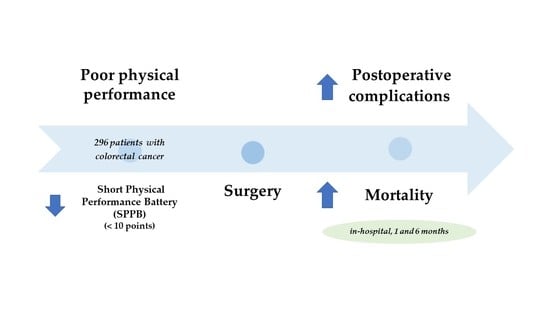
Poor Physical Performance Is Associated with Postoperative Complications and Mortality in Preoperative Patients with Colorectal Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Assessment of Nutritional Status
2.2. Physical Performance
2.3. Clinical Outcomes
2.4. Data Analysis
2.5. Ethics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sawicki, T.; Ruszkowska, M.; Danielewicz, A.; Niedźwiedzka, E.; Arłukowicz, T.; Przybyłowicz, K.E. A Review of Colorectal Cancer in Terms of Epidemiology, Risk Factors, Development, Symptoms and Diagnosis. Cancers 2021, 13, 2025. [Google Scholar] [CrossRef] [PubMed]
- Muscaritoli, M.; Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN practical guideline: Clinical Nutrition in cancer. Clin. Nutr. 2021, 40, 2898–2913. [Google Scholar] [CrossRef] [PubMed]
- Weimann, A.; Braga, M.; Carli, F.; Higashiguchi, T.; Hübner, M.; Klek, S.; Laviano, A.; Ljungqvist, O.; Lobo, D.N.; Martindale, R.; et al. ESPEN guideline: Clinical nutrition in surgery. Clin. Nutr. 2017, 36, 623–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martens, E.A.; Gonnissen, H.K.; Gatta-Cherifi, B.; Janssens, P.L.; Westerterp-Plantenga, M.S. ESPEN practical guideline: Clinical nutrition in surgery. Clin. Nutr. 2021, 40, 4745–4761. [Google Scholar] [CrossRef]
- Horowitz, M.; Neeman, E.; Sharon, E.; Ben-Eliyahu, S. Exploiting the critical perioperative period to improve long-term cancer outcomes. Nat. Rev. Clin. Oncol. 2015, 12, 213–226. [Google Scholar] [CrossRef] [Green Version]
- Tsai, H.-F.; Liu, C.-Y.; Yang, S.-H.; Chiou, A.-F. Factors Related to Frailty in Older Cancer Patients Undergoing Colorectal Surgery. Cancer Nurs. 2021. [Google Scholar] [CrossRef]
- Lee, L.; Schwartzman, K.; Carli, F.; Zavorsky, G.S.; Li, C.; Charlebois, P.; Stein, B.; Liberman, A.S.; Fried, G.M.; Feldman, L.S. The association of the distance walked in 6 min with pre-operative peak oxygen consumption and complications 1 month after colorectal resection. Anaesthesia 2013, 68, 811–816. [Google Scholar] [CrossRef]
- Minnella, E.M.; Liberman, A.S.; Charlebois, P.; Stein, B.; Scheede-Bergdahl, C.; Awasthi, R.; Gillis, C.; Bousquet-Dion, G.; Ramanakuma, A.V.; Pecorelli, N.; et al. The impact of improved functional capacity before surgery on postoperative complications: A study in colorectal cancer. Acta Oncol. 2019, 58, 573–578. [Google Scholar] [CrossRef] [Green Version]
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, R.J.; Berkman, L.F.; Blazer, D.G.; Scherr, P.A.; Wallace, R.B. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J. Gerontol. 1994, 49, M85–M94. [Google Scholar] [CrossRef]
- Zugasti Murillo, A.; Casas Herrero, Á. Frailty syndrome and nutritional status: Assessment, prevention and treatment. Nutr. Hosp. 2019, 36, 26–37. [Google Scholar] [CrossRef]
- Lohsiriwat, V. The influence of preoperative nutritional status on the outcomes of an enhanced recovery after surgery (ERAS) programme for colorectal cancer surgery. Tech. Coloproctology 2014, 18, 1075–1080. [Google Scholar] [CrossRef] [PubMed]
