Relation of Alcohol Intake to Kidney Function and Mortality Observational, Population-Based, Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Variables in Analyses
2.2. Calculations
2.3. Statistical Analyses
3. Results
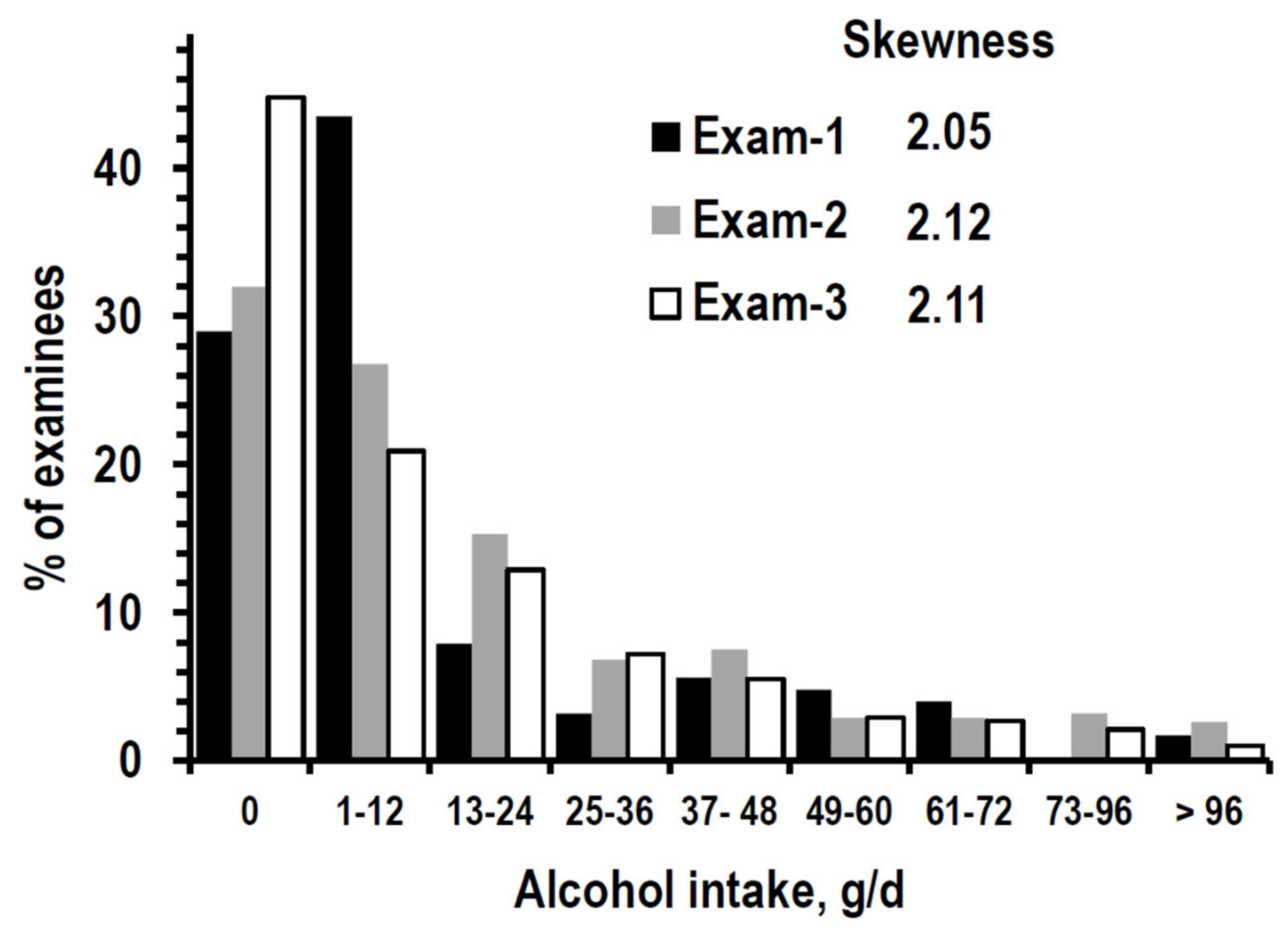
3.1. Descriptive Statistics
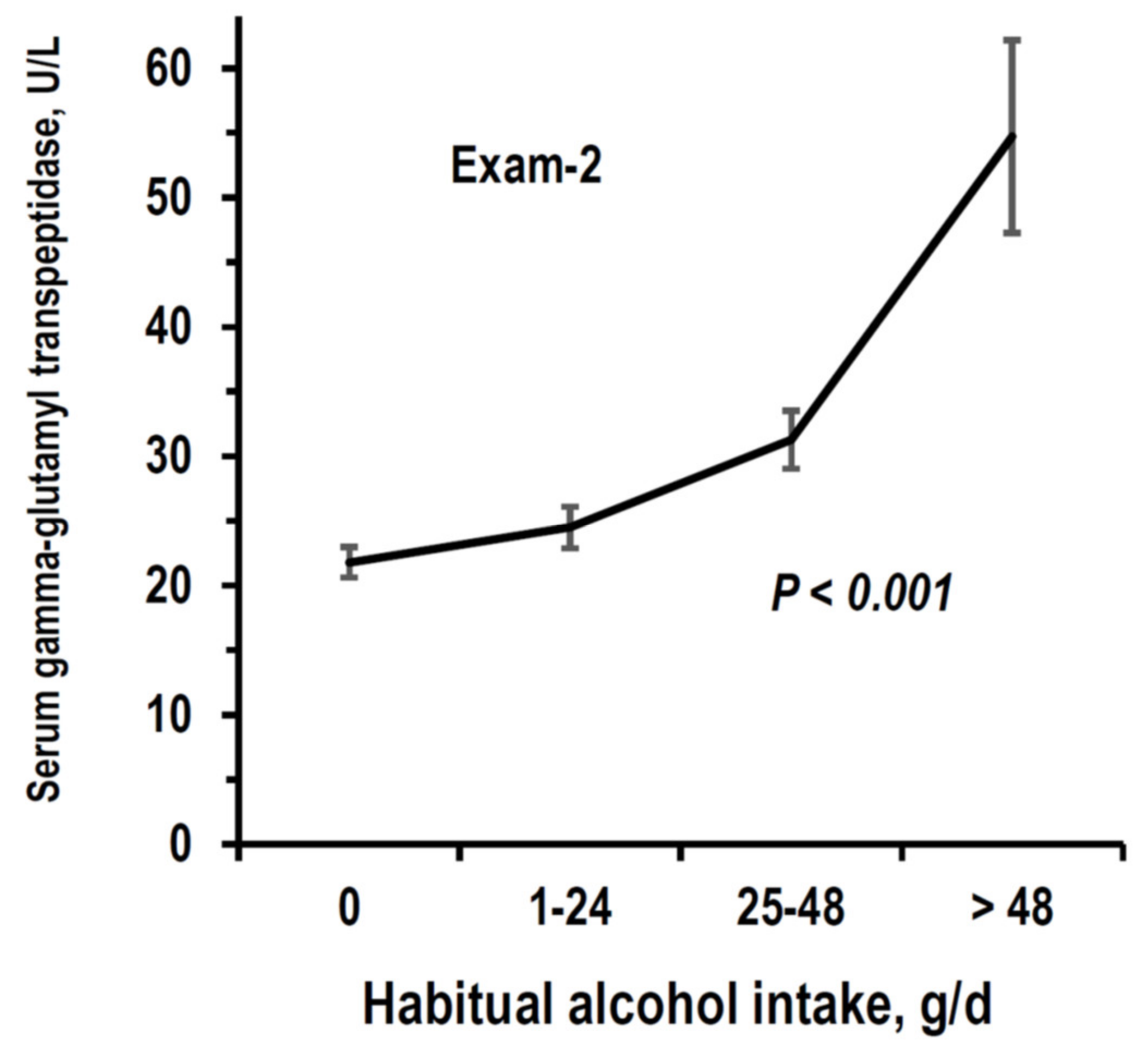
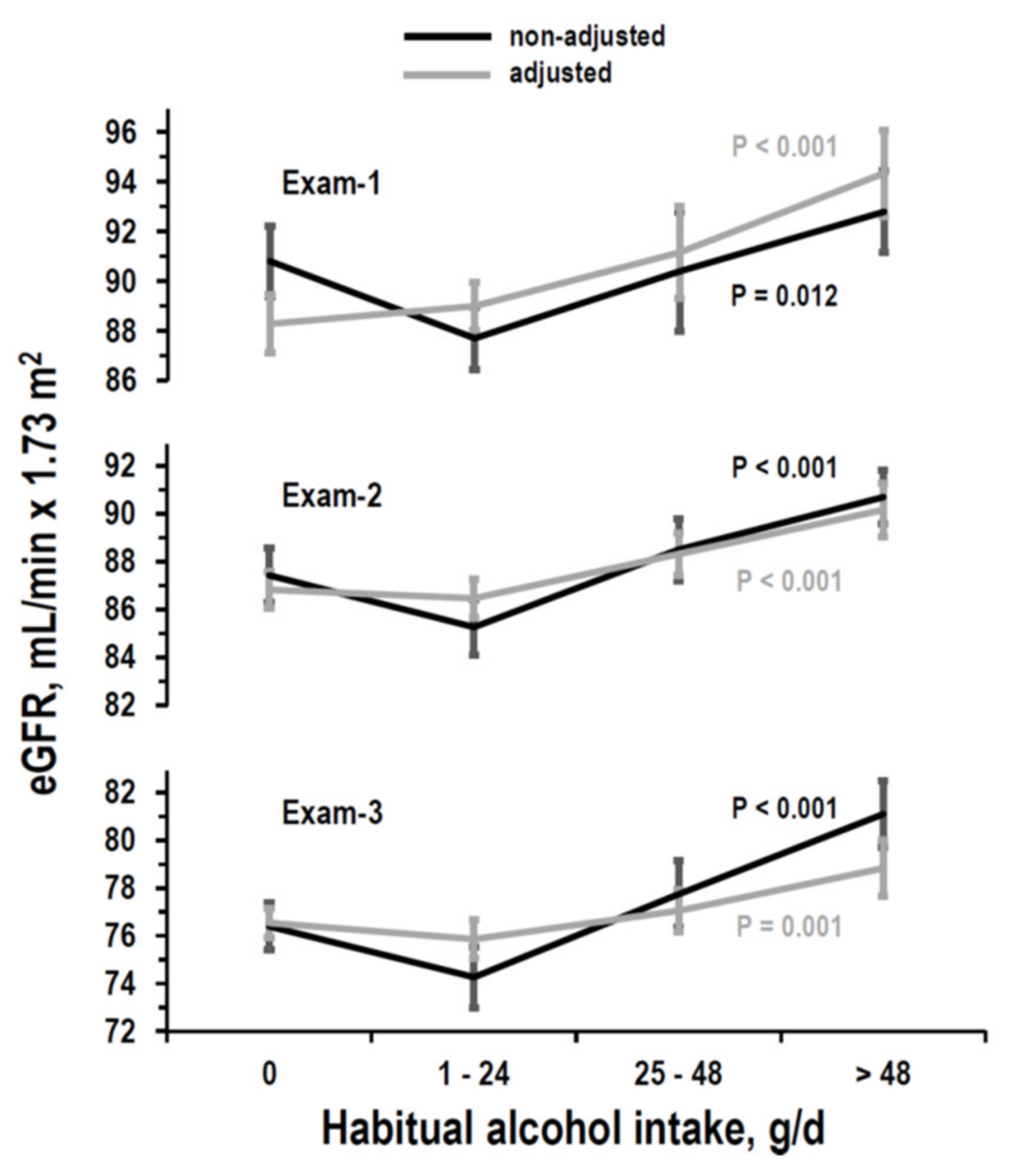
3.2. Cross-Sectional Analyses on eGFR
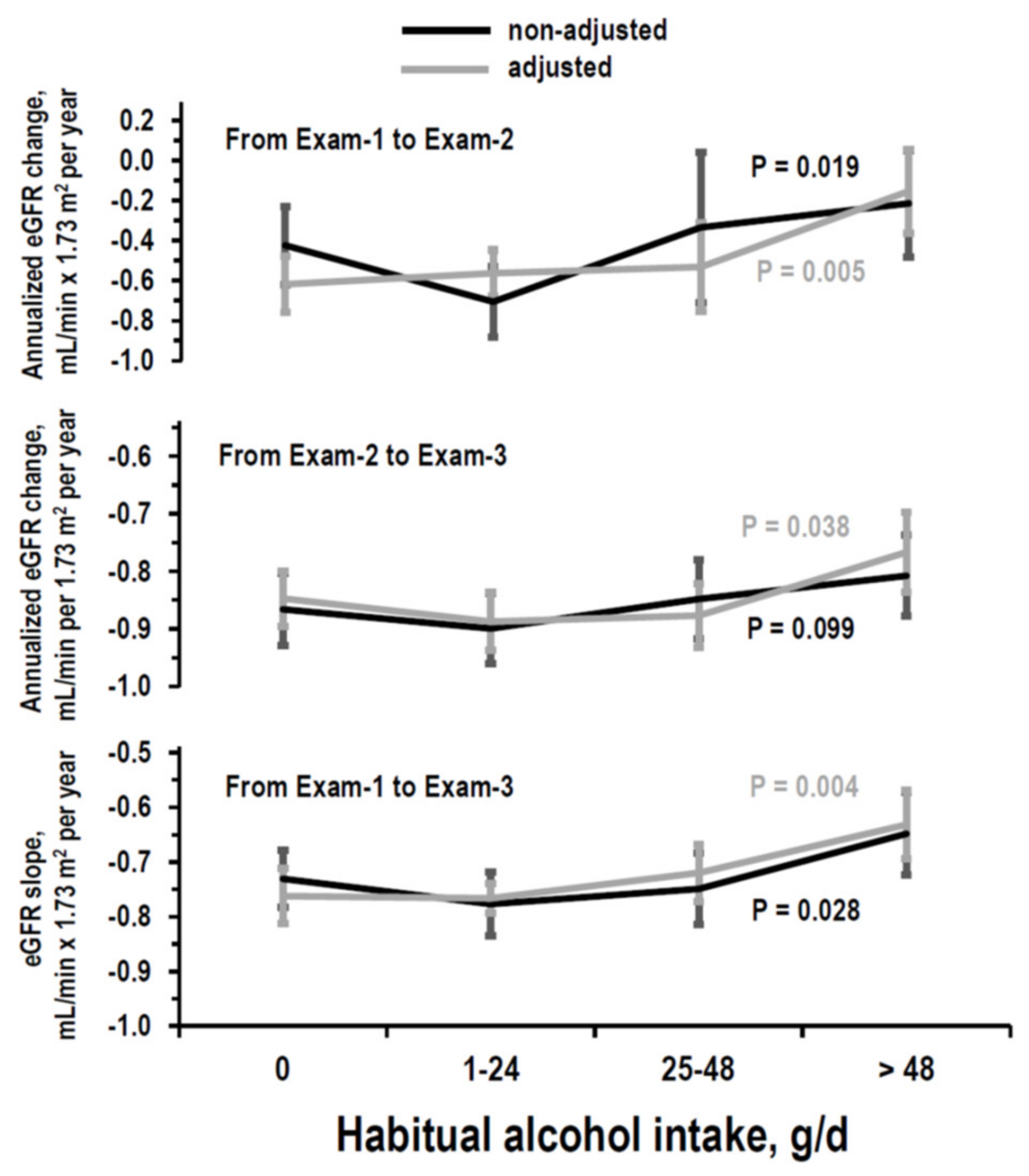
3.3. Longitudinal Analyses on eGFR
3.4. Analysis of Mortality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ikizler, T.A.; Burrowes, J.D.; Byham-Gray, L.D.; Campbell, K.L.; Carrero, J.-J.; Chan, W.; Fouque, D.; Friedman, A.N.; Ghaddar, S.; Goldstein-Fuchs, D.J.; et al. KDOQI Clinical Practice Guideline for Nutrition in CKD: 2020 Update. Am. J. Kidney Dis. 2020, 76 (Suppl. 1), S1–S107. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.T.; Su, G.; Zhang, L.; Qin, X.; Marshall, S.; González-Ortiz, A.; Clase, C.M.; Campbell, K.L.; Xu, H.; Carrero, J.-J. Modifiable Lifestyle Factors for Primary Prevention of CKD: A Systematic Review and Meta-Analysis. J. Am. Soc. Nephrol. 2020, 32, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Knight, E.L.; Stampfer, M.J.; Rimm, E.B.; Hankinson, S.E.; Curhan, G.C. Moderate alcohol intake and renal function decline in women: A prospective study. Nephrol. Dial. Transplant. 2003, 18, 1549–1554. [Google Scholar] [CrossRef] [PubMed]
- Stengel, B.; Tarver-Carr, M.E.; Powe, N.R.; Eberhardt, M.S.; Brancati, F.L. Lifestyle Factors, Obesity and the Risk of Chronic Kidney Disease. Epidemiology 2003, 14, 479–487. [Google Scholar] [CrossRef]
- White, S.L.; Polkinghorne, K.R.; Cass, A.; Shaw, J.E.; Atkins, R.C.; Chadban, S.J. Alcohol consumption and 5-year onset of chronic kidney disease: The AusDiab study. Nephrol. Dial. Transplant. 2009, 24, 2464–2472. [Google Scholar] [CrossRef]
- Menon, V.; Katz, R.; Mukamal, K.; Kestenbaum, B.; de Boer, I.H.; Siscovick, D.S.; Sarnak, M.J.; Shlipak, M.G. Alcohol consumption and kidney function decline in the elderly: Alcohol and Kidney Disease. Nephrol. Dial. Transplant. 2010, 25, 3301–3307. [Google Scholar] [CrossRef]
