Cows’ Milk Allergy-Associated Constipation: When to Look for It? A Narrative Review
Abstract
1. Introduction
1.1. Childhood Constipation
1.2. Cows’ Milk Allergy
1.3. Cows’ Milk Allergy-Associated Constipation
2. Critical Overview of Current Knowledge
2.1. CMA in Children with Constipation: A Literature Compendium
2.2. Diagnostic Work-Up for Food Allergy-Associated Constipation
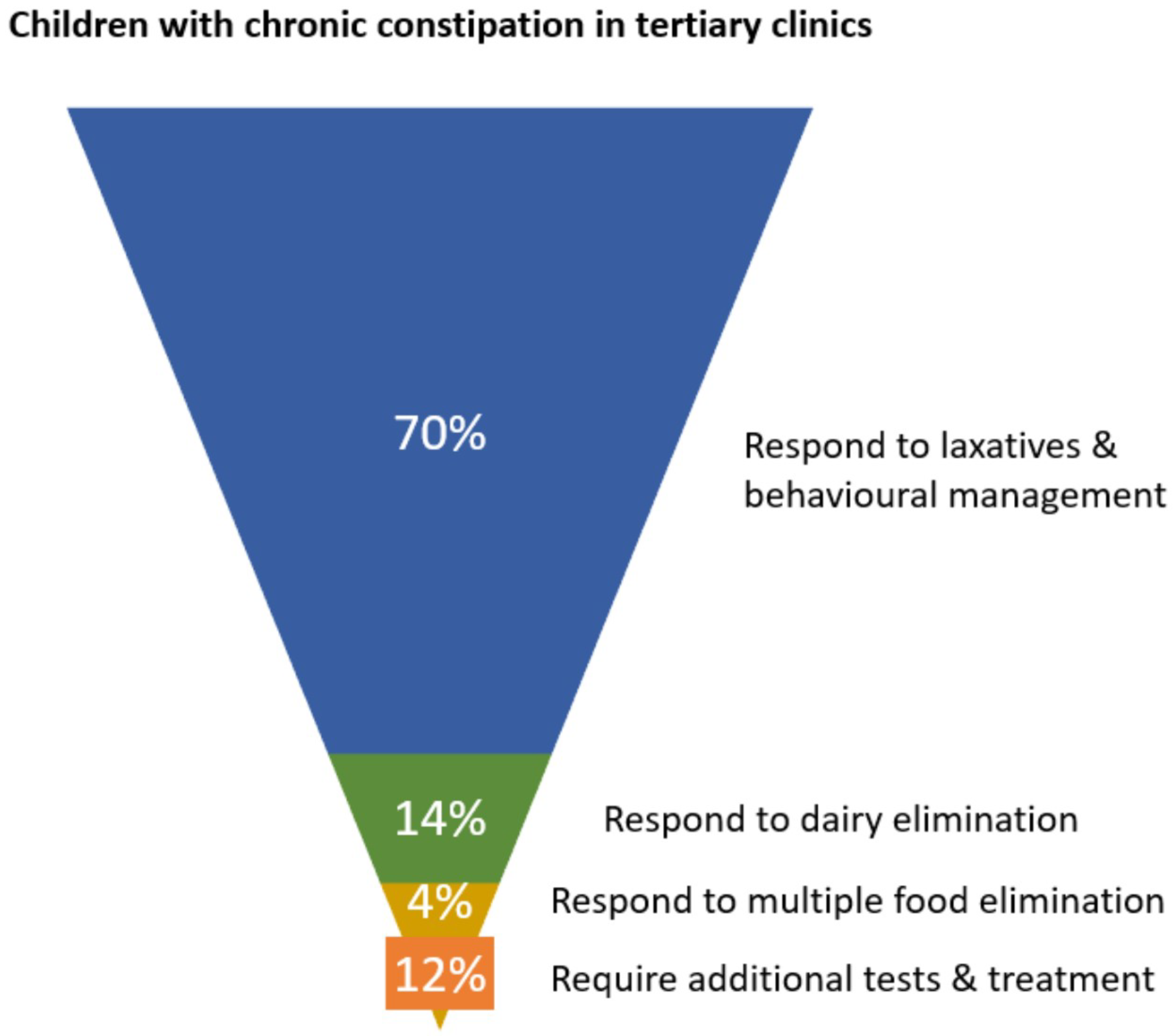
3. Food Allergy-Associated Constipation Management
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
References
- Robin, S.G.; Keller, C.; Zwiener, R.; Hyman, P.E.; Nurko, S.; Saps, M.; Di Lorenzo, C.; Shulman, R.J.; Hyams, J.S.; Palsson, O.; et al. Prevalence of Pediatric Functional Gastrointestinal Disorders Utilizing the Rome IV Criteria. J. Pediatr. 2018, 195, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Nwaru, B.I.; Hickstein, L.; Panesar, S.S.; Roberts, G.; Muraro, A.; Sheikh, A.; The EAACI Food Allergy and Anaphylaxis Guidelines Group. Prevalence of common food allergies in Europe: A systematic review and meta-analysis. Allergy 2014, 69, 992–1007. [Google Scholar] [CrossRef] [PubMed]
- Tabbers, M.M.; DiLorenzo, C.; Berger, M.Y.; Faure, C.; Langendam, M.W.; Nurko, S.; Staiano, A.; Vandenplas, Y.; Benninga, M.A.; European Society for Pediatric Gastroenterology, H.; et al. Evaluation and treatment of functional constipation in infants and children: Evidence-based recommendations from ESPGHAN and NASPGHAN. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 258–274. [Google Scholar] [CrossRef] [PubMed]
- Pensabene, L.; Salvatore, S.; D’Auria, E.; Parisi, F.; Concolino, D.; Borrelli, O.; Thapar, N.; Staiano, A.; Vandenplas, Y.; Saps, M. Cow’s Milk Protein Allergy in Infancy: A Risk Factor for Functional Gastrointestinal Disorders in Children? Nutrients 2018, 10, 1716. [Google Scholar] [CrossRef] [PubMed]
- Sopo, S.M.; Arena, R.; Greco, M.; Bergamini, M.; Monaco, S. Constipation and Cow’s Milk Allergy: A Review of the Literature. Int. Arch. Allergy Immunol. 2014, 164, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Meyer, R. Nutritional disorders resulting from food allergy in children. Pediatr. Allergy Immunol. 2018, 29, 689–704. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Hauser, B.; Salvatore, S. Functional Gastrointestinal Disorders in Infancy: Impact on the Health of the Infant and Family. Pediatr. Gastroenterol. Hepatol. Nutr. 2019, 22, 207–216. [Google Scholar] [CrossRef]
- Vernon-Roberts, A.; Alexander, I.; Day, A.S. Systematic Review of Pediatric Functional Gastrointestinal Disorders (Rome IV Criteria). J. Clin. Med. 2021, 10, 5087. [Google Scholar] [CrossRef]
- Peeters, B.; Benninga, M.; Hennekam, R. Childhood constipation; an overview of genetic studies and associated syndromes. Best Pract. Res. Clin. Gastroenterol. 2011, 25, 73–88. [Google Scholar] [CrossRef]
- Yamada, M.; Sekine, M.; Tatsuse, T.; Fujimura, Y. Lifestyle, psychological stress, and incidence of adolescent constipation: Results from the Toyama birth cohort study. BMC Public Health 2021, 21, 47. [Google Scholar] [CrossRef]
- Loening-Baucke, V. Chronic constipation in children. Gastroenterology 1993, 105, 1557–1564. [Google Scholar] [CrossRef]
- Di Lorenzo, C. Childhood constipation: Finally some hard data about hard stools! J. Pediatr. 2000, 136, 4–7. [Google Scholar] [CrossRef]
- Taitz, L.S.; Wales, J.; Urwin, O.M.; Molnar, D. Factors associated with outcome in management of defecation disorders. Arch. Dis. Child. 1986, 61, 472–477. [Google Scholar] [CrossRef]
- Loening-Baucke, V. Controversies in The Management of Chronic Constipation. J. Pediatr. Gastroenterol. Nutr. 2001, 32 (Suppl. 1), S38–S39. [Google Scholar] [CrossRef] [PubMed]
- Van Ginkel, R.; Reitsma, J.B.; A Büller, H.; van Wijk, M.; Taminiau, J.A.; Benninga, M.A. Childhood constipation: Longitudinal follow-up beyond puberty. Gastroenterology 2003, 125, 357–363. [Google Scholar] [CrossRef]
- Bongers, M.E.J.; van Wijk, M.P.; Reitsma, J.B.; Benninga, M.A. Long-Term Prognosis for Childhood Constipation: Clinical Outcomes in Adulthood. Pediatrics 2010, 126, e156–e162. [Google Scholar] [CrossRef] [PubMed]
- Hyams, J.S.; Di Lorenzo, C.; Saps, M.; Shulman, R.J.; Staiano, A.; van Tilburg, M. Childhood Functional Gastrointestinal Disorders: Child/Adolescent. Gastroenterology 2016, 150, 1456–1468. [Google Scholar] [CrossRef]
- Iacono, G.; Cavataio, F.; Montalto, G.; Florena, A.; Tumminello, M.; Soresi, M.; Notarbartolo, A.; Carroccio, A. Intolerance of Cow’s Milk and Chronic Constipation in Children. N. Engl. J. Med. 1998, 339, 1100–1104. [Google Scholar] [CrossRef]
- Carroccio, A.; Scalici, C.; Maresi, E.; Di Prima, L.; Cavataio, F.; Noto, D.; Porcasi, R.; Averna, M.R.; Iacono, G. Chronic constipation and food intolerance: A model of proctitis causing constipation. Scand. J. Gastroenterol. 2005, 40, 33–42. [Google Scholar] [CrossRef]
- Bourkheili, A.M.; Mehrabani, S.; Esmaelidoohi, M.; Ahmadi, M.H.; Moslemi, L. Effect of Cow’s-milk–free diet on chronic constipation in children; A randomized clinical trial. Casp. J. Intern. Med. 2021, 12, 91–96. [Google Scholar] [CrossRef]
- Benninga, M.A.; Nurko, S.; Faure, C.; Hyman, P.E.; Roberts, I.S.J.; Schechter, N.L. Childhood Functional Gastrointestinal Disorders: Neonate/Toddler. Gastroenterology 2016, 150, 1443–1455. [Google Scholar] [CrossRef] [PubMed]
- Laffolie, J.; Ibrahimi, G.; Zimmer, K.-P. Poor Perception of School Toilets and Increase of Functional Constipation. Klin. Padiatr. 2020, 233, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.-C.; Lee, C.-H.; Wang, H. Exploring the Association of Autism Spectrum Disorders and Constipation through Analysis of the Gut Microbiome. Int. J. Environ. Res. Public Health 2021, 18, 667. [Google Scholar] [CrossRef] [PubMed]
- Iacono, G.; Carroccio, A.; Cavataio, F.; Montalto, G.; Cantarero, M.; Notarbartolo, A. Chronic constipation as a symptom of cow milk allergy. J. Pediatr. 1995, 126, 34–39. [Google Scholar] [CrossRef]
- Shah, N.; Lindley, K.; Milla, P. Cow’s milk and chronic constipation in children. N. Engl. J. Med. 1999, 340, 891–892. [Google Scholar]
- Daher, S.; Tahan, S.; Sole, D.; Naspitz, C.K.; Da Silva Patricio, F.R.; Neto, U.F.; De Morais, M.B. Cow’s milk protein intolerance and chronic constipation in children. Pediatr. Allergy Immunol. 2001, 12, 339–342. [Google Scholar] [CrossRef]
- Turunen, S.; Karttunen, T.J.; Kokkonen, J. Lymphoid nodular hyperplasia and cow’s milk hypersensitivity in children with chronic constipation. J. Pediatr. 2004, 145, 606–611. [Google Scholar] [CrossRef]
- Iacono, G.; Bonventre, S.; Scalici, C.; Maresi, E.; Di Prima, L.; Soresi, M.; Di Gesù, G.; Noto, D.; Carroccio, A. Food intolerance and chronic constipation: Manometry and histology study. Eur. J. Gastroenterol. Hepatol. 2006, 18, 143–150. [Google Scholar] [CrossRef]
