Food Allergen Nitration Enhances Safety and Efficacy of Oral Immunotherapy in Food Allergy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Nitration of OVA
2.3. Mouse Treatment Protocols
2.4. Evaluation of OVA-Specific Antibody Titers in Sera and Intestinal Lavages
2.5. Analysis of Murine CD4+ and Regulatory T Cells by Flow Cytometry
2.6. Cytokine Measurements from Splenocyte Supernatants
2.7. Hematoxylin and Eosin Staining of Tissue Samples
2.8. Toluidine Blue Staining of Tissue Samples
2.9. Statistics
3. Results
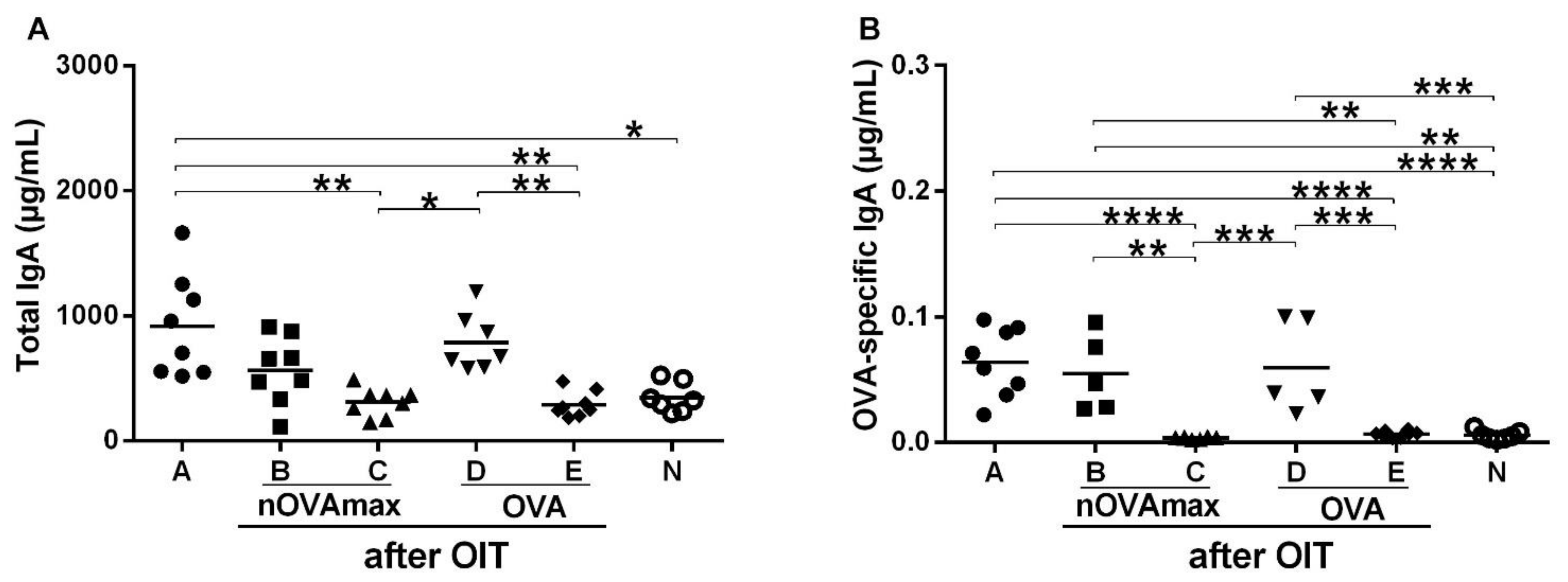
3.1. OIT Was Associated with Decreased OVA-Specific IgE while OVA-Specific Intestinal IgA Titers Remained Unchanged
3.2. Levels of Regulatory T Cells Are Comparable to Naïve Mice after Therapy
3.3. OIT with nOVAmax Protected against Systemic Allergic Reactions after Oral Immunotherapy
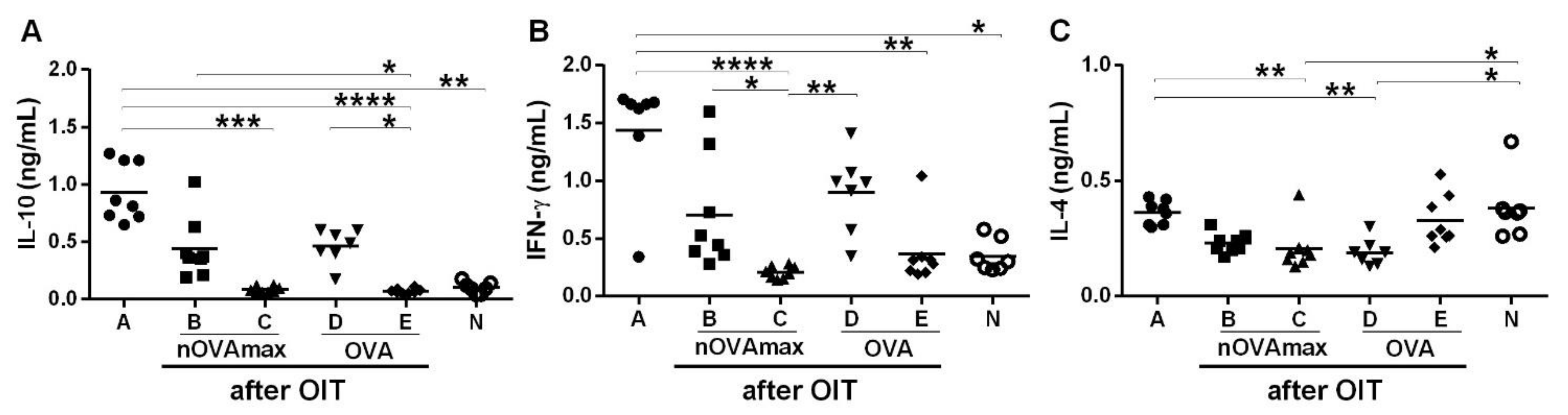
3.4. Unchanged Cytokine Levels after OIT with nOVAmax
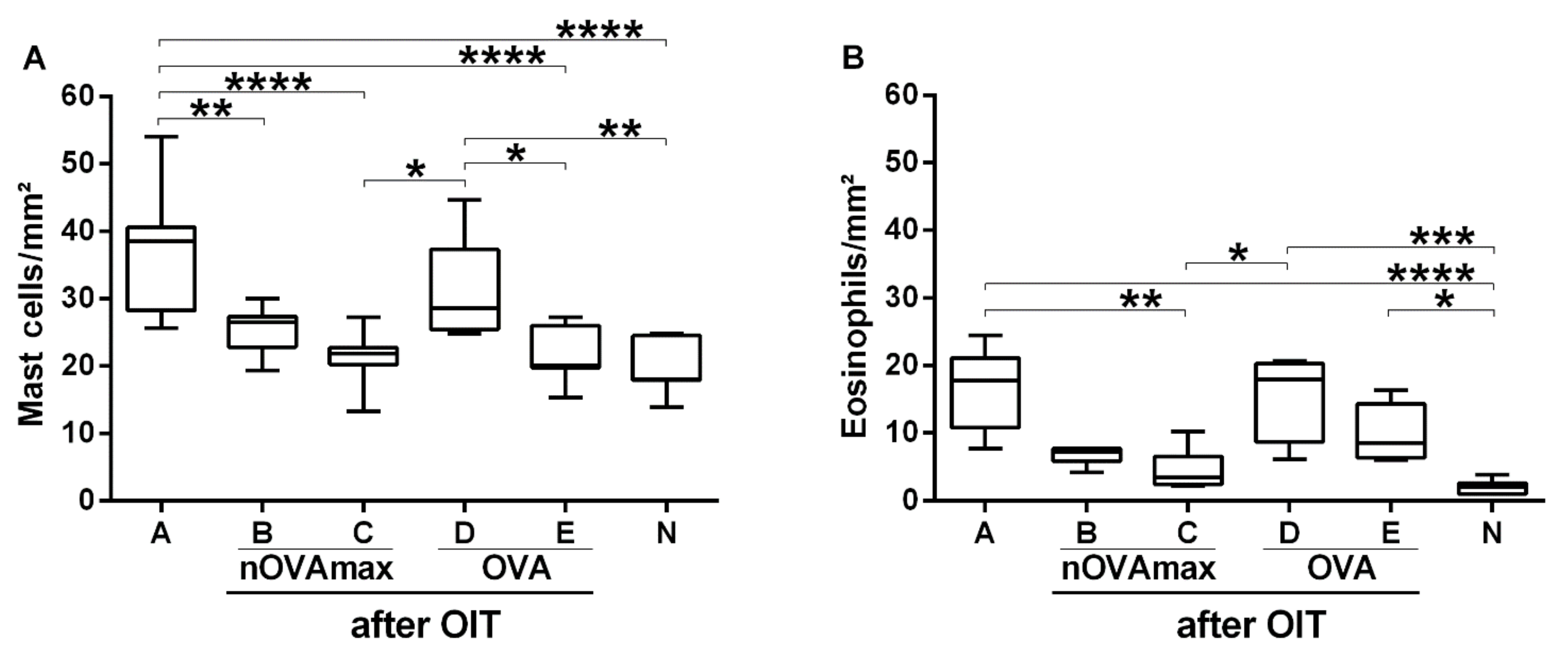
3.5. Reduced Allergy Effector Cell Influx Was Found in Gastric Mucosa of the nOVAmax-Treated Mice
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Noon, L. Prophylactic inoculation against hay fever. Lancet 1911, 1, 1572–1573. [Google Scholar] [CrossRef] [Green Version]
- Freeman, J. Further observations on the treatment of hay fever by hypodermic inoculations of pollen vaccine. Lancet 1911, 178, 814–817. [Google Scholar] [CrossRef] [Green Version]
- Frankland, A.W. High and low dosage pollen extract treatment in summer hay fever and asthma. Acta Allergol. 1955, 9, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Ring, J.; Gutermuth, J. 100 years of hyposensitization: History of allergen-specific immunotherapy (ASIT). Allergy 2011, 66, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Wood, R.A. Food allergen immunotherapy: Current status and prospects for the future. J. Allergy Clin. Immunol. 2016, 137, 973–982. [Google Scholar] [CrossRef] [Green Version]
