Maternal Obesity in Twin Pregnancy: The Role of Nutrition to Reduce Maternal and Fetal Complications
Abstract
1. Introduction
2. Materials and Methods
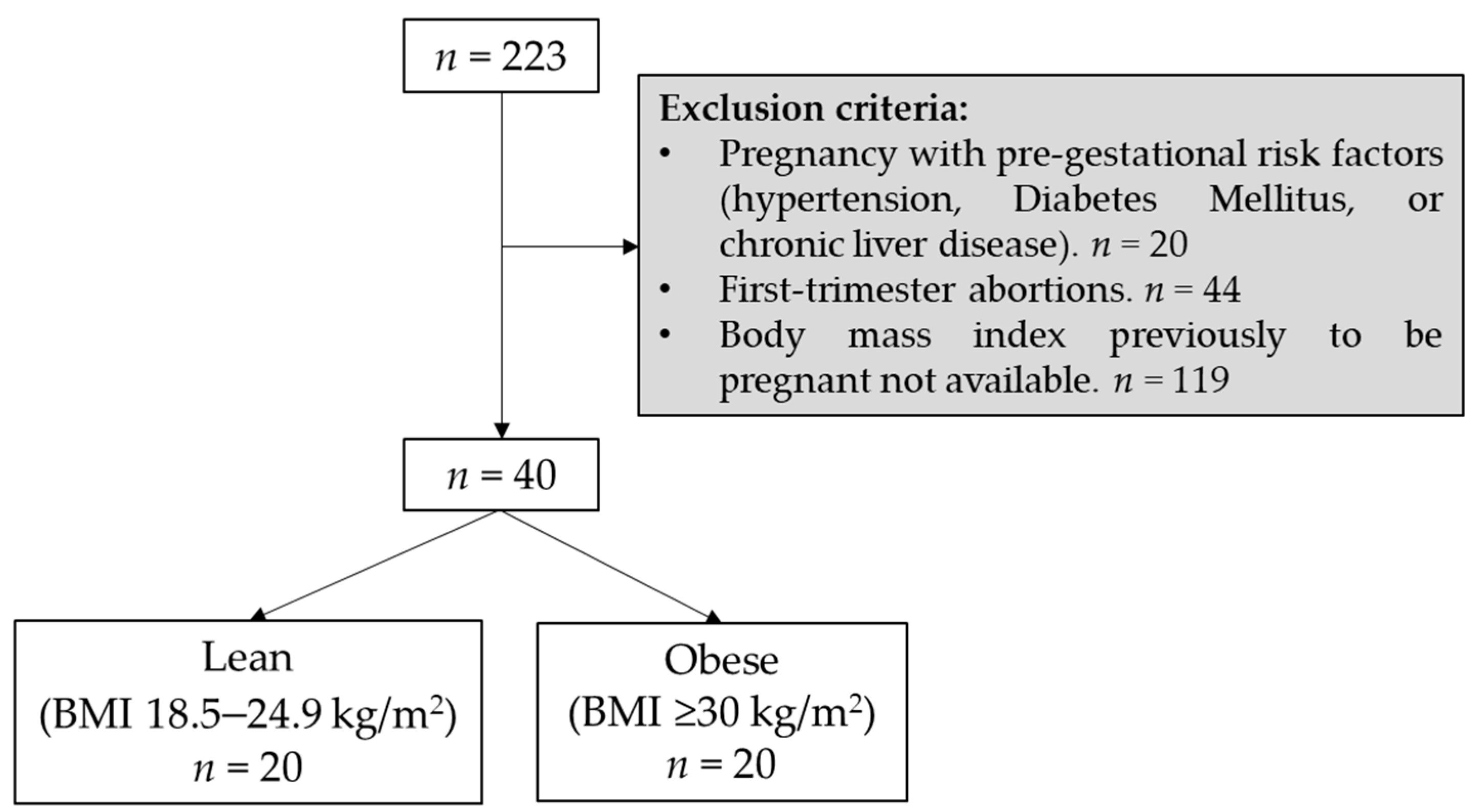
2.1. Study Design and Cohort Selection
2.2. Variable Collection
2.3. Statistical Analysis
3. Results
3.1. Maternal Characteristics of the Cohort
3.2. Biochemical and Hematological Parameters
3.3. Clinical Variables
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Doi, L.; Williams, A.J.; Marryat, L.; Frank, J. Cohort Study of High Maternal Body Mass Index and the Risk of Adverse Pregnancy and Delivery Outcomes in Scotland. BMJ Open 2020, 10, e026168. [Google Scholar] [CrossRef]
- INE; Instituto Nacional de Estadística. Índice De Masa Corporal Por Grupos De Edad Y Sexo. Available online: https://www.ine.es/jaxi/Datos.htm?path=/t00/mujeres_hombres/tablas_1/l0/&file=d06001.px#!tabs-tabla (accessed on 5 January 2022).