- Parekh, N.R.; Steiger, E. Percentage of Weight Loss as a Predictor of Surgical Risk: From the Time of Hiram Studley to Today. Nutr. Clin. Pract. 2004, 19, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Detsky, A.S.; McLaughlin, J.R.; Baker, J.P.; Johnston, N.; Whittaker, S.; Mendelson, R.A.; Jeejeebhoy, K.N. What is subjective global assessment of nutritional status? J. Parenter. Enter. Nutr. 1987, 11, 8–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dale, W.; Hemmerich, J.; Kamm, A.; Posner, M.C.; Matthews, J.B.; Rothman, R.; Palakodeti, A.; Roggin, K.K. Geriatric assessment improves prediction of surgical outcomes in older adults undergoing pancreaticoduodenectomy: A prospective cohort study. Ann. Surg. 2014, 259, 960–965. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Saitoh, M.; Kawamura, T.; Iwata, K.; Sakurada, K.; Okamura, D.; Tahara, M.; Yuguchi, S.; Kamisaka, K.; Oura, K.; et al. Postoperative atrial fibrillation is associated with delayed early rehabilitation after heart valve surgery: A multicenter study. Phys. Ther. Res. 2019, 22, E9957. [Google Scholar] [CrossRef] [PubMed]
- Nastasi, A.J.; Bryant, T.S.; Le, J.T.; Schrack, J.; Ying, H.; Haugen, C.E.; Fernández, M.G.; Segev, D.L.; McAdams-DeMarco, M.A. Pre-kidney transplant lower extremity impairment and transplant length of stay: A time-to-discharge analysis of a prospective cohort study. BMC Geriatr. 2018, 18, 246. [Google Scholar] [CrossRef]
- Kirchhoff, P.; Clavien, P.-A.; Hahnloser, D. Complications in colorectal surgery: Risk factors and preventive strategies. Patient Saf. Surg. 2010, 4, 5. [Google Scholar] [CrossRef] [Green Version]
- Pavasini, R.; Guralnik, J.; Brown, J.C.; Di Bari, M.; Cesari, M.; Landi, F.; Vaes, B.; Legrand, D.; Verghese, J.; Wang, C.; et al. Short Physical Performance Battery and all-cause mortality: Systematic review and meta-analysis. BMC Med. 2016, 14, 215. [Google Scholar] [CrossRef] [Green Version]
- Abizanda, P.; Romero, L.; Sanchez-Jurado, P.; Atienzar-Núñez, P.; Esquinas-Requena, J.; Garcia-Nogueras, I. Association between Functional Assessment Instruments and Frailty in Older Adults: The FRADEA Study. J. Frailty Aging 2012, 1, 162–168. [Google Scholar] [CrossRef]
- Bergland, A.; Strand, B.H. Norwegian reference values for the Short Physical Performance Battery (SPPB): The Tromsø Study. BMC Geriatr. 2019, 19, 216. [Google Scholar] [CrossRef] [Green Version]
- Yuguchi, S.; Saitoh, M.; Oura, K.; Tahara, M.; Kamisaka, K.; Kawamura, T.; Kato, M.; Morisawa, T.; Takahashi, T. Impact of preoperative frailty on regaining walking ability in patients after cardiac surgery: Multicenter cohort study in Japan. Arch. Gerontol. Geriatr. 2019, 83, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Verweij, N.M.; Schiphorst, A.H.W.; Pronk, A.; Bos, F.V.D.; Hamaker, M.E. Physical performance measures for predicting outcome in cancer patients: A systematic review. Acta Oncol. 2016, 55, 1386–1391. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Bolívar, V.; Sánchez-Torralvo, F.J.; Ruiz-Vico, M.; González-Almendros, I.; Barrios, M.; Padín, S.; Alba, E.; Olveira, G. GLIM criteria using hand grip strength adequately predict six-month mortality in cancer inpatients. Nutrients 2019, 11, 2043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torralvo, F.J.S.; Porras, N.; Fernandez, J.A.; Torres, F.G.; Tapia, M.J.; Lima, F.; Soriguer, F.; Gonzalo, M.; Martínez, G.R.; Olveira, G. Normative reference values for hand grip dynamometry in Spain. Association with lean mass. Nutr. Hosp. 2018, 35, 98–103. [Google Scholar]
- Syddall, H.; Cooper, C.; Martin, F.; Briggs, R.; Sayer, A.A. Is grip strength a useful single marker of frailty? Age Ageing 2003, 32, 650–656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gillis, C.; Richer, L.; Fenton, T.R.; Gramlich, L.; Keller, H.; Culos-Reed, S.N.; Sajobi, T.T.; Awasthi, R.; Carli, F. Colorectal cancer patients with malnutrition suffer poor physical and mental health before surgery. Surgery 2021, 170, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.J.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. J. Cachexia Sarcopenia Muscle 2019, 10, 207–217. [Google Scholar] [CrossRef] [Green Version]
- Prado, C.M.; Lieffers, J.R.; McCargar, L.J.; Reiman, T.; Sawyer, M.B.; Martin, L.; Baracos, V.E. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population-based study. Lancet Oncol. 2008, 9, 629–635. [Google Scholar] [CrossRef]
- Mintziras, I.; Miligkos, M.; Wächter, S.; Manoharan, J.; Maurer, E.; Bartsch, D.K. Sarcopenia and sarcopenic obesity are significantly associated with poorer overall survival in patients with pancreatic cancer: Systematic review and meta-analysis. Int. J. Surg. 2018, 59, 19–26. [Google Scholar] [CrossRef]
- Barbosa, L.R.L.S.; Lacerda-Filho, A.; Barbosa, L.C.L.S. Immediate preoperative nutritional status of patients with colorectal cancer: A warning. Arq. Gastroenterol. 2014, 51, 331–336. [Google Scholar] [CrossRef]
- Song, H.-N.; Wang, W.-B.; Luo, X.; Huang, D.-D.; Ruan, X.-J.; Xing, C.-G.; Chen, W.-Z.; Dong, Q.-T.; Chen, X.-L. Effect of GLIM-defined malnutrition on postoperative clinical outcomes in patients with colorectal cancer. Jpn. J. Clin. Oncol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.U.; Fan, G.H.; Hastie, D.J.; Addonizio, E.A.; Suh, J.; Prakasam, V.N.; Karagozian, R. The clinical impact of malnutrition on the postoperative outcomes of patients undergoing colorectal resection surgery for colon or rectal cancer: Propensity score matched analysis of 2011-2017 US hospitals. Surg. Oncol. 2021, 38, 101587. [Google Scholar] [CrossRef] [PubMed]
- Laur, C.V.; McNicholl, T.; Valaitis, R.; Keller, H.H. Malnutrition or frailty? Overlap and evidence gaps in the diagnosis and treatment of frailty and malnutrition. Appl. Physiol. Nutr. Metab. 2017, 42, 449–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, D.G.A.; Ohnuma, T.; Krishnamoorthy, V.; Raghunathan, K.; Sulo, S.; Cassady, B.A.; Hegazi, R.; Wischmeyer, P.E. Impact of early postoperative oral nutritional supplement utilization on clinical outcomes in colorectal surgery. Perioper. Med. 2020, 9, 29. [Google Scholar] [CrossRef]