- Buja, A.; Scafato, E.; Baggio, B.; Sergi, G.; Maggi, S.; Rausa, G.; Basile, A.; Manzato, E.; Ghirini, S.; Perissinotto, E. Renal impairment and moderate alcohol consumption in the elderly. Results from the Italian Longitudinal Study on Aging (ILSA). Public Heal. Nutr. 2011, 14, 1907–1918. [Google Scholar] [CrossRef]
- Foster, M.C.; Hwang, S.-J.; Massaro, J.M.; Jacques, P.F.; Fox, C.S.; Chu, A.Y. Lifestyle Factors and Indices of Kidney Function in the Framingham Heart Study. Am. J. Nephrol. 2015, 41, 267–274. [Google Scholar] [CrossRef]
- Shankar, A.; Klein, R.; Klein, B.E.K. The Association among Smoking, Heavy Drinking, and Chronic Kidney Disease. Am. J. Epidemiol. 2006, 164, 263–271. [Google Scholar] [CrossRef]
- Kanda, E.; Muneyuki, T.; Suwa, K.; Nakajima, K. Alcohol and Exercise Affect Declining Kidney Function in Healthy Males Regardless of Obesity: A Prospective Cohort Study. PLoS ONE 2015, 10, e0134937. [Google Scholar] [CrossRef]
- Pan, C.-S.; Ju, T.R.; Lee, C.C.; Chen, Y.-P.; Hsu, C.-Y.; Hung, D.-Z.; Chen, W.-K.; Wang, I.-K. Alcohol use disorder tied to development of chronic kidney disease: A nationwide database analysis. PLoS ONE 2018, 13, e0203410. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, K.; Ishida, K.; Sairenchi, T.; Takahashi, H.; Ohba, S.; Shiigai, T.; Narita, M.; Koyama, A. Risk factors for chronic kidney disease in a community-based population: A 10-year follow-up study. Kidney Int. 2007, 71, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Kronborg, J.; Solbu, M.; Njolstad, I.; Toft, I.; Eriksen, B.O.; Jenssen, T. Predictors of change in estimated GFR: A population-based 7-year follow-up from the Tromso study. Nephrol. Dial. Transplant. 2008, 23, 2818–2826. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, K.; Gu, D.; Chen, J.; Tang, X.; Yau, C.; Yu, L.; Chen, C.-S.; Wu, X.; Hamm, L.; He, J. Alcohol consumption and the risk of end-stage renal disease among Chinese men. Kidney Int. 2008, 73, 870–876. [Google Scholar] [CrossRef]