- Schubert, J.; Timmesfeld, N.; Noever, K.; Arabin, B. Challenges for Better Care Based on the Course of Maternal Body Mass Index, Weight Gain and Multiple Outcome in Twin Pregnancies: A Population-Based Retrospective Cohort Study in Hessen/Germany within 15 Years. Arch. Gynecol. Obstet. 2020, 301, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Poston, L.; Caleyachetty, R.; Cnattingius, S.; Corvalan, C.; Uauy, R.; Herring, S.; Gillman, M.W. Preconceptional and Maternal Obesity: Epidemiology and Health Consequences. Lancet Diabetes Endocrinol. 2016, 4, 1025–1036. [Google Scholar] [CrossRef]
- Schmatz, M.; Madan, J.; Marino, T.; Davis, J. Maternal Obesity: The Interplay between Inflammation, Mother and Fetus. J. Perinatol. 2010, 30, 441–446. [Google Scholar] [CrossRef]
- Catalano, P.M.; Shankar, K. Obesity and Pregnancy: Mechanisms of Short Term and Long Term Adverse Consequences for Mother and Child. BMJ 2017, 356, j1. [Google Scholar] [CrossRef]
- Catalano, P.; deMouzon, S.H. Maternal Obesity and Metabolic Risk to the Offspring: Why Lifestyle Interventions may have Not Achieved the Desired Outcomes. Int. J. Obes. 2015, 39, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Cinkajzlova, A.; Mraz, M.; Haluzik, M. Lymphocytes and Macrophages in Adipose Tissue in Obesity: Markers Or Makers of Subclinical Inflammation? Protoplasma 2017, 254, 1219–1232. [Google Scholar] [CrossRef]
- Rugina, C.; Marginean, C.O.; Melit, L.E.; Hutanu, A.; Ghiga, D.V.; Modi, V.; Marginean, C. Gestational Obesity and Subclinical Inflammation: The Pathway from Simple Assessment to Complex Outcome (STROBE-Compliant Article). Medicine 2021, 100, e26055. [Google Scholar] [CrossRef]
- Del Mar Bibiloni, M.; Maffeis, C.; Llompart, I.; Pons, A.; Tur, J.A. Dietary Factors Associated with Subclinical Inflammation among Girls. Eur. J. Clin. Nutr. 2013, 67, 1264–1270. [Google Scholar] [CrossRef]
- Esposito, K.; Giugliano, D. Diet and Inflammation: A Link to Metabolic and Cardiovascular Diseases. Eur. Heart J. 2006, 27, 15–20. [Google Scholar] [CrossRef]
- Yeh, K.L.; Kautz, A.; Lohse, B.; Groth, S.W. Associations between Dietary Patterns and Inflammatory Markers during Pregnancy: A Systematic Review. Nutrients 2021, 13, 834. [Google Scholar] [CrossRef] [PubMed]
- Arabin, B.; Stupin, J.H. Overweight and Obesity before, during and After Pregnancy: Part 2: Evidence-Based Risk Factors and Interventions. Geburtshilfe Frauenheilkd. 2014, 74, 646–655. [Google Scholar]
