Oxidative Stress and Left Ventricular Performance in Patients with Different Glycometabolic Phenotypes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Blood Pressure Measurement
2.3. Laboratory Determination
2.4. Serum Levels of Oxidative Stress Biomarkers
2.5. Echocardiographic Measurements
2.6. 2D Speckle Tracking Echocardiography
2.7. Statistical Methods
3. Results
3.1. Study Population
3.2. Oxidative Stress Serum Parameters
3.3. Echocardiographic Parameters According to Glucose Tolerance
3.4. Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abdul-Ghani, M.A.; Abdul-Ghani, T.; Ali, N.; DeFronzo, R.A. One-Hour Plasma Glucose Concentration and the Metabolic Syndrome Identify Subjects at High Risk for Future Type 2 Diabetes. Diabetes Care 2008, 31, 1650–1655. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, T.V.; Marini, M.A.; Succurro, E.; Andreozzi, F.; Perticone, M.; Hribal, M.L.; Sciacqua, A.; Perticone, F.; Sesti, G. One-Hour Postload Hyperglycemia: Implications for Prediction and Prevention of Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2018, 103, 3131–3143. [Google Scholar] [CrossRef] [PubMed]
- Succurro, E.; Arturi, F.; Lugarà, M.; Grembiale, A.; Fiorentino, T.V.; Caruso, V.; Andreozzi, F.; Sciacqua, A.; Hribal, M.L.; Perticone, F.; et al. One-Hour Postload Plasma Glucose Levels Are Associated with Kidney Dysfunction. Clin. J. Am. Soc. Nephrol. 2010, 5, 1922–1927. [Google Scholar] [CrossRef] [PubMed]
- Sciacqua, A.; Perticone, M.; Miceli, S.; Pinto, A.; Cassano, V.; Succurro, E.; Andreozzi, F.; Hribal, M.L.; Sesti, G.; Perticone, F. Elevated 1-h post-load plasma glucose is associated with right ventricular morphofunctional parameters in hypertensive patients. Endrocrine 2019, 34, 2291–2296. [Google Scholar] [CrossRef] [PubMed]
- Pastore, M.C.; De Carli, G.; Mandoli, G.E.; D’Ascenzi, F.; Focardi, M.; Contorni, F.; Mondillo, S.; Cameli, M. The prognostic role of speckle tracking echocardiography in clinical practice: Evidence and reference values from the literature. Heart Fail. Rev. 2021, 26, 1371–1381. [Google Scholar] [CrossRef]
- Adamu, U.; Schmitz, F.; Becker, M.; Kelm, M.; Hofmann, R. Advanced speckle tracking echocardiography allowing a three-myocardial layer-specifc analysis of deformation parameters. Eur. J. Echocardiogr. 2009, 10, 303–308. [Google Scholar] [CrossRef][Green Version]