- Arasi, S.; Passalacqua, G.; Caminiti, L.; Crisafulli, G.; Fiamingo, C.; Pajno, G.B. Efficacy and safety of sublingual immunotherapy in children. Expert Rev. Clin. Immunol. 2016, 12, 49–56. [Google Scholar] [CrossRef]
- Pajno, G.B.; Fernandez-Rivas, M.; Arasi, S.; Roberts, G.; Akdis, C.A.; Alvaro-Lozano, M.; Beyer, K.; Bindslev-Jensen, C.; Burks, W.; Ebisawa, M.; et al. EAACI Guidelines on allergen immunotherapy: IgE-mediated food allergy. Allergy 2018, 73, 799–815. [Google Scholar] [CrossRef] [Green Version]
- Schoos, A.M.; Bullens, D.; Chawes, B.L.; Costa, J.; de Vlieger, L.; DunnGalvin, A.; Epstein, M.M.; Garssen, J.; Hilger, C.; Knipping, K.; et al. Immunological Outcomes of Allergen-Specific Immunotherapy in Food Allergy. Front. Immunol. 2020, 11, 568598. [Google Scholar] [CrossRef]
- Kucuksezer, U.C.; Ozdemir, C.; Cevhertas, L.; Ogulur, I.; Akdis, M.; Akdis, C.A. Mechanisms of allergen-specific immunotherapy and allergen tolerance. Allergol. Int. 2020, 69, 549–560. [Google Scholar] [CrossRef]
- Sampson, H.A.; Aceves, S.; Bock, S.A.; James, J.; Jones, S.; Lang, D.; Nadeau, K.; Nowak-Wegrzyn, A.; Oppenheimer, J.; Perry, T.T.; et al. Food allergy: A practice parameter update-2014. J. Allergy Clin. Immunol. 2014, 134, 1016–1025.e1043. [Google Scholar] [CrossRef]
- Hofmann, A.M.; Scurlock, A.M.; Jones, S.M.; Palmer, K.P.; Lokhnygina, Y.; Steele, P.H.; Kamilaris, J.; Burks, A.W. Safety of a peanut oral immunotherapy protocol in children with peanut allergy. J. Allergy Clin. Immunol. 2009, 124, 286–291.e1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anagnostou, K.; Islam, S.; King, Y.; Foley, L.; Pasea, L.; Bond, S.; Palmer, C.; Deighton, J.; Ewan, P.; Clark, A. Assessing the efficacy of oral immunotherapy for the desensitisation of peanut allergy in children (STOP II): A phase 2 randomised controlled trial. Lancet 2014, 383, 1297–1304. [Google Scholar] [CrossRef] [Green Version]
