Low-Carbohydrate Diets and Mortality in Older Asian People: A 15-Year Follow-Up from a Prospective Cohort Study
Abstract
1. Introduction
2. Methods
2.1. Study Design and Sample
2.2. Assessment of LCD Score
2.3. Ascertainment of Mortality
2.4. Potential Confounders and Mediators
2.5. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Mortality and LCD Score
3.3. Subgroup and Sensitivity Analyses
4. Discussion
4.1. Comparison with Previous Studies
4.2. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ludwig, D.S.; Hu, F.B.; Tappy, L.; Brand-Miller, J. Dietary carbohydrates: Role of quality and quantity in chronic disease. BMJ 2018, 361, k2340. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Yang, X.; Fang, Y.; Zhang, J.; Yang, Z.; Wang, Z.; Liu, A.; He, L.; Sun, J.; Lian, Y.; et al. Trends and Disparities of Energy Intake and Macronutrient Composition in China: A Series of National Surveys, 1982–2012. Nutrients 2020, 12, 2168. [Google Scholar] [CrossRef] [PubMed]
- Shan, Z.; Rehm, C.D.; Rogers, G.; Ruan, M.; Wang, D.D.; Hu, F.B.; Mozaffarian, D.; Zhang, F.F.; Bhupathiraju, S.N. Trends in Dietary Carbohydrate, Protein, and Fat Intake and Diet Quality Among US Adults, 1999–2016. JAMA 2019, 322, 1178–1187. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Shi, Z. Dietary Pattern during 1991–2011 and Its Association with Cardio Metabolic Risks in Chinese Adults: The China Health and Nutrition Survey. Nutrients 2017, 9, 1218. [Google Scholar] [CrossRef]
- Seidelmann, S.B.; Claggett, B.; Cheng, S.; Henglin, M.; Shah, A.; Steffen, L.M.; Folsom, A.R.; Rimm, E.B.; Willett, W.C.; Solomon, S.D. Dietary carbohydrate intake and mortality: A prospective cohort study and meta-analysis. Lancet Public Health 2018, 3, e419–e428. [Google Scholar] [CrossRef]
- Gardner, C.D.; Trepanowski, J.F.; Del, G.L.; Hauser, M.E.; Rigdon, J.; Ioannidis, J.; Desai, M.; King, A.C. Effect of Low-Fat vs Low-Carbohydrate Diet on 12-Month Weight Loss in Overweight Adults and the Association With Genotype Pattern or Insulin Secretion: The DIETFITS Randomized Clinical Trial. JAMA 2018, 319, 667–679. [Google Scholar] [CrossRef]
- Foster, G.D.; Wyatt, H.R.; Hill, J.O.; Makris, A.P.; Rosenbaum, D.L.; Brill, C.; Stein, R.I.; Mohammed, B.S.; Miller, B.; Rader, D.J.; et al. Weight and metabolic outcomes after 2 years on a low-carbohydrate versus low-fat diet: A randomized trial. Ann. Intern. Med. 2010, 153, 147–157. [Google Scholar] [CrossRef]
- Shan, Z.; Guo, Y.; Hu, F.B.; Liu, L.; Qi, Q. Association of Low-Carbohydrate and Low-Fat Diets With Mortality among US Adults. JAMA Intern. Med. 2020, 180, 513–523. [Google Scholar] [CrossRef]
- Akter, S.; Mizoue, T.; Nanri, A.; Goto, A.; Noda, M.; Sawada, N.; Yamaji, T.; Iwasaki, M.; Inoue, M.; Tsugane, S. Low carbohydrate diet and all cause and cause-specific mortality. Clin. Nutr. 2021, 40, 2016–2024. [Google Scholar] [CrossRef]