- Liu, J.-H.; Chen, Y.; Yuen, M.; Zhen, Z.; Chan, C.W.-S.; Lam, K.S.-L.; Tse, H.-F.; Yiu, K.-H. Incremental prognostic value of global longitudinal strain in patients with type 2 diabetes mellitus. Cardiovasc. Diabetol. 2016, 15, 22. [Google Scholar] [CrossRef]
- Altiok, E.; Neizel, M.; Tiemann, S.; Krass, V.; Kuhr, K.; Becker, M.; Zwicker, C.; Koos, R.; Lehmacher, W.; Kelm, M. Quanti-tative analysis of endocardial and epicardial left ventricular myocardial deformation-comparison of strain-encoded car-diac magnetic resonance imaging with two-dimensional speckle-tracking echocardiography. J. Am. Soc. Echocardiogr. 2012, 25, 1179–1188. [Google Scholar] [CrossRef]
- Bu¨chi, M.; Hess, O.M.; Murakami, T.; Krayenbuehl, H.P. Left ventricular wall stress distribution in chronic pressure and volume overload: Effect of normal and depressed contractility on regional stress-velocity relations. Basic Res. Cardiol. 1990, 85, 367–383. [Google Scholar] [CrossRef]
- Tadic, M.; Cuspidi, C.; Vukomanovic, V.; Ilic, S.; Obert, P.; Kocijancic, V.; Celic, V. Layer-specific deformation of the left ventricle in uncomplicated patients with type 2 diabetes and arterial hypertension. Arch. Cardiovasc. Dis. 2018, 111, 17–24. [Google Scholar] [CrossRef]
- Mansour, M.; AlJaroudi, W.; Mansour, L.; Nehme, A.; Hamoui, O.; Ayoub, W.; Chammas, E. Value of myocardial work for assessment of myocardial adaptation to increased afterload in patients with high blood pressure at peak exercise. Int. J. Cardiovasc. Imaging 2020, 36, 1647–1656. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Huang, J.; Fan, L. Assessment of the subclinical myocardial dysfunction with preserved left ventricle ejection fraction in type 2 diabetes mellitus patients with global myocardial work. Diabetol. Metab. Syndr. 2022, 14, 17. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.H.L.; Bhat, A.; Chandrakumar, D.; Fernandez, F.; Kodsi, M.; Gan, G.; Tan, T.C. Key changes in indices of myo-cardial work in cardiometabolic disease states. Eur. Heart J. 2020, 41, ehaa946-0123. [Google Scholar] [CrossRef]
