Heat-Killed Enterococcus faecalis Prevents Adipogenesis and High Fat Diet-Induced Obesity by Inhibition of Lipid Accumulation through Inhibiting C/EBP-α and PPAR-γ in the Insulin Signaling Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Enterococcus faecalis 2001
2.2. Animal Experiments
2.3. Serological Analysis
2.4. 3T3-L1 Cell Culture and Differentiation
2.5. Oil Red O Staining of 3T3-L1 Adipocyte
2.6. Cell Cycle Analysis
2.7. Western Blotting Analysis
2.8. Confocal Microscopy
2.9. Statistical Analysis
3. Results
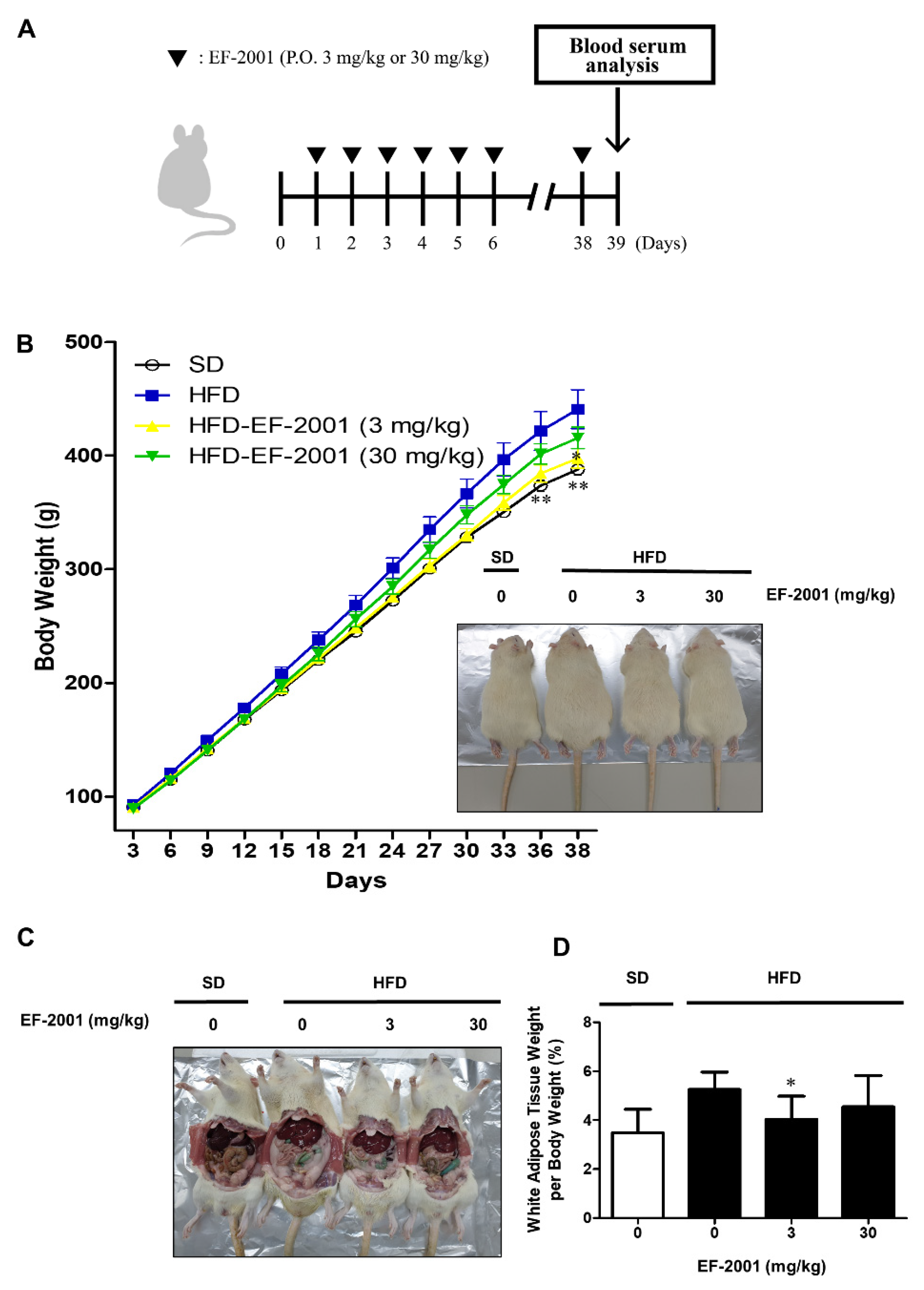
3.1. EF-2001 Intake Effectively Decreases White Adipose Tissue and Body Weights on HFD-Induced Obese Rats
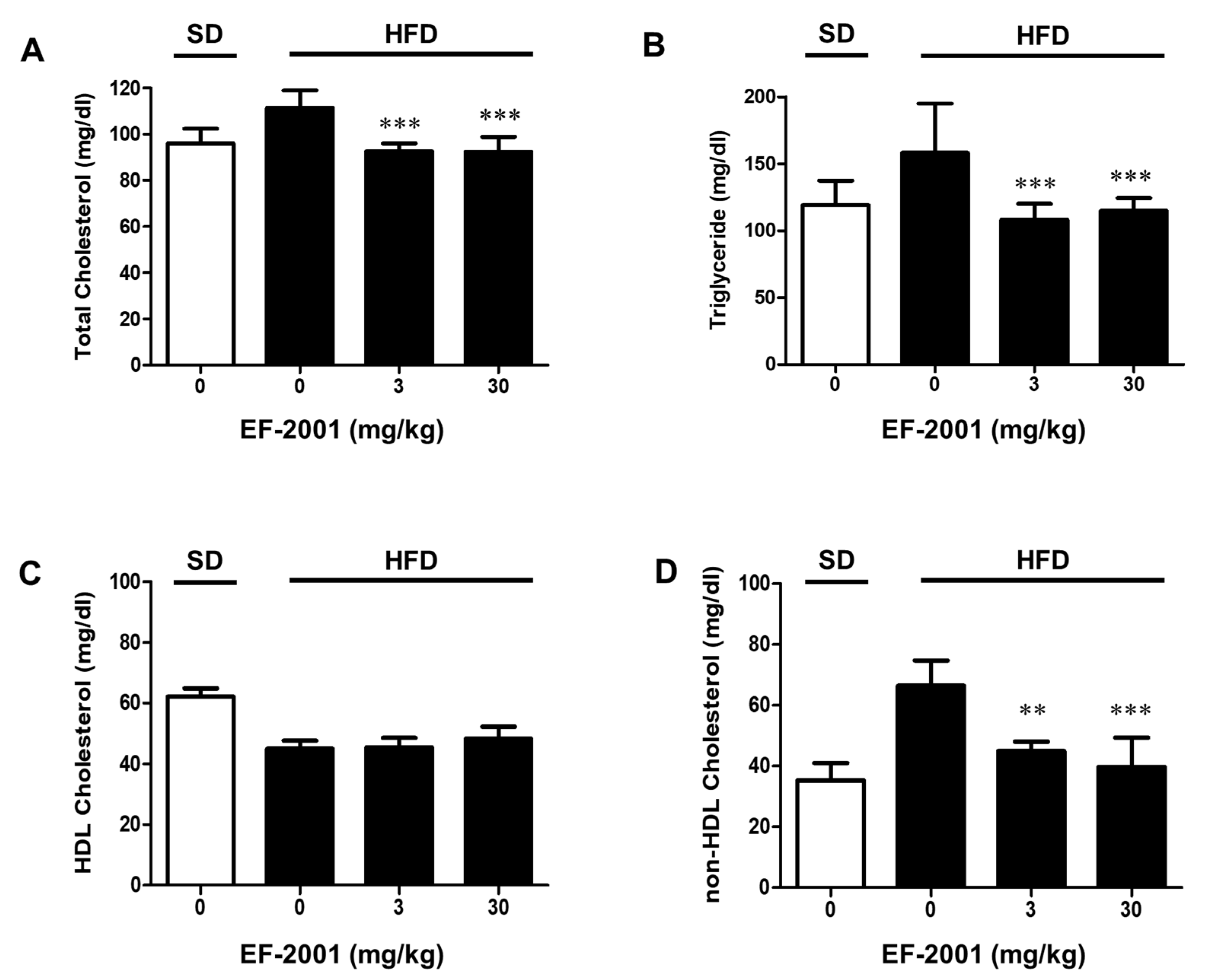
3.2. EF-2001 Down-Regulates the Levels of Total Cholesterol, TG, and Non-HDL in Serum of High Fat Diet-Induced Obese Rats
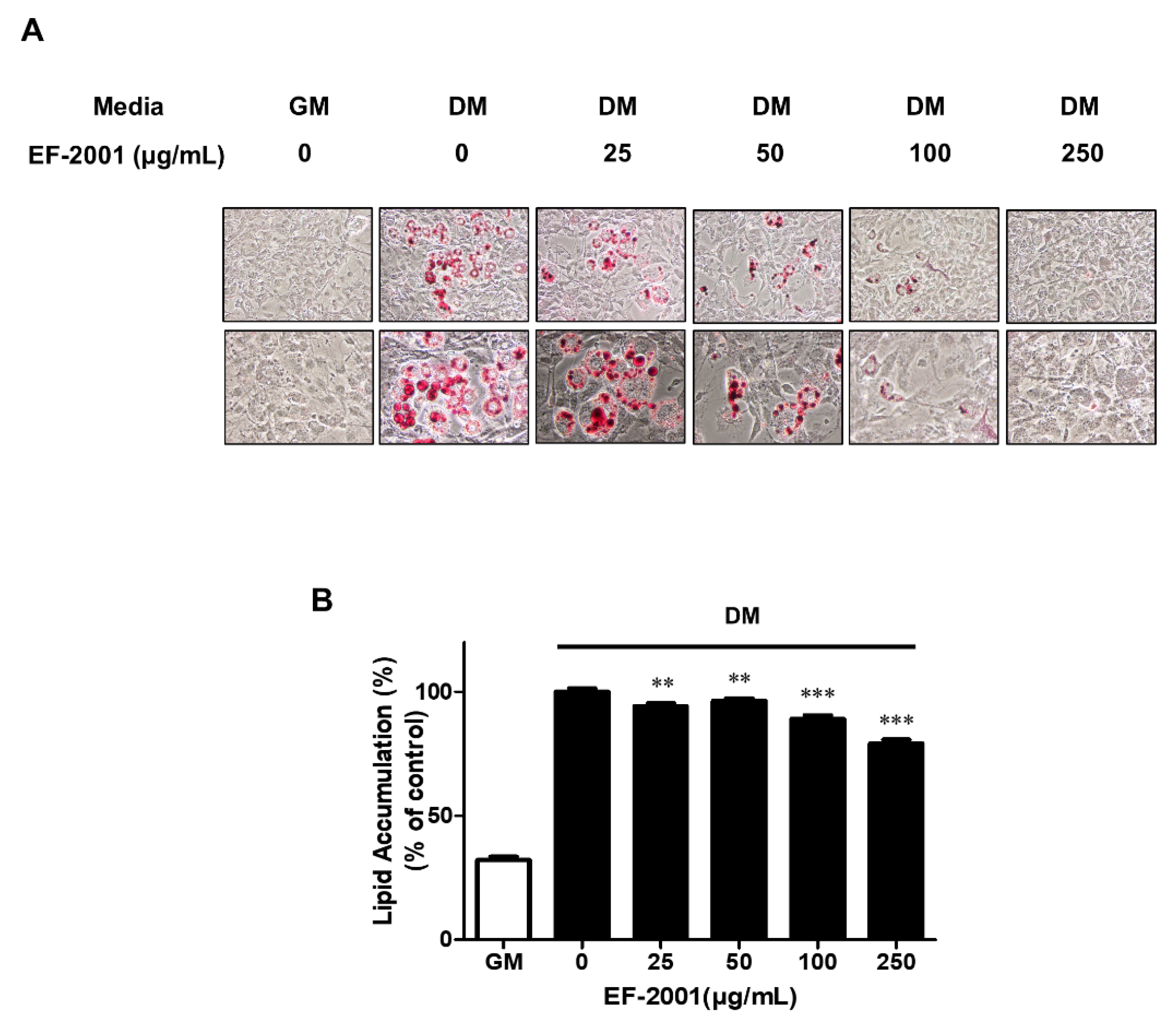
3.3. EF-2001 Inhibits Lipid Accumulation in Differentiated 3T3-L1 Adipocytes
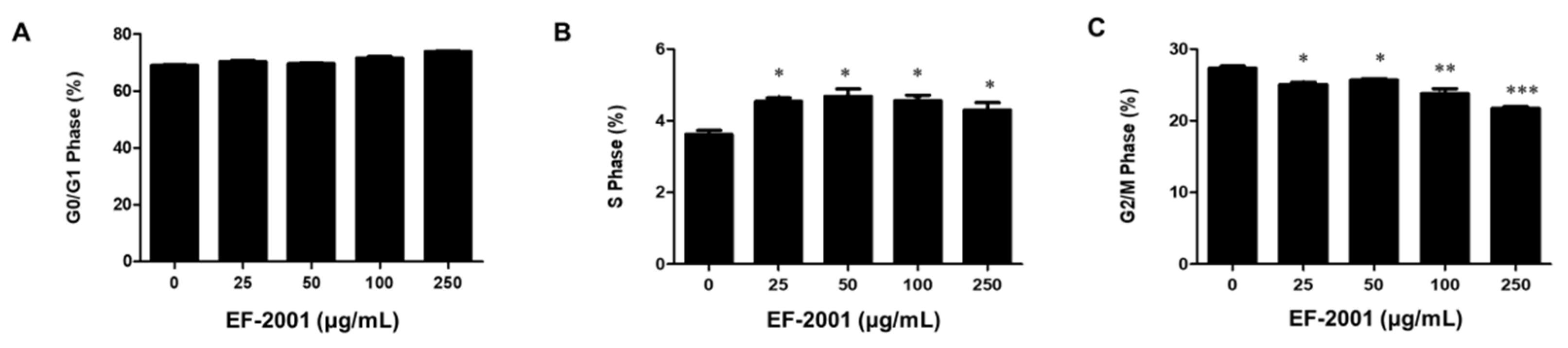
3.4. EF-2001 Delays MDI-Induced Cell Cycle Progression in 3T3-L1 Adipocytes
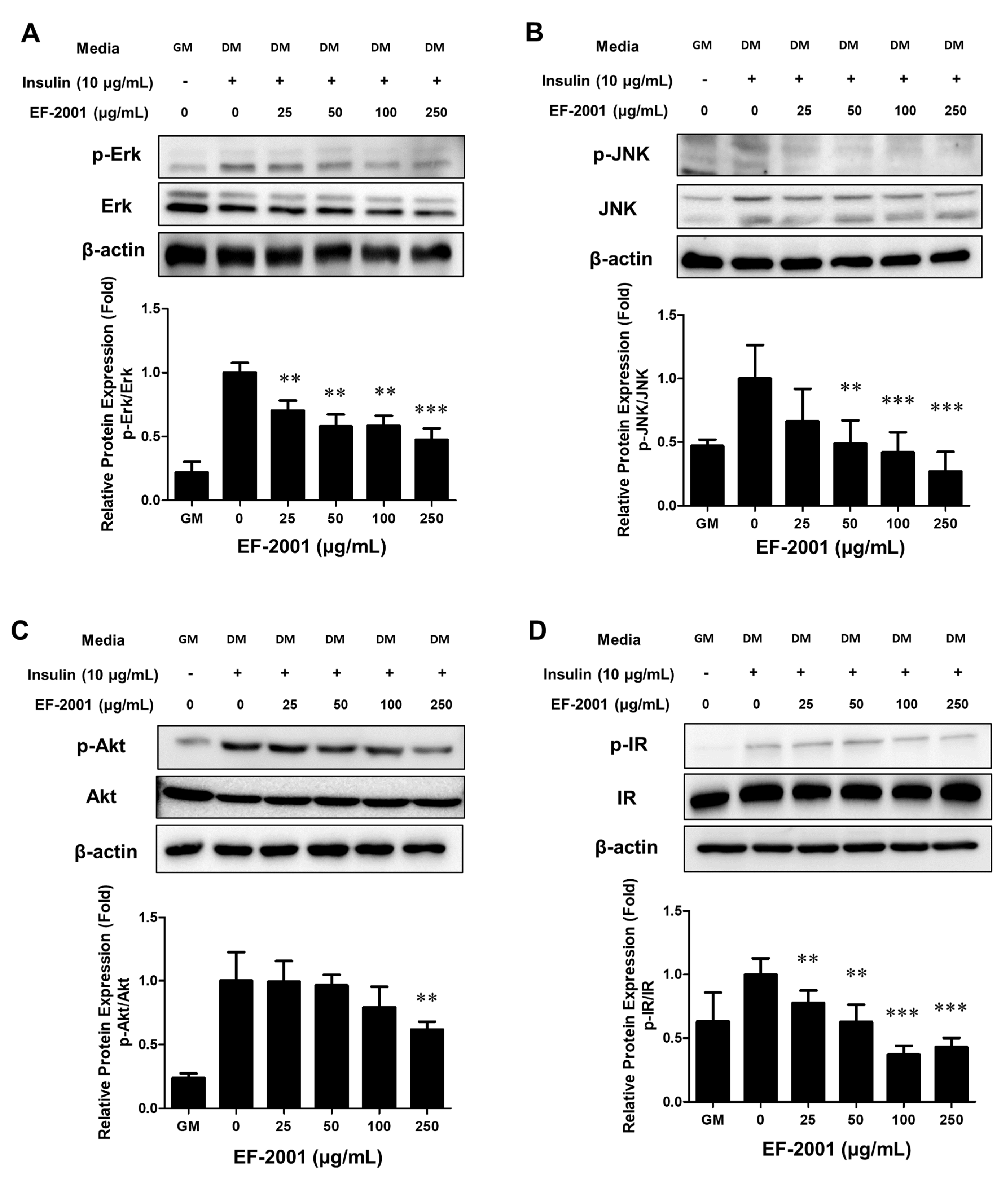
3.5. EF-2001 Inhibits MDI-Induced Insulin Receptor Pathways in 3T3-L1 Adipocytes
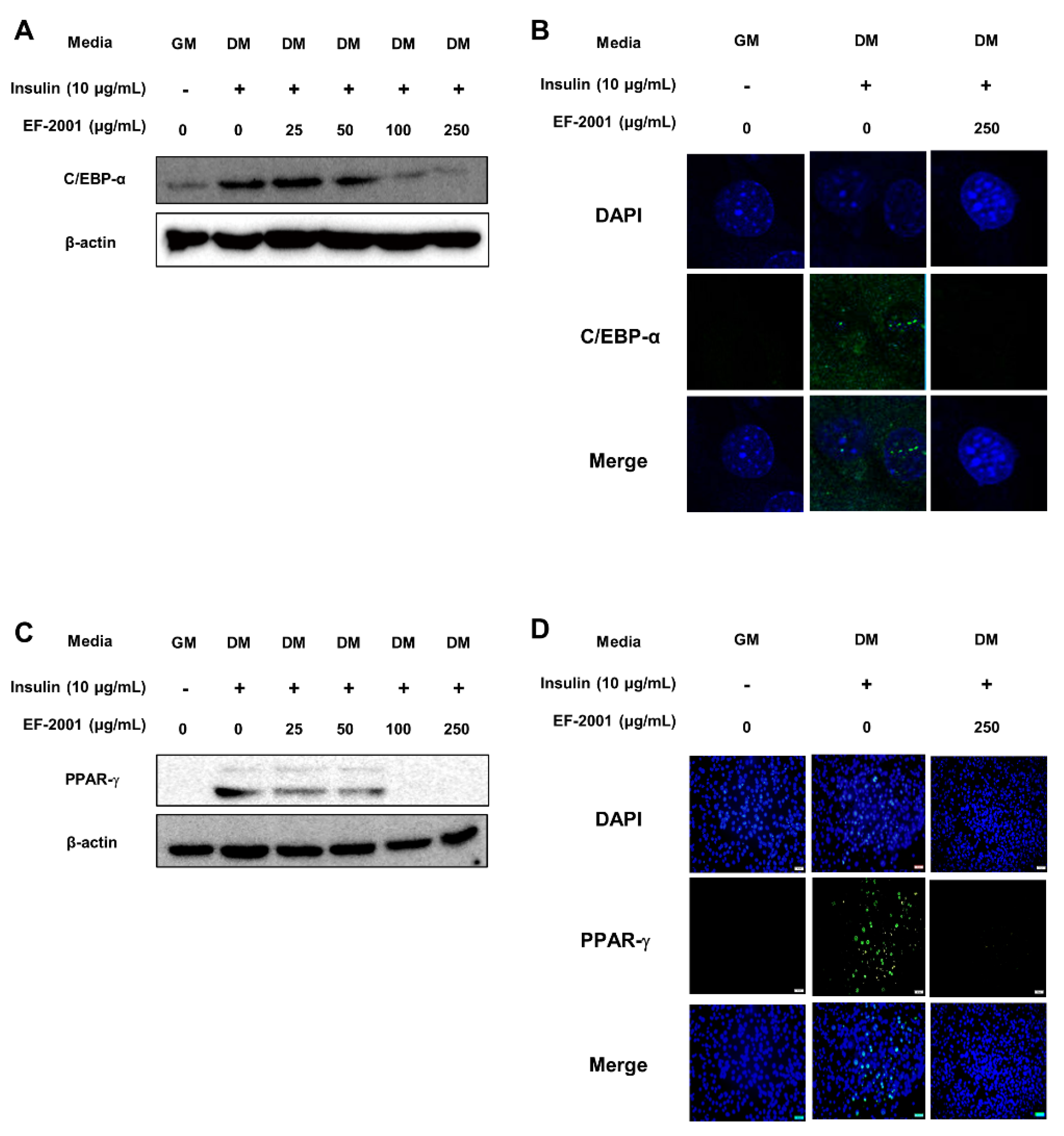
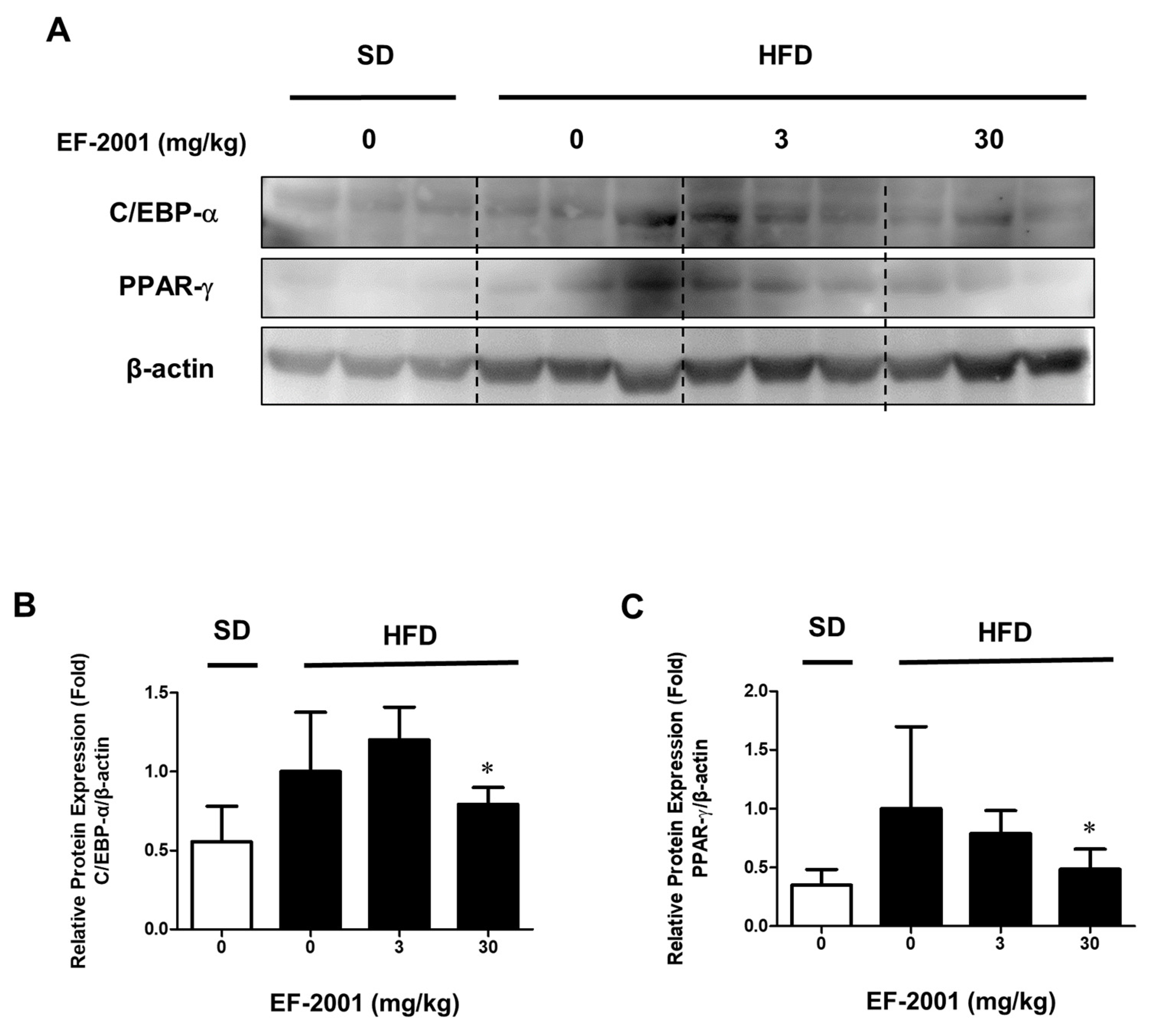
3.6. EF-2001 Inhibits the Protein Expression and Nuclar Translocation of C/EBP-α and PPAR-γ in 3T3-L1 Adipocytes and in HFD-Induced Adipose Tissues
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Les, F.; Cásedas, G.; Valero, M.S.; Arbonés-Mainar, J.M.; López, V. Rock tea (Jasonia glutinosa L. DC.) polyphenolic extract inhibits triglyceride accumulation in 3T3-L1 adipocyte-like cells and obesity related enzymes in vitro. Food Funct. 2020, 11, 8931–8938. [Google Scholar] [CrossRef] [PubMed]