- Palermi, S.; Iacono, O.; Sirico, F.; Modestino, M.; Ruosi, C.; Spera, R.; De Luca, M. The complex relationship between physical activity and diabetes: An overview. J. Basic Clin. Physiol. Pharmacol. 2021. [Google Scholar] [CrossRef]
- Berkel, A.E.M.; Bongers, B.C.; Kotte, H.; Weltevreden, P.; de Jongh, F.H.C.; Eijsvogel, M.M.M.; Wymenga, M.; Bigirwamungu-Bargeman, M.; van der Palen, J.; van Det, M.J.; et al. Effects of Community-based Exercise Prehabilitation for Patients Scheduled for Colorectal Surgery with High Risk for Postoperative Complications: Results of a Randomized Clinical Trial. Ann. Surg. 2021, 275, e299–e306. [Google Scholar] [CrossRef]
- Barberan-Garcia, A.; Ubré, M.; Roca, J.; Lacy, A.M.; Burgos, F.; Risco, R.; Momblán, D.; Balust, J.; Blanco, I.; Martínez-Pallí, G. Personalised Prehabilitation in High-risk Patients Undergoing Elective Major Abdominal Surgery: A Randomized Blinded Controlled Trial. Ann. Surg. 2018, 267, 50–56. [Google Scholar] [CrossRef]
- Daniels, S.L.; Lee, M.J.; George, J.; Kerr, K.; Moug, S.; Wilson, T.R.; Brown, S.R.; Wyld, L. Prehabilitation in elective abdominal cancer surgery in older patients: Systematic review and meta-analysis. BJS Open 2020, 4, 1022–1041. [Google Scholar] [CrossRef]
| n = 296 | ||
| Age (years) | mean ± SD (min–max) | 68.4 ± 10.2 (30–89) |
| Sex | n (%) | |
| Men | 175 (59.1) | |
| Women | 121 (40.9) | |
| Type of cancer | n (%) | |
| Colon | 180 (60.8) | |
| Rectum | 116 (39.2) | |
| Stage | n (%) | |
| I | 39 (13.1) | |
| II | 99 (33.5) | |
| III | 124 (41.9) | |
| IV | 34 (11.5) | |
| BMI (kg/m2) | mean ± SD (min–max) | |
| Men | 27.6 ± 5.1 (17.2–47.6) | |
| Women | 26.5 ± 5.3 (15.8–46.1) | |
| Surgical complications | n (%) | 131 (44.2) |
| Postoperative collection | 24 (18.3) | |
| Paralytic ileus | 23 (17.6) | |
| Surgical wound infection | 23 (17.6) | |
| Suture dehiscence | 16 (12.2) | |
| Febrile syndrome | 13 (9.9) | |
| Bleeding | 12 (9.2) | |
| Other | 20 (15.3) | |
| In-hospital exitus | n (%) | 8 (2.7) |
| 1-month exitus | n (%) | 9 (3) |
| 6-month exitus | n (%) | 14 (4.7) |
| n = 296 | ||
| SPPB | ||
| Balance (points) | mean ± SD | 3.81 ± 0.48 |
| 4 m gait speed (points) | mean ± SD | 3.60 ± 0.83 |
| Sit to stand (points) | mean ± SD | 3.14 ± 1.14 |
| Total (points) | mean ± SD | 10.57 ± 2.07 |
| SPPB < 10 (low physical performance) | n (%) | 69 (23.3%) |
| SPPB ≥ 10 | n (%) | 227 (76.7%) |
| Hand grip strength | ||
| Men | mean ± SD (min–max) | 34.01 ± 8.57 (13.3–57.8) |
| Women | mean ± SD (min–max) | 21.03 ± 5.09 (10.6–34) |
| Low hand grip strength | n (%) | 58 (19.6%) |
| Normal hand grip strength | n (%) | 238 (80.4%) |
| SPPB ≥ 10 (n = 227) Mean ± SD | SPPB < 10 (n = 69) Mean ± SD | p Value | Normal Hand Grip Strength (n = 238) Mean ± SD | Low Hand Grip Strength (n = 58) Mean ± SD | p Value | |
|---|---|---|---|---|---|---|
| Age (years) | 67 ± 9.9 | 73.2 ± 9.9 | <0.001 | 67 ± 10 | 74.3 ± 9.2 | <0.001 |
| BMI (kg/m2) | 26.9 ± 4.9 | 27.7 ± 6.3 | 0.29 | 27.5 ± 5.3 | 25.8 ± 5.1 | 0.033 |