- Kohata, Y.; Ohara, M.; Nagaike, H.; Fujikawa, T.; Osaka, N.; Goto, S.; Fukase, A.; Kushima, H.; Hiromura, M.; Terasaki, M. Association of Hemoglobin A1c, 1,5-Anhydro-d-Glucitol and Glycated Albumin with Oxidative Stress in Type 2 Dia-betes Mellitus Patients: A Cross-Sectional Study. Diabetes Ther. 2020, 11, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Pignatelli, P.; Carnevale, R.; Pastori, D.; Cangemi, R.; Napoleone, L.; Bartimoccia, S.; Nocella, C.; Basili, S.; Violi, F. Immediate Antioxidant and Antiplatelet Effect of Atorvastatin via Inhibition of Nox2. Circulation 2012, 126, 92–103. [Google Scholar] [CrossRef]
- Karam, B.S.; Chavez-Moreno, A.; Koh, W.; Akar, J.G.; Akar, F.G. Oxidative stress and inflammation as central mediators of atrial fibrillation in obesity and diabetes. Cardiovasc. Diabetol. 2017, 16, 120. [Google Scholar] [CrossRef]
- Trachanas, K.; Sideris, S.; Aggeli, C.; Poulidakis, E.; Gatzoulis, K.; Tousoulis, D.; Kallikazaros, I. Diabetic cardiomyopathy: From pathophysiology to treatment. Hell. J. Cardiol. 2014, 55, 411–421. [Google Scholar]
- Boudina, S.; Abel, E.D. Diabetic cardiomyopathy, causes and effects. Rev. Endocr. Metab. Disord. 2010, 11, 31–39. [Google Scholar] [CrossRef]
- Ahmed, S.S.; Jafer, G.A.; Narang, R.M.; Regan, T.J. Preclinical abnormality of left ventricular function in diabetes mellitus. Am. Heart J. 1975, 89, 153–158. [Google Scholar] [CrossRef]
- Hayat, S.A.; Patel, B.; Khattar, R.S.; Malik, R.A. Diabetic cardiomyopathy: Mechanisms, diagnosis and treatment. Clin. Sci. 2004, 107, 539–557. [Google Scholar] [CrossRef]
- Andreozzi, F.; Succurro, E.; Mancuso, M.R.; Perticone, M.; Sciacqua, A.; Perticone, F.; Sesti, G. Metabolic and cardiovas-cular risk factors in subjects with impaired fasting glucose: The 100 versus 110 mg/dL threshold. Diabetes Metab. Res. Rev. 2007, 23, 547–550. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hyper-tension (ESH). Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- American Diabetes Association. Classification and diagnosis of diabetes. Diabetes Care 2017, 39, S11–S24. [Google Scholar]
- Matsuda, M.; DeFronzo, R.A. Insulin sensitivity indices obtained from oral glucose tolerance testing: Comparison with the euglycemic insulin clamp. Diabetes Care 1999, 22, 1462–1470. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., III; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A New Equation to Estimate Glomerular Filtration Rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [CrossRef] [PubMed]