- Kopelman, P.G. Obesity as a medical problem. Nature 2000, 404, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Bays, H.E.; Toth, P.P.; Kris-Etherton, P.M.; Abate, N.; Aronne, L.J.; Brown, W.V.; Gonzalez-Campoy, J.M.; Jones, S.R.; Kumar, R.; La Forge, R. Obesity, adiposity, and dyslipidemia: A consensus statement from the National Lipid Association. J. Clin. Lipidol. 2013, 7, 304–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.S.; Hyun, I.K.; Seo, H.J.; Song, D.; Kim, M.Y.; Kang, S.S. Biotransformation of whey by Weissella cibaria suppresses 3T3-L1 adipocyte differentiation. Int. J. Dairy Sci. 2021, 104, 3876–3887. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Yun, J.M.; Kim, M.K.; Kwon, O.; Cho, B. Lactobacillus gasseri BNR17 supplementation reduces the visceral fat accumulation and waist circumference in obese adults: A randomized, double-blind, placebo-controlled trial. J. Med. food 2018, 21, 454–461. [Google Scholar] [CrossRef]
- Minami, J.; Iwabuchi, N.; Tanaka, M.; Yamauchi, K.; Xiao, J.-Z.; Abe, F.; Sakane, N. Effects of Bifidobacterium breve B-3 on body fat reductions in pre-obese adults: A randomized, double-blind, placebo-controlled trial. Biosci. Microbiota Food Health 2018, 37, 67–75. [Google Scholar] [CrossRef] [Green Version]
- Hsu, C.-L.; Hou, Y.-H.; Wang, C.-S.; Lin, S.-W.; Jhou, B.-Y.; Chen, C.-C.; Chen, Y.-L. Antiobesity and uric acid-lowering effect of Lactobacillus plantarum GKM3 in high-fat-diet-induced obese rats. J. Am. Coll. Nutr. 2019, 38, 623–632. [Google Scholar] [CrossRef]
- Ruiz-Ojeda, F.J.; Rupérez, A.I.; Gomez-Llorente, C.; Gil, A.; Aguilera, C.M. Cell models and their application for studying adipogenic differentiation in relation to obesity: A review. Int. J. Mol. Sci. 2016, 17, 1040. [Google Scholar] [CrossRef] [Green Version]
- Lwin, S.; Olechnowicz, S.; Fowler, J.; Edwards, C. Diet-induced obesity promotes a myeloma-like condition in vivo. Leukemia 2015, 29, 507–510. [Google Scholar] [CrossRef]
- Son, Y.H.; Ka, S.; Kim, A.Y.; Kim, J.B. Regulation of adipocyte differentiation via microRNAs. Endocrinol. Metab. 2014, 29, 122–135. [Google Scholar] [CrossRef] [Green Version]