- Borrelli, O.; Barbara, G.; Di Nardo, G.; Cremon, C.; Lucarelli, S.; Frediani, T.; Paganelli, M.; De Giorgio, R.; Stanghellini, V.; Cucchiara, S. Neuroimmune Interaction and Anorectal Motility in Children With Food Allergy-Related Chronic Constipation. Am. J. Gastroenterol. 2009, 104, 454–463. [Google Scholar] [CrossRef]
- El-Hodhod, M.A.; Younis, N.T.; Zaitoun, Y.A.; Daoud, S.D. Cow’s milk allergy related pediatric constipation: Appropriate time of milk tolerance. Pediatr. Allergy Immunol. 2010, 21, e407–e412. [Google Scholar] [CrossRef]
- Irastorza, I.; Ibáñez, B.; Delgado-Sanzonetti, L.; Maruri, N.; Vitoria, J. Cow’s-Milk–free Diet as a Therapeutic Option in Childhood Chronic Constipation. J. Pediatr. Gastroenterol. Nutr. 2010, 51, 171–176. [Google Scholar] [CrossRef]
- Dehghani, S.M.; Ahmadpour, B.; Haghighat, M.; Kashef, S.; Imanieh, M.H.; Soleimani, M. The Role of Cow’s Milk Allergy in Pediatric Chronic Constipation: A Randomized Clinical Trial. Iran. J. Pediatr. 2012, 22, 468–474. [Google Scholar] [PubMed]
- Walter, A.W.; Hovenkamp, A.; Devanarayana, N.M.; Solanga, R.; Rajindrajith, S.; Benninga, M.A. Functional constipation in infancy and early childhood: Epidemiology, risk factors, and healthcare consultation. BMC Pediatr. 2019, 19, 285. [Google Scholar] [CrossRef] [PubMed]
- Vriesman, M.H.; Koppen, I.J.N.; Camilleri, M.; Di Lorenzo, C.; Benninga, M.A. Management of functional constipation in children and adults. Nat. Rev. Gastroenterol. Hepatol. 2019, 17, 21–39. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, I.R.; E de Abreu, G.; Dourado, E.R.; Braga, A.A.N.M.; Lobo, V.A.; de Carvalho, I.W.B.; Netto, J.M.B.; Barroso, U., Jr. Emotional and behavioural problems in children and adolescents: The role of constipation. J. Paediatr. Child Health 2021, 57, 1003–1008. [Google Scholar] [CrossRef]
- Tappin, D.; Grzeda, M.; Joinson, C.; Heron, J. Challenging the view that lack of fibre causes childhood constipation. Arch. Dis. Child. 2020, 105, 864–868. [Google Scholar] [CrossRef]
- Carter, C.A.; Frischmeyer-Guerrerio, P.A. The Genetics of Food Allergy. Curr. Allergy Asthma Rep. 2018, 18, 2. [Google Scholar] [CrossRef]
- Park, J.; Jang, H.; Kim, M.; Hong, J.Y.; Kim, Y.H.; Sohn, M.H.; Park, S.-C.; Won, S.; Kim, K.W. Predicting allergic diseases in children using genome-wide association study (GWAS) data and family history. World Allergy Organ. J. 2021, 14, 100539. [Google Scholar] [CrossRef]
- Thorsteinsdottir, S.; Stokholm, J.; Thyssen, J.P.; Nørgaard, S.; Thorsen, J.; Chawes, B.; Bønnelykke, K.; Waage, J.; Bisgaard, H. Genetic, Clinical, and Environmental Factors Associated With Persistent Atopic Dermatitis in Childhood. JAMA Dermatol. 2019, 155, 50–57. [Google Scholar] [CrossRef]
- Wei, H.; Zhu, Y.; Wang, T.; Zhang, X.; Zhang, K.; Zhang, Z. Genetic risk factors for autism-spectrum disorders: A systematic review based on systematic reviews and meta-analysis. J. Neural Transm. 2021, 128, 717–734. [Google Scholar] [CrossRef]
- Xu, G.; Snetselaar, L.G.; Jing, J.; Liu, B.; Strathearn, L.; Bao, W. Association of Food Allergy and Other Allergic Conditions With Autism Spectrum Disorder in Children. JAMA Netw. Open 2018, 1, e180279. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, R.; Li, D.; Zhao, L.; Zhu, L. Role of gut microbiota in functional constipation. Gastroenterol. Rep. 2021, 9, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Liu, X.; An, Y.; Zhou, G.; Liu, Y.; Xu, M.; Dong, W.; Wang, S.; Yan, F.; Jiang, K.; et al. Dysbiosis contributes to chronic constipation development via regulation of serotonin transporter in the intestine. Sci. Rep. 2017, 7, 10322. [Google Scholar] [CrossRef] [PubMed]
- Fiocchi, A.; Brozek, J.; Schuenemann, H.; Bahna, S.L.; Von Berg, A.; Beyer, K.; Bozzola, M.; Bradsher, J.; Compalati, E.; Ebisawa, M.; et al. World Allergy Organization (WAO) Diagnosis and Rationale for Action against Cow’s Milk Allergy (DRACMA) Guidelines. Pediatr. Allergy Immunol. 2010, 21 (Suppl. 21), 1–125. [Google Scholar] [CrossRef]
- Sicherer, S.H.; Sampson, H.A. Food allergy: A review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. J. Allergy Clin. Immunol. 2018, 141, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Iacono, G.; Cavataio, F.; Montalto, G.; Soresi, M.; Notarbartolo, A.; Carroccio, A. Persistent cow’s milk protein intolerance in infants: The changing faces of the same disease. Clin. Exp. Allergy 1998, 28, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Carroccio, A.; Montalto, G.; Custro, N.; Notarbartolo, A.; Cavataio, F.; D’Amico, D.; Alabrese, D.; Iacono, G. Evidence of very delayed clinical reactions to cow’s milk in cow’s milk-intolerant patients. Allergy 2000, 55, 574–579. [Google Scholar]
- Caminero, A.; Meisel, M.; Jabri, B.; Verdu, E.F. Mechanisms by which gut microorganisms influence food sensitivities. Nat. Rev. Gastroenterol. Hepatol. 2018, 16, 7–18. [Google Scholar] [CrossRef]
- Sweeney, A.; Sampath, V.; Nadeau, K.C. Early intervention of atopic dermatitis as a preventive strategy for progression of food allergy. Allergy Asthma Clin. Immunol. 2021, 17, 30. [Google Scholar] [CrossRef]
- Gryboski, J.D.; Kocoshis, S. Immunoglobulin Deficiency in Gastrointestinal Allergies. J. Clin. Gastroenterol. 1980, 2, 71–76. [Google Scholar] [CrossRef]
- Abdel-Gadir, A.; Stephen-Victor, E.; Gerber, G.K.; Rivas, M.N.; Wang, S.; Harb, H.; Wang, L.; Li, N.; Crestani, E.; Spielman, S.; et al. Microbiota therapy acts via a regulatory T cell MyD88/RORgammat pathway to suppress food allergy. Nat. Med. 2019, 25, 1164–1174. [Google Scholar] [CrossRef] [PubMed]
- Stephen-Victor, E.; Crestani, E.; Chatila, T.A. Dietary and Microbial Determinants in Food Allergy. Immunity 2020, 53, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Venter, C.; Brown, T.; Shah, N.; Walsh, J.; Fox, A.T. Diagnosis and management of non-IgE-mediated cow’s milk allergy in infancy—A UK primary care practical guide. Clin. Transl. Allergy 2013, 3, 23. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Belohlavkova, S.; Enninger, A.; Frühauf, P.; Makwana, N.; Järvi, A. How Are Infants Suspected to Have Cow’s Milk Allergy Managed? A Real World Study Report. Nutrients 2021, 13, 3027. [Google Scholar] [CrossRef] [PubMed]
- Meyer, R.; Fleming, C.; Dominguez-Ortega, G.; Lindley, K.; Michaelis, L.; Thapar, N.; Elawad, M.; Chakravarti, V.; Fox, A.T.; Shah, N. Manifestations of food protein induced gastrointestinal allergies presenting to a single tertiary paediatric gastroenterology unit. World Allergy Organ. J. 2013, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Diaferio, L.; Caimmi, D.; Verga, M.C.; Palladino, V.; Trovè, L.; Giordano, P.; Verduci, E.; Miniello, V.L. May Failure to Thrive in Infants Be a Clinical Marker for the Early Diagnosis of Cow’s Milk Allergy? Nutrients 2020, 12, 466. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. NICE Guideline: Food Allergy in under 19s: Assessment and Diagnosis (CG116); NICE: London, UK, 2011. [Google Scholar]
- Cianferoni, A. Non-IgE Mediated Food Allergy. Curr. Pediatr. Rev. 2020, 16, 95–105. [Google Scholar] [CrossRef]