- Wood, R.A. Oral Immunotherapy for Food Allergy. J. Investig. Allergol. Clin. Immunol. 2017, 27, 151–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mills, E.N.; Sancho, A.I.; Rigby, N.M.; Jenkins, J.A.; Mackie, A.R. Impact of food processing on the structural and allergenic properties of food allergens. Mol. Nutr. Food Res. 2009, 53, 963–969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Untersmayr, E.; Diesner, S.C.; Oostingh, G.J.; Selzle, K.; Pfaller, T.; Schultz, C.; Zhang, Y.; Krishnamurthy, D.; Starkl, P.; Knittelfelder, R.; et al. Nitration of the egg-allergen ovalbumin enhances protein allergenicity but reduces the risk for oral sensitization in a murine model of food allergy. PLoS ONE 2010, 5, e14210. [Google Scholar] [CrossRef] [Green Version]
- Ondracek, A.S.; Heiden, D.; Oostingh, G.J.; Fuerst, E.; Fazekas-Singer, J.; Bergmayr, C.; Rohrhofer, J.; Jensen-Jarolim, E.; Duschl, A.; Untersmayr, E.; et al. Immune Effects of the Nitrated Food Allergen Beta-Lactoglobulin in an Experimental Food Allergy Model. Nutrients 2019, 11, 2463. [Google Scholar] [CrossRef] [Green Version]
- Samadi, N.; Klems, M.; Heiden, D.; Bauer, R.; Kitzmuller, C.; Weidmann, E.; Ret, D.; Ondracek, A.S.; Duschl, A.; Horejs-Hoeck, J.; et al. Nitrated food proteins induce a regulatory immune response associated with allergy prevention after oral exposure in a Balb/c mouse food allergy model. Allergy 2020, 75, 412–422. [Google Scholar] [CrossRef] [Green Version]
- Weidmann, E.; Samadi, N.; Klems, M.; Heiden, D.; Seppova, K.; Ret, D.; Untersmayr, E. Mouse Chow Composition Influences Immune Responses and Food Allergy Development in a Mouse Model. Nutrients 2018, 10, 1775. [Google Scholar] [CrossRef] [Green Version]