- Balakrishnan, B.B.; Krishnasamy, K.; Choi, K.C. Moringa concanensis Nimmo ameliorates hyperglycemia in 3T3-L1 adipocytes by upregulating PPAR-γ, C/EBP-α via Akt signaling pathway and STZ-induced diabetic rats. Biomed. Pharmacother. 2018, 103, 719–728. [Google Scholar] [CrossRef]
- Bost, F.; Aouadi, M.; Caron, L.; Binétruy, B. The role of MAPKs in adipocyte differentiation and obesity. Biochimie 2005, 87, 51–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vidal-Puig, A.; Jimenez-Liñan, M.; Lowell, B.B.; Hamann, A.; Hu, E.; Spiegelman, B.; Flier, J.S.; Moller, D.E. Regulation of PPAR gamma gene expression by nutrition and obesity in rodents. J. Clin. Investig. 1996, 97, 2553–2561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bastien, M.; Poirier, P.; Lemieux, I.; Després, J.P. Overview of epidemiology and contribution of obesity to cardiovascular disease. Prog. Cardiovasc. Dis. 2014, 56, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.J.; Lee, M.H.; Kim, W.J.; Chae, Y.; Iwasa, M.; Han, K.I.; Kim, W.J.; Kim, T.-J. Effect of heat-killed Enterococcus faecalis, EF-2001 on C2C12 myoblast damage induced by oxidative stress and muscle volume decreased by sciatic denervation in C57BL/6 mice. J. Life Sci. 2019, 29, 215–222. [Google Scholar]
- Choi, E.J.; Iwasa, M.; Han, K.I.; Kim, W.J.; Tang, Y.; Han, W.C.; Kim, E.K.; Park, Z.Y. Effect of Enterococcus faecalis EF-2001 on experimentally induced atopic eczema in mice. Food Sci. Biotechnol. 2016, 25, 1087–1093. [Google Scholar] [CrossRef]
- Gu, Y.H.; Choi, H.; Yamashita, T.; Kang, K.M.; Iwasa, M.; Lee, M.J.; Lee, K.H.; Kim, C.H. Pharmaceutical production of anti-tumor and immune-potentiating Enterococcus faecalis-2001 β-glucans: Enhanced activity of macrophage and lymphocytes in tumor-implanted mice. Curr. Pharm. Biotechnol. 2017, 18, 653–661. [Google Scholar] [CrossRef]
- Satonaka, K.; Ohashi, K.; Nohmi, T.; Yamamoto, T.; Abe, S.; Uchida, K.; Yamaguchi, H. Prophylactic Effect of Enterococcus faecalis FK-23 Preparation on Experimental Candidiasis in Mice. Microbiol. Immunol. 1996, 40, 217–222. [Google Scholar] [CrossRef]