- Reynolds, A.; Mann, J.; Cummings, J.; Winter, N.; Mete, E.; Te, M.L. Carbohydrate quality and human health: A series of systematic reviews and meta-analyses. Lancet 2019, 393, 434–445. [Google Scholar] [CrossRef]
- Muraki, I.; Wu, H.; Imamura, F.; Laden, F.; Rimm, E.B.; Hu, F.B.; Willett, W.C.; Sun, Q. Rice consumption and risk of cardiovascular disease: Results from a pooled analysis of 3 U.S. cohorts. Am. J. Clin. Nutr. 2015, 101, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Hardy, D.S.; Garvin, J.T.; Xu, H. Carbohydrate quality, glycemic index, glycemic load and cardiometabolic risks in the US, Europe and Asia: A dose-response meta-analysis. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 853–871. [Google Scholar] [CrossRef] [PubMed]
- Bolla, A.M.; Caretto, A.; Laurenzi, A.; Scavini, M.; Piemonti, L. Low-Carb and Ketogenic Diets in Type 1 and Type 2 Diabetes. Nutrients 2019, 11, 962. [Google Scholar] [CrossRef] [PubMed]
- Sacks, F.M.; Lichtenstein, A.H.; Wu, J.; Appel, L.J.; Creager, M.A.; Kris-Etherton, P.M.; Miller, M.; Rimm, E.B.; Rudel, L.L.; Robinson, J.G.; et al. Dietary Fats and Cardiovascular Disease: A Presidential Advisory From the American Heart Association. Circulation 2017, 136, e1–e23. [Google Scholar] [CrossRef]
- Zhong, V.W.; Allen, N.B.; Greenland, P.; Carnethon, M.R.; Ning, H.; Wilkins, J.T.; Lloyd-Jones, D.M.; Van Horn, L. Protein foods from animal sources, incident cardiovascular disease and all-cause mortality: A substitution analysis. Int. J. Epidemiol. 2021, 50, 223–233. [Google Scholar] [CrossRef]
- Jiang, C.; Thomas, G.N.; Lam, T.H.; Schooling, C.M.; Zhang, W.; Lao, X.; Adab, P.; Liu, B.; Leung, G.M.; Cheng, K.K. Cohort profile: The Guangzhou Biobank Cohort Study, a Guangzhou-Hong Kong-Birmingham collaboration. Int. J. Epidemiol. 2006, 35, 844–852. [Google Scholar] [CrossRef]
- Woo, J.; Leung, S.S.; Ho, S.C.; Lam, T.H.; Janus, E.D. A food frequency questionnaire for use in the Chinese population in Hong Kong: Description and examination of validity. Nutr. Res. 1997, 17, 1633–1641. [Google Scholar] [CrossRef]
- Lam, T.H.; Xu, L.; Jiang, C.Q.; Zhang, W.S.; Zhu, F.; Jin, Y.L.; Thomas, G.N.; Cheng, K.K. High relative risk of all-cause mortality attributed to smoking in China: Guangzhou Biobank Cohort Study. PLoS ONE 2018, 13, e196610. [Google Scholar] [CrossRef]
- Vathesatogkit, P.; Batty, G.D.; Woodward, M. Socioeconomic disadvantage and disease-specific mortality in Asia: Systematic review with meta-analysis of population-based cohort studies. J. Epidemiol. Community Health 2014, 68, 375–383. [Google Scholar] [CrossRef]