- Yanagida, N.; Sato, S.; Asaumi, T.; Nagakura, K.; Ogura, K.; Ebisawa, M. Safety and Efficacy of Low-Dose Oral Immunotherapy for Hen’s Egg Allergy in Children. Int. Arch. Allergy Immunol. 2016, 171, 265–268. [Google Scholar] [CrossRef]
- Barni, S.; Mori, F.; Piccorossi, A.; Sarti, L.; Pucci, N.; Maresca, M.; Giovannini, M.; Liccioli, G.; Novembre, E. Low-Dose Oral Food Challenge with Hazelnut: Efficacy and Tolerability in Children. Int. Arch. Allergy Immunol. 2019, 178, 97–100. [Google Scholar] [CrossRef]
- Blumchen, K.; Trendelenburg, V.; Ahrens, F.; Gruebl, A.; Hamelmann, E.; Hansen, G.; Heinzmann, A.; Nemat, K.; Holzhauser, T.; Roeder, M.; et al. Efficacy, Safety, and Quality of Life in a Multicenter, Randomized, Placebo-Controlled Trial of Low-Dose Peanut Oral Immunotherapy in Children with Peanut Allergy. J. Allergy Clin. Immunol. Pract. 2019, 7, 479–491.e410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burks, A.W.; Jones, S.M.; Wood, R.A.; Fleischer, D.M.; Sicherer, S.H.; Lindblad, R.W.; Stablein, D.; Henning, A.K.; Vickery, B.P.; Liu, A.H.; et al. Oral immunotherapy for treatment of egg allergy in children. N. Engl. J. Med. 2012, 367, 233–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vonk, M.M.; Wagenaar, L.; Pieters, R.H.H.; Knippels, L.M.J.; Willemsen, L.E.M.; Smit, J.J.; van Esch, B.C.A.M.; Garssen, J. The efficacy of oral and subcutaneous antigen-specific immunotherapy in murine cow’s milk- and peanut allergy models. Clin. Transl. Allergy 2017, 7, 35. [Google Scholar] [CrossRef] [PubMed]
- Pajno, G.B.; Cox, L.; Caminiti, L.; Ramistella, V.; Crisafulli, G. Oral Immunotherapy for Treatment of Immunoglobulin E-Mediated Food Allergy: The Transition to Clinical Practice. Pediatr. Allergy Immunol. Pulmonol. 2014, 27, 42–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potaczek, D.P.; Kabesch, M. Current Concepts of IgE Regulation and Impact of Genetic Determinants. Clin. Exp. Allergy 2012, 42, 852–871. [Google Scholar] [CrossRef]