- Yanagisawa, T.; Gu, Y.; Tsuchihashi, E.; Umekawa, M.; Yamamoto, H.; Iwasa, T.; Suzuki, I. Analgesic and anti-neoplastic effects of the immunization-active fraction of Enterococcus faecalis 2001. J. Orient. Med. 2000, 5, 97–102. [Google Scholar]
- Baureder, M.; Reimann, R.; Hederstedt, L. Contribution of catalase to hydrogen peroxide resistance in Enterococcus faecalis. FEMS microbiol. Lett. 2012, 331, 160–164. [Google Scholar] [CrossRef]
- Fisher, K.; Phillips, C. The ecology, epidemiology and virulence of Enterococcus. Microbiology 2009, 155, 1749–1757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, K.; Kurokawa, K.; Hong, L.; Miyagawa, K.; Mochida-Saito, A.; Iwasa, M.; Iwasa, H.; Nakagawasai, O.; Tadano, T.; Takeda, H. Antidepressant effects of Enterococcus faecalis 2001 through the regulation of prefrontal cortical myelination via the enhancement of CREB/BDNF and NF-κB p65/LIF/STAT3 pathways in olfactory bulbectomized mice. J. Psychiatr. Res. 2022, 148, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Choi, Y.J.; Wedamulla, N.E.; Tang, Y.; Han, K.I.; Hwang, J.Y.; Kim, E.K. Heat-Killed Enterococcus faecalis EF-2001 attenuate lipid accumulation in diet-induced obese (DIO) mice by activating AMPK signaling in liver. Foods 2022, 11, 575. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Fan, M.; Tang, Y.; Iwasa, M.; Han, K.I.; Lee, H.; Hwang, J.Y.; Lee, B.; Kim, E.K. Heat-Killed and Live Enterococcus faecalis Attenuates Enlarged Prostate in an Animal Model of Benign Prostatic Hyperplasia. J. Microbiol. Biotechnol. 2021, 31, 1134–1143. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D. The Role of Secreted Bacterial Metabolites in Regulating Adipose Biology: The Effect of the Lab4 Consortium of Probiotics on Adipogenesis; Cardiff University: Cardiff, UK, 28 March 2018. [Google Scholar]