- Salvatore, S.; Vandenplas, Y. Gastroesophageal Reflux and Cow Milk Allergy: Is There a Link? Pediatrics 2002, 110, 972–984. [Google Scholar] [CrossRef]
- Nomura, I.; Morita, H.; Ohya, Y.; Saito, H.; Matsumoto, K. Non–IgE-Mediated Gastrointestinal Food Allergies: Distinct Differences in Clinical Phenotype Between Western Countries and Japan. Curr. Allergy Asthma Rep. 2012, 12, 297–303. [Google Scholar] [CrossRef]
- Luyt, D.; Ball, H.; Makwana, N.; Green, M.R.; Bravin, K.; Nasser, S.M.; Clark, A.T. BSACI guideline for the diagnosis and management of cow’s milk allergy. Clin. Exp. Allergy 2014, 44, 642–672. [Google Scholar] [CrossRef]
- Lucarelli, S.; Di Nardo, G.; Frediani, S.; Frediani, T.; Cozzi, D.A.; Cucchiara, S. Rectal prolapse in a child with cow’s milk allergy. Int. J. Color. Dis. 2009, 24, 1239. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Koletzko, S.; Niggemann, B.; Arato, A.; Dias, J.A.; Heuschkel, R.; Husby, S.; Mearin, M.L.; Papadopoulou, A.; Ruemmele, F.M.; Staiano, A.; et al. Diagnostic Approach and Management of Cow’s-Milk Protein Allergy in Infants and Children: Espghan gi committee practical guidelines. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, S.; Agosti, M.; Baldassarre, M.; D’Auria, E.; Pensabene, L.; Nosetti, L.; Vandenplas, Y. Cow’s Milk allergy or Gastroesophageal Reflux Disease—Can We Solve the Dilemma in Infants? Nutrients 2021, 13, 297. [Google Scholar] [CrossRef] [PubMed]
- Fox, A.; Brown, T.; Walsh, J.; Venter, C.; Meyer, R.; Nowak-Wegrzyn, A.; Levin, M.; Spawls, H.; Beatson, J.; Lovis, M.-T.; et al. An update to the Milk Allergy in Primary Care guideline. Clin. Transl. Allergy 2019, 9, 40. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Salvatore, S.; Hauser, B. The diagnosis and management of gastro-oesophageal reflux in infants. Early Hum. Dev. 2005, 81, 1011–1024. [Google Scholar] [CrossRef]
- Heine, R.G. Allergic gastrointestinal motility disorders in infancy and early childhood. Pediatr. Allergy Immunol. 2008, 19, 383–391. [Google Scholar] [CrossRef]
- Singh, H.; Connor, F. Paediatric constipation: An approach and evidence-based treatment regimen. Aust. J. Gen. Pract. 2018, 47, 273–277. [Google Scholar] [CrossRef]
- Fritscher-Ravens, A.; Pflaum, T.; Mösinger, M.; Ruchay, Z.; Röcken, C.; Milla, P.J.; Das, M.; Böttner, M.; Wedel, T.; Schuppan, D. Many Patients With Irritable Bowel Syndrome Have Atypical Food Allergies Not Associated with Immunoglobulin E. Gastroenterology 2019, 157, 109–118.e105. [Google Scholar] [CrossRef]
- Aguilera-Lizarraga, J.; Florens, M.V.; Viola, M.F.; Jain, P.; Decraecker, L.; Appeltans, I.; Cuende-Estevez, M.; Fabre, N.; Van Beek, K.; Perna, E.; et al. Local immune response to food antigens drives meal-induced abdominal pain. Nature 2021, 590, 151–156. [Google Scholar] [CrossRef]
- Kamer, B.; Dółka, E.; Pyziak, K.; Blomberg, A. Food allergy as a cause of constipation in children in the first three years of life—Own observations. Med. Wieku Rozw. 2011, 15, 157–161. [Google Scholar]
- Yang, Q.H.; Zheng, B.S.; Zhou, S.M. Clinical features of cow’s milk protein allergy in infants presenting mainly with gastrointestinal symptoms: An analysis of 280 cases. Zhongguo Dang Dai Er Ke Za Zhi 2019, 21, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Vanderhoof, J.A.; Perry, D.; Hanner, T.L.; Young, R.J. Allergic Constipation: Association with Infantile Milk Allergy. Clin. Pediatr. 2001, 40, 399–402. [Google Scholar] [CrossRef] [PubMed]
- Andiran, F.; Dayi, S.; Mete, E. Cows milk consumption in constipation and anal fissure in infants and young children. J. Paediatr. Child Health 2003, 39, 329–331. [Google Scholar] [CrossRef]
- De Oliveira, M.B.B.; Jardim-Botelho, A.; de Morais, M.B.; Melo, I.R.d.C.; Maciel, J.F.; Gurgel, R.Q. Impact of Infant Milk-Type and Childhood Eating Behaviors on Functional Constipation in Preschool Children. J. Pediatr. Gastroenterol. Nutr. 2021, 73, e50–e56. [Google Scholar] [CrossRef] [PubMed]
- Andreoli, C.S.; Vieira-Ribeiro, S.A.; Fonseca, P.C.A.; Moreira, A.V.B.; Ribeiro, S.M.R.; De Morais, M.B.; Franceschini, S.C.C. Eating habits, lifestyle and intestinal constipation in children aged four to seven years. Nutr. Hosp. 2019, 36, 25–31. [Google Scholar] [PubMed]
- Appak, Y.; Karakoyun, M.; Koru, T.; Baran, M. Dietary properties and anthropometric findings of children with functional constipation: A cross-sectional study. Arch. Argent. Pediatr. 2019, 117, e224–e231. [Google Scholar] [CrossRef]
- Chin, K.C.; Tarlow, M.J.; Allfree, A.J. Allergy to cows’ milk presenting as chronic constipation. Br. Med. J. 1983, 287, 1593. [Google Scholar] [CrossRef][Green Version]
- Carroccio, A.; Iacono, G. Review article: Chronic constipation and food hypersensitivity? An intriguing relationship. Aliment. Pharmacol. Ther. 2006, 24, 1295–1304. [Google Scholar] [CrossRef]
- Syrigou, E.I.; Pitsios, C.; Panagiotou, I.; Chouliaras, G.; Kitsiou, S.; Kanariou, M.; Roma-Giannikou, E. Food allergy-related paediatric constipation: The usefulness of atopy patch test. Eur. J. Pediatr. 2011, 170, 1173–1178. [Google Scholar] [CrossRef]
- Southwell, B.R.; King, S.; Hutson, J. Chronic constipation in children: Organic disorders are a major cause. J. Paediatr. Child Health 2005, 41, 1–15. [Google Scholar] [CrossRef]
- Hynes, M.C.; Yik, Y.I.; Veysey, D.; Cook, D.; Tudball, C.; Hutson, J.M.; Southwell, B.R. Gastrointestinal Transit Patterns Identified in Children with Intractable Chronic Constipation using Scintigraphy: Experience of Over 1000 Cases. Gastroenterology 2017, 152, S515. [Google Scholar] [CrossRef]
- Yik, Y.I.; Cain, T.M.; Tudball, C.F.; Cook, D.J.; Southwell, B.R.; Hutson, J.M. Nuclear transit studies of patients with intractable chronic constipation reveal a subgroup with rapid proximal colonic transit. J. Pediatr. Surg. 2011, 46, 1406–1411. [Google Scholar] [CrossRef] [PubMed]
- Friess, M.; Zizmor, J. Roentgen studies of children with alimentary disturbances due to food allergy. Am. J. Dis. Child. 1937, 54, 1239–1251. [Google Scholar] [CrossRef]
- Liu, H.-Y.; Whitehouse, W.M.; Giday, Z. Proximal Small Bowel Transit Pattern in Patients with Malabsorption Induced by Bovine Milk Protein Ingestion. Radiology 1975, 115, 415–420. [Google Scholar] [CrossRef]
- El-Hodhod, M.A.-A.; Hamdy, A.M.; El-Deeb, M.T.; Elmaraghy, M.O. Cow’s Milk Allergy Is a Major Contributor in Recurrent Perianal Dermatitis of Infants. ISRN Pediatr. 2012, 2012, 108769. [Google Scholar] [CrossRef]
- Bloom, D.A.; Buonomo, C.; Fishman, S.J.; Furuta, G.; Nurko, S. Allergic colitis: A mimic of Hirschsprung disease. Pediatr. Radiol. 1999, 29, 37–41. [Google Scholar] [CrossRef]
- Kawai, M.; Kubota, A.; Ida, S.; Yamamura, Y.; Yoshimura, N.; Takeuchi, M.; Nakayama, M.; Okuyama, H.; Oue, T.; Kawahara, H.; et al. Cow’s milk allergy presenting Hirschsprung’s disease-mimicking symptoms. Pediatr. Surg. Int. 2005, 21, 850–852. [Google Scholar] [CrossRef]
