Fitness, Food, and Biomarkers: Characterizing Body Composition in 19,634 Early Adolescents
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Anthropometric Measures and Body Composition
2.3. Cardiorespiratory Measures
2.4. Blood Sampling for Adolescents with BMI ≥ 90th Percentile
2.5. Parental Questionnaire
2.6. Statistical Analyses
3. Results
3.1. Sample Description
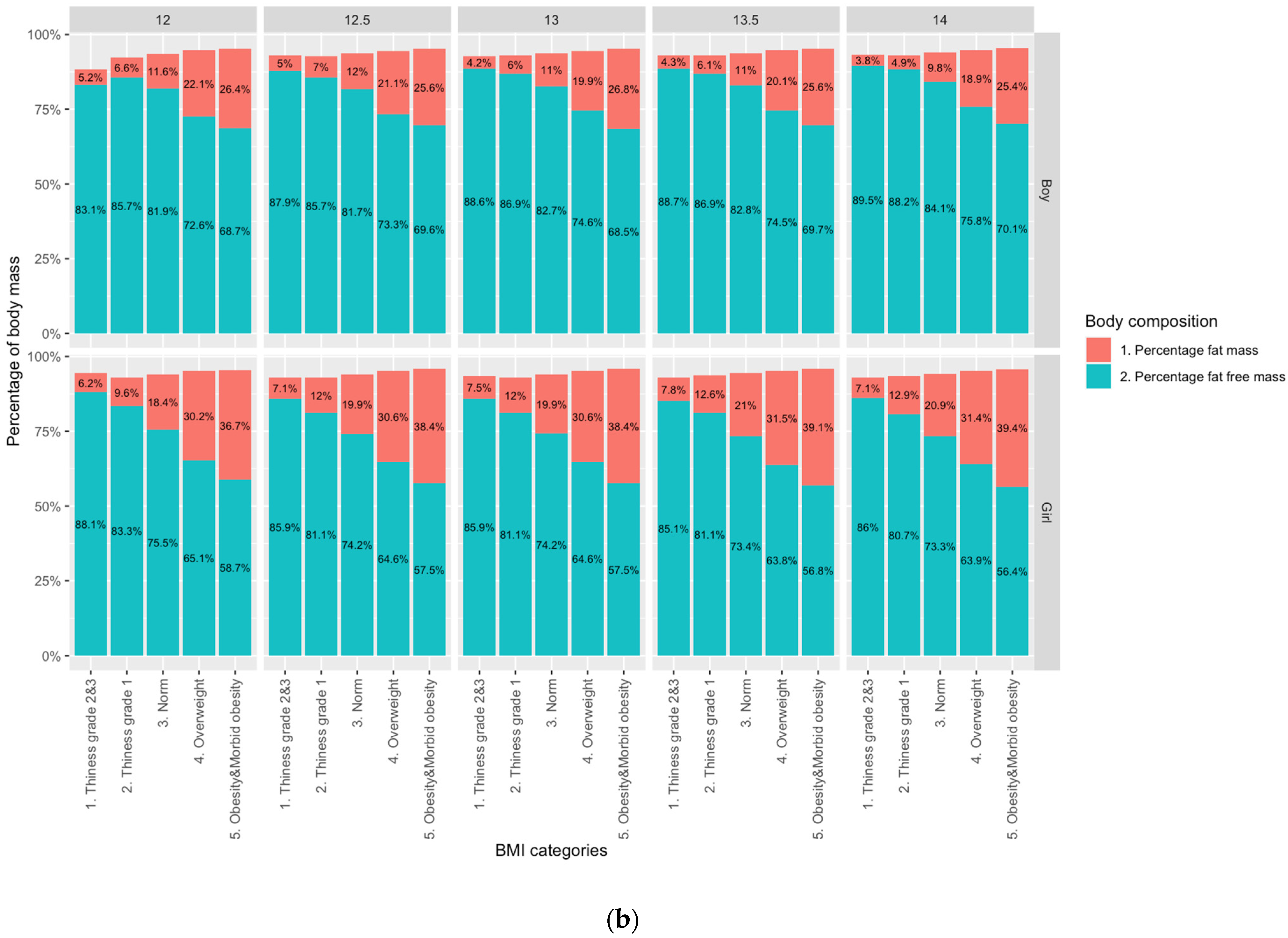
3.2. Sex Differences in Adiposity
3.3. CRF
3.4. Lifestyle and Family
3.5. Biomarkers of Disease Risk
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kobza, J.; Geremek, M. Exploring the Life Expectancy Increase in Poland in the Context of CVD Mortality Fall. INQUIRY J. Health Care Organ. Provis. Financ. 2015, 52. [Google Scholar] [CrossRef] [PubMed]
- OECD/European Observatory on Health Systems and Policies. Poland: Country Health Profile 2017. In State of Health in the EU; OECD Publishing: Paris, France; European Observatory on Health Systems and Policies: Brussels, Belgium, 2017. [Google Scholar] [CrossRef]
- Leon, D.A. Trends in European life expectancy: A salutary view. Int. J. Epidemiol. 2011, 40, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Movsisyan, N.K.; Vinciguerra, M.; Medina-Inojosa, J.R.; Lopez-Jimenez, F. Cardiovascular Diseases in Central and Eastern Europe: A Call for More Surveillance and Evidence-Based Health Promotion. Ann. Glob. Health 2020, 86, 21. [Google Scholar] [CrossRef] [PubMed]
- Stefler, D.; Brett, D.; Sarkadi-Nagy, E.; Kopczynska, E.; Detchev, S.; Bati, A.; Scrob, M.; Koenker, D.; Aleksov, B.; Douarin, E.; et al. Traditional Eastern European diet and mortality: Prospective evidence from the HAPIEE study. Eur. J. Nutr. 2021, 60, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Jackson-Leach, R.; Lobstein, T. Estimated burden of paediatric obesity and co-morbidities in Europe. Part 1. The increase in the prevalence of child obesity in Europe is itself increasing. Int. J. Pediatric Obes. 2006, 1, 26–32. [Google Scholar] [CrossRef]
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in diabetes since 1980: A pooled analysis of 751 population-based studies with 4·4 million participants. Lancet 2016, 387, 1513–1530. [Google Scholar] [CrossRef]
- Ahluwalia, N.; Dalmasso, P.; Rasmussen, M.; Lipsky, L.; Currie, C.; Haug, E.; Kelly, C.; Damsgaard, M.T.; Due, P.; Tabak, I.; et al. Trends in overweight prevalence among 11-, 13- and 15-year-olds in 25 countries in Europe, Canada and USA from 2002 to 2010. Eur. J. Public Health 2015, 25, 28–32. [Google Scholar] [CrossRef]
- Knai, C.; Suhrcke, M.; Lobstein, T. Obesity in Eastern Europe: An overview of its health and economic implications. Econ. Hum. Biol. 2007, 5, 392–408. [Google Scholar] [CrossRef]
- Chrzanowska, M.; Koziel, S.; Ulijaszek, S.J. Changes in BMI and the prevalence of overweight and obesity in children and adolescents in Cracow, Poland, 1971–2000. Econ. Hum. Biol. 2007, 5, 370–378. [Google Scholar] [CrossRef]
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef]
- NCD Risk Factor Collaboration (NCD-RisC). Height and body-mass index trajectories of school-aged children and adolescents from 1985 to 2019 in 200 countries and territories: A pooled analysis of 2181 population-based studies with 65 million participants. Lancet 2020, 396, 1511–1524. [Google Scholar] [CrossRef]
- The Global Strategy for Women’s, Children’s and Adolescents’ Health 2016–2030. Every Woman Every Child. 2015. Available online: http://www.everywomaneverychild.org/globalstrategy (accessed on 7 January 2022).
- Tirosh, A.; Shai, I.; Afek, A.; Dubnov-Raz, G.; Ayalon, N.; Gordon, B.; Derazne, E.; Tzur, D.; Shamis, A.; Vinker, S.; et al. Adolescent BMI Trajectory and Risk of Diabetes versus Coronary Disease. N. Engl. J. Med. 2011, 364, 1315–1325. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, S.M.; Afifi, R.A.; Bearinger, L.H.; Blakemore, S.-J.; Dick, B.; Ezeh, A.C.; Patton, G.C. Adolescence: A foundation for future health. Lancet 2012, 379, 1630–1640. [Google Scholar] [CrossRef]
- Spear, B.A. Adolescent growth and development. J. Am. Diet. Assoc. 2002, 102 (Suppl. 3), S23–S29. [Google Scholar] [CrossRef]