- Thangaratinam, S.; Rogozinska, E.; Jolly, K.; Glinkowski, S.; Roseboom, T.; Tomlinson, J.W.; Kunz, R.; Mol, B.W.; Coomarasamy, A.; Khan, K.S. Effects of Interventions in Pregnancy on Maternal Weight and Obstetric Outcomes: Meta-Analysis of Randomised Evidence. BMJ 2012, 344, e2088. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.Y.; Zafman, K.B.; Fox, N.S. Weight Gain and Pregnancy Outcomes in Overweight Or Obese Women with Twin Gestations. J. Matern. Fetal. Neonatal Med. 2021, 34, 1774–1779. [Google Scholar] [CrossRef]
- Deriba, B.S.; Bulto, G.A.; Bala, E.T. Nutritional-Related Predictors of Anemia among Pregnant Women Attending Antenatal Care in Central Ethiopia: An Unmatched Case-Control Study. Biomed Res. Int. 2020, 2020, 8824291. [Google Scholar] [CrossRef]
- Chasen, S.T.; Chervenar, F.A. Twin Pregnancy: Prenatal Issues; UpToDate: Waltham, MA, USA, 2017. [Google Scholar]
- Summers, J.; Ecker, J.L.; Hearns-Stokes, R. Perinatal Risks Associated with Assisted Reproductive Technology. Obs. Gynecol. 2016, 128, e61–e68. [Google Scholar]
- Stephenson, J.; Heslehurst, N.; Hall, J.; Schoenaker, D.A.; Hutchinson, J.; Cade, J.E.; Poston, L.; Barrett, G.; Crozier, S.R.; Barker, M.; et al. Before the Beginning: Nutrition and Lifestyle in the Preconception Period and its Importance for Future Health. Lancet 2018, 391, 1830–1841. [Google Scholar] [CrossRef]
- Kasum, M.; Oreskovic, S.; Cehic, E.; Lila, A.; Ejubovic, E.; Soldo, D. The Role of Female Obesity on in Vitro Fertilization Outcomes. Gynecol. Endocrinol. 2018, 34, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Pandey, S.; Maheshwari, A.; Bhattacharya, S. The Impact of Female Obesity on the Outcome of Fertility Treatment. J. Hum. Reprod. Sci. 2010, 3, 62–67. [Google Scholar] [PubMed]
- Pajuelo Ramírez, J.; Muñoz, C.; Ayquipa, A.; Ponciano, W.; López, R. El Sobrepeso, La Obesidad Y La Anemia Nutricional En La Mujer Adulta. An. Fac. Med. 2014, 61, 265–270. [Google Scholar] [CrossRef][Green Version]
- Vricella, L.K. Emerging Understanding and Measurement of Plasma Volume Expansion in Pregnancy. Am. J. Clin. Nutr. 2017, 106, 1620S–1625S. [Google Scholar] [CrossRef] [PubMed]
- Kiely, M.E.; Wagner, C.L.; Roth, D.E. Vitamin D in Pregnancy: Where we are and Where we should Go. J. Steroid Biochem. Mol. Biol. 2020, 201, 105669. [Google Scholar] [CrossRef]
- Bozkus, F.; Dikmen, N.; Samur, A.; Bilal, N.; Atilla, N.; Arpag, H. Does the Neutrophil-to-Lymphocyte Ratio have any Importance between Subjects with Obstructive Sleep Apnea Syndrome with Obesity and without Obesity? Tuberk. Toraks 2018, 66, 8–15. [Google Scholar] [PubMed]