- Richards, D.G.; Somers, S.; Issenman, R.M.; Stevenson, G.W. Cow’s milk protein/soy protein allergy: Gastrointestinal imaging. Radiology 1988, 167, 721–723. [Google Scholar] [CrossRef]
- Swischuk, L.E.; Hayden, C.K., Jr. Barium enema findings (? segmental colitis) in four neonates with bloody diarrhea--possible cow’s milk allergy. Pediatr. Radiol. 1985, 15, 34–37. [Google Scholar] [CrossRef]
- Lee, J.H.; Choe, Y.H.; Lee, S.-K.; Seo, J.M.; Kim, J.H.; Suh, Y.-L. Allergic proctitis and abdominal distention mimicking Hirschsprung’s disease in infants. Acta Paediatr. 2007, 96, 1784–1789. [Google Scholar] [CrossRef]
- Burgers, R.; Bonanno, E.; Madarena, E.; Graziano, F.; Pensabene, L.; Gardner, W.; Mousa, H.; Benninga, M.A.; Di Lorenzo, C. The care of constipated children in primary care in different countries. Acta Paediatr. 2012, 101, 677–680. [Google Scholar] [CrossRef] [PubMed]
- Clein, N.W. Cow’s milk allergy in infants. Pediatr. Clin. N. Am. 1954, 4, 949–962. [Google Scholar] [CrossRef]
- McGrath, J. Allergy to cow’s milk presenting as chronic constipation. Br. Med. J. 1984, 288, 236. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Crowley, E.T.; Williams, L.T.; Roberts, T.K.; Dunstan, R.H.; Jones, P.D. Does Milk Cause Constipation? A Crossover Dietary Trial. Nutrients 2013, 5, 253–266. [Google Scholar] [CrossRef]
- Gelsomino, M.; Del Vescovo, E.; Bersani, G.; Sopo, S.M. Functional constipation related to cow’s milk allergy in children. A management proposal. Allergol. Immunopathol. 2021, 49, 17–20. [Google Scholar] [CrossRef]
- Connor, F.L. Allergy and neurogastroenterology. In Pediatric Neurogastroenterology; Faure, C., di Lorenzo, C., Thapar, N., Eds.; Springer Nature: New York, NY, USA, in press.
- Simeone, D.; Miele, E.; Boccia, G.; Marino, A.; Troncone, R.; Staiano, A. Prevalence of atopy in children with chronic constipation. Arch. Dis. Child. 2008, 93, 1044–1047. [Google Scholar] [CrossRef]
- Iacono, G.; Ravelli, A.; Di Prima, L.; Scalici, C.; Bolognini, S.; Chiappa, S.; Pirrone, G.; Licastri, G.; Carroccio, A. Colonic Lymphoid Nodular Hyperplasia in Children: Relationship to Food Hypersensitivity. Clin. Gastroenterol. Hepatol. 2007, 5, 361–366. [Google Scholar] [CrossRef]
- Schäppi, M.G.; Borrelli, O.; Knafelz, D.; Williams, S.; Smith, V.V.; Milla, P.J.; Lindley, K.J. Mast Cell–Nerve Interactions in Children With Functional Dyspepsia. J. Pediatr. Gastroenterol. Nutr. 2008, 47, 472–480. [Google Scholar] [CrossRef]
- Andrews, C.N.; Storr, M. The pathophysiology of chronic constipation. Can. J. Gastroenterol. 2011, 25 (Suppl. B), 16B–21B. [Google Scholar] [CrossRef]
- Walker, W. Cow’s milk protein-sensitive enteropathy at school age: A new entity or a spectrum of mucosal immune responses with age. J. Pediatr. 2001, 139, 765–766. [Google Scholar] [CrossRef]
- Sekkidou, M.; Muhardi, L.; Constantinou, C.; Kudla, U.; Vandenplas, Y.; Nicolaou, N. Nutritional Management With a Casein-Based Extensively Hydrolysed Formula in Infants With Clinical Manifestations of Non-IgE-Mediated CMPA Enteropathies and Constipation. Front. Allergy 2021, 2, 17. [Google Scholar] [CrossRef]
- De Boissieu, D.; Waguet, J.; Dupont, C. The atopy patch tests for detection of cow’s milk allergy with digestive symptoms. J. Pediatr. 2003, 142, 203–205. [Google Scholar] [CrossRef] [PubMed]
- Cudowska, B.; Kaczmarski, M. Atopy patch test in the diagnosis of food allergy in children with gastrointestinal symptoms. Adv. Med. Sci. 2010, 55, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Kalach, N.; Kapel, N.; Waligora-Dupriet, A.-J.; Castelain, M.-C.; Cousin, M.O.; Sauvage, C.; Ba, F.; Nicolis, I.; Campeotto, F.; Butel, M.J.; et al. Intestinal permeability and fecal eosinophil-derived neurotoxin are the best diagnosis tools for digestive non-IgE-mediated cow’s milk allergy in toddlers. Clin. Chem. Lab. Med. 2012, 51, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Mowszet, K.; Matusiewicz, K.; Iwańczak, B. Value of the Atopy Patch Test in the Diagnosis of Food Allergy in Children with Gastrointestinal Symptoms. Adv. Clin. Exp. Med. 2014, 23, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Majamaa, H.; Moisio, P.; Holm, K.; Kautiainen, H.; Turjanmaa, K. Cow’s milk allergy: Diagnostic accuracy of skin prick and patch tests and specific IgE. Allergy 1999, 54, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Vanto, T.; Juntunen-Backman, K.; Kalimo, K.; Klemola, T.; Koivikko, A.; Koskinen, P.; Syvanen, P.; Valovirta, E.; Varjonen, E. The patch test, skin prick test, and serum milk-specific IgE as diagnostic tools in cow’s milk allergy in infants. Allergy 1999, 54, 837–842. [Google Scholar] [CrossRef]
- Saarinen, K.M.; Suomalainen, H.; Savilahti, E. Diagnostic value of skin-prick and patch tests and serum eosinophil cationic protein and cow’s milk-specific IgE in infants with cow’s milk allergy. Clin. Exp. Allergy 2001, 31, 423–429. [Google Scholar] [CrossRef]
- Kalach, N.; Soulaines, P.; Deboissieu, D.; Dupont, C. A pilot study of the usefulness and safety of a ready-to-use atopy patch test (Diallertest) versus a comparator (Finn Chamber) during cow’s milk allergy in children. J. Allergy Clin. Immunol. 2005, 116, 1321–1326. [Google Scholar] [CrossRef]
- Keskin, O.; Tuncer, A.; Adalioglu, G.; Sekerel, B.E.; Sackesen, C.; Kalayci, O. Evaluation of the utility of atopy patch testing, skin prick testing, and total and specific IgE assays in the diagnosis of cow’s milk allergy. Ann. Allergy Asthma Immunol. 2005, 94, 553–560. [Google Scholar] [CrossRef]
- Mehl, A.; Rolinck-Werninghaus, C.; Staden, U.; Verstege, A.; Wahn, U.; Beyer, K.; Niggemann, B. The atopy patch test in the diagnostic workup of suspected food-related symptoms in children. J. Allergy Clin. Immunol. 2006, 118, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Canani, R.B.; Ruotolo, S.; Auricchio, L.; Caldore, M.; Porcaro, F.; Manguso, F.; Terrin, G.; Troncone, R. Diagnostic accuracy of the atopy patch test in children with food allergy-related gastrointestinal symptoms. Allergy 2007, 62, 738–743. [Google Scholar] [CrossRef] [PubMed]
- Dupont, C.; Soulaines, P.; Lapillonne, A.; Donne, N.; Kalach, N.; Benhamou, P. Atopy Patch Test for Early Diagnosis of Cow’s Milk Allergy in Preterm Infants. J. Pediatr. Gastroenterol. Nutr. 2010, 50, 463–464. [Google Scholar] [CrossRef] [PubMed]
- Canani, R.B.; Buongiovanni, A.; Nocerino, R.; Cosenza, L.; Troncone, R. Toward a standardized reading of the atopy patch test in children with suspected cow’s milk allergy-related gastrointestinal symptoms. Allergy 2011, 66, 1499–1500. [Google Scholar] [CrossRef]
- Costa, A.J.F.; Sarinho, E.S.C.; Motta, M.E.F.A.; Gomes, P.N.; Melo, S.M.d.O.d.; Da Silva, G.A.P. Allergy to cow’s milk proteins: What contribution does hypersensitivity in skin tests have to this diagnosis? Pediatr. Allergy Immunol. 2011, 22, e133–e138. [Google Scholar] [CrossRef]