- Granados, A.; Gebremariam, A.; Lee, J.M. Relationship between Timing of Peak Height Velocity and Pubertal Staging in Boys and Girls. J. Clin. Res. Pediatric Endocrinol. 2015, 7, 235–237. [Google Scholar] [CrossRef]
- Takamura, T.; Kita, Y.; Nakagen, M.; Sakurai, M.; Isobe, Y.; Takeshita, Y.; Kawai, K.; Urabe, T.; Kaneko, S. Weight-adjusted lean body mass and calf circumference are protective against obesity-associated insulin resistance and metabolic abnormalities. Heliyon 2017, 3, e00347. [Google Scholar] [CrossRef]
- Bel-Serrat, S.; Ojeda-Rodríguez, A.; Heinen, M.M.; Buoncristiano, M.; Abdrakhmanova, S.; Duleva, V.; Sant’Angelo, V.F.; Fijałkowska, A.; Hejgaard, T.; Huidumac, C.; et al. Clustering of Multiple Energy Balance-Related Behaviors in School Children and its Association with Overweight and Obesity—WHO European Childhood Obesity Surveillance Initiative (COSI 2015–2017). Nutrients 2019, 11, 511. [Google Scholar] [CrossRef]
- Bába, B.; Ráthonyi, G.; Müller, A.; Ráthonyi-Odor, K.; Balogh, P.; Ádány, R.; Bács, Z. Physical Activity of the Population of the Most Obese Country in Europe, Hungary. Front. Public Health 2020, 8, 203. [Google Scholar] [CrossRef]
- Sawyer, S.M.; Azzopardi, P.S.; Wickremarathne, D.; Patton, G.C. The age of adolescence. Lancet Child Adolesc. Health 2018, 2, 223–228. [Google Scholar] [CrossRef]
- Barreira, T.; Staiano, A.; Katzmarzyk, P.T. Validity assessment of a portable bioimpedance scale to estimate body fat percentage in White and African-American children and adolescents. Pediatric Obes. 2012, 8, e29–e32. [Google Scholar] [CrossRef]
- Cole, T.J.; Lobstein, T. Extended international (IOTF) body mass index cut-offs for thinness, overweight and obesity. Pediatric Obes. 2012, 7, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Jankowski, M.; Niedzielska, A.; Brzezinski, M.; Drabik, J. Cardiorespiratory Fitness in Children: A Simple Screening Test for Population Studies. Pediatric Cardiol. 2014, 36, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Bruggeman, B.S.; Vincent, H.K.; Chi, X.; Filipp, S.L.; Mercado, R.; Modave, F.; Guo, Y.; Gurka, M.J.; Bernier, A. Simple tests of cardiorespiratory fitness in a pediatric population. PLoS ONE 2020, 15, e0238863. [Google Scholar] [CrossRef]
- Jacks, D.E.; Topp, R.; Moore, J.B. Prediction of VO2 Peak Using a Sub-maximal Bench Step Test in Children. Clin. Kinesiol. 2012, 65, 68–75. [Google Scholar] [CrossRef]
- Martinsson, A.; Andersson, C.; Andell, P.; Koul, S.; Engström, G.; Smith, J.G. Anemia in the general population: Prevalence, clinical correlates and prognostic impact. Eur. J. Epidemiol. 2014, 29, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Alleyne, M.; Horne, M.K.; Miller, J.L. Individualized Treatment for Iron-deficiency Anemia in Adults. Am. J. Med. 2008, 121, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Andes, L.J.; Cheng, Y.J.; Rolka, D.B.; Gregg, E.W.; Imperatore, G. Prevalence of Prediabetes among Adolescents and Young Adults in the United States, 2005–2016. JAMA Pediatric 2020, 174. [Google Scholar] [CrossRef]
- Rokholm, B.; Baker, J.L.; Sørensen, T.I.A. The levelling off of the obesity epidemic since the year 1999—A review of evidence and perspectives. Obes. Rev. 2010, 11, 835–846. [Google Scholar] [CrossRef]
- Wang, Y.; Beydoun, M.A.; Min, J.; Xue, H.; Kaminsky, L.A.; Cheskin, L.J. Has the prevalence of overweight, obesity and central obesity levelled off in the United States? Trends, patterns, disparities, and future projections for the obesity epidemic. Int. J. Epidemiol. 2020, 49, 810–823. [Google Scholar] [CrossRef]
- Schaefer, F.; Georgi, M.; Wühl, E.; Schärer, K. Body mass index and percentage fat mass in healthy German schoolchildren and adolescents. Int. J. Obes. 1998, 22, 461–469. [Google Scholar] [CrossRef]