- Koning, S.H.; Gansevoort, R.T.; Mukamal, K.J.; Rimm, E.B.; Bakker, S.J.; Joosten, M.M.; PREVEND Study Group. Alcohol consumption is inversely associated with the risk of developing chronic kidney disease. Kidney Int. 2015, 87, 1009–1016. [Google Scholar] [CrossRef]
- Hu, E.A.; Lazo, M.; Rosenberg, S.D.; Grams, M.E.; Steffen, L.M.; Coresh, J.; Rebholz, C.M. Alcohol Consumption and Incident Kidney Disease: Results from the Atherosclerosis Risk in Communities Study. J. Ren. Nutr. 2020, 30, 22–30. [Google Scholar] [CrossRef]
- Alcohol Research: Current Reviews Editorial Staff. Drinking Patterns and Their Definitions. Alcohol Res. 2018, 39, 17–18. [Google Scholar]
- Bloomfield, K.; Stockwell, T.; Gmel, G.; Rehn, N. International Comparisons of Alcohol Consumption. Alcohol Res. Health 2003, 27, 95–109. [Google Scholar]
- Myers, G.L.; Miller, W.G.; Coresh, J.; Fleming, J.; Greenberg, N.; Greene, T.; Hostetter, T.; Levey, A.S.; Panteghini, M.; Welch, M.; et al. Recommendations for improving serum creatinine measurement: A report from the Laboratory Working Group of the National Kidney Disease Education Program. Clin. Chem. 2006, 52, 5–18. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., III; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A New Equation to Estimate Glomerular Filtration Rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- WHO. Global Status Report on Alcohol and Health 2018; World Health Organization: Geneva, Switzerland, 2018; ISBN 978-4-156563-9. [Google Scholar]
- Holmes, A.J.; Anderson, K. Convergence in National Alcohol Consumption Patterns: New Global Indicators. J. Wine Econ. 2017, 12, 117–148. [Google Scholar] [CrossRef]
- Laurenzi, M.; Cirillo, M.; Angeletti, M.; Buongiorno, A.; Morisi, G.; Panarelli, W.; Panfili, M.; Stamler, J.; Terradura-Vagnarelli, O.; Trevisan, M. Guest Editor: Stamler R. Nutr. Metab. Cardiovasc. Dis. 1991, 1, S1–S18. [Google Scholar]