- Zhu, N.; Yu, C.; Guo, Y.; Bian, Z.; Han, Y.; Yang, L.; Chen, Y.; Du, H.; Li, H.; Liu, F.; et al. Adherence to a healthy lifestyle and all-cause and cause-specific mortality in Chinese adults: A 10-year prospective study of 0.5 million people. Int J. Behav. Nutr. Phys. Act. 2019, 16, 98. [Google Scholar] [CrossRef]
- Castro-Espin, C.; Agudo, A.; Bonet, C.; Katzke, V.; Turzanski-Fortner, R.; Aleksandrova, K.; Schulze, M.B.; Tjønneland, A.; Dahm, C.C.; Quirós, J.R.; et al. Inflammatory potential of the diet and risk of breast cancer in the European Investigation into Cancer and Nutrition (EPIC) study. Eur. J. Epidemiol. 2021, 36, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Mazidi, M.; Katsiki, N.; Mikhailidis, D.P.; Sattar, N.; Banach, M. Lower carbohydrate diets and all-cause and cause-specific mortality: A population-based cohort study and pooling of prospective studies. Eur. Heart J. 2019, 40, 2870–2879. [Google Scholar] [CrossRef]
- Fung, T.T.; van Dam, R.M.; Hankinson, S.E.; Stampfer, M.; Willett, W.C.; Hu, F.B. Low-carbohydrate diets and all-cause and cause-specific mortality: Two cohort studies. Ann. Intern. Med. 2010, 153, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, S.; Dehghan, M.; Raj, J.M.; Thomas, T.; Rangarajan, S.; Jenkins, D.; Mony, P.; Mohan, V.; Lear, S.A.; Avezum, A.; et al. Associations of cereal grains intake with cardiovascular disease and mortality across 21 countries in Prospective Urban and Rural Epidemiology study: Prospective cohort study. BMJ 2021, 372, m4948. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Keum, N.; Giovannucci, E.; Fadnes, L.T.; Boffetta, P.; Greenwood, D.C.; Tonstad, S.; Vatten, L.J.; Riboli, E.; Norat, T. Whole grain consumption and risk of cardiovascular disease, cancer, and all cause and cause specific mortality: Systematic review and dose-response meta-analysis of prospective studies. BMJ 2016, 353, i2716. [Google Scholar] [CrossRef]
- Xu, X.; Byles, J.E.; Shi, Z.; Hall, J.J. Evaluation of older Chinese people’s macronutrient intake status: Results from the China Health and Nutrition Survey. Br. J. Nutr. 2015, 113, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; McLerran, D.F.; Rolland, B.; Chen, Y.; Grant, E.J.; Vedanthan, R.; Inoue, M.; Tsugane, S.; Gao, Y.T.; Tsuji, I.; et al. Meat intake and cause-specific mortality: A pooled analysis of Asian prospective cohort studies. Am. J. Clin. Nutr. 2013, 98, 1032–1041. [Google Scholar] [CrossRef]
- Shao, M.Y.; Jiang, C.Q.; Zhang, W.S.; Zhu, F.; Jin, Y.L.; Woo, J.; Cheng, K.K.; Lam, T.H.; Xu, L. Association of fish consumption with risk of all-cause and cardiovascular disease mortality: An 11-year follow-up of the Guangzhou Biobank Cohort Study. Eur. J. Clin. Nutr. 2021, 76, 389–396. [Google Scholar] [CrossRef]