- Radwan, H.; Hashim, M.; Hasan, H.; Abbas, N.; Obaid, R.R.S.; Al Ghazal, H.; Naja, F. Adherence to the Mediterranean Diet during Pregnancy is Associated with Lower Odds of Excessive Gestational Weight Gain and Postpartum Weight Retention: Results of the Mother-Infant Study Cohort. Br. J. Nutr. 2021, 1–12. [Google Scholar] [CrossRef]
- Koutelidakis, A.E.; Alexatou, O.; Kousaiti, S.; Gkretsi, E.; Vasios, G.; Sampani, A.; Tolia, M.; Kiortsis, D.N.; Giaginis, C. Higher Adherence to Mediterranean Diet Prior to Pregnancy is Associated with Decreased Risk for Deviation from the Maternal Recommended Gestational Weight Gain. Int. J. Food Sci. Nutr. 2018, 69, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Al-Obaidly, S.; Parrish, J.; Murphy, K.E.; Maxwell, C. Maternal Pre-Gravid Body Mass Index and Obstetric Outcomes in Twin Gestations. J. Perinatol. 2014, 34, 425–428. [Google Scholar] [CrossRef]
- Fox, N.S.; Roman, A.S.; Saltzman, D.H.; Klauser, C.K.; Rebarber, A. Obesity and Adverse Pregnancy Outcomes in Twin Pregnancies. J. Matern. Fetal. Neonatal Med. 2014, 27, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Mack, L.R.; Tomich, P.G. Gestational Diabetes: Diagnosis, Classification, and Clinical Care. Obstet. Gynecol. Clin. N. Am. 2017, 44, 207–217. [Google Scholar] [CrossRef]
- O’Brien, T.E.; Ray, J.G.; Chan, W.S. Maternal Body Mass Index and the Risk of Preeclampsia: A Systematic Overview. Epidemiology 2003, 14, 368–374. [Google Scholar] [CrossRef]
- Suzuki, S.; Yoneyama, Y.; Sawa, R.; Shin, S.; Araki, T. Clinical Usefulness of Maternal Body Mass Index in Twin Pregnancies. Hypertens. Pregnancy 2000, 19, 273–279. [Google Scholar] [CrossRef]
- Finneran, M.M.; Gonzalez-Brown, V.M.; Smith, D.D.; Landon, M.B.; Rood, K.M. Obesity and Laboratory Aspirin Resistance in High-Risk Pregnant Women Treated with Low-Dose Aspirin. Am. J. Obstet. Gynecol. 2019, 220, e1–e385. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Inde, Y.; Miyake, H. Maternal Obesity as a Risk Factor for very Pre-Term Delivery in Dichorionic Twin Pregnancies. J. Obstet. Gynaecol. 2010, 30, 354–356. [Google Scholar] [CrossRef] [PubMed]
- McDonald, S.D.; Han, Z.; Mulla, S.; Beyene, J.; Knowledge Synthesis Group. Overweight and Obesity in Mothers and Risk of Preterm Birth and Low Birth Weight Infants: Systematic Review and Meta-Analyses. BMJ 2010, 341. [Google Scholar] [CrossRef]
- Nohr, E.A.; Bech, B.H.; Vaeth, M.; Rasmussen, K.M.; Henriksen, T.B.; Olsen, J. Obesity, Gestational Weight Gain and Preterm Birth: A Study within the Danish National Birth Cohort. Paediatr. Perinat. Epidemiol. 2007, 21, 5–14. [Google Scholar] [CrossRef]
