topical kinetin 0.1% lotion for improving the signs and symptoms of rosacea. Clin. Exp. Dermatol. 2007, 32, 693–695. [Google Scholar] [CrossRef]
- Naseem, M.; Othman, E.M.; Fathy, M.; Iqbal, J.; Howari, F.M.; AlRemeithi, F.A.; Kodandaraman, G.; Stopper, H.; Bencurova, E.; Vlachakis, D.; et al. Integrated structural and functional analysis of the protective effects of kinetin against oxidative stress in mammalian cellular systems. Sci. Rep. 2020, 10, 13330. [Google Scholar] [CrossRef]
- Othman, E.M.; Fathy, M.; Bekhit, A.A.; Abdel-Razik, A.-H.; Jamal, A.; Nazzal, Y.; Shams, S.; Dandekar, T.; Naseem, M. Modulatory and Toxicological Perspectives on the Effects of the Small Molecule Kinetin. Molecules 2021, 26, 670. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Z.; Yang, X. Kinetin protects against lipid peroxidation and improves antioxidant status in cultured astrocytes and mouse brain exposed to D-galactose. Afr. J. Biotechnol. 2011, 10, 11721. [Google Scholar]
- Milne, G.R.; Palmer, T.M. Anti-inflammatory and immunosuppressive effects of the A2A adenosine receptor. Sci. World J. 2011, 11, 320–339. [Google Scholar] [CrossRef] [PubMed]
- Dolezal, K.; Popa, I.; Hauserová, E.; Spíchal, L.; Chakrabarty, K.; Novák, O.; Kryštof, V.; Voller, J.; Holub, J.; Strnad, M. Preparation, biological activity and endogenous occurrence of N6-benzyladenosines. Bioorg. Med. Chem. 2007, 15, 3737–3747. [Google Scholar] [CrossRef] [PubMed]
- Okada, Y.; Okajima, H. Scavenging Activity of Furan Derivatives against Hydroxyl Radical Generated by Fenton System. Yakugaku Zasshi 1998, 118, 226–230. [Google Scholar] [CrossRef][Green Version]
- Okada, Y.; Kaneko, M.; Okajima, H. Hydroxyl Radical Scavenging Activity of Naturally Occurring Furan Fatty Acids. Biol. Pharm. Bull. 1996, 19, 1607–1610. [Google Scholar] [CrossRef]
- Parvez, M.; Birdsall, W. Structure of a copper (II) kinetin complex. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1990, 46, 1775–1778. [Google Scholar] [CrossRef]
- Olsen, A.; Siboska, G.E.; Clark, B.F.; Rattan, S.I. N6-furfuryladenine, kinetin, protects against fenton reaction-mediated oxidative damage to DNA. Biochem. Biophys. Res. Commun. 1999, 265, 499–502. [Google Scholar] [CrossRef]
- Verbeke, P.; Siboska, G.E.; Clark, B.F.; Rattan, S.I. Kinetin Inhibits Protein Oxidation and Glycoxidation in Vitro. Biochem. Biophys. Res. Commun. 2000, 276, 1265–1270. [Google Scholar] [CrossRef]
- Othman, E.M.; Naseem, M.; Awad, E.; Dandekar, T.; Stopper, H. The Plant Hormone Cytokinin Confers Protection against Oxidative Stress in Mammalian Cells. PLoS ONE 2016, 11, e0168386. [Google Scholar] [CrossRef]
- Alena Kadlecová, Barbara Maková, Marta Artal-Sanz, Miroslav Strnad, Jiří Voller, The plant hormone kinetin in disease therapy and healthy aging. Ageing Res. Rev. 2019, 55, 100958. [CrossRef]
- Brizzolari, A.; Marinello, C.; Carini, M.; Santaniello, E.; Biondi, P.A. Evaluation of the antioxidant activity and capacity of some natural N6-substituted adenine derivatives (cytokinins) by fluorimetric and spectrophotometric assays. J. Chromatogr. B 2016, 1019, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Liu, D.; Zheng, Y.; Li, H.; Hao, C.; Ouyang, W. Protective effects of kinetin against aluminum chloride and D-galactose induced cognitive impairment and oxidative damage in mouse. Brain Res. Bull. 2017, 134, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, G.; Shen, M.-Y.; Lin, K.-H.; Chou, C.-Y.; Tzu, N.-H.; Lin, C.-H.; Chou, D.-S.; Chen, T.-F.; Sheu, J.-R. Inhibitory activity of kinetin on free radical formation of activated platelets in vitro and on thrombus formation in vivo. Eur. J. Pharmacol. 2003, 465, 281–287. [Google Scholar] [CrossRef]