- Zhong, V.W.; Van Horn, L.; Greenland, P.; Carnethon, M.R.; Ning, H.; Wilkins, J.T.; Lloyd-Jones, D.M.; Allen, N.B. Associations of Processed Meat, Unprocessed Red Meat, Poultry, or Fish Intake with Incident Cardiovascular Disease and All-Cause Mortality. JAMA Intern. Med. 2020, 180, 503–512. [Google Scholar] [CrossRef]
- Godfray, H.; Aveyard, P.; Garnett, T.; Hall, J.W.; Key, T.J.; Lorimer, J.; Pierrehumbert, R.T.; Scarborough, P.; Springmann, M.; Jebb, S.A. Meat consumption, health, and the environment. Science 2018, 361, eaam5324. [Google Scholar] [CrossRef]
- Shridhar, K.; Dhillon, P.K.; Bowen, L.; Kinra, S.; Bharathi, A.V.; Prabhakaran, D.; Reddy, K.S.; Ebrahim, S. The association between a vegetarian diet and cardiovascular disease (CVD) risk factors in India: The Indian Migration Study. PLoS ONE 2014, 9, e110586. [Google Scholar]
- Jayedi, A.; Soltani, S.; Abdolshahi, A.; Shab-Bidar, S. Fish consumption and the risk of cardiovascular disease and mortality in patients with type 2 diabetes: A dose-response meta-analysis of prospective cohort studies. Crit Rev. Food Sci. Nutr. 2021, 61, 1640–1650. [Google Scholar] [CrossRef] [PubMed]
- Miller, V.; Mente, A.; Dehghan, M.; Rangarajan, S.; Zhang, X.; Swaminathan, S.; Dagenais, G.; Gupta, R.; Mohan, V.; Lear, S.; et al. Fruit, vegetable, and legume intake, and cardiovascular disease and deaths in 18 countries (PURE): A prospective cohort study. Lancet 2017, 390, 2037–2049. [Google Scholar] [CrossRef]
- Kim, H.; Lee, K.; Rebholz, C.M.; Kim, J. Plant-based diets and incident metabolic syndrome: Results from a South Korean prospective cohort study. PLoS Med. 2020, 17, e1003371. [Google Scholar] [CrossRef]
- Cui, Y.; Hao, P.; Liu, B.; Meng, X. Effect of traditional Chinese cooking methods on fatty acid profiles of vegetable oils. Food Chem. 2017, 233, 77–84. [Google Scholar] [CrossRef]
- de Souza, R.J.; Mente, A.; Maroleanu, A.; Cozma, A.I.; Ha, V.; Kishibe, T.; Uleryk, E.; Budylowski, P.; Schünemann, H.; Beyene, J.; et al. Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: Systematic review and meta-analysis of observational studies. BMJ 2015, 351, h3978. [Google Scholar] [CrossRef]
- Strain, W.D.; Paldánius, P.M. Diabetes, cardiovascular disease and the microcirculation. Cardiovasc. Diabetol. 2018, 17, 57. [Google Scholar] [CrossRef] [PubMed]
- Tobias, D.K.; Chen, M.; Manson, J.E.; Ludwig, D.S.; Willett, W.; Hu, F.B. Effect of low-fat diet interventions versus other diet interventions on long-term weight change in adults: A systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015, 3, 968–979. [Google Scholar] [CrossRef]
- Musa-Veloso, K.; Poon, T.; Harkness, L.S.; O’Shea, M.; Chu, Y. The effects of whole-grain compared with refined wheat, rice, and rye on the postprandial blood glucose response: A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2018, 108, 759–774. [Google Scholar] [CrossRef] [PubMed]