- Boonyaviwat, O.; Pacharn, P.; Jirapongsananuruk, O.; Vichyanond, P.; Visitsunthorn, N. Role of atopy patch test for diagnosis of food allergy-related gastrointestinal symptoms in children. Pediatr. Allergy Immunol. 2015, 26, 737–741. [Google Scholar] [CrossRef]
- Kose, S.S.; Asilsoy, S.; Tezcan, D.; Atakul, G.; Al, S.; Atay, O.; Boyacioglu, O.K.; Uzuner, N.; Anal, O.; Karaman, O. Atopy patch test in children with cow’s milk and hen’s egg allergy: Do clinical symptoms matter? Allergol. Immunopathol. 2020, 48, 323–331. [Google Scholar] [CrossRef]
- Sopo, S.M.; Arena, R.; Scala, G. Functional Constipation and Cow’s-Milk Allergy. J. Pediatr. Gastroenterol. Nutr. 2014, 59, e34. [Google Scholar] [CrossRef]
- El-Hodhod, M.A.; El-Shabrawi, M.H.F.; AlBadi, A.; Hussein, A.; Almehaidib, A.; Nasrallah, B.; AlBassam, E.M.; El Feghali, H.; Isa, H.M.; Al Saraf, K.; et al. Consensus statement on the epidemiology, diagnosis, prevention, and management of cow’s milk protein allergy in the Middle East: A modified Delphi-based study. World J. Pediatr. 2021, 17, 576–589. [Google Scholar] [CrossRef]
- Meyer, R.; Lozinsky, A.C.; Fleischer, D.M.; Vieira, M.C.; Du Toit, G.; Vandenplas, Y.; Dupont, C.; Knibb, R.; Uysal, P.; Cavkaytar, O.; et al. Diagnosis and management of Non-IgE gastrointestinal allergies in breastfed infants—An EAACI Position Paper. Allergy 2019, 75, 14–32. [Google Scholar] [CrossRef]
- Allen, K.J.; Davidson, G.P.; Day, A.S.; Hill, D.J.; Kemp, A.S.; E Peake, J.; Prescott, S.L.; Shugg, A.; Sinn, J.K.; Heine, R.G. Management of cow’s milk protein allergy in infants and young children: An expert panel perspective. J. Paediatr. Child Health 2009, 45, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Matthai, J.; Sathiasekharan, M.; Poddar, U.; Sibal, A.; Srivastava, A.; Waikar, Y.; Malik, R.; Ray, G.; Geetha, S.; Yachha, S.K.; et al. Guidelines on Diagnosis and Management of Cow’s Milk Protein Allergy. Indian Pediatr. 2020, 57, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Guler, N.; Cokugras, F.; Sapan, N.; Selimoglu, A.; Turktas, I.; Cokugras, H.C.; Aydogan, M.; Beser, O. Diagnosis and management of cow’s milk protein allergy in Turkey: Region-specific recommendations by an expert-panel. Allergol. Immunopathol. 2020, 48, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Putnam, P.E. The milk of human constipation. J. Pediatr. 2004, 145, 578–580. [Google Scholar] [CrossRef] [PubMed]
- Canani, R.B.; Rapacciuolo, L.; Romano, M.; de Horatio, L.T.; Terrin, G.; Manguso, F.; Cirillo, P.; Paparo, F.; Troncone, R. Diagnostic value of faecal calprotectin in paediatric gastroenterology clinical practice. Dig. Liver Dis. 2004, 36, 467–470. [Google Scholar] [CrossRef]
- Chang, M.-H.; Chou, J.-W.; Chen, S.-M.; Tsai, M.-C.; Sun, Y.-S.; Lin, C.-C.; Lin, C.-P. Faecal calprotectin as a novel biomarker for differentiating between inflammatory bowel disease and irritable bowel syndrome. Mol. Med. Rep. 2014, 10, 522–526. [Google Scholar] [CrossRef]
- Degraeuwe, P.L.; Beld, M.P.; Ashorn, M.; Canani, R.B.; Day, A.S.; Diamanti, A.; Fagerberg, U.L.; Henderson, P.; Kolho, K.-L.; Van de Vijver, E.; et al. Faecal Calprotectin in Suspected Paediatric Inflammatory Bowel Disease. J. Pediatr. Gastroenterol. Nutr. 2015, 60, 339–346. [Google Scholar] [CrossRef]
- Beşer, Ö.F.; Sancak, S.; Erkan, T.; Kutlu, T.; Cokugras, H.C.; Çokuğraş, F.Ç. Can Fecal Calprotectin Level Be Used as a Markers of Inflammation in the Diagnosis and Follow-Up of Cow’s Milk Protein Allergy? Allergy Asthma Immunol. Res. 2014, 6, 33–38. [Google Scholar] [CrossRef]
- Belizón, C.T.; Páez, E.O.; Claros, A.F.M.; Sánchez, I.R.; González, A.R.; Medialdea, R.V.; Salguero, J.M.R. Calprotectina fecal como apoyo al diagnóstico en la alergia a las proteínas de leche de vaca no IgE mediada. An. Pediatría 2016, 84, 318–323. [Google Scholar] [CrossRef]
- Lendvai-Emmert, D.; Emmert, V.; Fusz, K.; Prémusz, V.; Boncz, I.; Tóth, G. Quantitative Assay of Fecal Calprotectin in Children with Cow’s Milk Protein Allergy. Value Health 2018, 21, S128. [Google Scholar] [CrossRef]
- Xiong, L.-J.; Xie, X.-L.; Li, Y.; Deng, X.-Z. Current status of fecal calprotectin as a diagnostic or monitoring biomarker for cow’s milk protein allergy in children: A scoping review. World J. Pediatr. 2020, 17, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Calvani, M.; Anania, C.; Cuomo, B.; D’Auria, E.; Decimo, F.; Indirli, G.; Marseglia, G.; Mastrorilli, V.; Sartorio, M.; Santoro, A.; et al. Non–IgE- or Mixed IgE/Non–IgE-Mediated Gastrointestinal Food Allergies in the First Years of Life: Old and New Tools for Diagnosis. Nutrients 2021, 13, 226. [Google Scholar] [CrossRef] [PubMed]
- Winberg, A.; Nagaeva, O.; Nagaev, I.; Lundell, C.; Arencibia, I.; Mincheva-Nilsson, L.; Rönmark, E.; West, C.E. Dynamics of cytokine mRNA expression and fecal biomarkers in school-children undergoing a double-blind placebo-controlled food challenge series. Cytokine 2016, 88, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Zhang, G.-Q.; Li, Z.-Y. The diagnostic value of APT for food allergy in children: A systematic review and meta-analysis. Pediatr. Allergy Immunol. 2019, 30, 451–461. [Google Scholar] [CrossRef]
- Kokkonen, T.R.J.; Ruuska, T.; Karttunen, T.; Niinimäki, A. Mucosal pathology of the foregut associated with food allergy and recurrent abdominal pains in children. Acta Paediatr. 2001, 90, 16–21. [Google Scholar] [CrossRef]
- Turjanmaa, K.; Darsow, U.; Niggemann, B.; Rancé, F.; Vanto, T.; Werfel, T. EAACI/GA2LEN Position paper: Present status of the atopy patch test. Allergy 2006, 61, 1377–1384. [Google Scholar] [CrossRef]
- Fiocchi, A.; Schünemann, H.J.; Brozek, J.; Restani, P.; Beyer, K.; Troncone, R.; Martelli, A.; Terracciano, L.; Bahna, S.L.; Rancé, F.; et al. Diagnosis and Rationale for Action against Cow’s Milk Allergy (DRACMA): A summary report. J. Allergy Clin. Immunol. 2010, 126, 1119–1128.e1112. [Google Scholar] [CrossRef]
- Ikeda, K.; Ida, S.; Kawahara, H.; Kawamoto, K.; Etani, Y.; Kubota, A. Importance of evaluating for cow’s milk allergy in pediatric surgical patients with functional bowel symptoms. J. Pediatr. Surg. 2011, 46, 2332–2335. [Google Scholar] [CrossRef]
- Matsumoto, K. Non-IgE-Related Diagnostic Methods (LST, Patch Test). Chem. Immunol. Allergy 2015, 101, 79–86. [Google Scholar] [CrossRef]
- Yagi, H.; Sato, K.; Arakawa, N.; Inoue, T.; Nishida, Y.; Yamada, S.; Ishige, T.; Yamada, Y.; Arakawa, H.; Takizawa, T. Expression of Leucine-rich Repeat-containing Protein 32 Following Lymphocyte Stimulation in Patients with Non-IgE-mediated. Gastrointest. Food Allerg. 2020, 93, 645–655. [Google Scholar]
- Vandenplas, Y.; A Brough, H.; Fiocchi, A.; Miqdady, M.; Munasir, Z.; Salvatore, S.; Thapar, N.; Venter, C.; Vieira, M.C.; Meyer, R. Current Guidelines and Future Strategies for the Management of Cow’s Milk Allergy. J. Asthma Allergy 2021, 14, 1243–1256. [Google Scholar] [CrossRef] [PubMed]
- NICE Quality Standard [QS118]; Quality Statement 1: Allergy-Focused Clinical History; National Institute for Health and Care Excellence: London, UK, 2016.