- Suliga, E. Visceral adipose tissue in children and adolescents: A review. Nutr. Res. Rev. 2009, 22, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Tumilowicz, A.; Beal, T.; Neufeld, L.M.; Frongillo, E.A. Perspective: Challenges in Use of Adolescent Anthropometry for Understanding the Burden of Malnutrition. Adv. Nutr. Int. Rev. J. 2019, 10, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Freedman, D.S.; Wang, J.; Maynard, L.M.; Thornton, J.C.; Mei, Z.; Pierson, R.N., Jr.; Dietz, W.H.; Horlick, M. Relation of BMI to fat and fat-free mass among children and adolescents. Int. J. Obes. 2005, 29, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y. Epidemiology of childhood obesity—Methodological aspects and guidelines: What is new? Int. J. Obes. 2004, 28, S21–S28. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.C.K. A Hattori chart analysis of body mass index in infants and children. Int. J. Obes. 2000, 24, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.C.K.; Fewtrell, M.S. Is body composition important for paediatricians? Arch. Dis. Child. 2007, 93, 168–172. [Google Scholar] [CrossRef]
- Kaminsky, L.A.; Arena, R.; Beckie, T.M.; Brubaker, P.H.; Church, T.S.; Forman, D.E.; Franklin, B.A.; Gulati, M.; Lavie, C.J.; Myers, J.; et al. The Importance of Cardiorespiratory Fitness in the United States: The Need for a National Registry. Circulation 2013, 127, 652–662. [Google Scholar] [CrossRef]
- Farooq, A.; Martin, A.; Janssen, X.; Wilson, M.G.; Gibson, A.; Hughes, A.; Reilly, J.J. Longitudinal changes in moderate-to-vigorous-intensity physical activity in children and adolescents: A systematic review and meta-analysis. Obes. Rev. 2020, 21, e12953. [Google Scholar] [CrossRef]
- Kwon, S.; Janz, K.F.; Letuchy, E.M.; Burns, T.L.; Levy, S.M. Developmental Trajectories of Physical Activity, Sports, and Television Viewing During Childhood to Young Adulthood. JAMA Pediatrics 2015, 169, 666–672. [Google Scholar] [CrossRef]
- O’Malley, G.; Ring-Dimitriou, S.; Nowicka, P.; Vania, A.; Frelut, M.-L.; Farpour-Lambert, N.; Weghuber, D.; Thivel, D. Physical Activity and Physical Fitness in Pediatric Obesity: What are the First Steps for Clinicians? Expert Conclusion from the 2016 ECOG Workshop. Int. J. Exerc. Sci. 2017, 10, 487–496. [Google Scholar]
- Alves, A.C.; De Silva, N.M.G.; Karhunen, V.; Sovio, U.; Das, S.; Taal, H.R.; Warrington, N.M.; Lewin, A.M.; Kaakinen, M.; Cousminer, D.L.; et al. GWAS on longitudinal growth traits reveals different genetic factors influencing infant, child, and adult BMI. Sci. Adv. 2019, 5, eaaw3095. [Google Scholar] [CrossRef] [PubMed]
- Ruiter, E.L.M.; Saat, J.J.E.H.; Molleman, G.R.M.; Fransen, G.A.J.; Van Der Velden, K.; Van Jaarsveld, C.H.M.; Engels, R.; Assendelft, W.J.J. Parents’ underestimation of their child’s weight status. Moderating factors and change over time: A cross-sectional study. PLoS ONE 2020, 15, e0227761. [Google Scholar] [CrossRef]
- Solmi, F.; Sharpe, H.; Gage, S.H.; Maddock, J.; Lewis, G.; Patalay, P. Changes in the Prevalence and Correlates of Weight-Control Behaviors and Weight Perception in Adolescents in the UK, 1986-2015. JAMA Pediatrics 2021, 175, 267. [Google Scholar] [CrossRef]
- Madsen, K.A.; Thompson, H.R.; Linchey, J.; Ritchie, L.D.; Gupta, S.; Neumark-Sztainer, D.; Crawford, P.B.; McCulloch, C.E.; Ibarra-Castro, A. Effect of School-Based Body Mass Index Reporting in California Public Schools. JAMA Pediatrics 2021, 175, 251. [Google Scholar] [CrossRef] [PubMed]
- Laird, R.D.; Pettit, G.S.; Bates, J.E.; Dodge, K.A. Parents’ Monitoring-Relevant Knowledge and Adolescents’ Delinquent Behavior: Evidence of Correlated Developmental Changes and Reciprocal Influences. Child Dev. 2003, 74, 752–768. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.S.; Benson, M.J. Parent-adolescent relations and adolescent functioning: Self-esteem, substance abuse, and delin-quency. Adolescence 2004, 39, 519–530. [Google Scholar] [PubMed]
- Kivimäki, M.; Kuosma, E.; Ferrie, J.E.; Luukkonen, R.; Nyberg, S.T.; Alfredsson, L.; Batty, G.; Brunner, E.; Fransson, E.; Goldberg, M.; et al. Overweight, obesity, and risk of cardiometabolic multimorbidity: Pooled analysis of individual-level data for 120 813 adults from 16 cohort studies from the USA and Europe. Lancet Public Health 2017, 2, e277–e285. [Google Scholar] [CrossRef]
- Di Bonito, P.; Valerio, G.; Licenziati, M.R.; Campana, G.; del Giudice, E.M.; Di Sessa, A.; Morandi, A.; Maffeis, C.; Chiesa, C.; Pacifico, L.; et al. Uric acid, impaired fasting glucose and impaired glucose tolerance in youth with overweight and obesity. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 675–680. [Google Scholar] [CrossRef]