- Cirillo, M.; Terradura-Vagnarelli, O.; Mancini, M.; Menotti, A.; Zanchetti, A.; Laurenzi, M. Cohort profile: The Gubbio Population Study. Int. J. Epidemiol. 2013, 43, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Laurenzi, M.; Cirillo, M.; Vagnarelli, O.T.; Giampaoli, S. A report on the Gubbio Study thirty-eight years after its inception. Panminerva Med. 2021, 63, 410–415. [Google Scholar] [CrossRef]
- Cirillo, M.; Lombardi, C.; Chiricone, D.; De Santo, N.G.; Zanchetti, A.; Bilancio, G. Protein intake and kidney function in the middle-age population: Contrast between cross-sectional and longitudinal data. Nephrol. Dial. Transplant. 2014, 29, 1733–1740. [Google Scholar] [CrossRef]
- Cirillo, M.; Cavallo, P.; Bilancio, G.; Lombardi, C.; Vagnarelli, O.T.; Laurenzi, M. Low Protein Intake in the Population: Low Risk of Kidney Function Decline but High Risk of Mortality. J. Ren. Nutr. 2018, 28, 235–244. [Google Scholar] [CrossRef]
- Cirillo, M.; Bilancio, G.; Cavallo, P.; Palladino, R.; Terradura-Vagnarelli, O.; Laurenzi, M. Sodium intake and kidney function in the general population—Observational, population-based study. Clin. Kidney J. 2021, 14, 647–655. [Google Scholar] [CrossRef]
- Cirillo, M.; Bilancio, G.; Cavallo, P.; Palladino, R.; Zulli, E.; Villa, R.; Veneziano, R.; Laurenzi, M. Urinary Potassium and Kidney Function Decline in the Population—Observational Study. Nutrients 2021, 13, 2747. [Google Scholar] [CrossRef]
- Cirillo, M.; Cavallo, P.; Palladino, R.; Terradura-Vagnarelli, O.; Zulli, E.; Villa, R.; Veneziano, R.; Laurenzi, M. Relationship of the Intake of Water and Other Beverages with Renal Endpoints: Cross-Sectional and Longitudinal Data—Observational, Population-Based Study. J. Ren. Nutr. 2022, 32, 68–77. [Google Scholar] [CrossRef]
- Cirillo, M.; Ciacci, C.; De Santo, N.G. Age, Renal Tubular Phosphate Reabsorption, and Serum Phosphate Levels in Adults. N. Engl. J. Med. 2008, 359, 864–866. [Google Scholar] [CrossRef]
- Chick, J.; Kreitman, N.; Plant, M. Mean cell volume and gamma-glutamyl-transpeptidase as markers of drinking in working men. Lancet 1981, 317, 1249–1251. [Google Scholar] [CrossRef]