- Vandenplas, Y.; Mukherjee, R.; Dupont, C.; Eigenmann, P.; Høst, A.; Kuitunen, M.; Ribes-Koninkx, C.; Shah, N.; Szajewska, H.; Von Berg, A.; et al. Protocol for the validation of sensitivity and specificity of the Cow’s Milk-related Symptom Score (CoMiSS) against open food challenge in a single-blinded, prospective, multicentre trial in infants. BMJ Open 2018, 8, e019968. [Google Scholar] [CrossRef] [PubMed]
- Vandenplas, Y.; Dupont, C.; Eigenmann, P.; Host, A.; Kuitunen, M.; Ribes-Koninckx, C.; Shah, N.; Shamir, R.; Staiano, A.; Szajewska, H.; et al. A workshop report on the development of the Cow’s Milk-related Symptom Score awareness tool for young children. Acta Paediatr. 2014, 104, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, S.; Bertoni, E.; Bogni, F.; Bonaita, V.; Armano, C.; Moretti, A.; Baù, M.; Luini, C.; D’Auria, E.; Marinoni, M.; et al. Testing the Cow’s Milk-Related Symptom Score (CoMiSSTM) for the Response to a Cow’s Milk-Free Diet in Infants: A Prospective Study. Nutrients 2019, 11, 2402. [Google Scholar] [CrossRef] [PubMed]
- Vandenplas, Y.; Salvatore, S.; Ribes-Koninckx, C.; Carvajal, E.; Szajewska, H.; Huysentruyt, K. The Cow Milk Symptom Score (CoMiSSTM) in presumed healthy infants. PLoS ONE 2018, 13, e0200603. [Google Scholar] [CrossRef]
- Kokkonen, J.; Haapalahti, M.; Tikkanen, S.; Karttunen, R.; Savilahti, E. Gastrointestinal complaints and diagnosis in children: A population-based study. Acta Paediatr. 2004, 93, 880–886. [Google Scholar] [CrossRef]
- Royal College of Paediatrics and Child Health. Taking an Allergy Focused Clinical History. Available online: https://www.rcpch.ac.uk/sites/default/files/Taking_an_Allergy_Focused_Clinical_History_-_Allergy_Care_Pathways_Project.pdf (accessed on 15 January 2022).
- Salvatore, S.; Abkari, A.; Cai, W.; Catto-Smith, A.; Cruchet, S.; Gottrand, F.; Hegar, B.; Lifschitz, C.; Ludwig, T.; Shah, N.; et al. Review shows that parental reassurance and nutritional advice help to optimise the management of functional gastrointestinal disorders in infants. Acta Paediatr. 2018, 107, 1512–1520. [Google Scholar] [CrossRef]
- Labrosse, R.; Graham, F.; Caubet, J.-C. Non-IgE-Mediated Gastrointestinal Food Allergies in Children: An Update. Nutrients 2020, 12, 2086. [Google Scholar] [CrossRef]
- Ball, H.B.; Luyt, D. Home-based cow’s milk reintroduction using a milk ladder in children less than 3 years old with IgE-mediated cow’s milk allergy. Clin. Exp. Allergy 2019, 49, 911–920. [Google Scholar] [CrossRef]
- Van Wering, H.M.; Tabbers, M.M.; A Benninga, M. Are constipation drugs effective and safe to be used in children?: A review of the literature. Expert Opin. Drug Saf. 2011, 11, 71–82. [Google Scholar] [CrossRef]
- Gordon, M.; Macdonald, J.K.; E Parker, C.; Akobeng, A.K.; Thomas, A.G. Osmotic and stimulant laxatives for the management of childhood constipation. Cochrane Database Syst. Rev. 2016, 2016, CD009118. [Google Scholar] [CrossRef] [PubMed]
- Pijpers, M.A.M.; Tabbers, M.M.; Benninga, M.A.; Berger, M.Y. Currently recommended treatments of childhood constipation are not evidence based: A systematic literature review on the effect of laxative treatment and dietary measures. Arch. Dis. Child. 2008, 94, 117–131. [Google Scholar] [CrossRef] [PubMed]
- Pijpers, M.; Bongers, M.; Benninga, M.; Berger, M. Functional Constipation in Children: A Systematic Review on Prognosis and Predictive Factors. J. Pediatr. Gastroenterol. Nutr. 2010, 50, 256–268. [Google Scholar] [CrossRef] [PubMed]
- Vandenplas, Y.; Alturaiki, M.A.; Al-Qabandi, W.; Alrefaee, F.; Bassil, Z.; Eid, B.; El Beleidy, A.; AlMehaidib, A.I.; Mouawad, P.; Sokhn, M. Middle East Consensus Statement on the Diagnosis and Management of Functional Gastrointestinal Disorders in <12 Months Old Infants. Pediatr. Gastroenterol. Hepatol. Nutr. 2016, 19, 153–161. [Google Scholar] [CrossRef]
- Nowak-Węgrzyn, A.; Chehade, M.; Groetch, M.E.; Spergel, J.; Wood, R.A.; Allen, K.; Atkins, D.; Bahna, S.; Barad, A.V.; Berin, C.; et al. International consensus guidelines for the diagnosis and management of food protein–induced enterocolitis syndrome: Executive summary—Workgroup Report of the Adverse Reactions to Foods Committee, American Academy of Allergy, Asthma & Immunology. J. Allergy Clin. Immunol. 2017, 139, 1111–1126.e4. [Google Scholar] [CrossRef]
- Dupont, C.; Chouraqui, J.-P.; Linglart, A.; Bocquet, A.; Darmaun, D.; Feillet, F.; Frelut, M.-L.; Girardet, J.-P.; Hankard, R.; Rozé, J.-C.; et al. Nutritional management of cow’s milk allergy in children: An update. Arch. Pédiatrie 2018, 25, 236–243. [Google Scholar] [CrossRef]
- Jaime, B.E.; Martín, J.J.D.; Baviera, L.C.B.; Monzón, A.C.; Hernández, A.H.; Burriel, J.I.G.; Mérida, M.J.G.; Fernández, C.P.; Rodríguez, C.C.; Riechmann, E.R.; et al. Alergia a las proteínas de leche de vaca no mediada por IgE: Documento de consenso de la Sociedad Española de Gastroenterología, Hepatología y Nutrición Pediátrica (SEGHNP), la Asociación Española de Pediatría de Atención Primaria (AEPAP), la Sociedad Española de Pediatría Extrahospitalaria y Atención Primaria (SEPEAP) y la Sociedad Española de Inmunología Clínica, Alergología y Asma Pediátrica (SEICAP). An. Pediatría 2019, 90, 193.e111–193.e191. [Google Scholar] [CrossRef]
- D’Auria, E.; Salvatore, S.; Acunzo, M.; Peroni, D.; Pendezza, E.; Di Profio, E.; Fiore, G.; Zuccotti, G.V.; Verduci, E. Hydrolysed Formulas in the Management of Cow’s Milk Allergy: New Insights, Pitfalls and Tips. Nutrients 2021, 13, 2762. [Google Scholar] [CrossRef]
- Meyer, R.; Groetch, M.; Venter, C. When Should Infants with Cow’s Milk Protein Allergy Use an Amino Acid Formula? A Practical Guide. J. Allergy Clin. Immunol. Pract. 2018, 6, 383–399. [Google Scholar] [CrossRef]