- Mireku, M.O.; Rodriguez, A. Family Income Gradients in Adolescent Obesity, Overweight and Adiposity Persist in Extremely Deprived and Extremely Affluent Neighbourhoods but Not in Middle-Class Neighbourhoods: Evidence from the UK Millennium Cohort Study. Int. J. Environ. Res. Public Health 2020, 17, 418. [Google Scholar] [CrossRef]
- Smetkowski, M.; Płoszaj, A.; Rok, J. Local Concentration of Deprivation in Poland; EUROREG Reports and Analyses 8/2016; Center for European Regional and Local Studies EURORE: Warsaw, Poland, 2016; p. 45. [Google Scholar] [CrossRef]
- Gomula, A.; Nowak-Szczepanska, N.; Danel, D.; Koziel, S. Overweight trends among Polish schoolchildren before and after the transition from communism to capitalism. Econ. Hum. Biol. 2015, 19, 246–257. [Google Scholar] [CrossRef]
- Popławska, H.; Wilczewski, A.; Dmitruk, A.; Hołub, W. The timing of sexual maturation among boys and girls in east-ern Poland, 1980–2000: A rural–urban comparison. Econ. Hum. Biol. 2013, 11, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Pikel, T.R.; Malus, T.; Starc, G.; Golja, P. Changes in the Growth and Development of Adolescents in a Country in Socio-Economic Transition 1993–2013. Slov. J. Public Health 2020, 59, 164–171. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Risk Factors Collaborators. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1223–1249. [Google Scholar] [CrossRef]
- LBD Double Burden of Malnutrition Collaborators. Mapping local patterns of childhood overweight and wasting in low- and middle-income countries between 2000 and 2017. Nat. Med. 2020, 26, 750–759. [Google Scholar] [CrossRef]
- Johnson, W.; William, J.; Kuh, D.; Hardy, R. How Has the Age-Related Process of Overweight or Obesity Development Changed over Time? Co-ordinated Analyses of Individual Participant Data from Five United Kingdom Birth Cohorts. PLoS Med. 2015, 12, e1001828. [Google Scholar] [CrossRef]
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abera, S.F.; Gakidou, E.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the global burden of disease study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef]
- Skinner, A.C.; Ravanbakht, S.N.; Skelton, J.A.; Perrin, E.M.; Armstrong, S.C. Prevalence of Obesity and Severe Obesity in US Children, 1999–2016. Pediatrics 2018, 141, e20173459. [Google Scholar] [CrossRef]
- NHS Digital. Health Survey for England 2019: Overweight and Obesity in Adults and Children. Available online: https://digital.nhs.uk/data-and-information/publications/statistical/health-survey-for-england/2019 (accessed on 22 May 2021).
- Garrido-Miguel, M.; Cavero-Redondo, I.; Alvarez-Bueno, C.; Rodríguez-Artalejo, F.; Moreno, L.A.; Ruiz, J.R.; Ahrens, W.; Martínez-Vizcaíno, V. Prevalence and Trends of Overweight and Obesity in European Children From 1999 to 2016. JAMA Pediatrics 2019, 173, e192430. [Google Scholar] [CrossRef]
- Hanson, M.; Gluckman, P. Commentary: Developing the future: Life course epidemiology, DOHaD and evolutionary medicine. Int. J. Epidemiol. 2016, 45, 993–996. [Google Scholar] [CrossRef][Green Version]
- Godfrey, K.; Gluckman, P.D.; Hanson, M. Developmental origins of metabolic disease: Life course and intergenerational perspectives. Trends Endocrinol. Metab. 2010, 21, 199–205. [Google Scholar] [CrossRef]
- Jacob, C.M.; Baird, J.; Barker, M.; Cooper, C.; Hanson, M. The Importance of a Life Course Approach to Health: Chronic Disease Risk from Preconception through Adolescence and Adulthood; 2017 White Paper; World Health Organization: Geneva, Switzerland, 2017; p. 41. [Google Scholar]
- Khalife, N.; Kantomaa, M.; Glover, V.; Tammelin, T.; Laitinen, J.; Ebeling, H.; Hurtig, T.; Jarvelin, M.-R.; Rodriguez, A. Childhood Attention-Deficit/Hyperactivity Disorder Symptoms Are Risk Factors for Obesity and Physical Inactivity in Adolescence. J. Am. Acad. Child Adolesc. Psychiatry 2014, 53, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Brooks, S.J.; Parks, S.M.; Stamoulis, C. Widespread Positive Direct and Indirect Effects of Regular Physical Activity on the Developing Functional Connectome in Early Adolescence. Cereb. Cortex 2021, 31, 4840–4852. [Google Scholar] [CrossRef] [PubMed]
- Eurostat, Births and Fertility in 2016. Available online: https://ec.europa.eu/eurostat/documents/2995521/8774296/3-28032018-AP-EN.pdf/fdf8ebdf-a6a4-4153-9ee9-2f05652d8ee0 (accessed on 22 May 2021).