- Vanegas, S.M.; Meydani, M.; Barnett, J.B.; Goldin, B.; Kane, A.; Rasmussen, H.; Brown, C.; Vangay, P.; Knights, D.; Jonnalagadda, S.; et al. Substituting whole grains for refined grains in a 6-wk randomized trial has a modest effect on gut microbiota and immune and inflammatory markers of healthy adults. Am. J. Clin. Nutr. 2017, 105, 635–650. [Google Scholar] [CrossRef]
- Welsh, J.A.; Sharma, A.; Abramson, J.L.; Vaccarino, V.; Gillespie, C.; Vos, M.B. Caloric sweetener consumption and dyslipidemia among US adults. JAMA 2010, 303, 1490–1497. [Google Scholar] [CrossRef] [PubMed]
- Chan, R.; Leung, J.; Woo, J. High Protein Intake Is Associated with Lower Risk of All-Cause Mortality in Community-Dwelling Chinese Older Men and Women. J. Nutr. Health Aging 2019, 23, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Fung, T.T.; Hu, F.B.; Willett, W.C.; Longo, V.D.; Chan, A.T.; Giovannucci, E.L. Association of Animal and Plant Protein Intake With All-Cause and Cause-Specific Mortality. JAMA Intern. Med. 2016, 176, 1453–1463. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Lam, T.H.; Jiang, C.Q.; Zhang, W.S.; Zhu, F.; Jin, Y.L.; Woo, J.; Cheng, K.K.; Thomas, G.N. Egg consumption and the risk of cardiovascular disease and all-cause mortality: Guangzhou Biobank Cohort Study and meta-analyses. Eur. J. Nutr. 2019, 58, 785–796. [Google Scholar] [CrossRef]
- Schooling, C.M.; Ho, S.Y.; Leung, G.M.; Thomas, G.N.; McGhee, S.M.; Mak, K.H.; Lam, T.H. Diet synergies and mortality—A population-based case-control study of 32,462 Hong Kong Chinese older adults. Int. J. Epidemiol. 2006, 35, 418–426. [Google Scholar] [CrossRef][Green Version]
- Goldenberg, J.Z.; Day, A.; Brinkworth, G.D.; Sato, J.; Yamada, S.; Jönsson, T.; Beardsley, J.; Johnson, J.A.; Thabane, L.; Johnston, B.C. Efficacy and safety of low and very low carbohydrate diets for type 2 diabetes remission: Systematic review and meta-analysis of published and unpublished randomized trial data. BMJ 2021, 372, m4743. [Google Scholar] [CrossRef]
- Lee, H.; Cashin, A.G.; Lamb, S.E.; Hopewell, S.; Vansteelandt, S.; VanderWeele, T.J.; MacKinnon, D.P.; Mansell, G.; Collins, G.S.; Golub, R.M.; et al. A Guideline for Reporting Mediation Analyses of Randomized Trials and Observational Studies. JAMA J. Am. Med. Assoc. 2021, 326, 1045–1056. [Google Scholar] [CrossRef]
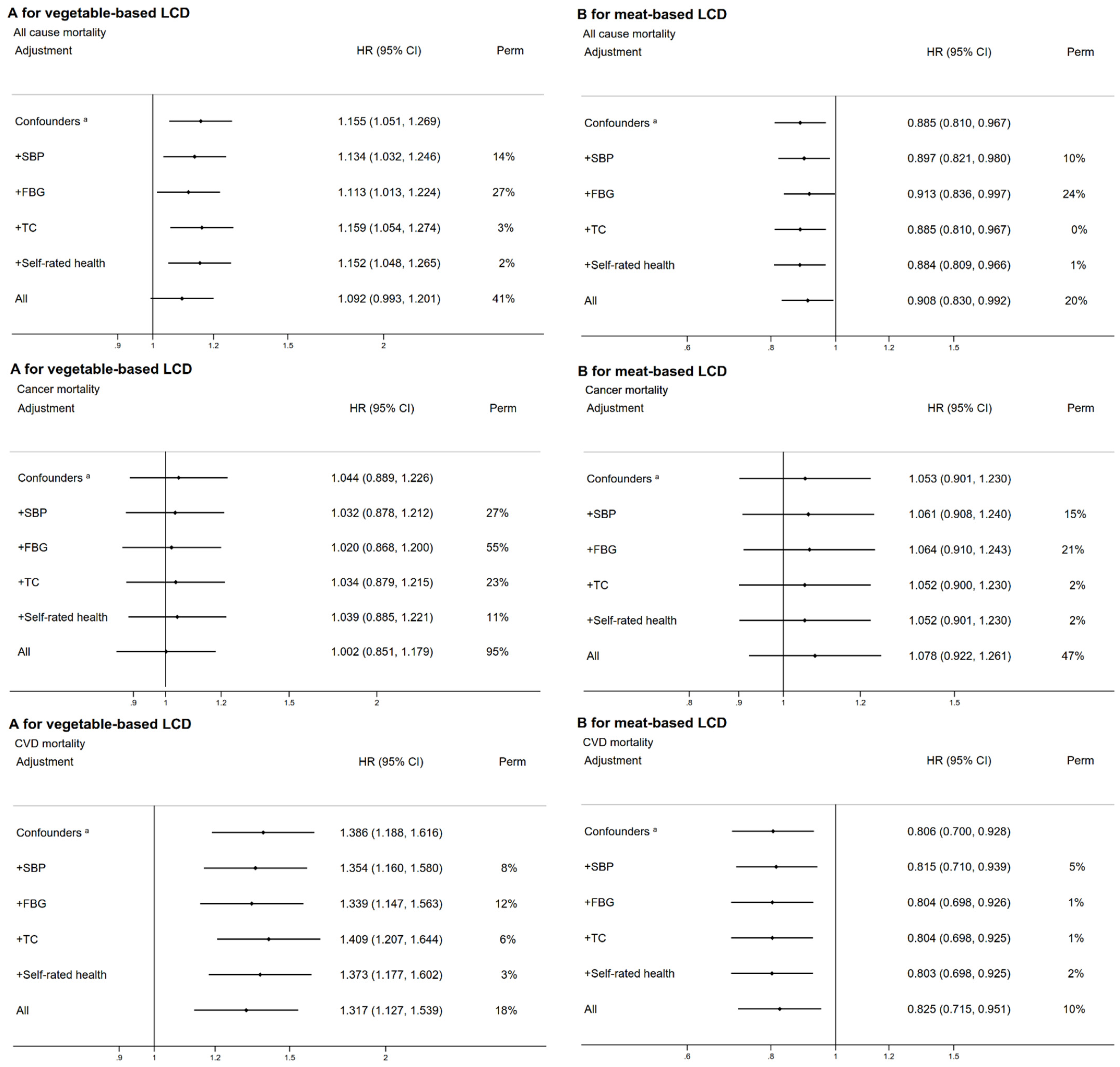
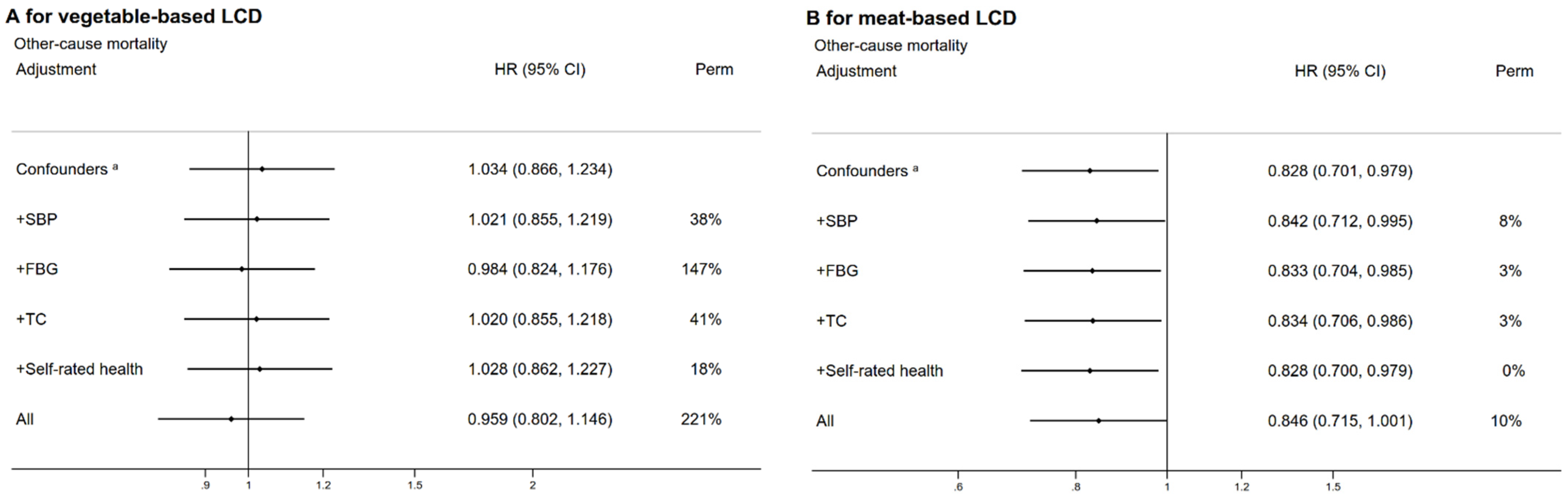


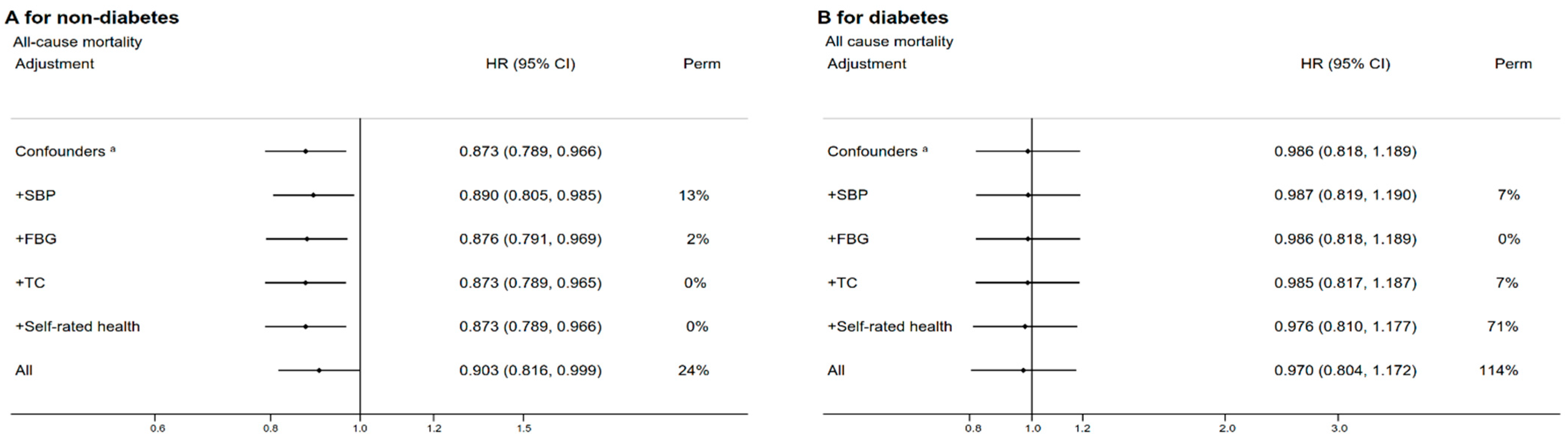
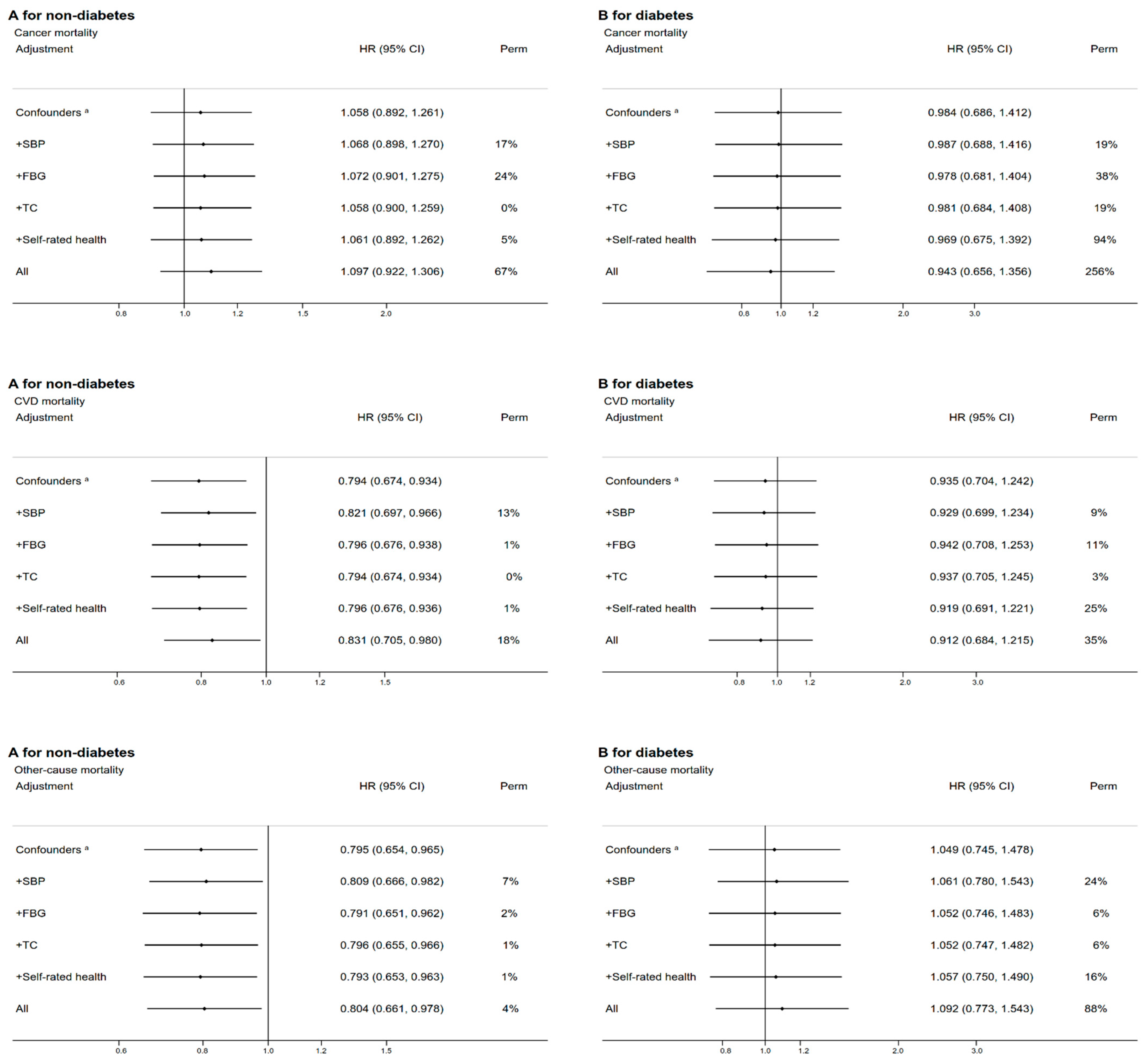
| Characteristic | Overall Low-Carbohydrate-Diet Score | p-Value | Vegetable-Based Low-Carbohydrate-Diet Score | p-Value | Meat-Based Low-Carbohydrate-Diet Score | p-Value | |||
|---|---|---|---|---|---|---|---|---|---|
| Quartile 1 | Quartile 4 | Quartile 1 | Quartile 4 | Quartile 1 | Quartile 4 | ||||