- Han, J.H.; Jang, K.W.; Park, M.H.; Myung, C.S. Garcinia cambogia suppresses adipogenesis in 3T3-L1 cells by inhibiting p90RSK and Stat3 activation during mitotic clonal expansion. J. Cell. Physiol. 2021, 236, 1822–1839. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, S.; Yasuda, K.; Kitahara, R.; Tsujimoto, K.; Yamashita, K.; Hoshino, N.; Fujimoto, Y.; Okuhira, K. Comparative effects of sulforaphane and allyl isothiocyanate on 3T3-L1 adipogenesis. J. Nutr. Metab. 2022, 2022, 8705163. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.C.; Tsai, S.H.; Chang, E.Y.C.; Hee, S.W.; Chen, W.H.; Lee, S.C.; Chuang, L.M. Cytoskeletal protein vimentin interacts with and regulates peroxisome proliferator-activated receptor gamma via a proteasomal degradation process. J. Cell. Biochem. 2013, 114, 1559–1567. [Google Scholar] [CrossRef]
- Karimi, G.; Sabran, M.R.; Jamaluddin, R.; Parvaneh, K.; Mohtarrudin, N.; Ahmad, Z.; Khazaai, H.; Khodavandi, A. The anti-obesity effects of Lactobacillus casei strain shirota versus orlistat on high fat diet-induced obese rats. Food Nutr. Res. 2015, 59, 29273. [Google Scholar] [CrossRef] [Green Version]
- Hong, S.-M.; Chung, E.-C.; Kim, C.-H. Anti-obesity effect of fermented whey beverage using lactic acid bacteria in diet-induced obese rats. Korean J. Food Sci. Anim. Resour. 2015, 35, 653. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Nie, S.-P.; Ding, Q.; Zhu, K.-X.; Wang, Z.-J.; Xiong, T.; Gong, J.; Xie, M.-Y. Cholesterol-lowering effect of Lactobacillus plantarum NCU116 in a hyperlipidaemic rat model. J. Funct. Foods 2014, 8, 340–347. [Google Scholar] [CrossRef]
- Yang, R.; Le, G.; Li, A.; Zheng, J.; Shi, Y. Effect of antioxidant capacity on blood lipid metabolism and lipoprotein lipase activity of rats fed a high-fat diet. Nutrition 2006, 22, 1185–1191. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Wang, Z.; Zhang, Y.; Zhang, J.; Wang, L.; Dong, X.; Su, F.; Yao, G.; Wang, S.; Zhang, H. Effect of Lactobacillus plantarum P-8 on lipid metabolism in hyperlipidemic rat model. Eur. J. Lipid Sci. Technol. 2012, 114, 1230–1236. [Google Scholar] [CrossRef]