- Rodriguez, A.; Miettunen, J.; Henriksen, T.B.; Olsen, J.; Obel, C.; Taanila, A.; Ebeling, H.; Linnet, K.M.; Moilanen, I.; Jarvelin, M.-R. Maternal adiposity prior to pregnancy is associated with ADHD symptoms in offspring: Evidence from three prospective pregnancy cohorts. Int. J. Obes. 2007, 32, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A. Maternal pre-pregnancy obesity and risk for inattention and negative emotionality in children. J. Child Psychol. Psychiatry 2010, 51, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Voerman, E.; Santos, S.; Golab, B.P.; Amiano, P.; Ballester, F.; Barros, H.; Bergström, A.; Charles, M.-A.; Chatzi, L.; Chevrier, C.; et al. Maternal body mass index, gestational weight gain, and the risk of overweight and obesity across childhood: An individual participant data meta-analysis. PLoS Med. 2019, 16, e1002744. [Google Scholar] [CrossRef] [PubMed]
- Kelsey, M.M.; Zeitler, P.S. Insulin Resistance of Puberty. Curr. Diabetes Rep. 2016, 16, 64. [Google Scholar] [CrossRef]
- Cousminer, D.L.; Stergiakouli, E.; Berry, D.J.; Ang, W.; Groen-Blokhuis, M.M.; Körner, A.; Siitonen, N.; Ntalla, I.; Marinelli, M.; Perry, J.R.; et al. Genome-wide association study of sexual maturation in males and females highlights a role for body mass and menarche loci in male puberty. Hum. Mol. Genet. 2014, 23, 4452–4464. [Google Scholar] [CrossRef]
- Buxton, J.L.; Das, S.; Rodríguez, A.; Kaakinen, M.; Alves, A.C.; Sebért, S.; Millwood, I.Y.; Laitinen, J.; O’Reilly, P.F.; Järvelin, M.-R.; et al. Multiple Measures of Adiposity Are Associated with Mean Leukocyte Telomere Length in the Northern Finland Birth Cohort 1966. PLoS ONE 2014, 9, e99133. [Google Scholar] [CrossRef]
- Henriksson, P.; Henriksson, H.; Tynelius, M.P.; Berglind, D.; Löf, M.; Lee, M.I.-M.; Shiroma, S.E.J.; Ortega, F.B. Fitness and Body Mass Index During Adolescence and Disability Later in Life: A Cohort Study. Ann. Intern. Med. 2019, 170, 230–239. [Google Scholar] [CrossRef]
- Kantomaa, M.T.; Stamatakis, E.; Kankaanpää, A.; Kaakinen, M.; Rodriguez, A.; Taanila, A.; Ahonen, T.; Järvelin, M.-R.; Tammelin, T. Physical activity and obesity mediate the association between childhood motor function and adolescents’ academic achievement. Proc. Natl. Acad. Sci. USA 2012, 110, 1917–1922. [Google Scholar] [CrossRef]
- Dahl, R.E.; Allen, N.B.; Wilbrecht, L.; Suleiman, A.B. Importance of investing in adolescence from a developmental science perspective. Nature 2018, 554, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Cardel, M.I.; Atkinson, M.A.; Taveras, E.M.; Holm, J.-C.; Kelly, A.S. Obesity Treatment Among Adolescents: A Review of Current Evidence and Future Directions. JAMA Pediatrics 2020, 174, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Patton, G.C.; Sawyer, S.M.; Santelli, J.S.; Ross, D.A.; Afifi, R.; Allen, N.B.; Arora, M.; Azzopardi, P.; Baldwin, W.; Bonell, C.; et al. Our future: A Lancet commission on adolescent health and wellbeing. Lancet 2016, 387, 2423–2478. [Google Scholar] [CrossRef]
- Azzopardi, P.S.; Hearps, S.J.C.; Francis, K.L.; Kennedy, E.C.; Mokdad, A.H.; Kassebaum, N.J.; Lim, S.; Irvine, C.M.S.; Vos, T.; Brown, A.D.; et al. Progress in adolescent health and wellbeing: Tracking 12 headline indicators for 195 countries and territories, 1990–2016. Lancet 2019, 393, 1101–1118. [Google Scholar] [CrossRef]

| Characteristic | All Participants N = 19,634 | Males N = 9556 (48.7%) | Females N = 10,078 (51.3%) | Male-Female Comparison χ2 or t-Test | p-Value |
|---|---|---|---|---|---|
| Study Site (n, %) | 10.37 | p = 0.0157 | |||
| Gdynia | 3914 (19.9) | 1898 (48.5) | 2016 (51.5) | 3.56 | p = 0.0593 |