- Host, A.; Halken, S. Hypoallergenic formulas—When, to whom and how long: After more than 15 years we know the right indication! Allergy 2004, 59, 45–52. [Google Scholar] [CrossRef]
- American Academy of Pediatrics. Committee on Nutrition. Hypoallergenic infant formulas. Pediatrics 2000, 106, 346–349. [Google Scholar] [CrossRef]
- Kemp, A.S.; Hill, D.J.; Allen, K.; Anderson, K.; Davidson, G.P.; Day, A.S.; Heine, R.G.; E Peake, J.; Prescott, S.L.; Shugg, A.W.; et al. Guidelines for the use of infant formulas to treat cows milk protein allergy: An Australian consensus panel opinion. Med. J. Aust. 2008, 188, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Merritt, R.J.; Fleet, S.E.; Fifi, A.; Jump, C.; Schwartz, S.; Sentongo, T.; Duro, D.; Rudolph, J.; Turner, J. North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition Position Paper: Plant-based Milks. J. Pediatr. Gastroenterol. Nutr. 2020, 71, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Katz, Y.; Gutierrez-Castrellon, P.; González, M.G.; Rivas, R.; Lee, B.W.; Alarcon, P. A Comprehensive Review of Sensitization and Allergy to Soy-Based Products. Clin. Rev. Allergy Immunol. 2014, 46, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Meyer, R.; Foong, R.-X.M.; Thapar, N.; Kritas, S.; Shah, N. Systematic review of the impact of feed protein type and degree of hydrolysis on gastric emptying in children. BMC Gastroenterol. 2015, 15, 137. [Google Scholar] [CrossRef] [PubMed]
- Dalziel, J.; Peters, J.; Dunstan, K.; McKenzie, C.; Spencer, N.; Haggarty, N.; Roy, N. Alteration in propagating colonic contractions by dairy proteins in isolated rat large intestine. J. Dairy Sci. 2019, 102, 9598–9604. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-L.; Ding, D.; Fang, A.-P.; Chen, P.-Y.; Chen, S.; Jing, L.-P.; Chen, Y.-M.; Zhu, H.-L. Growth, Gastrointestinal Tolerance and Stool Characteristics of Healthy Term Infants Fed an Infant Formula Containing Hydrolyzed Whey Protein (63%) and Intact Casein (37%): A Randomized Clinical Trial. Nutrients 2017, 9, 1254. [Google Scholar] [CrossRef]
- Kearsey, I.; Hutson, J.M.; Southwell, B.R. The effect of food withdrawal in children with rapid-transit constipation. Pediatr. Surg. Int. 2016, 32, 683–689. [Google Scholar] [CrossRef]
- D’Auria, E.; Pendezza, E.; Leone, A.; Riccaboni, F.; Bosetti, A.; Borsani, B.; Zuccotti, G.; Bertoli, S. Nutrient intake in school-aged children with food allergies: A case-control study. Int. J. Food Sci. Nutr. 2021, 1–8. [Google Scholar] [CrossRef]
- Saps, M.; Lu, P.; Bonilla, S. Cow’s-Milk Allergy Is a Risk Factor for the Development of FGIDs in Children. J. Pediatr. Gastroenterol. Nutr. 2011, 52, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Olén, O.; Neuman, A.; Koopmann, B.; Ludvigsson, J.F.; Ballardini, N.; Westman, M.; Melen, E.; Kull, I.; Simren, M.; Bergström, A. Allergy-related diseases and recurrent abdominal pain during—A birth cohort study. Aliment. Pharmacol. Ther. 2014, 40, 1349–1358. [Google Scholar] [CrossRef] [PubMed]
- Di Nardo, G.; Cremon, C.; Frediani, S.; Lucarelli, S.; Villa, M.P.; Stanghellini, V.; La Torre, G.; Martemucci, L.; Barbara, G. Allergic Proctocolitis Is a Risk Factor for Functional Gastrointestinal Disorders in Children. J. Pediatr. 2018, 195, 128–133.e121. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.-K.; Chen, A.-C.; Lin, C.-L.; Shen, T.-C.; Li, T.-C.; Wei, C.-C. Preschoolers With Allergic Diseases Have an Increased Risk of Irritable Bowel Syndrome When Reaching School Age. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 26–30. [Google Scholar] [CrossRef]
- Koloski, N.; Jones, M.; Walker, M.M.; Veysey, M.; Zala, A.; Keely, S.; Holtmann, G.; Talley, N.J. Population based study: Atopy and autoimmune diseases are associated with functional dyspepsia and irritable bowel syndrome, independent of psychological distress. Aliment. Pharmacol. Ther. 2019, 49, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Nocerino, R.; Di Costanzo, M.; Bedogni, G.; Cosenza, L.; Maddalena, Y.; Di Scala, C.; Della Gatta, G.; Carucci, L.; Voto, L.; Coppola, S.; et al. Dietary Treatment with Extensively Hydrolyzed Casein Formula Containing the Probiotic Lactobacillus rhamnosus GG Prevents the Occurrence of Functional Gastrointestinal Disorders in Children with Cow’s Milk Allergy. J. Pediatr. 2019, 213, 137–142.e2. [Google Scholar] [CrossRef] [PubMed]
- Berni Canani, R.; Nocerino, R.; Terrin, G.; Coruzzo, A.; Cosenza, L.; Leone, L.; Troncone, R. Effect of Lactobacillus GG on tolerance acquisition in infants with cow’s milk allergy: A randomized trial. J. Allergy Clin. Immunol. 2012, 129, 580–582.e5. [Google Scholar] [CrossRef] [PubMed]
- Paparo, L.; Nocerino, R.; Bruno, C.; Di Scala, C.; Cosenza, L.; Bedogni, G.; Di Costanzo, M.; Mennini, M.; D’Argenio, V.; Salvatore, F.; et al. Randomized controlled trial on the influence of dietary intervention on epigenetic mechanisms in children with cow’s milk allergy: The EPICMA study. Sci. Rep. 2019, 9, 2828. [Google Scholar] [CrossRef]
- Sánchez-Valverde, F.; Etayo, V.; Gil, F.; Aznal, E.; Martínez, D.; Amézqueta, A.; Mendizábal, M.; Galbete, A.; Pastor, N.; Vanderhoof, J. Factors Associated with the Development of Immune Tolerance in Children with Cow’s Milk Allergy. Int. Arch. Allergy Immunol. 2019, 179, 290–296. [Google Scholar] [CrossRef]
- Carroccio, A.; Di Prima, L.; Iacono, G.; Florena, A.M.; D’Arpa, F.; Sciumè, C.; Cefalu, A.B.; Noto, D.; Averna, M. Multiple food hypersensitivity as a cause of refractory chronic constipation in adults. Scand. J. Gastroenterol. 2006, 41, 498–504. [Google Scholar] [CrossRef][Green Version]