- Kitadokoro, K.; Tanaka, M.; Hikima, T.; Okuno, Y.; Yamamoto, M.; Kamitani, S. Crystal structure of pathogenic Staphylococcus aureus lipase complex with the anti-obesity drug orlistat. Sci. Rep. 2020, 10, 1–13. [Google Scholar]
- Choi, W.J.; Dong, H.J.; Jeong, H.U.; Ryu, D.W.; Song, S.M.; Kim, Y.R.; Jung, H.H.; Kim, T.H.; Kim, Y.H. Lactobacillus plantarum LMT1-48 exerts anti-obesity effect in high-fat diet-induced obese mice by regulating expression of lipogenic genes. Sci. Rep. 2020, 10, 1–9. [Google Scholar]
- Won, S.M.; Chen, S.; Park, K.W.; Yoon, J.H. Isolation of lactic acid bacteria from kimchi and screening of Lactobacillus sakei ADM14 with anti-adipogenic effect and potential probiotic properties. LWT 2020, 126, 109296. [Google Scholar] [CrossRef]
- Park, J.E.; Oh, S.H.; Cha, Y.S. Lactobacillus Brevis OPK-3 from kimchi prevents obesity and modulates the expression of adipogenic and pro-inflammatory genes in adipose tissue of diet-Induced obese mice. Nutrients 2020, 12, 604. [Google Scholar] [CrossRef] [Green Version]
- Patel, Y.M.; Lane, M.D. Mitotic clonal expansion during preadipocyte differentiation: Calpain-mediated turnover of p27. J. Biol. Chem. 2000, 275, 17653–17660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, J.Y.; Seo, S.G.; Yue, S.; Cheng, J.X.; Lee, K.W.; Kim, K.H. An inhibitory effect of resveratrol in the mitotic clonal expansion and insulin signaling pathway in the early phase of adipogenesis. Nutr. Res. Rev. 2012, 32, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, H.; Kamikado, K.; Aoki, R.; Suganuma, N.; Nishijima, T.; Nakatani, A.; Kimura, I. Bifidobacterium animalis subsp. lactis GCL2505 modulates host energy metabolism via the short-chain fatty acid receptor GPR43. Sci. Rep. 2020, 10, 1–8. [Google Scholar]