- Cogswell, M.E.; Maalouf, J.; Elliott, P.; Loria, C.M.; Patel, S.; Bowman, B.A. Use of Urine Biomarkers to Assess Sodium Intake: Challenges and Opportunities. Annu. Rev. Nutr. 2015, 35, 349–387. [Google Scholar] [CrossRef] [PubMed]
- Bingham, S.A. Urine Nitrogen as a Biomarker for the Validation of Dietary Protein Intake. J. Nutr. 2003, 133 (Suppl. 3), 921S–924S. [Google Scholar] [CrossRef] [PubMed]
- Heymsfield, S.B.; Arteaga, C.; McManus, C.; Smith, J.; Moffitt, S. Measurement of muscle mass in humans: Validity of the 24-hour urinary creatinine method. Am. J. Clin. Nutr. 1983, 37, 478–494. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, M.; Lanti, M.; Menotti, A.; Laurenzi Mancini, M.; Zanchetti, A.; De Santo, N.G. Definition of kidney dysfunction as a cardi-ovascular risk factor: Use of urinary albumin excretion and estimated glomerular filtration. Arch. Intern. Med. 2008, 168, 617–624. [Google Scholar] [CrossRef][Green Version]
- Cirillo, M.; Lombardi, C.; Luciano, M.G.; Bilancio, G.; Anastasio, P.; De Santo, N.G. Estimation of GFR: A Comparison of New and Established Equations. Am. J. Kidney Dis. 2010, 56, 802–804. [Google Scholar] [CrossRef]
- Società Italiana di Nutrizione Umana (SINU) [Italian Society of Human Nutrition (SINU)]. Livelli di Assunzione di Riferimento di Nutrienti (LARN)–IV Revisione [Levels of Reference Consumption of Nutrients (LARN)–Fourth Revision]; Società Italiana di Comunicazione Scientifica e Sanitaria: Rome, Italy, 2014; ISBN 9788890685224. [Google Scholar]
- Ix, J.H.; Wassel, C.L.; Stevens, L.A.; Beck, G.J.; Froissart, M.; Navis, G.; Rodby, R.; Torres, V.E.; Zhang, Y.; Greene, T.; et al. Equations to Estimate Creatinine Excretion Rate: The CKD Epidemiology Collaboration. Clin. J. Am. Soc. Nephrol. 2010, 6, 184–191. [Google Scholar] [CrossRef]
- Naimi, T.S.; Stadtmueller, L.A.; Chikritzhs, T.; Stockwell, T.; Zhao, J.; Britton, A.; Saitz, R.; Sherk, A. Alcohol, Age, and Mortality: Es-timating Selection Bias Due to Premature Death. J. Stud. Alcohol Drugs 2019, 80, 63–68. [Google Scholar] [CrossRef]