| Lublin | 4709 (24.0) | 2339 (49.7) | 2370 (50.3) | 0.20 | p = 0.6514 |
| Warsaw | 6662 (33.9) | 3290 (49.4) | 3372 (50.6) | 1.01 | p = 0.3151 |
| Wrocław | 4349 (22.2) | 2029 (46.7) | 2320 (53.3) | 19.47 | p < 0.0001 |
| Age (n, %) | 27.936 | p < 0.0001 | |||
| 12.0 | 606 (3.1) | 248 (40.9) | 358 (59.1) | 19.97 | p < 0.0000 |
| 12.5 | 2271 (11.6) | 1047 (46.1) | 1224 (53.9) | 13.80 | p = 0.0002 |
| 13.0 | 7598 (38.7) | 3677 (48.4) | 3921 (51.6) | 7.84 | p = 0.0051 |
| 13.5 | 7411 (37.7) | 3701 (49.9) | 3710 (50.1) | 0.01 | p = 0.9167 |
| 14.0 | 1748 (8.9) | 883 (50.5) | 865 (49.5) | 0.19 | p = 0.6668 |
| Height, cm | 163.5 ± 7.8 | 165.4 ± 8.7 | 161.8 ± 6.4 | 33.02 | p < 0.0001 |
| Weight, kg | 54.1 ± 12.1 | 55.6 ± 13.1 | 52.6 ± 10.7 | 17.46 | p < 0.0001 |
| BMI c | 20.1 ± 3.6 | 20.2 ± 3.8 | 20.0 ± 3.5 | 2.75 | p = 0.0060 |
| IOTF d BMI, % | 139.84 | p < 0.0001 | |||
| Thinness grade 2 & 3 | 2.0 | 1.7 | 2.4 | 15.01 | p < 0.0001 |
| Thinness grade 1 | 7.6 | 6.5 | 8.5 | 38.72 | p < 0.0001 |
| Normal | 68.9 | 67.1 | 70.6 | 38.24 | p < 0.0001 |
| Overweight | 17.4 | 19.6 | 15.4 | 29.58 | p < 0.0001 |
| Obesity and Morbid obesity | 4.1 | 5.1 | 3.2 | 33.79 | p < 0.0001 |
| Waist girth, cm | 69.06 ± 9.17 | 71.09 ± 9.70 | 67.12 ± 8.19 | 30.80 | p < 0.0001 |
| Hip girth, cm | 86.94 ± 8.77 | 86.36 ± 9.16 | 87.48 ± 8.36 | 8.93 | p < 0.0001 |
| Waist–hip ratio (WHR) | 0.795 ± 0.068 | 0.823 ± 0.64 | 0.768 ± 0.061 | 61.92 | p < 0.0001 |
| Waist-to-height ratio (WHtR) | 0.422 ± 0.052 | 0.430 ± 0.054 | 0.415 ± 0.048 | 20.18 | p < 0.0001 |
| Percent Fat Mass (FM) | 17.6 ± 8.3 | 13.3 ± 6.8 | 21.7 ± 7.5 | 81.89 | p < 0.0001 |
| Percent Fat-Free Mass (FFM) | 76.5 ± 7.8 | 80.6 ± 6.5 | 72.7 ± 6.9 | 83.15 | p < 0.0001 |
| Systolic blood pressure | 115.1 ± 11.4 | 116.9 ± 11.8 | 113.5 ± 10.7 | 20.61 | p < 0.0001 |
| Diastolic blood pressure | 69.3 ± 8.0 | 68.6 ± 8.0 | 70.0 ± 7.8 | 12.63 | p < 0.0001 |
| Cardiorespiratory Fitness (CRF) e Classification f % | 163.7 | p < 0.0001 | |||
| Excellent | 2.8 | 2.6 | 3.0 | 1.71 | p = 0.1913 |
| Very good | 9.7 | 9.9 | 9.5 | 0.39 | p = 0.5322 |
| Good | 19.3 | 17.7 | 20.9 | 19.94 | p < 0.0001 |
| Satisfactory | 29.6 | 28.1 | 31.2 | 13.32 | p = 0.0002 |
| Weak | 31.8 | 32.5 | 31.0 | 2.46 | p = 0.1169 |
| Very weak | 6.8 | 9.2 | 4.4 | 125.9 | p < 0.0001 |
| Heart rate (HR) post exertion | 126.2 ± 15.9 | 121.3 ± 15.2 | 131.2 ± 15.0 | 40.21 | p < 0.0001 |
| Perceived adolescent BMI (parental report), % | 175.61 | p < 0.0001 | |||
| severe underweight | 1.8 | 2.2 | 1.4 | 10.71 | p = 0.0011 |
| underweight | 13.1 | 15.4 | 11.0 | 42.10 | p < 0.0001 |
| normal | 67.2 | 62.4 | 71.7 | 117.80 | p < 0.0001 |
| overweight | 14.3 | 15.9 | 12.7 | 16.57 | p < 0.0001 |
| obesity | 3.6 | 4.0 | 3.2 | 5.53 | p = 0.0210 |
| Reported maternal BMI | 23.90 ± 4.03 | 23.87 ± 4.05 | 23.92 ± 4.01 | 0.80 | p = 0.4263 |
| Reported paternal BMI | 26.91 ± 3.79 | 26.91 ± 3.76 | 26.90 ± 3.83 | 0.26 | p = 0.7942 |
| Fast-food consumption, % | 188.57 | p < 0.0001 | |||
| >5 times per week | 0.3 | 0.4 | 0.3 | 2.48 | p = 0.1151 |
| 3–5 times per week | 1.3 | 1.6 | 0.9 | 14.07 | p = 0.0002 |
| 1–3 times per week | 49.8 | 54.5 | 45.3 | 33.18 | p < 0.0001 |
| no consumption | 42.9 | 37.8 | 47.8 | 151.53 | p < 0.0001 |
| unknown | 5.7 | 5.6 | 5.7 | 1.27 | p = 0.2594 |
| Junk food snacks, % | 9.74 | p = 0.08 | |||
| few times per day | 13.2 | 12.5 | 13.9 | 15.85 | p = 0.0001 |
| ≤1 per day | 27.5 | 27.7 | 27.2 | 2.07 | p = 0.1504 |
| few times per week | 46.1 | 46.2 | 45.9 | 5.92 | p = 0.0150 |
| ≤1 per week | 11.3 | 11.7 | 11.0 | 0.03 | p = 0.8579 |
| no consumption | 0.7 | 0.7 | 0.7 | 0.03 | p = 0.8608 |
| unknown | 1.1 | 1.1 | 1.2 | 1.27 | p = 0.2603 |