- Seo, M.J.; Lee, Y.J.; Hwang, J.H.; Kim, K.J.; Lee, B.Y. The inhibitory effects of quercetin on obesity and obesity-induced inflammation by regulation of MAPK signaling. J. Nutr. Biochem. 2015, 26, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Darlington, G.J.; Ross, S.E.; MacDougald, O.A. The role of C/EBP genes in adipocyte differentiation. J. Biol. Chem. 1998, 273, 30057–30060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ross, S.R.; Graves, R.A.; Greenstein, A.; Platt, K.A.; Shyu, H.-L.; Mellovitz, B.; Spiegelman, B.M. A fat-specific enhancer is the primary determinant of gene expression for adipocyte P2 in vivo. Proc. Natl. Acad. Sci. USA. 1990, 87, 9590–9594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olsen, K.; Danielsen, K.; Wilsgaard, T.; Sangvik, M.; Sollid, J.U.; Thune, I.; Eggen, A.E.; Simonsen, G.S.; Furberg, A.-S. Obesity and Staphylococcus aureus nasal colonization among women and men in a general population. PLoS ONE 2013, 8, e63716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, L.; Li, X.; Tang, Q.-Q. Transcriptional regulation of adipocyte differentiation: A central role for CCAAT/enhancer-binding protein (C/EBP) β. J. Biol. Chem. 2015, 290, 755–761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsabisa, M.; Chukwuma, C.; Chaudhary, S.; Kumar, C.; Baleni, R.; Javu, M.; Oyedemi, S. Dicoma anomala (Sond.) abates glycation and DPP-IV activity and modulates glucose utilization in Chang liver cells and 3T3-L1 adipocytes. S. Afr. J. Bot. 2020, 128, 182–188. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, L.; Xing, M.; Xu, X.; Wei, J.; Wang, J.; Kang, W. Trelagliptin succinate: DPP-4 inhibitor to improve insulin resistance in adipocytes. Biomed. Pharmacother. 2020, 125, 109952. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Gao, S.; Guan, H.; Zhang, X.; Gao, Y.; Su, Y.; Song, Y.; Jiang, Y.; Li, N. Naringin protects human nucleus pulposus cells against TNF-α-induced inflammation, oxidative stress, and loss of cellular homeostasis by enhancing autophagic flux via AMPK/SIRT1 activation. Oxid. Med. Cell. Longev. 2022, 2022, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.; Ladilov, Y. Emerging Role of cAMP/AMPK Signaling. Cells 2022, 11, 308. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Nakagawasai, O.; Nemoto, W.; Odaira, T.; Sakuma, W.; Onogi, H.; Nishijima, H.; Furihata, R.; Nemoto, Y.; Iwasa, H. Effect of Enterococcus faecalis 2001 on colitis and depressive-like behavior in dextran sulfate sodium-treated mice: Involvement of the brain–gut axis. J. Neuroinflamm. 2019, 16, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, Y.H.; Yamasita, T.; Kang, K.M. Subchronic oral dose toxicity study of Enterococcus faecalis 2001 (EF 2001) in mice. Toxicol. Res. 2018, 34, 55–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-H.; Woo, K.-J.; Kim, M.-A.; Hong, J.; Kim, J.; Kim, S.-H.; Han, K.-I.; Iwasa, M.; Kim, T.-J. Heat-Killed Enterococcus faecalis Prevents Adipogenesis and High Fat Diet-Induced Obesity by Inhibition of Lipid Accumulation through Inhibiting C/EBP-α and PPAR-γ in the Insulin Signaling Pathway. Nutrients 2022, 14, 1308. https://doi.org/10.3390/nu14061308
Lee J-H, Woo K-J, Kim M-A, Hong J, Kim J, Kim S-H, Han K-I, Iwasa M, Kim T-J. Heat-Killed Enterococcus faecalis Prevents Adipogenesis and High Fat Diet-Induced Obesity by Inhibition of Lipid Accumulation through Inhibiting C/EBP-α and PPAR-γ in the Insulin Signaling Pathway. Nutrients. 2022; 14(6):1308. https://doi.org/10.3390/nu14061308
Chicago/Turabian StyleLee, Jin-Ho, Keun-Jung Woo, Min-Ah Kim, Joonpyo Hong, Jihee Kim, Sun-Hong Kim, Kwon-Il Han, Masahiro Iwasa, and Tack-Joong Kim. 2022. "Heat-Killed Enterococcus faecalis Prevents Adipogenesis and High Fat Diet-Induced Obesity by Inhibition of Lipid Accumulation through Inhibiting C/EBP-α and PPAR-γ in the Insulin Signaling Pathway" Nutrients 14, no. 6: 1308. https://doi.org/10.3390/nu14061308
APA StyleLee, J.-H., Woo, K.-J., Kim, M.-A., Hong, J., Kim, J., Kim, S.-H., Han, K.-I., Iwasa, M., & Kim, T.-J. (2022). Heat-Killed Enterococcus faecalis Prevents Adipogenesis and High Fat Diet-Induced Obesity by Inhibition of Lipid Accumulation through Inhibiting C/EBP-α and PPAR-γ in the Insulin Signaling Pathway. Nutrients, 14(6), 1308. https://doi.org/10.3390/nu14061308