- Devereux, R.B.; Alonso, D.R.; Lutas, E.M.; Gottlieb, G.J.; Campo, E.; Sachs, I.; Reichek, N. Echocardiographic assessment of left ventricular hypertrophy: Comparison to necropsy findings. Am. J. Cardiol. 1986, 57, 450–458. [Google Scholar] [CrossRef]
- Manganaro, R.; Marchetta, S.; Dulgheru, R.; Ilardi, F.; Sugimoto, T.; Robinet, S.; Cimino, S.; Go, Y.Y.; Bernard, A.; Kacharava, G.; et al. Echocardiographic reference ranges for normal non-invasive myocardial work indices: Results from the EACVI NORRE study. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, T.V.; Sesti, F.; Andreozzi, F.; Pedace, E.; Sciacqua, A.; Hribal, M.L.; Perticone, F.; Sesti, G. One-hour post-load hyperglycemia combined with HbA1c identifies pre-diabetic individuals with a higher cardio-metabolic risk burden. Atherosclerosis 2016, 253, 61–69. [Google Scholar] [CrossRef]
- Bergman, M.; Chetrit, A.; Roth, J.; Dankner, R. One-hour post-load plasma glucose level during the OGTT predicts mor-tality: Observations from the Israel Study of Glucose Intolerance, Obesity and Hypertension. Diabet. Med. 2016, 33, 1060–1066. [Google Scholar] [CrossRef]
- Saunajoki, A.; Auvinen, J.; Bloigu, A.; Ukkola, O.; Keinänen-Kiukaanniemi, S.; Timonen, M. One-hour post-load glucose improves the prediction of cardiovascular events in the OPERA study. Ann. Med. 2021, 53, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.L.; Pareek, M.; Leósdóttir, M.; Eriksson, K.-F.; Nilsson, P.M.; Olsen, M.H. One-hour glucose value as a long-term predictor of cardiovascular morbidity and mortality: The Malmö Preventive Project. Eur. J. Endocrinol. 2018, 178, 225–236. [Google Scholar] [CrossRef]
- Ozedr, A. Lipid profile abnormalities seen in T2DM patients in primary healthcare in Turkey: A cross-sectional study. Lipids Health Dis. 2014, 13, 183. [Google Scholar] [CrossRef] [PubMed]
- Luc, K.; Schramm-Luc, A.; Guzik, T.J.; Mikolajczyk, T.P. Oxidative stress and inflammatory markers in prediabetes and diabetes. J. Physiol. Pharmacol. 2019, 70, 809–824. [Google Scholar]
- Davalli, P.; Mitic, T.; Caporali, A.; Lauriola, A.; D’Arca, D. ROS, Cell Senescence, and Novel Molecular Mechanisms in Aging and Age-Related Diseases. Oxidative Med. Cell. Longev. 2016, 2016, 3565127. [Google Scholar] [CrossRef]