- Qamar, A.Y.; Fang, X.; Bang, S.; Kim, M.J.; Cho, J. Effects of kinetin supplementation on the post-thaw motility, viability, and structural integrity of dog sperm. Cryobiology 2020, 95, 90–96. [Google Scholar] [CrossRef]
- Hashem, E.Z.; Eslami, M. Kinetin improves motility, viability and antioxidative parameters of ram semen during storage at refrigerator temperature. Cell Tissue Bank. 2016, 19, 97–111. [Google Scholar] [CrossRef]
- Rattan, S.; Clark, B. Kinetin delays the onset of aging characteristics in human fibroblasts. Biochem. Biophys. Res. Commun. 1994, 201, 665–672. [Google Scholar] [CrossRef]
- Rattan, S.I.; Sodagam, L. Gerontomodulatory and youth-preserving effects of zeatin on human skin fibroblasts undergoing aging in vitro. Rejuvenation Res. 2005, 8, 46–57. [Google Scholar] [CrossRef]
- Berge, U.; Kristensen, P.; Rattan, S.I.S. Kinetin-Induced Differentiation of Normal Human Keratinocytes Undergoing Aging in Vitro. Ann. N. Y. Acad. Sci. 2006, 1067, 332–336. [Google Scholar] [CrossRef]
- Berge, U.; Kristensen, P.; Rattan, S.I. Hormetic modulation of differentiation of normal human epidermal keratinocytes undergoing replicative senescence in vitro. Exp. Gerontol. 2008, 43, 658–662. [Google Scholar] [CrossRef]
- Jabłońska-Trypuć, A.; Matejczyk, M.; Czerpak, R. N6-benzyladenine and kinetin influence antioxidative stress parameters in human skin fibroblasts. Mol. Cell. Biochem. 2016, 413, 97–107. [Google Scholar] [CrossRef]
- Bolund, L.; Jensen, P.K.; Bjerring, P. Method and Composition for Treating Hyperproliferative Skin Diseases Using 6-Aminopurine Cytokinins. US Patent 5,021,422, 4 June 1991. [Google Scholar]
- McDaniel, D.H.; Neudecker, B.A.; Dinardo, J.C.; Lewis, J.A.; Maibach, H.I. Idebenone: A new antioxidant—Part I. Relative assessment of oxidative stress protection capacity compared to commonly known antioxidants. J. Cosmet. Dermatol. 2005, 4, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Bi, Z.; Yang, B.; Ji, C.; Kang, J.; Chen, W.; Wan, Y. Trans-Zeatin inhibits UVB-induced matrix metalloproteinase-1 expression via MAP kinase signaling in human skin fibroblasts. Int. J. Mol. Med. 2009, 23, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Bi, Z.; Ji, C.; Yang, Y.; Yang, B.; Xia, J.; Sun, W.; Su, Z.; Yu, L.; Shan, S.; He, S.; et al. Trans-Zeatin attenuates ultraviolet induced down-regulation of aquaporin-3 in cultured human skin keratinocytes. Int. J. Mol. Med. 2010, 26, 257–263. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McCullough, J.L.; Weinstein, G.D. Clinical study of safety and efficacy of using topical kinetin 0.1% (Kinerase) to treat photo-damaged skin. Cosmet Dermatol. 2002, 15, 29–32. [Google Scholar]
- Thornfeldt, C.R.; Rizer, R.L. Superior Efficacy of an Herbal-based Cosmeceutical Compared with Common Prescription and Cos-metic Antiaging Therapies. J. Drugs Dermatol. 2016, 15, 218–223. [Google Scholar]
- Wanitphakdeedecha, R.; Meeprathom, W.; Manuskiatti, W. Efficacy and safety of 0.1% kinetin cream in the treatment of pho-toaging skin. Indian J. Dermatol. Venereol. Leprol. 2015, 81, 547. [Google Scholar] [CrossRef]