- Presti, R.L.; Carollo, C.; Caimi, G. Wine consumption and renal diseases: New perspectives. Nutrition 2007, 23, 598–602. [Google Scholar] [CrossRef]
- Sailer, C.O.; Refardt, J.; Bissig, S.; Bologna, K.; Imber, C.; Christ-Crain, M. Effects of alcohol consumption on copeptin levels and sodium-water homeostasis. Am. J. Physiol. Renal Physiol. 2020, 318, F702–F709. [Google Scholar] [CrossRef]
- Meijer, E.; Bakker, S.J.; Halbesma, N.; de Jong, P.E.; Struck, J.; Gansevoort, R.T. Copeptin, a surrogate marker of vasopressin, is associated with microalbuminuria in a large population cohort. Kidney Int. 2010, 77, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Fragopoulou, E.; Choleva, M.; Antonopoulou, S.; Demopoulos, C.A. Wine and its metabolic effects. A comprehensive review of clinical trials. Metabolism 2018, 83, 102–119. [Google Scholar] [CrossRef] [PubMed]
- Haseeb, S.; Alexander, B.; Baranchuk, A. Wine and Cardiovascular Health: A Comprehensive Review. Circulation 2017, 136, 1434–1448. [Google Scholar] [CrossRef] [PubMed]


| Exam-1 | Exam-2 | Exam-3 | ||
|---|---|---|---|---|
| Date of exam, year | 1983–1985 | 1989–1992 | 2001–2007 | |
| Number of examinees | 2069 | 2069 | 2069 | |
| % men | 42.8% | 42.8% | 42.8% | |
| Age, years | 43 ± 13 | 49 ± 13 | 62 ± 12 | |
| Alcohol intake | g/day | 12 (0/25) | 12 (0/36) | 12 (0/24) |
| % reporting 0 g/day | 29.0% (601) | 32.0% (663) | 44.8% (927) | |
| % reporting 1–24 g/day | 43.5% (899) | 27.3% (565) | 22.8% (472) | |
| % reporting 25–48 g/day | 11.1% (230) | 21.0% (454) | 19.4% (401) | |
| % reporting > 48 g/day | 16.4% (339) | 18.7% (387) | 13.0% (269) | |
| eGFR, mL/min × 1.73 m2 | 91 ± 18 | 88 ± 14 | 77 ± 14 | |
| Covariates | ||||
| Urinary sodium/creatinine, mmol/g | 107 (74/151) | 103 (68/145) | not assessed | |
| Urinary potassium/creatinine, mmol/g | 29 (22/38) | 24 (19/33) | not assessed | |
| Urinary urea nitrogen/creatinine, g/g | not assessed | 9.9 (7.6/12.1) | not assessed | |
| Education, year | 7.6 ± 4.3 | 7.9 ± 4.4 | not assessed | |
| Urinary creatinine, g/24-h | 1.26 ± 0.31 | 1.25 ± 0.31 | 1.18 ± 0.33 | |