| Sugar-sweetened beverages, % | 167.46 | p < 0.0001 | |||
| ≥5 times per week | 15.5 | 17.0 | 14.1 | 11.11 | p = 0.0009 |
| 3–5 times per week | 16.1 | 17.2 | 15.0 | 3.44 | p = 0.0635 |
| 1–3 times per week | 41.2 | 42.8 | 39.8 | 0.25 | p = 0.6195 |
| no consumption | 25.8 | 21.4 | 29.8 | 167.43 | p < 0.0001 |
| unknown | 1.4 | 1.6 | 1.3 | 1.44 | p = 0.2304 |
| Number of hours of active sport activity per week, % | 319.64 | p < 0.0001 | |||
| ≥5 h | 29.0 | 34.7 | 23.7 | 127.26 | p < 0.0001 |
| 3–5 h | 24.3 | 24.2 | 24.4 | 5.17 | p = 0.02 |
| 1–3 h | 32.8 | 30.3 | 35.2 | 62.87 | p < 0.0001 |
| <1 h | 13.9 | 10.9 | 16.7 | 139.97 | p < 0.0001 |
| Sedentary behavior, % | 110.62 | p < 0.0001 | |||
| >3 h | 32.9 | 36.0 | 29.9 | 22.03 | p < 0.0001 |
| 1–3 h | 53.6 | 52.7 | 54.6 | 22.01 | p < 0.0001 |
| <1 h | 13.5 | 11.3 | 15.5 | 83.32 | p < 0.0001 |
| FM | FFM | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Models: | Unadj Model Adj R2 | Adjusted a Model Adj R2 | β | CI | RSME | Unadj Model Adj R2 | Adjusted a Model Adj R2 | β | CI | RSME |
| Testing BMI | ||||||||||
| Boys | 0.76 | 0.76 | 0.032 | 0.69 | 0.69 | 0.035 | ||||
| BMI | 0.60 | 0.60–0.60 | −0.52 | −0.52–−0.52 | ||||||
| WHtR | 0.31 | 0.28–0.33 | −0.34 | −0.37–−0.31 | ||||||
| Girls | 0.89 | 0.89 | 0.024 | 0.86 | 0.85 | 0.026 | ||||
| BMI | 0.93 | 0.93–0.94 | −0.91 | −0.91–−0.91 | ||||||
| WHtR | 0.02 | 0.01–0.03 | −0.01 | −0.04–−0.01 | ||||||
| Testing IOTF BMI b | ||||||||||
| Boys | 0.72 | 0.72 | 0.035 | 0.66 | 0.66 | 0.037 | ||||
| IOTF BMI | ||||||||||
| Thin | −0.13 | −0.13–−0.13 | 0.10 | 0.10–0.11 | ||||||
| Overweight | 0.26 | 0.25–0.26 | −0.23 | −0.23–−0.23 | ||||||
| Obese | 0.18 | 0.18–0.19 | −0.16 | −0.16–−0.15 | ||||||
| Morbid obesity | 0.10 | 0.09–0.11 | −0.09 | −0.10–−0.08 | ||||||
| WHtR | 0.54 | 0.52–0.57 | −0.54 | −0.57–−0.52 | ||||||
| Girls | 0.77 | 0.77 | 0.035 | 0.74 | 0.73 | 0.035 | ||||
| IOTF BMI | ||||||||||
| Thin | −0.30 | −0.31–−0.30 | 0.30 | 0.30–0.30 | ||||||
| Overweight | 0.30 | 0.30–0.31 | −0.29 | −0.29–−0.28 | ||||||
| Obese | 0.22 | 0.22–0.23 | −0.22 | −0.22–−0.21 | ||||||
| Morbid obesity | 0.11 | 0.10–0.13 | −0.11 | −0.12–−0.10 | ||||||
| WHtR | 0.43 | 0.40–0.45 | −0.02 | −0.04–0.01 | ||||||
| Unadjusted Model | Fully Adjusted Model a | ||||
|---|---|---|---|---|---|
| Adj R2 | Adj R2 | F for Model t | p< | RSME | |
| Model: Boys | |||||
| (Including FM) | 0.17 | 0.18 | 81.25 | <0.0001 | 1.11 |
| FM | 145.67 | <0.0001 | |||
| Sports activity | 124.88 | <0.0001 | |||
| Systolic blood pressure | 80.49 | <0.0001 | |||
| WHtR | 28.24 | <0.0001 | |||
| BMI | 28.18 | <0.0001 | |||
| Sedentary activity | 10.0 | <0.0001 | |||
| Consumption of junk food | 3.30 | 0.06 | |||
| Model: Boys | |||||
| (Including FFM) | 0.16 | 0.18 | 67.74 | <0.0001 | 1.11 |
| Sports activity | 127.26 | <0.0001 | |||
| FFM | 117.89 | <0.0001 | |||
| Systolic blood pressure | 79.90 | <0.0001 | |||
| WHtR | 33.67 | 0.02 | |||
| BMI | 10.72 | 0.001 | |||
| Sedentary activity | 9.69 | <0.0001 | |||
| Consumption of junk food | 3.25 | 0.07 | |||
| Model: Girls | |||||
| (Including FM) | 0.11 | 0.12 | 66.58 | <0.0001 | 1.07 |
| Systolic blood pressure | 127.01 | <0.0001 | |||
| Sports activity | 77.67 | <0.0001 | |||
| FM | 60.69 | <0.0001 | |||
| Consumption of junk food | 13.19 | 0.0003 | |||
| BMI | 10.37 | 0.002 | |||
| WHtR | 4.90 | 0.02 | |||
| Model: Girls | |||||
| (including FFM) | 0.11 | 0.12 | 61.20 | <0.0001 | 1.08 |
| Systolic blood pressure | 129.59 | <0.0001 | |||
| Sports activity | 79.79 | <0.0001 | |||
| FFM | 37.87 | <0.0001 | |||
| Consumption of junk food | 14.61 | <0.0001 | |||
| WHtR | 4.95 | <0.0001 | |||
| BMI | 2.41 | 0.02 | |||