| Number of participants | 5100 | 4936 | 5278 | 3775 | 5163 | 4418 | |||
| Age, mean (SD), year | 63.5 (6.5) | 62.1 (6.8) | 0.04 | 62.7 (6.7) | 62.8 (6.6) | 0.70 | 63.4 (6.6) | 62.0 (6.8) | 0.02 |
| Sex | <0.001 | 0.56 | <0.001 | ||||||
| Women | 1696 (33.3) | 3707 (75.1) | 3740 (70.9) | 2677 (70.9) | 3398 (65.8) | 3432 (77.7) | |||
| Men | 3404 (66.7) | 1229 (24.9) | 1538 (29.1) | 1098 (29.1) | 1765 (34.2) | 986 (22.3) | |||
| Education level | <0.001 | <0.001 | <0.001 | ||||||
| Less than primary school | 2678 (52.5) | 1988 (40.3) | 2231 (42.3) | 1878 (49.8) | 2826 (54.8) | 1619 (36.6) | |||
| Middle school | 2009 (39.4) | 2445 (49.5) | 2463 (46.7) | 1609 (42.6) | 2003 (38.8) | 2305 (52.2) | |||
| College or above | 413 (8.1) | 503 (10.2) | 584 (11.0) | 288 (7.6) | 330 (6.4) | 493 (11.2) | |||
| Family income, RMB/year | <0.001 | 0.21 | <0.001 | ||||||
| <20,000 | 1261 (24.7) | 820 (16.6) | 992 (18.8) | 764 (20.2) | 1297 (25.2) | 688 (15.6) | |||
| 20,000–30,000 | 986 (19.3) | 935 (18.9) | 1062 (20.1) | 756 (20.0) | 1004 (19.5) | 839 (19.0) | |||
| 30,000–50,000 | 781 (15.3) | 1063 (21.5) | 979 (18.6) | 689 (18.3) | 812 (15.7) | 943 (21.4) | |||
| ≥50,000 | 473 (9.3) | 902 (18.3) | 768 (14.5) | 550 (14.6) | 443 (8.6) | 880 (20.0) | |||
| Do not know | 1599 (31.4) | 1216 (24.7) | 1474 (28.0) | 1016 (26.9) | 1598 (31.0) | 1060 (24.0) | |||
| BMI, mean (SD), kg/m2 | 23.8 (3.3) | 23.7 (3.3) | 0.71 | 23.7 (3.2) | 23.8 (3.3) | 0.63 | 23.9 (3.3) | 23.7 (3.3) | 0.02 |
| Physical activity | <0.001 | <0.001 | <0.001 | ||||||
| Low | 305 (6.0) | 479 (9.7) | 285 (5.4) | 498 (13.2) | 378 (7.4) | 397 (9.0) | |||
| Moderate | 2113 (41.4) | 2630 (53.3) | 2416 (45.8) | 1808 (47.9) | 2155 (41.7) | 2347 (53.1) | |||
| High | 2682 (52.6) | 1827 (37.0) | 2577 (48.8) | 1469 (38.9) | 2630 (50.9) | 1674 (37.9) | |||
| Smoking | <0.001 | <0.001 | <0.001 | ||||||
| Never | 3944 (77.4) | 4045 (82.0) | 4301 (81.5) | 2890 (76.6) | 3915 (75.9) | 3731 (84.5) | |||
| Past | 581 (11.4) | 393 (7.9) | 532 (10.1) | 380 (10.0) | 600 (11.6) | 327 (7.4) | |||
| Current | 575 (11.3) | 498 (10.1) | 445 (8.4) | 506 (13.4) | 648 (12.5) | 360 (8.1) | |||
| Drinking | 0.001 | 0.24 | 0.004 | ||||||
| Never | 4139 (81.4) | 3919 (79.5) | 4170 (79.2) | 3052 (81.0) | 4164 (80.8) | 3547 (80.5) | |||
| Current | 823 (16.1) | 915 (18.5) | 991 (18.7) | 639 (16.9) | 869 (16.8) | 785 (17.7) | |||
| Past | 138 (2.6) | 102 (2.0) | 116 (2.0) | 84 (2.1) | 130 (2.4) | 86 (1.8) | |||
| Dietary intake, mean (SD) | |||||||||
| Total energy, kcal/d | 1853 (520) | 1730 (502) | 0.003 | 1817 (530) | 1783 (488) | <0.001 | 1835 (506) | 1781.0 (520) | <0.001 |
| Total carbohydrate, % of total energy intake | 67.6 (4.7) | 46.5 (5.4) | <0.001 | 60.9 (8.0) | 52.2 (8.7) | <0.001 | 65.9 (6.1) | 48.0 (7.4) | <0.001 |