| Body mass index, kg/m2 | 26.1 ± 4.2 | 26.8 ± 4.2 | 27.2 ± 4.3 | |
| Systolic pressure, mm Hg | 127 ± 18 | 125 ± 18 | 134 ± 19 | |
| Diastolic pressure, mm Hg | 76 ± 11 | 75 ± 10 | 77 ± 10 | |
| On antihypertensive drug, % (n) | 8.8% (183) | 14.8% (306) | 43.7% (905) | |
| Serum total cholesterol, mg/dL | 206 ± 44 | 219 ± 40 | 216 ± 38 | |
| Smoking, % (n) | 15.6% (322) | 31.7% (656) | 22.2% (459) | |
| Diabetes, % (n) | 1.1% (22) | 3.2% (67) | 8.2% (170) | |
| Number of Examinees | 2069 | |
|---|---|---|
| Men, n (%) | 42.8% (886) | |
| Follow-up duration, years | from Exam-1 to Exam-2 | 5.94 ± 0.97 |
| from Exam-2 to Exam-3 | 13.34 ± 2.08 | |
| from Exam-1 to Exam-3 | 19.3 ± 2.05 | |
| Annualized eGFR change * | from Exam-1 to Exam-2 | −0.51 ± 0.84 |
| from Exam-2 to Exam-3 | −0.85 ± 0.76 | |
| eGFR slope * | from Exam-1 to Exam-3 | −0.74 ± 0.71 |
| Dependent Variable | ||||
|---|---|---|---|---|
| Exam-1 eGFR | Exam-2 eGFR | Exam-3 eGFR | ||
| Model 1 | Model 2 | Model 3 | ||
| Habitual alcohol intake, g/day | 0 (non-drinker) | 0 (reference) | 0 (reference) | 0 (reference) |
| 1–24 | B = 0.707 (−1.53/2.94) p = 0.535 | B = −0.360 (−1.45/0.73) p = 0.515 | B = −0.686 (−1.68/0.31) p = 0.177 | |
| 25–48 | B = 2.869 (1.36/4.38) p < 0.001 | B = 1.491 (0.30/2.68) p = 0.014 | B = 0.505 (−0.61/1.62) p = 0.376 | |
| >48 | B = 6.056 (3.87/8.24) p < 0.001 | B = 3.336 (1.89/4.78) p < 0.001 | B = 2.284 (0.88/3.69) p = 0.001 | |
| Dependent Variable | ||||
|---|---|---|---|---|
| Annualized eGFR Change from Exam-1 to Exam-2 | Annualized eGFR Change from Exam-2 to Exam-3 | eGFR Slope from Exam-1 to Exam-3 | ||
| Model 1 | Model 2 | Model 3 | ||
| Habitual alcohol intake, g/day | 0 (non-drinker) | 0 (reference) | 0 (reference) | 0 (reference) |
| 1–24 | B = 0.055 (−0.13/0.24) p = 0.549 | B = −0.040 (−0.11/0.03) p = 0.249 | B = −0.006 (−0.06/0.05) p = 0.841 | |
| 25–48 | B = 0.086 (−0.18/0.35) p = 0.525 | B = −0.029 (−0.10/0.05) p = 0.437 | B = 0.037 (−0.04/0.11) p = 0.336 | |
| >48 | B = 0.464 (0.20/0.73) p = 0.001 | B = 0.158 (0.07/0.25) p = 0.016 | B = 0.136 (0.05/0.22) p = 0.002 | |