- Sheiner, E.; Levy, A.; Menes, T.S.; Silverberg, D.; Katz, M.; Mazor, M. Maternal Obesity as an Independent Risk Factor for Caesarean Delivery. Paediatr. Perinat. Epidemiol. 2004, 18, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.M.; McAuliffe, F.M. Prediction and Prevention of the Macrosomic Fetus. Eur. J. Obstet. Gynecol. Reprod. Biol. 2012, 162, 125–130. [Google Scholar] [CrossRef]
- Kitahara, C.M.; Platz, E.A.; Ladenson, P.W.; Mondul, A.M.; Menke, A.; Berrington de Gonzalez, A. Body Fatness and Markers of Thyroid Function among U.S. Men and Women. PLoS ONE 2012, 7, e34979. [Google Scholar] [CrossRef]
- Pearce, E.N. Thyroid Hormone and Obesity. Curr. Opin. Endocrinol. Diabetes Obes. 2012, 19, 408–413. [Google Scholar] [CrossRef]
- Godoy, G.A.; Korevaar, T.I.; Peeters, R.P.; Hofman, A.; de Rijke, Y.B.; Bongers-Schokking, J.J.; Tiemeier, H.; Jaddoe, V.W.; Gaillard, R. Maternal Thyroid Hormones during Pregnancy, Childhood Adiposity and Cardiovascular Risk Factors: The Generation R Study. Clin. Endocrinol. 2014, 81, 117–125. [Google Scholar] [CrossRef]
- Kahr, M.K.; Antony, K.M.; DelBeccaro, M.; Hu, M.; Aagaard, K.M.; Suter, M.A. Increasing Maternal Obesity is Associated with Alterations in both Maternal and Neonatal Thyroid Hormone Levels. Clin. Endocrinol. 2016, 84, 551–557. [Google Scholar] [CrossRef]
- Chu, S.Y.; Kim, S.Y.; Lau, J.; Schmid, C.H.; Dietz, P.M.; Callaghan, W.M.; Curtis, K.M. Maternal Obesity and Risk of Stillbirth: A Metaanalysis. Am. J. Obstet. Gynecol. 2007, 197, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Balki, I.; Sheth, H.; Shafey, A.; Maxwell, C.; Stephens, D.; Shah, V. Maternal BMI in Twin Pregnancies and Impact on Neonatal Outcomes in the Level I Unit: A Retrospective Cohort Study. J. Obstet. Gynaecol. Can. 2019, 41, 1150–1156. [Google Scholar] [CrossRef] [PubMed]
| Lean (n = 20) | Obesity (n = 20) | p-Value | |
|---|---|---|---|
| Maternal age (years) | 37.0 [35.8; 43.0] | 36.0 [32.8; 38.5] | 0.097 a |
| BMI (kg/m2) | 21.4 [20.3; 22.8] | 32.9 [31.2; 34.5] | <0.001 a |
| Ethnicity | 0.999 b | ||
| Caucasian | 4 (20.0%) | 4 (20.0%) | |
| Latin | 16 (80.0%) | 15 (75.0%) | |
| Black | 0 (0.0%) | 1 (5.0%) | |
| Smoking habits | 2 (10.0%) | 1 (5.0%) | 0.999 b |
| Parity | 1.5 [1.0; 2.0] | 2.0 [1.0; 3.2] | 0.173 a |
| Miscarriages | 0.0 [0.0; 1.0] | 0.0 [0.0; 1.0] | 0.659 a |
| Weight pre-pregnancy (kg) | 58.0 [55.0; 63.5] | 89.7 [80.8; 94.5] | <0.001 a |
| Weight gain during pregnancy (kg) | 14.8 (5.9) | 8.8 (6.1) | 0.003 c |
| Twin | 0.273 b | ||
| Dichorionic diamniotic | 13 (65.0%) | 17 (85.0%) | |