| High-quality carbohydrate | 10.1 (7.3) | 10.0 (6.1) | <0.001 | 15.1 (7.2) | 5.6 (3.8) | <0.001 | 7.4 (4.4) | 13.8 (8.5) | <0.001 |
| Low-quality carbohydrate | 57.4 (8.7) | 36.4 (7.1) | <0.001 | 45.8 (10.2) | 46.5 (9.7) | <0.001 | 58.4 (6.6) | 34.0 (6.9) | <0.001 |
| Total protein, % of total energy intake | 14.2 (1.9) | 18.0 (2.8) | <0.001 | 16.5 (2.9) | 15.3 (3.1) | <0.001 | 14.0 (2.0) | 18.3 (2.8) | <0.001 |
| Animal protein | 5.2 (1.8) | 10.1 (2.9) | <0.001 | 8.1 (3.1) | 6.8 (2.8) | <0.001 | 5.1 (1.7) | 10.1 (2.9) | <0.001 |
| Plant protein | 9.0 (1.3) | 7.9 (1.8) | <0.001 | 8.4 (1.6) | 8.5 (1.8) | <0.001 | 8.9 (1.3) | 8.2 (2.0) | <0.001 |
| Total fat, % of total energy intake | 12.9 (5.6) | 26.1 (7.2) | <0.001 | 14.5 (6.0) | 26.1 (7.8) | <0.001 | 13.2 (6.0) | 26.6 (7.3) | <0.001 |
| Saturated fat | 3.4 (1.5) | 6.2 (1.7) | <0.001 | 4.3 (1.8) | 5.6 (1.8) | <0.001 | 3.0 (1.0) | 7.0 (1.5) | <0.001 |
| Monounsaturated fat | 5.6 (2.5) | 11.4 (3.4) | <0.001 | 6.2 (2.7) | 11.4 (3.7) | <0.001 | 5.8 (2.7) | 11.4 (3.7) | <0.001 |
| Polyunsaturated fat | 3.9 (2.4) | 8.5 (3.5) | <0.001 | 4.0 (2.5) | 9.2 (3.7) | <0.001 | 4.4 (2.8) | 8.2 (3.7) | <0.001 |
| History of CVD | 2058 (40.8) | 2.052 (42.1) | 0.20 | 2171 (41.7) | 1581 (42.4) | 0.70 | 2013 (39.4) | 1908 (43.7) | <0.001 |
| History of cancer | 98 (1.9) | 108 (2.2) | 0.63 | 136 (2.6) | 57 (1.5) | 0.005 | 98 (1.9) | 104 (2.4) | 0.004 |
| Fasting plasma-glucose, mmol/L | 5.9 (1.6) | 5.7 (1.9) | <0.001 | 5.9 (1.6) | 5.7 (2.0) | <0.001 | 5.8 (1.7) | 5.8 (1.9) | <0.001 |
| Systolic blood pressure, mmHg | 133.4 (22.4) | 130.1 (22.2) | <0.001 | 130.3 (22.2) | 132.6 (22.3) | <0.001 | 133.4 (22.4) | 129.6 (22.2) | <0.001 |
| Total cholesterol, mmol/L | 3.7 (1.0) | 3.7 (0.9) | 0.51 | 3.7 (1.0) | 3.6 (1.0) | <0.001 | 3.7 (1.0) | 3.7 (1.0) | 0.90 |
| Self-rated health | |||||||||
| Good/very good | 4296 (85.1) | 4409 (83.0) | 0.02 | 4450 (85.5) | 3096 (83.1) | 0.004 | 4353 (85.2) | 3604 (82.6) | 0.005 |
| Poor/very poor | 752 (14.9) | 827 (17.0) | 758 (14.6) | 632 (16.9) | 754 (14.8) | 760 (17.4) | |||
| Characteristic | Quartiles of LCD Scores | p for Trend | p for Non-Linear | |||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |||
| Overall LCD score a | ||||||
| Median score (IQR) | 6 (4, 8) | 13 (11, 14) | 18 (17, 19) | 24 (22, 26) | ||
| Person-years of follow-up | 74,195 | 77,724 | 71,289 | 71,640 | ||
| Mortality rate (per 1000 person-years) | 182.1 | 156.3 | 140.1 | 147.8 | ||
| Crude HR (95% CI) | 1.00 | 0.87 (0.80–0.94) * | 0.79 (0.72–0.85) ** | 0.83 (0.77–0.90) ** | <0.001 | 0.07 |
| Adjusted HR (95% CI) d | 1.00 | 0.92 (0.85–0.99) * | 0.89 (0.82–0.96) * | 0.96 (0.88–1.04) | 0.17 | 0.008 |
| Adjusted HR (95% CI) e | 1.00 | 0.95 (0.87–1.02) | 0.92 (0.85–1.02) | 0.99 (0.91–1.08) | 0.73 | 0.04 |