- Fiorentino, T.V.; Prioletta, A.; Zuo, P.; Folli, F. Hyperglycemia-induced oxidative stress and its role in diabetes mellitus re-lated cardiovascular diseases. Curr. Pharm. Des. 2013, 19, 5695–5703. [Google Scholar] [CrossRef]
- Bogdanović, J.; Ašanin, M.; Krljanac, G.; Lalić, N.M.; Jotić, A.; Stanković, S.; Rajković, N.; Stošić, L.; Rasulić, I.; Milin, J.; et al. Impact of acute hyperglycemia on layer-specific left ventricular strain in asymptomatic diabetic patients: An analysis based on two-dimensional speckle tracking echocardiography. Cardiovasc. Diabetol. 2019, 18, 68. [Google Scholar] [CrossRef]
- Ghosh, S.; Lertwattanarak, R.; Lefort, N.; Molina-Carrion, M.; Joya-Galeana, J.; Bowen, B.P.; de Garduno-Garcia, J.J.; Abdul-Ghani, M.; Richardson, A.; DeFronzo, R.A.; et al. Reduction in reactive oxygen species production by mitochon-dria from elderly subjects with normal and impaired glucose tolerance. Diabetes 2011, 60, 2051–2060. [Google Scholar] [CrossRef]
- Aasum, E.; Hafstad, A.D.; Severson, D.L.; Larsen, T.S. Age-Dependent Changes in Metabolism, Contractile Function, and Ischemic Sensitivity in Hearts From db/db Mice. Diabetes 2003, 52, 434–441. [Google Scholar] [CrossRef]
- Boardman, N.; Hafstad, A.D.; Larsen, T.S.; Severson, D.L.; Aasum, E. Increased O2 cost of basal metabolism and excita-tion-contraction coupling in hearts from type 2 diabetic mice. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H1373–H1379. [Google Scholar] [CrossRef]
- Belke, D.D.; Larsen, T.S.; Gibbs, E.M.; Severson, D.L. Altered metabolismcauses cardiac dysfunction in perfused hearts from diabetic (db/db) mice. Am. J. Physiol. Endocrinol. Metab. 2000, 279, E1104–E1113. [Google Scholar] [CrossRef] [PubMed]
- Peterson, L.R.; Herrero, P.; Schechtman, K.B.; Racette, S.; Waggoner, A.D.; Kisrieva-Ware, Z.; Dence, C.; Klein, S.; Marsala, J.; Meyer, T.; et al. Effect of Obesity and Insulin Resistance on Myocardial Substrate Metabolism and Efficiency in Young Women. Circulation 2004, 109, 2191–2196. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.; Khan, A.; Rehman, A. Physiology, Coronary Circulation. In Statpearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Kim, S.A.; Park, S.M.; Kim, M.N.; Shim, W.J. Assessment of left ventricular function by layer-specific strain and its rela-tionship to structural remodelling in patients with hypertension. Can. J. Cardiol. 2016, 32, 211–216. [Google Scholar] [CrossRef] [PubMed]