- Hellman, L. Regulation of OgE homeostasis, and the identification of potential tragets for therapeutic intervention. Biomed. Pharmacother. 2007, 61, 34–49. [Google Scholar] [CrossRef]
- Uermosi, C.; Zabel, F.; Manolova, V.; Bauer, M.; Beerli, R.R.; Senti, G.; Kündig, T.M.; Saudan, P.; Bachmann, M.F. IgG-mediated down-regulation of IgE bound to mast cells: A potential novel mechanism of allergen-specific desensitization. Allergy 2014, 69, 338–347. [Google Scholar] [CrossRef]
- Stone, K.D.; Prussin, C.; Metcalfe, D.D. IgE, mast cells, basophils, and eosinophils. J. Allergy Clin. Immunol. 2010, 125 (Suppl. 2), S73–S80. [Google Scholar] [CrossRef]
- Kraneveld, A.D.; Sagar, S.; Garssen, J.; Folkerts, G. The two faces of mast cells in food allergy and allergic asthma: The possible concept of Yin Yang. Biochim. Biophys. Acta 2012, 1822, 93–99. [Google Scholar] [CrossRef] [Green Version]
- Blanchard, C.; Rothenberg, M.E. Biology of the eosinophil. Adv. Immunol. 2009, 101, 81–121. [Google Scholar]
- Untersmayr, E.; Scholl, I.; Swoboda, I.; Beil, W.J.; Forster-Waldl, E.; Walter, F.; Riemer, A.; Kraml, G.; Kinaciyan, T.; Spitzauer, S.; et al. Antacid medication inhibits digestion of dietary proteins and causes food allergy: A fish allergy model in BALB/c mice. J. Allergy Clin. Immunol. 2003, 112, 616–623. [Google Scholar] [CrossRef]
- Untersmayr, E.; Ellinger, A.; Beil, W.J.; Jensen-Jarolim, E. Eosinophils accumulate in the gastric mucosa of food-allergic mice. Int. Arch. Allergy Immunol. 2004, 135, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Alashkar Alhamwe, B.; Meulenbroek, L.A.P.M.; Veening-Griffioen, D.H.; Wehkamp, T.; Alhamdan, F.; Miethe, S.; Harb, H.; Hogenkamp, A.; Knippels, L.M.J.; von Strandmann, E.P.; et al. Decreased Histone Acetylation Levels at Th1 and Regulatory Loci after Induction of Food Allergy. Nutrients 2020, 12, 3193. [Google Scholar] [CrossRef] [PubMed]