- An, S.; Cha, H.J.; Ko, J.-M.; Han, H.; Kim, S.Y.; Kim, K.-S.; Lee, S.J.; An, I.-S.; Kim, S.; Youn, H.J.; et al. Kinetin improves barrier function of the skin by modulating keratinocyte differentiation markers. Ann. Dermatol. 2017, 29, 6–12. [Google Scholar] [CrossRef]
- McCullough, J.L.; Garcia, R.L.; Reece, B. A clinical study of topical Pyratine 6 for improving the appearance of photodamaged skin. J. Drugs Dermatol. 2008, 7, 131–135. [Google Scholar]
- Tremaine, A.M.; Ortiz, A.; Elkeeb, L.; Tran, M.; Weinstein, G. Long-term efficacy and safety of topical PRK 124 (0.125%) lotion (Pyratine-XR) in the treatment of mild-to-moderate rosacea. J. Drugs Dermatol. 2010, 9, 647–650. [Google Scholar]
- Kimura, T.; Doi, K. Depigmentation and rejuvenation effects of kinetin on the aged skin of hairless descendants of Mexican hair-less dogs. Rejuvenation Res. 2004, 7, 32–39. [Google Scholar] [CrossRef]
- Mielcarek, M.; Isalan, M. Kinetin stimulates differentiation of C2C12 myoblasts. PLoS ONE 2021, 16, e0258419. [Google Scholar] [CrossRef] [PubMed]
- Janahmadi, Z.; Nekooeian, A.A.; Moaref, A.R.; Emamghoreishi, M. Oleuropein offers cardioprotection in rats with acute myocar-dial infarction. Cardiovasc. Toxicol. 2015, 15, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Mijikovic, D.; Hranisavljevic, J.; Pietrzkowski, Z. Pharmaceutical Compositions and Methods for Metabolic Modulation. U.S. Patent 10/567,875, 12 July 2007. [Google Scholar]
- Orr, M.F.; McSwain, B. The effect of kinetin, kinetin ribofuranoside and gibberellic acid upon cultures of skin and mammary car-cinoma and cystic disease. Cancer Res. 1960, 20, 1362–1364. [Google Scholar] [PubMed]
- Voller, J.; Zatloukal, M.; Lenobel, R.; Doležal, K.; Béreš, T.; Kryštof, V.; Spíchal, L.; Niemann, P.; Džubák, P.; Hajdúch, M. Anti-cancer activity of natural cytokinins: A structure–activity relationship study. Phytochemistry 2010, 71, 1350–1359. [Google Scholar] [CrossRef]
- Voller, J.; Béres, T.; Zatloukal, M.; Kaminski, P.A.; Niemann, P.; Doležal, K.; Džubák, P.; Hajdúch, M.; Strnad, M. The natural cytokinin 2OH3MeOBAR induces cell death by a mechanism that is different from that of the “classical” cytokinin ribosides. Phytochemistry 2017, 136, 156–164. [Google Scholar] [CrossRef]
- Ishii, Y.; Hori, Y.; Sakai, S.; Honma, Y. Control of differentiation and apoptosis of human myeloid leukemia cells by cytokinins and cytokinin nucleosides, plant redifferentiation-inducing hormones. Cell Growth Differ. Mol. Biol. J. Am. Assoc. Cancer Res. 2002, 13, 19–26. [Google Scholar]
- Ishii, Y.; Sakai, S.; Honma, Y. Cytokinin-induced differentiation of human myeloid leukemia HL-60 cells is associated with the formation of nucleotides, but not with incorporation into DNA or RNA. Biochim. Biophys. Acta 2003, 1643, 11–24. [Google Scholar] [CrossRef]
- Ishii, Y.; Kasukabe, T.; Honma, Y. Immediate up-regulation of the calcium-binding protein S100P and its involvement in the cyto-kinin-induced differentiation of human myeloid leukemia cells. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2005, 1745, 156–165. [Google Scholar] [CrossRef]
- Orlicka-Płocka, M.; Gurda-Wozna, D.; Fedoruk-Wyszomirska, A.; Wyszko, E. Circumventing the Crabtree effect: Forcing oxidative phosphorylation (OXPHOS) via galactose medium increases sensitivity of HepG2 cells to the purine derivative kinetin riboside. Apoptosis 2020, 25, 835–852. [Google Scholar] [CrossRef]