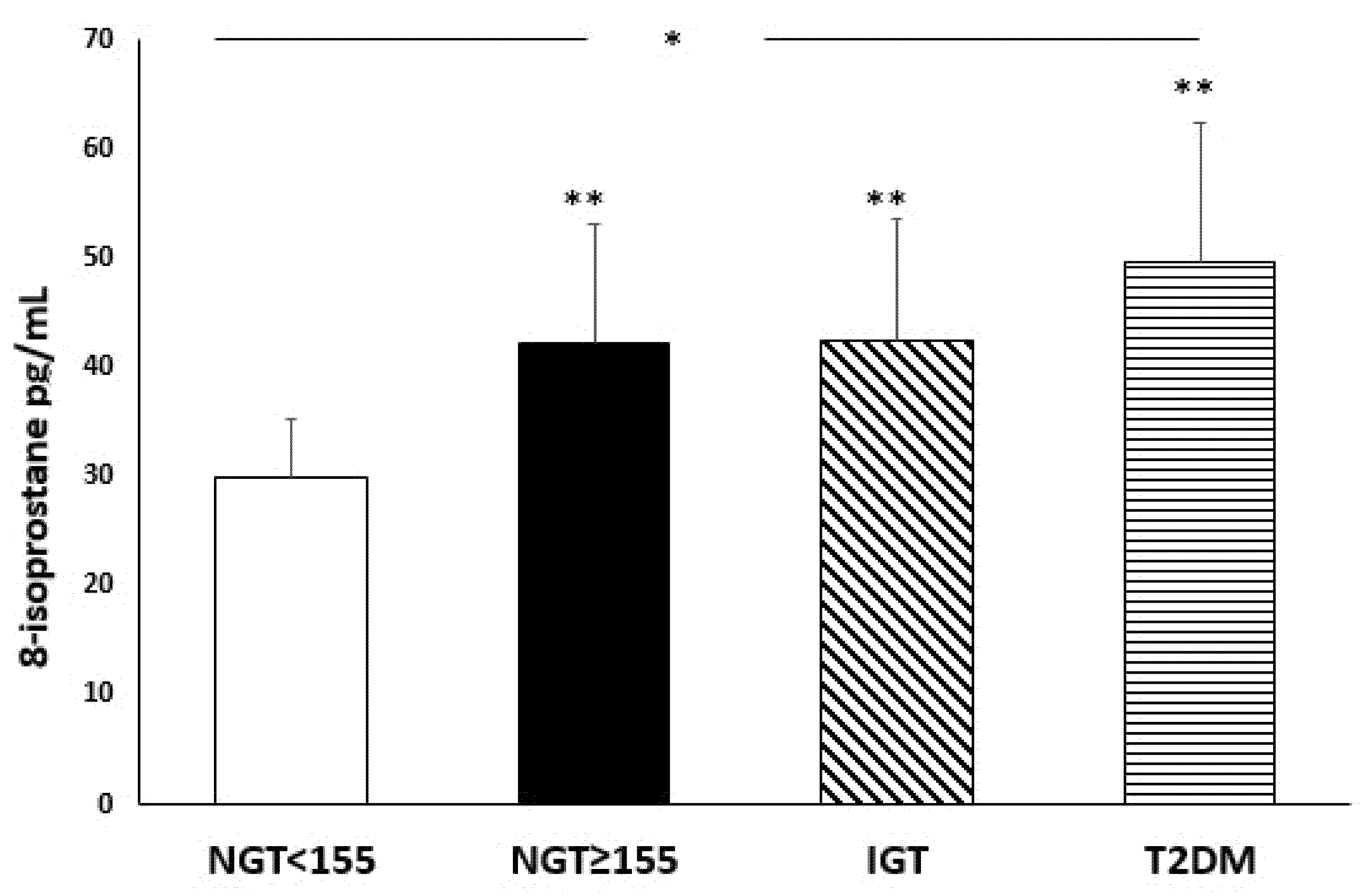
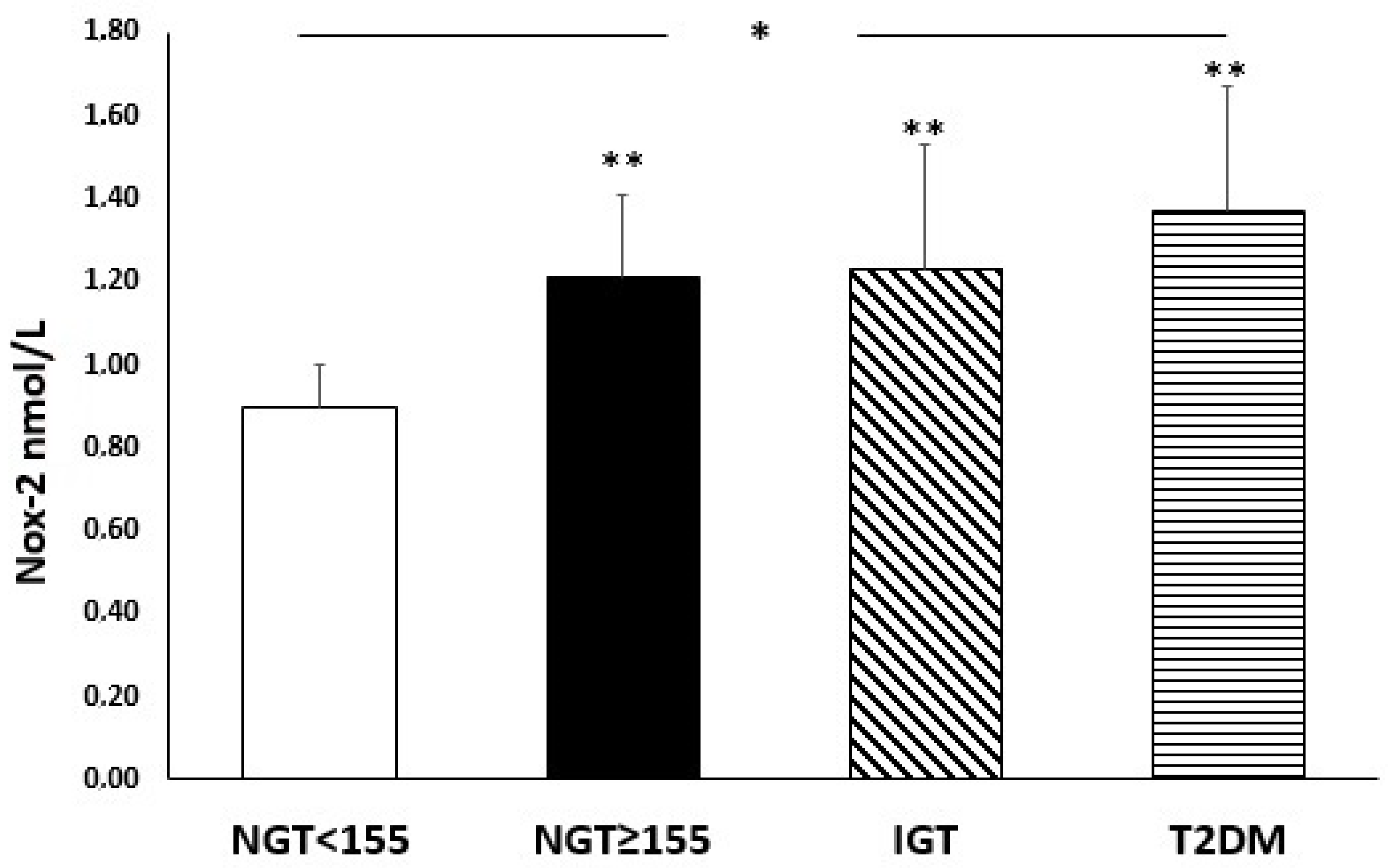
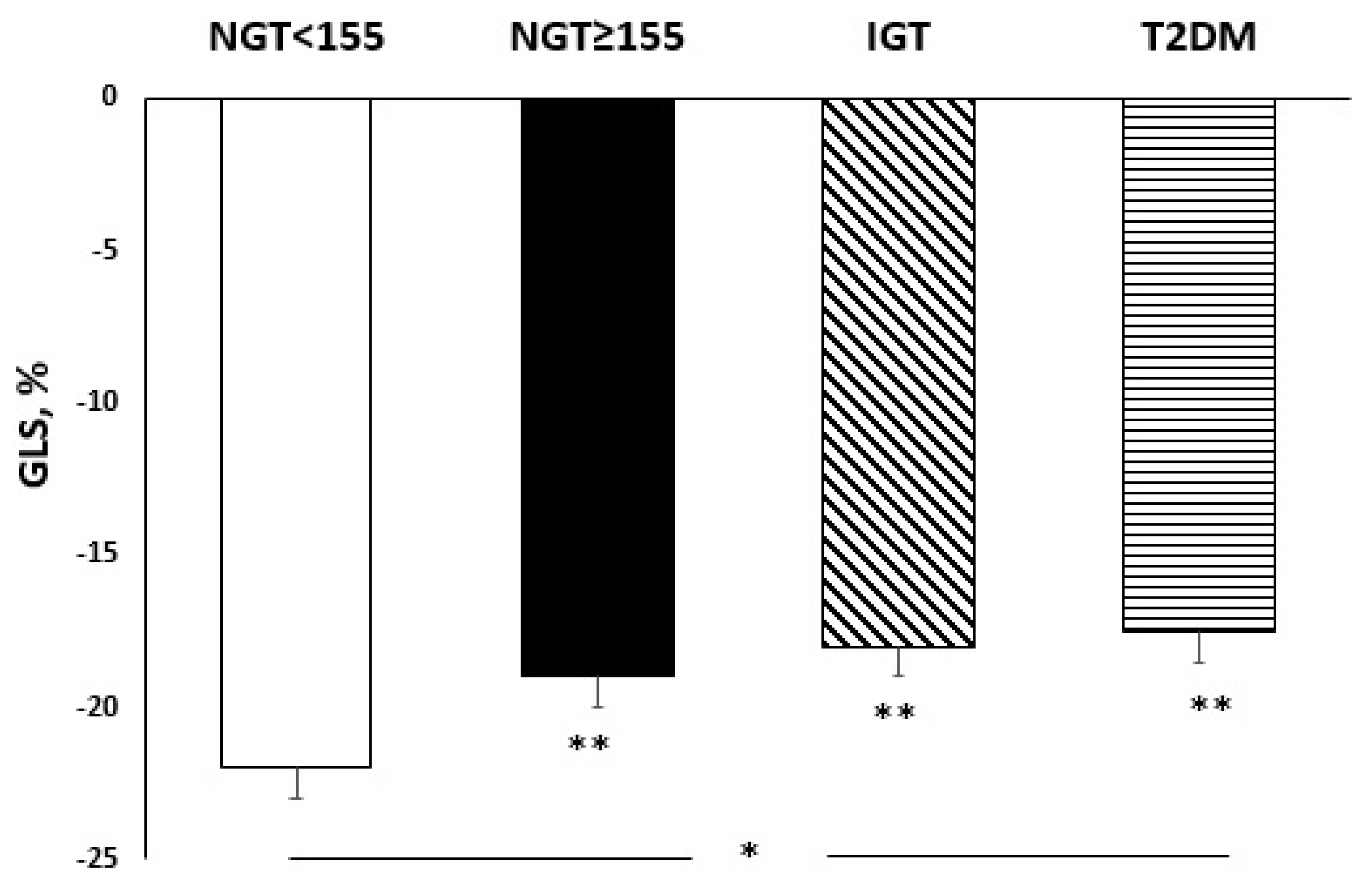
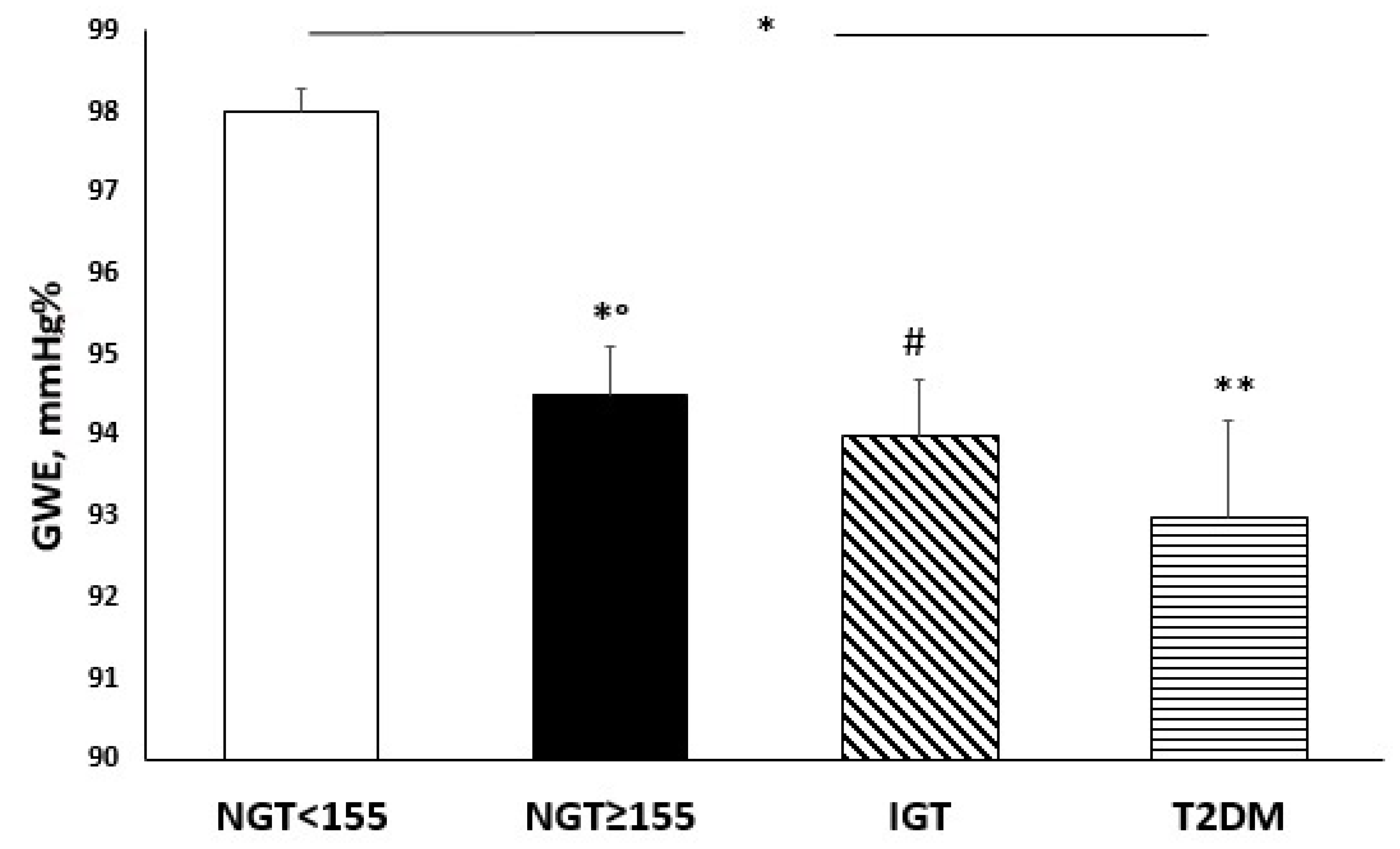
| Variables | All (n = 100) | NGT < 155 (n = 30) | NGT ≥ 155 (n = 24) | IGT (n = 28) | T2DM (ND) (n = 18) | pa |
|---|---|---|---|---|---|---|
| Gender, m/f | 61/39 | 13/17 | 15/9 | 21/7 | 12/6 | 0.080 |
| Age, years | 61.4 ± 10.7 | 58.0 ± 12.2 | 65.0 ± 8.0 | 61.6 ± 11.2 | 62.2 ± 9.4 | 0.120 |
| BMI, Kg/m2 | 30.5 ± 5.0 | 29.6 ± 5.7 | 31.6 ± 4.6 | 31.1 ± 5.1 | 29.7 ± 4.0 | 0.402 |
| SBP, mmHg | 134.4 ± 11.4 | 130 ± 13.0 | 134.6 ± 10.0 | 135.3 ± 9.0 | 139.8 ± 12.0 | 0.031 |
| DBP, mmHg | 79.9 ± 9.6 | 79.0 ± 8.8 | 81.2 ± 10.9 | 79.1 ± 9.8 | 80.8 ± 9.1 | 0.766 |
| PP, mmHg | 54.7 ± 12.2 | 51.1 ± 13.1 | 53.4 ± 13.2 | 56.2 ± 11.1 | 60.4 ± 8.8 | 0.061 |
| HR, bpm | 69.9 ± 6.2 | 69.2 ± 5.3 | 68.6 ± 5.9 | 69.6 ± 5.6 | 73.5 ± 7.8 | 0.054 |
| FPG, mg/dL | 99.2 ± 17.0 | 88.1 ± 11.7 | 95.9 ± 18.7 | 101.5 ± 12.5 | 118.4 ± 9.8 | <0.0001 |
| 1 h glucose, mg/dL | 180.7 ± 47.7 | 127.1 ± 24.1 | 183.9 ± 31.7 | 197.9 ± 21.5 | 238.8 ± 31.0 | <0.0001 |
| 2 h glucose, mg/dL | 154.6 ± 48.3 | 115.9 ± 29.6 | 125.0 ± 27.6 | 174.7 ± 14.6 | 227.4 ± 23.5 | <0.0001 |
| Fasting insulin, µU/mL | 15.5 ± 6.0 | 11.2 ± 3.4 | 14.4 ± 4.3 | 18.7 ± 5.9 | 19.2 ± 6.6 | <0.0001 |
| 1h-insulin, µU/mL | 100.6 ± 50.8 | 79.6 ± 38.4 | 119 ± 31.1 | 113.8 ± 66.3 | 90.4 ± 50.7 | 0.001 |
| 2h-insulin, µU/mL | 98.1 ± 42.0 | 76.8 ± 24.0 | 80.9 ± 18.5 | 119.7 ± 48.1 | 122.8 ± 52.2 | <0.0001 |
| Matsuda/ISI | 50.6 ± 20.9 | 73 ± 15.3 | 49.5 ± 11.2 | 38.0 ± 13.4 | 34.6 ± 16.5 | <0.0001 |
| Total cholesterol, mg/dL | 179.2 ± 39.0 | 189.7 ± 40.0 | 178.8 ± 35.3 | 169.9 ± 34.5 | 176.5 ± 47.0 | 0.280 |
| Triglyceride, mg/dL | 138.1 ± 61.1 | 115.8 ± 31.0 | 123.0 ± 54.1 | 132.5 ± 71.4 | 203.9 ± 46.7 | <0.0001 |
| HDL, mg/dL | 47.9 ± 12.1 | 50.5 ± 12.4 | 50.3 ± 11.9 | 45.5 ± 10.1 | 44.3 ± 13.5 | 0.170 |
| LDL, mg/dL | 118 ± 36.7 | 124.9 ± 41.5 | 118.4 ± 33.5 | 111.4 ± 29.0 | 116.2 ± 43.5 | 0.576 |
| hs-CRP, mg/L | 3.2 ± 2.4 | 1.5 ± 1.1 | 3.4 ± 1.9 | 4.2 ± 3.0 | 4.3 ± 1.7 | <0.0001 |