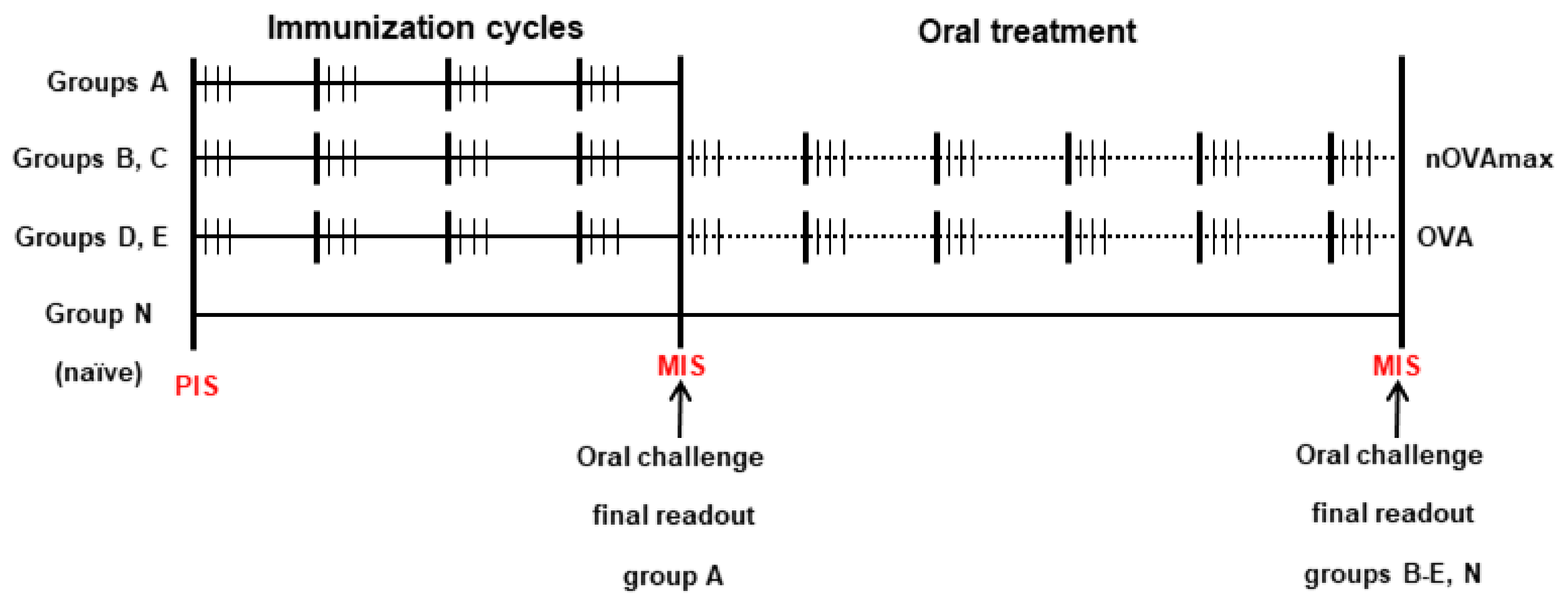




| Group | Immunizations | Oral Immunotherapy |
|---|---|---|
| A | 116 µg omeprazole, 2 mg sucralfate + 200 µg OVA | No therapy |
| B | 116 µg omeprazole, 2 mg sucralfate + 200 µg OVA | 200 µg nOVAmax |
| C | Naïve | 200 µg nOVAmax |
| D | 116 µg omeprazole, 2 mg sucralfate + 200 µg OVA | 200 µg OVA |
| E | Naïve | 200 µg OVA |
| N | Naïve | No therapy |
| Group | Mean | SD | p |
|---|---|---|---|
| A | 3363 | 310.6 | 0.2 |
| B | 3400 | 445.1 | 0.2 |
| C | 3588 | 284.2 | 0.2 |
| D | 3600 | 262.7 | 0.2 |
| E | 3751 | 278.2 | 0.088 |
| Naïve | 3707 | 433.8 | 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samadi, N.; Koidl, L.; Salzmann, M.; Klems, M.; Komatitsch, N.; Schaffer, D.; Weidmann, E.; Duschl, A.; Horejs-Hoeck, J.; Untersmayr, E. Food Allergen Nitration Enhances Safety and Efficacy of Oral Immunotherapy in Food Allergy. Nutrients 2022, 14, 1373. https://doi.org/10.3390/nu14071373
Samadi N, Koidl L, Salzmann M, Klems M, Komatitsch N, Schaffer D, Weidmann E, Duschl A, Horejs-Hoeck J, Untersmayr E. Food Allergen Nitration Enhances Safety and Efficacy of Oral Immunotherapy in Food Allergy. Nutrients. 2022; 14(7):1373. https://doi.org/10.3390/nu14071373
Chicago/Turabian StyleSamadi, Nazanin, Larissa Koidl, Martina Salzmann, Martina Klems, Natalie Komatitsch, Denise Schaffer, Eleonore Weidmann, Albert Duschl, Jutta Horejs-Hoeck, and Eva Untersmayr. 2022. "Food Allergen Nitration Enhances Safety and Efficacy of Oral Immunotherapy in Food Allergy" Nutrients 14, no. 7: 1373. https://doi.org/10.3390/nu14071373
APA StyleSamadi, N., Koidl, L., Salzmann, M., Klems, M., Komatitsch, N., Schaffer, D., Weidmann, E., Duschl, A., Horejs-Hoeck, J., & Untersmayr, E. (2022). Food Allergen Nitration Enhances Safety and Efficacy of Oral Immunotherapy in Food Allergy. Nutrients, 14(7), 1373. https://doi.org/10.3390/nu14071373