| Monochorionic diamniotic | 7 (35.0%) | 3 (15.0%) | |
| Assisted reproduction techniques | 14 (70.0%) | 3 (15.0%) | 0.001 b |
| Trimester 1 | Trimester 3 | p-Value 3 | |||||
|---|---|---|---|---|---|---|---|
| Lean (n = 17) | Obesity (n = 15) | p-Value 1 | Lean (n = 20) | Obesity (n = 19) | p-Value 2 | ||
| Glucose (mg/dL) | 78.2 (7.50) | 82.7 (6.60) | 0.096 a | 75.7 (7.50) | 75.9 (7.90) | 0.932 a | 0.438 |
| Hemoglobin (g/dL) | 12.6 (0.70) | 12.1 (0.80) | 0.048 a | 12.3 (1.00) | 12.3 (0.80) | 0.858 a | 0.224 |
| Fibrinogen (mg/dL) | 494.1 (101.8) | 592.5 (182.2) | 0.373 a | 604.5 [580.8; 650.8] | 651.0 [590.0; 708.0] | 0.190 b | 0.905 |
| Ferritin (g/mL) | 22.0 [14.5; 31.5] | 15.5 [10.0; 37.2] | 0.565 b | 24.0 [15.5; 32.0] | 19.0 [16.0; 29.0] | 0.884 b | 0.145 |
| TSH (mUI/L) | 1.30 (1.10) | 2.20 (1.30) | 0.051 a | 1.70 (1.00) | 3.30 (1.60) | 0.009 a | 0.048 |
| Vitamin D (ng/mL) | 20.7 (8.40) | 13.0 (6.40) | 0.070 a | 21.6 (5.70) | 17.4 (7.30) | 0.323 a | 0.675 |
| Hematocrit (%) | 38.0 (2.1) | 37.4 (2.10) | 0.469 a | 37.4 [35.8; 38.7] | 37.1 [35.5; 39.0] | 0.725 b | 0.919 |
| Platelets (106/mL) | 245.0 [201.5; 267.5] | 289.0 [241.0; 331.0] | 0.056 b | 206.5 [188.5; 250.5] | 253.0 [204.0; 309.0] | 0.074 b | 0.912 |
| Leukocytes (106/mL) | 8.90 (1.90) | 8.80 (2.20) | 0.892 a | 8.90 [8.10; 9.10] | 8.50 [7.80; 1.10] | 0.955 b | 0.703 |
| Lymphocytes (103/μL) | 2.13 (0.82) | 2.22 (0.66) | 0.737 a | 1.96 (0.59) | 2.28 (0.57) | 0.091 a | 0.588 |
| Neutrophils (103/μL) | 6.00 (1.42) | 5.66 (1.61) | 0.546 a | 5.87 (1.04) | 6.04 (1.43) | 0.670 a | 0.689 |
| Monocytes (103/μL) | 0.49 (0.16) | 0.48 (0.13) | 0.860 a | 0.49 [0.43; 0.60] | 0.44 [0.38; 0.60] | 0.800 b | 0.515 |
| Eosinophils (103/μL) | 0.08 [0.06; 0.20] | 0.12 [0.09; 0.24] | 0.183 b | 0.10 [0.07; 0.14] | 0.10 [0.07; 0.21] | 0.473 b | 0.943 |
| Basophils (103/μL) | 0.03 [0.02; 0.04] | 0.03 [0.03; 0.05] | 0.410 b | 0.03 [0.02; 0.04] | 0.03 [0.02; 0.05] | 0.689 b | 0.555 |
| Lean (n = 20) | Obesity (n = 20) | p-Value | |
|---|---|---|---|
| Maternal complications | |||
| Preeclampsia | 4 (20.0%) | 2 (10.0%) | 0.661 a |
| Pregnancy-induced hypertension | 1 (5.0%) | 5 (25.0%) | 0.182 a |
| Gestational diabetes mellitus | 1 (5.0%) | 4 (20.0%) | 0.342 a |
| Anemia | 18 (90.0%) | 15 (75.0%) | 0.407 a |
| Urinary infection | 1 (5.0%) | 2 (10.0%) | 0.999 a |
| Fetal complications | |||
| Intrauterine growth restriction–Fetus 1 | 0 (0.0%) | 2 (10.0%) | 0.487 a |
| Intrauterine growth restriction–Fetus 2 | 2 (10.0%) | 1 (5.0%) | 0.999 a |