| e-GFR, mL/min/1.73 m2 | 103.5 ± 25.7 | 119.6 ± 28.0 | 102.3 ± 20.0 | 93.2 ± 21.3 | 94.4 ± 23.1 | <0.0001 |
| Hb, g/dL | 14.0 ± 1.5 | 14.0 ± 1.3 | 14.0 ± 1.6 | 13.4 ± 1.6 | 13.4 ± 1.8 | 0.464 |
| Variables | All (n = 100) | NGT < 155 (n = 30) | NGT ≥ 155 (n = 24) | IGT (n = 28) | T2DM (ND) (n = 18) | pa |
|---|---|---|---|---|---|---|
| LVDD, cm | 5.1 ± 0.4 | 5.1 ± 0.4 | 5.1 ± 0.4 | 5.2 ± 0.3 | 5.2 ± 0.4 | 0.347 |
| dIVS, cm | 1.22 ± 0.1 | 1.1 ± 0.1 | 1.2 ± 0.1 | 1.2 ± 0.1 | 1.3 ± 0.1 | <0.0001 |
| dPW, cm | 1.0 ± 0.1 | 0.9 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 | <0.0001 |
| LVM, g | 198.5 ± 37.2 | 172.7 ± 22.1 | 193.7 ± 30.3 | 209.8 ± 32.4 | 230.2 ± 42.6 | <0.0001 |
| LVMI, g/m2 | 109 ± 19.7 | 94.2 ± 10.8 | 107.3 ± 17.6 | 116.8 ± 15.7 | 124 ± 22.7 | <0.0001 |
| Stroke volume, mL | 78.9 ± 16.4 | 77.6 ± 12.3 | 74.5 ± 16.0 | 82 ± 16.4 | 81.5 ± 22.2 | 0.325 |
| LVEF% | 61.1 ± 4.2 | 62.7 ± 4.4 | 61 ± 4.7 | 60.3 ± 3.3 | 60 ± 3.5 | 0.052 |
| GLS% | −19.4 ± 2.4 | −21.1 ± 1.8 | −19.1 ± 2.2 | −18 ± 1.4 | −18.1 ± 2.1 | <0.0001 |
| GLSendo% | −21.6 ± 2.9 | −24.0 ± 1.5 | −21.7 ± 2.2 | −20.1 ± 2.3 | −19.7 ± 3.7 | <0.0001 |
| GLSepi % | −16.1 ± 2 | −16.1 ± 1.3 | −16.5 ± 2 | −15.5 ± 2 | −16.3 ± 2.8 | 0.340 |
| GLS endo/epi ratio | 1.34 ± 0.2 | 1.5 ± 0.1 | 1.3 ± 0.2 | 1.3 ± 0.1 | 1.2 ± 0.2 | <0.0001 |
| GWI, mmHg % | 2045.7 ± 370.5 | 2096.6 ± 491.4 | 1954.6 ± 285.5 | 2068.4 ± 311.9 | 2047.4 ± 325.0 | <0.0001 |
| GCW, mmHg % | 2114.2 ± 407.1 | 2457.5 ± 419.8 | 2074.4 ± 220.2 | 1944.9 ± 350.2 | 1858.4 ± 279.2 | <0.0001 |
| GWW, mmHg% | 93 ± 46.7 | 55.6 ± 25.6 | 100.3 ± 36.6 | 106.3 ± 44.7 | 125.3 ± 51.3 | <0.0001 |
| GWE, mmHg % | 95.5 (93–97.75) * | 98.0 ± 0.3 | 94.5 ± 0.6 | 94.0 ± 0.7 | 93.0 ± 1.2 | <0.0001 |
| TAPSE, mm | 21.6 ± 3.0 | 24.0 ± 2.8 | 21.5 ± 2.5 | 20.9 ± 2.3 | 19.2 ± 1.9 | <0.0001 |
| s-PAP, mmHg | 31.9 ± 6.8 | 26.4 ± 5.7 | 33.1 ± 6.5 | 33.9 ± 5.4 | 36.3 ± 5.4 | <0.0001 |
| TAPSE/s-PAP, mm/mmHg | 0.7 ± 0.2 | 0.9 ± 0.2 | 0.7 ± 0.2 | 0.6 ± 0.1 | 0.5 ± 0.1 | <0.0001 |
| E, m/s | 0.7 ± 0.2 | 0.9 ± 0.2 | 0.7 ± 0.2 | 0.6 ± 0.1 | 0.7 ± 0.2 | <0.0001 |
| A, m/s | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.8 ± 0.1 | 0.8 ± 0.2 | 1.0 ± 0.2 | <0.0001 |
| E/A | 0.9 ± 0.3 | 1.2 ± 0.3 | 0.9 ± 0.3 | 0.8 ± 0.1 | 0.7 ± 0.1 | <0.0001 |
| E/e’ | 12.9 ± 3.4 | 9.3 ± 2.0 | 13.2 ± 1.5 | 14 ± 2.2 | 16.2 ± 3.2 | <0.0001 |