- Choi, B.-H.; Kim, W.; Wang, Q.C.; Kim, D.-C.; Tan, S.N.; Yong, J.W.H.; Kim, K.-T.; Yoon, H.S. Kinetin riboside preferentially induces apoptosis by modulating Bcl-2 family proteins and caspase-3 in cancer cells. Cancer Lett. 2008, 261, 37–45. [Google Scholar] [CrossRef]
- Zhang, Z.-G.; Zou, J.; Huang, Y.; Wu, L. Kinetin inhibits proliferation of hepatic stellate cells by interrupting cell cycle and induces apoptosis by down-regulating ratio of Bcl-2/Bax. J. Huazhong Univ. Sci. Technol. 2015, 35, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Aoki, M.M.; Seegobin, M.; Kisiala, A.; Noble, A.; Brunetti, C.; Emery, R.J.N. Phytohormone metabolism in human cells: Cytokinins are taken up and interconverted in HeLa cell culture. FASEB BioAdv. 2019, 1, 320–331. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Liu, P.; Guo, G.; Wang, L. Combination of cytokinin and auxin induces apoptosis, cell cycle progression arrest and blockage of the Akt pathway in HeLa cells. Mol. Med. Rep. 2012, 12, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Vedenicheva, N.P.; Al-Maali, G.A.; Bisko, N.A.; Kosakivska, I.V.; Ostrovska, G.V.; Khranovska, N.M.; Gorbach, O.I.; Garmanchuk, L.V.; Ostapchenko, L.I. Effect of Cytokinin-Containing Extracts from Some Medicinal Mushroom Mycelia on HepG2 Cells In Vitro. Int. J. Med. Mushrooms 2021, 23, 15–28. [Google Scholar] [CrossRef]
- Dolezal, K.; Popa, I.; Krystof, V.; Spíchal, L.; Fojtíková, M.; Holub, J.; Lenobel, R.; Schmülling, T.; Strnad, M. Preparation and biological activity of 6-benzylaminopurine derivatives in plants and human cancer cells. Bioorg. Med. Chem. 2006, 14, 875–884. [Google Scholar] [CrossRef]
- Slaugenhaupt, S.A.; Mull, J.; Leyne, M.; Cuajungco, M.P.; Gill, S.P.; Hims, M.M.; Quintero, F.; Axelrod, F.B.; Gusella, J.F. Rescue of a human mRNA splicing defect by the plant cytokinin kinetin. Hum. Mol. Genet. 2003, 13, 429–436. [Google Scholar] [CrossRef]
- Hims, M.M.; Ibrahim, E.C.; Leyne, M.; Mull, J.; Liu, L.; Lazaro, C.; Shetty, R.S.; Gill, S.; Gusella, J.F.; Reed, R.; et al. Therapeutic potential and mechanism of kinetin as a treatment for the human splicing disease familial dysautonomia. Klin. Wochenschr. 2007, 85, 149–161. [Google Scholar] [CrossRef]
- Sinha, R.; Kim, Y.J.; Nomakuchi, T.; Sahashi, K.; Hua, Y.; Rigo, F.; Bennett, C.F.; Krainer, A.R. Antisense oligonucleotides correct the familial dysautonomia splicing defect in IKBKAP transgenic mice. Nucleic Acids Res. 2018, 46, 4833–4844. [Google Scholar] [CrossRef]
- Chohan, H.; Esfandiarei, M.; Arman, D.; Van Raamsdonk, C.D.; van Breemen, C.; Friedman, J.M.; Jett, K.A. Neurofibromin hap-loinsufficiency results in altered spermatogenesis in a mouse model of neurofibromatosis type 1. PLoS ONE 2018, 13, e0208835. [Google Scholar] [CrossRef]
- Bowie, L.E.; Maiuri, T.; Alpaugh, M.; Gabriel, M.; Arbez, N.; Galleguillos, D.; Hung, C.L.K.; Patel, S.; Xia, J.; Hertz, N.T.; et al. N6-Furfuryladenine is protective in Huntington’s disease models by signaling huntingtin phosphorylation. Proc. Natl. Acad. Sci. USA 2018, 115, E7081–E7090. [Google Scholar] [CrossRef]
- Gonzalez, G.; Grúz, J.; D’Acunto, C.W.; Kaňovský, P.; Strnad, M. Cytokinin Plant Hormones Have Neuroprotective Activity in In Vitro Models of Parkinson’s Disease. Molecules 2021, 26, 361. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-C.; Yang, Y.-C.; Huang, C.-L.; Kuo, T.-Y.; Lin, J.-H.; Yang, D.-M.; Huang, N.-K. When cytokinin, a plant hormone, meets the adenosine A2A receptor: A novel neuroprotectant and lead for treating neurodegenerative disorders? PLoS ONE 2012, 7, e38865. [Google Scholar] [CrossRef]