| Malformations–Fetus 1 | 3 (15.0%) | 0 (0.0%) | 0.231 a |
| Malformations–Fetus 2 | 3 (15.0%) | 2 (10.0%) | 0.999 a |
| Obstetrical complications | |||
| Premature rupture of membrane | 1 (5.0%) | 4 (20.0%) | 0.342 a |
| Threat of premature labor | 1 (5.0%) | 2 (10.0%) | 0.999 a |
| C-section | 13 (65.0%) | 16 (80.0%) | 0.479 a |
| Puerperal hemorrhage | 1 (5.0%) | 1 (5.0%) | 0.999 a |
| Neonatal variables and complications | |||
| Gestational age (weeks) | 37.5 [36.5; 38.0] | 37.1 [35.0; 37.4] | 0.083 b |
| Prematurity | 6 (30.0%) | 9 (45.0%) | 0.514 a |
| Birth body weight-neonate 1 (g) | 2400.0 [2216.2; 2712.5] | 2467.5 [1867.0; 3011.2] | 0.705 b |
| LBW-neonate 1 | 2 (10.0%) | 6 (30.0%) | 0.235 a |
| Birth body weight-neonate 2 (g) | 2602.5 [2098.8; 2783.8] | 2500.0 [2141.2; 2887.5] | 0.725 b |
| LBW-neonate 2 | 3 (15.0%) | 4 (20.0%) | 0.999 a |
| Apgar 5 min-neonate 1 | 10.0 [9.0; 10.0] | 10.0 [9.0; 10.0] | 0.890 b |
| Apgar 5 min-neonate 2 | 10.0 [9.0; 10.0] | 10.0 [9.0; 10.0] | 0.436 b |
| Artery pH-neonate 1 | 7.3 [7.3; 7.3] | 7.3 [7.3; 7.3] | 0.211 b |
| Artery pH-neonate 2 | 7.3 [7.3; 7.3] | 7.3 [7.3; 7.3] | 0.507 b |
| NICU admission-neonate 1 | 8 (40.0%) | 8 (40.0%) | 0.999 a |
| RDS-neonate 1 | 1 (5.0%) | 4 (20.0%) | 0.342 a |
| NICU admission-neonate 2 | 8 (40.0%) | 7 (35.0%) | 0.999 a |
| RDS-neonate 2 | 3 (15.0%) | 4 (20.0%) | 0.999 a |
| Neonatal death | 0 (0.0%) | 1 (5.0%) | 0.999 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de la Calle, M.; Bartha, J.L.; Marín, C.; Rus, J.C.; Córcoles, G.; Ruvira, S.; Ramiro-Cortijo, D. Maternal Obesity in Twin Pregnancy: The Role of Nutrition to Reduce Maternal and Fetal Complications. Nutrients 2022, 14, 1326. https://doi.org/10.3390/nu14071326
de la Calle M, Bartha JL, Marín C, Rus JC, Córcoles G, Ruvira S, Ramiro-Cortijo D. Maternal Obesity in Twin Pregnancy: The Role of Nutrition to Reduce Maternal and Fetal Complications. Nutrients. 2022; 14(7):1326. https://doi.org/10.3390/nu14071326
Chicago/Turabian Stylede la Calle, María, Jose L. Bartha, Clara Marín, Juan Carlos Rus, Guillermo Córcoles, Santiago Ruvira, and David Ramiro-Cortijo. 2022. "Maternal Obesity in Twin Pregnancy: The Role of Nutrition to Reduce Maternal and Fetal Complications" Nutrients 14, no. 7: 1326. https://doi.org/10.3390/nu14071326
APA Stylede la Calle, M., Bartha, J. L., Marín, C., Rus, J. C., Córcoles, G., Ruvira, S., & Ramiro-Cortijo, D. (2022). Maternal Obesity in Twin Pregnancy: The Role of Nutrition to Reduce Maternal and Fetal Complications. Nutrients, 14(7), 1326. https://doi.org/10.3390/nu14071326