| Hand grip strength (kg) | ||||||
| Men | 35.2 ± 7.7 | 25.4 ± 6.5 | <0.001 | 37.3 ± 6.1 | 21.8 ± 4 | <0.001 |
| Women | 22.7 ± 4.5 | 17.1 ± 4.2 | <0.001 | 22.6 ± 4 | 13.3 ± 1.6 | <0.001 |
| Brachial circumference (cm) | 28.8 ± 3.8 | 28.4 ± 4.8 | 0.49 | 29.1 ± 3.8 | 27 ± 4.6 | <0.001 |
| Albumin (g/dL) | 3.7 ± 0.5 | 3.5 ± 0.5 | 0.003 | 3.7 ± 0.4 | 3.5 ± 0.6 | 0.006 |
| CRP (mg/dL) | 7.7 ± 11.8 | 17.9 ± 26 | 0.003 | 7.1 ± 9 | 19.5 ± 29 | <0.001 |
| CRP/albumin ratio | 2.3 ± 3.9 | 6.7 ± 11.7 | 0.001 | 2 ± 2.7 | 7.4 ± 12.4 | <0.001 |
| Length of stay (days) | 11.1 ± 8.9 | 14.1 ± 9.4 | 0.018 | 11.2 ± 9.2 | 13.8 ± 8.1 | 0.058 |
| Crude | Adjusted | |||||||
|---|---|---|---|---|---|---|---|---|
| Odds Ratio | 95% CI | p Value | Odds Ratio | 95% CI | p Value | |||
| Lower | Upper | Lower | Upper | |||||
| Low SPPB | 2.37 | 1.35 | 4.17 | 0.003 | 2.52 | 1.35 | 4.70 | 0.004 |
| Low hand grip strength | 2.77 | 1.51 | 5.07 | 0.001 | 2.62 | 1.33 | 5.13 | 0.005 |
| In-Hospital Mortality | 1-Month Mortality | 6-Month Mortality | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Normal | Low | p Value | Normal | Low | p Value | Normal | Low | p Value | |
| SPPB | 2 (0.9%) | 6 (8.7%) | 0.021 | 3 (1.3%) | 6 (8.7%) | 0.002 | 2 (2.2%) | 9 (13.1%) | <0.001 |
| Hand grip strength | 5 (2.1%) | 3 (5.3%) | 0.187 | 6 (2.5%) | 3 (5.3%) | 0.280 | 8 (3.3%) | 6 (10.5%) | 0.022 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Torralvo, F.J.; González-Poveda, I.; García-Olivares, M.; Porras, N.; Gonzalo-Marín, M.; Tapia, M.J.; Mera-Velasco, S.; Toval-Mata, J.A.; Ruiz-López, M.; Carrasco-Campos, J.; et al. Poor Physical Performance Is Associated with Postoperative Complications and Mortality in Preoperative Patients with Colorectal Cancer. Nutrients 2022, 14, 1484. https://doi.org/10.3390/nu14071484
Sánchez-Torralvo FJ, González-Poveda I, García-Olivares M, Porras N, Gonzalo-Marín M, Tapia MJ, Mera-Velasco S, Toval-Mata JA, Ruiz-López M, Carrasco-Campos J, et al. Poor Physical Performance Is Associated with Postoperative Complications and Mortality in Preoperative Patients with Colorectal Cancer. Nutrients. 2022; 14(7):1484. https://doi.org/10.3390/nu14071484
Chicago/Turabian StyleSánchez-Torralvo, Francisco José, Iván González-Poveda, María García-Olivares, Nuria Porras, Montserrat Gonzalo-Marín, María José Tapia, Santiago Mera-Velasco, José Antonio Toval-Mata, Manuel Ruiz-López, Joaquín Carrasco-Campos, and et al. 2022. "Poor Physical Performance Is Associated with Postoperative Complications and Mortality in Preoperative Patients with Colorectal Cancer" Nutrients 14, no. 7: 1484. https://doi.org/10.3390/nu14071484
APA StyleSánchez-Torralvo, F. J., González-Poveda, I., García-Olivares, M., Porras, N., Gonzalo-Marín, M., Tapia, M. J., Mera-Velasco, S., Toval-Mata, J. A., Ruiz-López, M., Carrasco-Campos, J., Santoyo-Santoyo, J., & Olveira, G. (2022). Poor Physical Performance Is Associated with Postoperative Complications and Mortality in Preoperative Patients with Colorectal Cancer. Nutrients, 14(7), 1484. https://doi.org/10.3390/nu14071484