| LAVI, mL/mq | 33.9 ± 5.0 | 30.4 ± 4.5 | 34.2 ± 3.5 | 34.5 ± 4.9 | 38.7 ± 3.1 | <0.0001 |
| GLS Endo/Epi Ratio | GLS | GWE | |
|---|---|---|---|
| r/p | r/p | r/p | |
| e-GFR, ml/min/1.73 m2 | 0.280/0.002 | −0.184/0.034 | 0.199/0.023 |
| Matsuda/ISI | 0.541/<0.0001 | −0.538/<0.0001 | 0.496/<0.0001 |
| Hb, g/dL | 0.029/0.388 | −0.195/0.026 | 0.347/0.001 |
| 1 h glucose, mg/dL | −0.559/<0.0001 | 0.564/<0.0001 | −0.420/<0.0001 |
| PP, mmHg | −0.086/0.198 | 0.160/0.056 | −0.237/0.009 |
| 8-isoprostane, pg/mL | −0.537/<0.0001 | 0.445/<0.0001 | −0.279/0.002 |
| hs-CRP, mg/L | −0.276/0.003 | 0.370/<0.0001 | −0.465/<0.0001 |
| Nox-2, nmol/L | −0.551/<0.0001 | 0.468/<0.0001 | −0.336/<0.0001 |
| HR, bpm | −0.069/0.249 | 0.140/0.082 | −0.086/0.198 |
| E/e’ | −0.494/<0.0001 | 0.473/<0.0001 | −0.359/<0.0001 |
| LVMI, g/m2 | −0.401/<0.0001 | 0.408/<0.0001 | −0.302/0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cassano, V.; Miceli, S.; Armentaro, G.; Mannino, G.C.; Fiorentino, V.T.; Perticone, M.; Succurro, E.; Hribal, M.L.; Andreozzi, F.; Perticone, F.; et al. Oxidative Stress and Left Ventricular Performance in Patients with Different Glycometabolic Phenotypes. Nutrients 2022, 14, 1299. https://doi.org/10.3390/nu14061299
Cassano V, Miceli S, Armentaro G, Mannino GC, Fiorentino VT, Perticone M, Succurro E, Hribal ML, Andreozzi F, Perticone F, et al. Oxidative Stress and Left Ventricular Performance in Patients with Different Glycometabolic Phenotypes. Nutrients. 2022; 14(6):1299. https://doi.org/10.3390/nu14061299
Chicago/Turabian StyleCassano, Velia, Sofia Miceli, Giuseppe Armentaro, Gaia Chiara Mannino, Vanessa Teresa Fiorentino, Maria Perticone, Elena Succurro, Marta Letizia Hribal, Francesco Andreozzi, Francesco Perticone, and et al. 2022. "Oxidative Stress and Left Ventricular Performance in Patients with Different Glycometabolic Phenotypes" Nutrients 14, no. 6: 1299. https://doi.org/10.3390/nu14061299
APA StyleCassano, V., Miceli, S., Armentaro, G., Mannino, G. C., Fiorentino, V. T., Perticone, M., Succurro, E., Hribal, M. L., Andreozzi, F., Perticone, F., Sesti, G., & Sciacqua, A. (2022). Oxidative Stress and Left Ventricular Performance in Patients with Different Glycometabolic Phenotypes. Nutrients, 14(6), 1299. https://doi.org/10.3390/nu14061299