| Unadj. Model a | Adjusted Model b | BMI | WHtR | FM | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Adj R2 | Adj R2 | F | df | p value | Β c | 95% CI | β c | 95% CI | β c | 95% CI | |
| Fasting Glucose | |||||||||||
| Boy | 0.01 | 0.06 | 5.66 | 10,683 | <0.0001 | 0.07 | 0.05–0.08 | 0.01 | −0.83–0.83 | 0.01 | −0.65–0.62 |
| Girl | 0.01 | 0.11 | 8.70 | 10,613 | <0.0001 | 0.01 | −0.02–0.05 | 0.04 | −0.02–0.05 | 0.09 | −0.86–0.94 |
| Insulin | |||||||||||
| Boy | 0.12 | 0.14 | 12.05 | 10,672 | <0.0001 | 0.16 | 0.14–0.17 | 0.21 | −0.84–1.26 | 0.04 | −0.76–−0.84 |
| Girl | 0.05 | 0.08 | 6.34 | 10,599 | <0.0001 | 0.12 | 0.07–0.17 | 0.16 | −1.11–1.42 | −0.03 | −3.07–3.01 |
| Uric Acid | |||||||||||
| Boy | 0.09 | 0.13 | 11.73 | 10,682 | <0.0001 | 0.40 | 0.36–0.43 | 0.03 | −2.34–2.40 | 0.21 | −2.03–1.61 |
| Girl | 0.07 | 0.09 | 7.30 | 10,615 | <0.09 | 0.77 | 0.68–0.86 | 0.03 | −2.26–2.31 | 0.51 | −5.89–4.88 |
| Total Cholesterol | |||||||||||
| Boy | 0.04 | 0.04 | 3.53 | 10,680 | <0.0001 | −0.21 | −0.23–−0.18 | 0.21 | −1.46–1.88 | 0.11 | −1.17–1.39 |
| Girl | 0.01 | 0.02 | 2.11 | 10,607 | 0.02 | 0.19 | 0.13–0.26 | 0.12 | 0.12–0.26 | 0.28 | −1.69–1.93 |
| HDL | |||||||||||
| Boy | 0.04 | 0.05 | 4.56 | 10,681 | <0.0001 | −0.28 | −0.29–−0.27 | 0.10 | −0.59–0.79 | 0.02 | −0.55–0.51 |
| Girl | 0.03 | 3.26 | 10,613 | 0.03 | 0.21 | −0.66–0.66 | −0.11 | −0.23–−0.18 | 0.14 | −0.85–0.62 | |
| LDL | |||||||||||
| Boy | 0.03 | 0.03 | 3.02 | 10,669 | 0.0009 | −0.16 | −0.18–−0.13 | 0.13 | −1.33–1.58 | 0.16 | −0.96–1.28 |
| Girl | 0.01 | 0.01 | 1.32 | 10,609 | 0.21 | 0.24 | −1.40–1.40 | 0.05 | −1.52–1.63 | −0.27 | −3.97–3.43 |
| Triglycerides | |||||||||||
| Boy | 0.03 | 0.03 | 3.24 | 10,675 | 0.0004 | 0.05 | −0.92–1.25 | 0.16 | −0.82–0.86 | 0.02 | −0.15–0.06 |
| Girl | 0.06 | 0.08 | 6.66 | 10,610 | 0.08 | 0.32 | 0.28–0.36 | 0.33 | −0.70–1.36 | 0.44 | −2.88–1.93 |
| Hemoglobin | |||||||||||
| Boy | 0.07 | 0.09 | 8.15 | 10,684 | <0.0001 | 0.31 | 0.27–0.34 | −0.27 | −2.58–2.03 | −0.11 | −1.87–1.66 |
| Girl | 0.00 | 0.03 | 3.24 | 10,612 | 0.0004 | 0.05 | −2.12–2.12 | 0.14 | −0.04–0.14 | 0.17 | −2.24–2.52 |
| MCHC | |||||||||||
| Boy | 0.01 | 0.15 | 13.68 | 10,684 | <0.0001 | 0.02 | −0.02–0.05 | −0.96 | −2.46–2.26 | −0.08 | −1.89–1.73 |
| Girl | 0.00 | 0.15 | 11.38 | 10,612 | <0.0001 | −0.26 | −0.36–−0.16 | 0.11 | −2.41–2.63 | 0.11 | −5.83–6.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodriguez, A.; Korzeniowska, K.; Szarejko, K.; Borowski, H.; Brzeziński, M.; Myśliwiec, M.; Czupryniak, L.; Berggren, P.-O.; Radziwiłł, M.; Soszyński, P. Fitness, Food, and Biomarkers: Characterizing Body Composition in 19,634 Early Adolescents. Nutrients 2022, 14, 1369. https://doi.org/10.3390/nu14071369
Rodriguez A, Korzeniowska K, Szarejko K, Borowski H, Brzeziński M, Myśliwiec M, Czupryniak L, Berggren P-O, Radziwiłł M, Soszyński P. Fitness, Food, and Biomarkers: Characterizing Body Composition in 19,634 Early Adolescents. Nutrients. 2022; 14(7):1369. https://doi.org/10.3390/nu14071369
Chicago/Turabian StyleRodriguez, Alina, Katarzyna Korzeniowska, Kamila Szarejko, Hubert Borowski, Michał Brzeziński, Małgorzata Myśliwiec, Leszek Czupryniak, Per-Olof Berggren, Marcin Radziwiłł, and Piotr Soszyński. 2022. "Fitness, Food, and Biomarkers: Characterizing Body Composition in 19,634 Early Adolescents" Nutrients 14, no. 7: 1369. https://doi.org/10.3390/nu14071369
APA StyleRodriguez, A., Korzeniowska, K., Szarejko, K., Borowski, H., Brzeziński, M., Myśliwiec, M., Czupryniak, L., Berggren, P.-O., Radziwiłł, M., & Soszyński, P. (2022). Fitness, Food, and Biomarkers: Characterizing Body Composition in 19,634 Early Adolescents. Nutrients, 14(7), 1369. https://doi.org/10.3390/nu14071369