| All Exam-1 Participants | Exam-1 Participants with eGFR = 89–60 mL/min × 1.73 m2 | Exam-1 Participants with eGFR < 60 mL/min × 1.73 m2 | ||
|---|---|---|---|---|
| Number of examinees | 4524 | 2201 | 392 | |
| eGFR range, mL/min × 1.73 m2 | 195–21 | 89–60 | 59–21 | |
| Number of deaths | 992 | 609 | 214 | |
| Patients years product | 91832 | 43241 | 6208 | |
| Habitual alcohol intake, g/day | 0 (non-drinker) | 1 (reference) | 1 (reference) | 1 (reference) |
| 1–24 | HR = 0.767 (0.64/0.91) p = 0.003 | HR = 0.820 (0.65/1.03) p = 0.090 | HR = 0.687 (0.49/0.97) p = 0.033 | |
| 25–48 | HR = 1.100 (0.88/1.37) p = 0.394 | HR = 1.24 (0.95/1.63) 0.112 | HR = 0.802 (0.44/1.46) p = 0.0.941 | |
| >48 | HR = 0.916 (0.74/1.14) p = 0.431 | HR = 0.889 (0.67/1.17) p = 0.405 | HR = 0.973 (0.48/1.99) p = 0.941 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cirillo, M.; Bilancio, G.; Secondulfo, C.; Iesce, G.; Ferrara, C.; Terradura-Vagnarelli, O.; Laurenzi, M. Relation of Alcohol Intake to Kidney Function and Mortality Observational, Population-Based, Cohort Study. Nutrients 2022, 14, 1297. https://doi.org/10.3390/nu14061297
Cirillo M, Bilancio G, Secondulfo C, Iesce G, Ferrara C, Terradura-Vagnarelli O, Laurenzi M. Relation of Alcohol Intake to Kidney Function and Mortality Observational, Population-Based, Cohort Study. Nutrients. 2022; 14(6):1297. https://doi.org/10.3390/nu14061297
Chicago/Turabian StyleCirillo, Massimo, Giancarlo Bilancio, Carmine Secondulfo, Gennaro Iesce, Carmela Ferrara, Oscar Terradura-Vagnarelli, and Martino Laurenzi. 2022. "Relation of Alcohol Intake to Kidney Function and Mortality Observational, Population-Based, Cohort Study" Nutrients 14, no. 6: 1297. https://doi.org/10.3390/nu14061297
APA StyleCirillo, M., Bilancio, G., Secondulfo, C., Iesce, G., Ferrara, C., Terradura-Vagnarelli, O., & Laurenzi, M. (2022). Relation of Alcohol Intake to Kidney Function and Mortality Observational, Population-Based, Cohort Study. Nutrients, 14(6), 1297. https://doi.org/10.3390/nu14061297