| Adjusted HR (95% CI) f | 1.00 | 0.95 (0.88–1.03) | 0.92 (0.84–1.00) | 0.97 (0.89–1.06) | 0.38 | 0.08 |
| Vegetable-based LCD score b | ||||||
| Median score (IQR) | 11 (9, 12) | 14 (13, 15) | 17 (16, 18) | 20 (19, 21) | ||
| Person-years of follow-up | 77,358 | 85,584 | 77,518 | 54,388 | ||
| Mortality rate (per 1000 person-years) | 151.0 | 148.2 | 161.8 | 171.7 | ||
| Crude HR (95% CI) | 1.00 | 0.98 (0.91–1.07) | 1.08 (1.00–1.17) * | 1.16 (1.06–1.26) ** | <0.001 | 0.15 |
| Adjusted HR (95% CI) d | 1.00 | 0.99 (0.91–1.07) | 1.12 (1.04–1.22) ** | 1.18 (1.09–1.29) ** | <0.001 | 0.23 |
| Adjusted HR (95% CI) e | 1.00 | 0.99 (0.91–1.07) | 1.11 (1.02–1.21) * | 1.16 (1.05–1.27) * | <0.001 | 0.18 |
| Adjusted HR (95% CI) f | 1.00 | 0.98 (0.90–1.06) | 1.09 (1.00–1.18) | 1.09 (0.99–1.20) | 0.01 | 0.20 |
| Meat-based LCD score c | ||||||
| Median score (IQR) | 6 (3, 8) | 13 (11, 14) | 19 (17, 20) | 24 (23, 27) | ||
| Person-years of follow-up | 74,816 | 75,838 | 79,936 | 64,259 | ||
| Mortality rate (per 1000 person-years) | 186.9 | 155.7 | 143.2 | 140.1 | ||
| Crude HR (95% CI) | 1.00 | 0.84 (0.77–0.90) *** | 0.78 (0.72–0.84) *** | 0.77 (0.71–0.84) *** | <0.001 | 0.15 |
| Adjusted HR (95% CI) d | 1.00 | 0.89 (0.82–0.96) ** | 0.87 (0.80–0.94) ** | 0.88 (0.81–0.96) ** | 0.001 | 0.12 |
| Adjusted HR (95% CI) e | 1.00 | 0.89 (0.83–0.97) ** | 0.90 (0.83–0.97) ** | 0.89 (0.81–0.97) ** | 0.007 | 0.06 |
| Adjusted HR (95% CI) f | 1.00 | 0.89 (0.82–0.97) ** | 0.91 (0.84–0.98) * | 0.91 (0.83–0.99) * | 0.01 | 0.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, C.; Zhang, W.-S.; Jiang, C.-Q.; Jin, Y.-L.; Deng, X.-Q.; Woo, J.; Cheng, K.-K.; Lam, T.-H.; Thomas, G.N.; Xu, L. Low-Carbohydrate Diets and Mortality in Older Asian People: A 15-Year Follow-Up from a Prospective Cohort Study. Nutrients 2022, 14, 1406. https://doi.org/10.3390/nu14071406
Sun C, Zhang W-S, Jiang C-Q, Jin Y-L, Deng X-Q, Woo J, Cheng K-K, Lam T-H, Thomas GN, Xu L. Low-Carbohydrate Diets and Mortality in Older Asian People: A 15-Year Follow-Up from a Prospective Cohort Study. Nutrients. 2022; 14(7):1406. https://doi.org/10.3390/nu14071406
Chicago/Turabian StyleSun, Ce, Wei-Sen Zhang, Chao-Qiang Jiang, Ya-Li Jin, Xue-Qing Deng, Jean Woo, Kar-Keung Cheng, Tai-Hing Lam, G. Neil Thomas, and Lin Xu. 2022. "Low-Carbohydrate Diets and Mortality in Older Asian People: A 15-Year Follow-Up from a Prospective Cohort Study" Nutrients 14, no. 7: 1406. https://doi.org/10.3390/nu14071406
APA StyleSun, C., Zhang, W.-S., Jiang, C.-Q., Jin, Y.-L., Deng, X.-Q., Woo, J., Cheng, K.-K., Lam, T.-H., Thomas, G. N., & Xu, L. (2022). Low-Carbohydrate Diets and Mortality in Older Asian People: A 15-Year Follow-Up from a Prospective Cohort Study. Nutrients, 14(7), 1406. https://doi.org/10.3390/nu14071406