- Ercan, N.; Adıgüzel, K.T. Effect of early childhood cow’s milk elimination diet on eating behaviours, nutrition and growth status at age 2–6 years. J. Hum. Nutr. Diet. 2021. [Google Scholar] [CrossRef]
- Meyer, R.; De Koker, C.; Dziubak, R.; Skrapac, A.; Godwin, H.; Reeve, K.; Chebar-Lozinsky, A.; Shah, N. A practical approach to vitamin and mineral supplementation in food allergic children. Clin. Transl. Allergy 2015, 5, 11. [Google Scholar] [CrossRef] [PubMed]
- Canani, R.B.; Leone, L.; D’Auria, E.; Riva, E.; Nocerino, R.; Ruotolo, S.; Terrin, G.; Cosenza, L.; Di Costanzo, M.; Passariello, A.; et al. The Effects of Dietary Counseling on Children with Food Allergy: A Prospective, Multicenter Intervention Study. J. Acad. Nutr. Diet. 2014, 114, 1432–1439. [Google Scholar] [CrossRef] [PubMed]
- D’Auria, E.; Pendezza, E.; Zuccotti, G.V. Personalized Nutrition in Food Allergy: Tips for Clinical Practice. Front. Pediatr. 2020, 8, 113. [Google Scholar] [CrossRef]
- Wróblewska, B.; Szyc, A.M.; Markiewicz, L.H.; Zakrzewska, M.; Romaszko, E. Increased prevalence of eating disorders as a biopsychosocial implication of food allergy. PLoS ONE 2018, 13, e0198607. [Google Scholar] [CrossRef] [PubMed]
- Wiklund, C.A.; Kuja-Halkola, R.; Thornton, L.M.; Hübel, C.; Leppä, V.; Bulik, C.M. Prolonged constipation and diarrhea in childhood and disordered eating in adolescence. J. Psychosom. Res. 2019, 126, 109797. [Google Scholar] [CrossRef] [PubMed]
- Ms, H.K.M.; Wagner, C.; Becker, K.; Bühren, K.; Correll, C.U.; Egberts, K.M.; Ehrlich, S.; Fleischhaker, C.; Föcker, M.; Hahn, F.; et al. Incontinence and constipation in adolescent patients with anorexia nervosa—Results of a multicenter study from a German web-based registry for children and adolescents with anorexia nervosa. Int. J. Eat. Disord. 2019, 53, 219–228. [Google Scholar] [CrossRef]
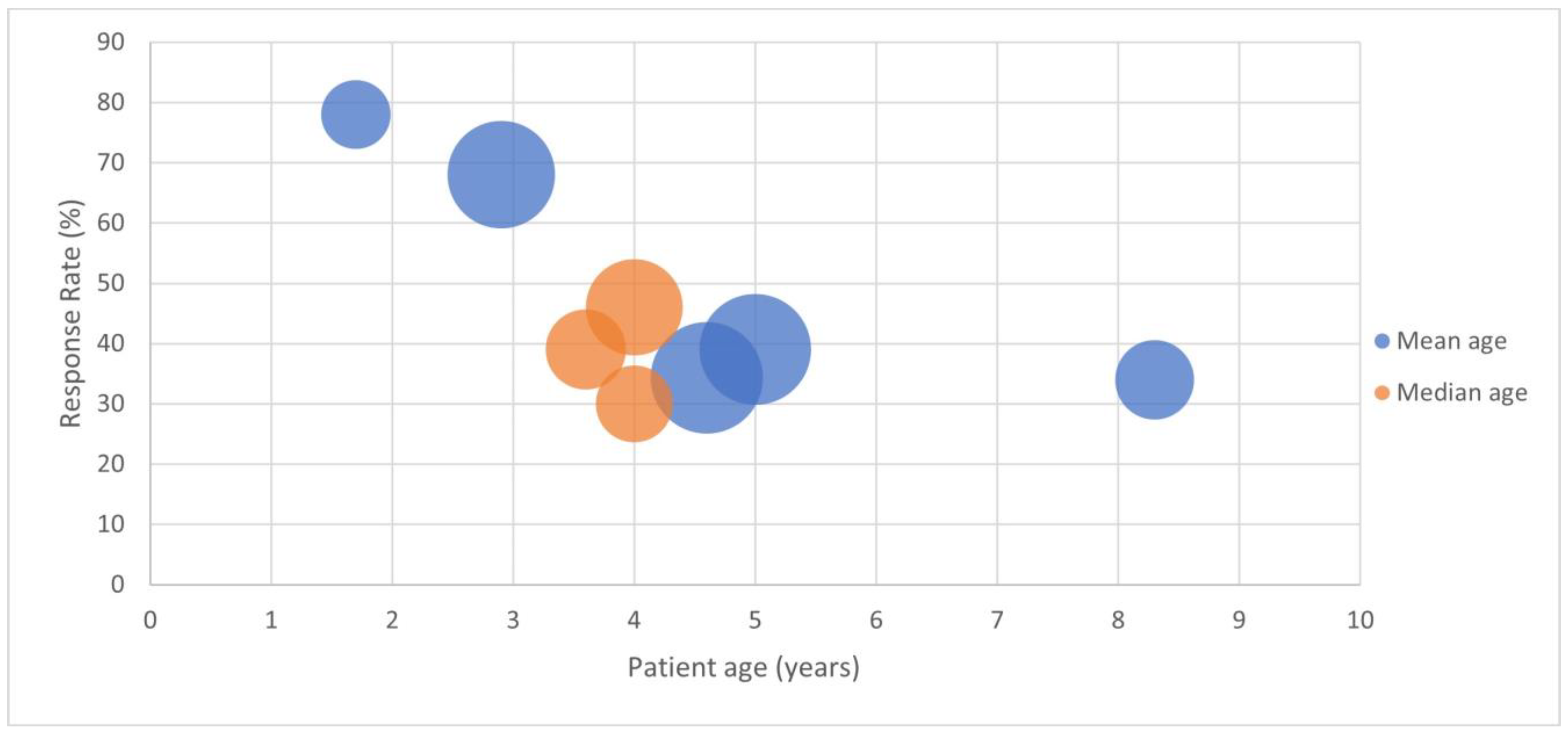
| Year | Author | Country | Number of Cases | Study Type | Age Range (years) | Mean () or Median Age (years) | Responders to Dairy Elimination (%) | Response Confirmed with Challenge (%) | Treatment Dependent or Resistant | Setting (Primary Care, Secondary, Gastro) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1995 | Iacono G. et al. [24] | Italy | 27 | Prospective, open challenge | 0.5–3 | 1.7 | 78% | 78% | either | ped GE |
| 1998 | Iacono G. et al. [18] | Italy | 65 | Prospective crossover RCT DBPCFC | 1–6 | 2.9 | 68% | 68% | resistant | ped GE |
| 1999 | Shah N. et al. [25] | United Kingdom | 14 | Prospective | 0.5–6.7 | 3.1 (med) | 79% * | n/a | resistant | ped GE |
| 2001 | Daher S. et al. [26] | Brazil | 25 | Prospective, open challenge | 0.25–11 | nr | 28% | 28% | resistant | ped GE |
| 2004 | Turunen S. et al. [27] | Finland | 35 | Prospective, open challenge | 3–15 | 8.3 | 83% | 34% | either | ped GE |
| 2005 | Carroccio A. et al. [19] | Italy | 52 | Prospective with DBPCFC | 4.25+/−1.5 SD | 4 (med) | 46% * | 46% | resistant | ped GE |
| 2006 | Iacono G. et al. [28] | Italy | 36 | Prospective with DBPCFC | 0.75–10 | 3.6 (med) | 39% * | 39% | resistant | ped GE |
| 2008 | Simeone D. et al. [98] | Italy | 11 | Prospective | nr | nr | 0 | n/a | resistant | primary |
| 2009 | Borrelli O. et al. [29] | Italy | 33 | Prospective with DBPCFC | 1–10.8 | 4 (med) | 30% * | 30% | resistant | ped GE |
| 2009 | El-Hodhod M.A. et al. [30] | Egypt | 27 | Prospective, long follow up | 0.7–4 | nr | 78% | 78% | resistant | ped GE |
| 2010 | Irastorza I. et al. [31] | Spain | 69 | Prospective, open challenge | 0.5–14 | 5 | 51% | 39% | either | ped GE |
| 2012 | Dehghani S.M. et al. [32] | Iran | 70 | Prospective, case controlled RCT | 1–13 | 4.6 | 80% | 34% | resistant | ped GE |
| 2013 | Crowley E.T. et al. [95] | Australia | 30 | Prospective crossover RCT with DBPCFC | 1.5–12 | nr | 81% | 33% | resistant | secondary |
| 2021 | Mohamma-di Bourkheili A. et al. [20] | Iran | 35 | Prospective case controlled RCT | 4–14 | 5 | 71% | n/a | resistant | ped GE |
| Author | Year | Age Range (months) | Number in Study | Number with Constipation Prior to CMP-Free Diet | Constipation Responders to Dairy Free Diet/Challenge: Number (%) |
|---|---|---|---|---|---|
| De Boissieu, D. et al. [104] | 2003 | 2–57 | 35 | 4 | 3 (75%) |
| Cudowska, B. et al. [105] | 2010 | 6–144 | 28 | 7 | 3 (43%) |
| Kalach, N. et al. [106] | 2013 | 1–18 | 25 | 5 | 2(40%) |
| Mowszet, K. et al. [107] | 2014 | 3–36 | 39 | not reported | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Connor, F.; Salvatore, S.; D’Auria, E.; Baldassarre, M.E.; Acunzo, M.; Di Bella, G.; Farella, I.; Sestito, S.; Pensabene, L. Cows’ Milk Allergy-Associated Constipation: When to Look for It? A Narrative Review. Nutrients 2022, 14, 1317. https://doi.org/10.3390/nu14061317
Connor F, Salvatore S, D’Auria E, Baldassarre ME, Acunzo M, Di Bella G, Farella I, Sestito S, Pensabene L. Cows’ Milk Allergy-Associated Constipation: When to Look for It? A Narrative Review. Nutrients. 2022; 14(6):1317. https://doi.org/10.3390/nu14061317
Chicago/Turabian StyleConnor, Frances, Silvia Salvatore, Enza D’Auria, Maria Elisabetta Baldassarre, Miriam Acunzo, Gaia Di Bella, Ilaria Farella, Simona Sestito, and Licia Pensabene. 2022. "Cows’ Milk Allergy-Associated Constipation: When to Look for It? A Narrative Review" Nutrients 14, no. 6: 1317. https://doi.org/10.3390/nu14061317
APA StyleConnor, F., Salvatore, S., D’Auria, E., Baldassarre, M. E., Acunzo, M., Di Bella, G., Farella, I., Sestito, S., & Pensabene, L. (2022). Cows’ Milk Allergy-Associated Constipation: When to Look for It? A Narrative Review. Nutrients, 14(6), 1317. https://doi.org/10.3390/nu14061317