- Radhakrishna, V.; Nanilu, S.K.; Sanjeev, G.; Shetty, J.; Somyaji, Y.T.; Moodithaya, S.S. Evaluation of the potency of kinetin on ra-diation induced behavioural changes in Swiss albino mice. J. Clin. Diagn. Res. 2017, 11, TF01. [Google Scholar] [PubMed]
- Wei, Y.; Liu, D.; Zheng, Y.; Hao, C.; Li, H.; Ouyang, W. Neuroprotective effects of kinetin against glutamate-induced oxidative cytotoxicity in HT22 cells: Involvement of Nrf2 and heme oxygenase-1. Neurotox. Res. 2017, 33, 725–737. [Google Scholar] [CrossRef] [PubMed]
- Yannai, S.; Zonszain, J.; Donyo, M.; Ast, G. Combinatorial treatment increases IKAP levels in human cells generated from familial dysautonomia patients. PLoS ONE 2019, 14, e0211602. [Google Scholar] [CrossRef] [PubMed]
- Hönig, M.; Plíhalová, L.; Spíchal, L.; Grúz, J.; Kadlecová, A.; Voller, J.; Svobodová, A.R.; Vostálová, J.; Ulrichová, J.; Doležal, K.; et al. New cytokinin derivatives possess UVA and UVB photoprotective effect on human skin cells and prevent oxidative stress. Eur. J. Med. Chem. 2018, 150, 946–957. [Google Scholar] [CrossRef] [PubMed]
- Lappas, C.M. The plant hormone zeatin riboside inhibits T lymphocyte activity via adenosine A2A receptor activation. Cell. Mol. Immunol. 2014, 12, 107–112. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Choi, S.J.; Jeong, C.-H.; Choi, S.-G.; Chun, J.-Y.; Kim, Y.J.; Lee, J.; Shin, D.-H.; Heo, H.J. Zeatin prevents amyloid β-induced neurotoxicity and scopolamine-induced cognitive deficits. J. Med. Food 2009, 12, 271–277. [Google Scholar] [CrossRef]
| Activity | Mechanism |
|---|---|
| Antioxidant [81,86,87,88,89,90,91,92,93,94,95] | Kinetin controls the level of ROS via
Kinetin and BA protect against oxidative damage via
|
| Antithrombotic [93] | Kinetin suppressed hydroxyl radical formation |
| Anti-aging [52,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114] | Kinetin
|
| Anticancer [77,98,115,116,117,118,119,120,121,122,123,124,125,126,127] | Kinetin, 2iP and BA show antiproliferative activity via
|
| Neuroprotective [43,128,129,130,131,132,133,134,135,136,137,138] | Kinetin
|
| Anti-inflammatory and immunomodulatory [128,129,139,140] | Zeatin riboside modulated mammalian T lymphocyte and immune system activity via an A2AR-dependent mechanism by
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fathy, M.; Saad Eldin, S.M.; Naseem, M.; Dandekar, T.; Othman, E.M. Cytokinins: Wide-Spread Signaling Hormones from Plants to Humans with High Medical Potential. Nutrients 2022, 14, 1495. https://doi.org/10.3390/nu14071495
Fathy M, Saad Eldin SM, Naseem M, Dandekar T, Othman EM. Cytokinins: Wide-Spread Signaling Hormones from Plants to Humans with High Medical Potential. Nutrients. 2022; 14(7):1495. https://doi.org/10.3390/nu14071495
Chicago/Turabian StyleFathy, Moustafa, Sahar M. Saad Eldin, Muhammad Naseem, Thomas Dandekar, and Eman M. Othman. 2022. "Cytokinins: Wide-Spread Signaling Hormones from Plants to Humans with High Medical Potential" Nutrients 14, no. 7: 1495. https://doi.org/10.3390/nu14071495
APA StyleFathy, M., Saad Eldin, S. M., Naseem, M., Dandekar, T., & Othman, E. M. (2022). Cytokinins: Wide-Spread Signaling Hormones from Plants to Humans with High Medical Potential. Nutrients, 14(7), 1495. https://doi.org/10.3390/nu14071495









