Maternal Vitamin D Status Correlates to Leukocyte Antigenic Responses in Breastfeeding Infants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Clinical Study
2.2. Sample Collection and Vitamin D Measurements
2.3. Plasma Cytokine Measurements
2.4. In Vitro Antigenic Stimulation and Cytokine Measurements
2.5. Statistics and Data Analyses
3. Results
3.1. Clinical Study of vitD Supplementation
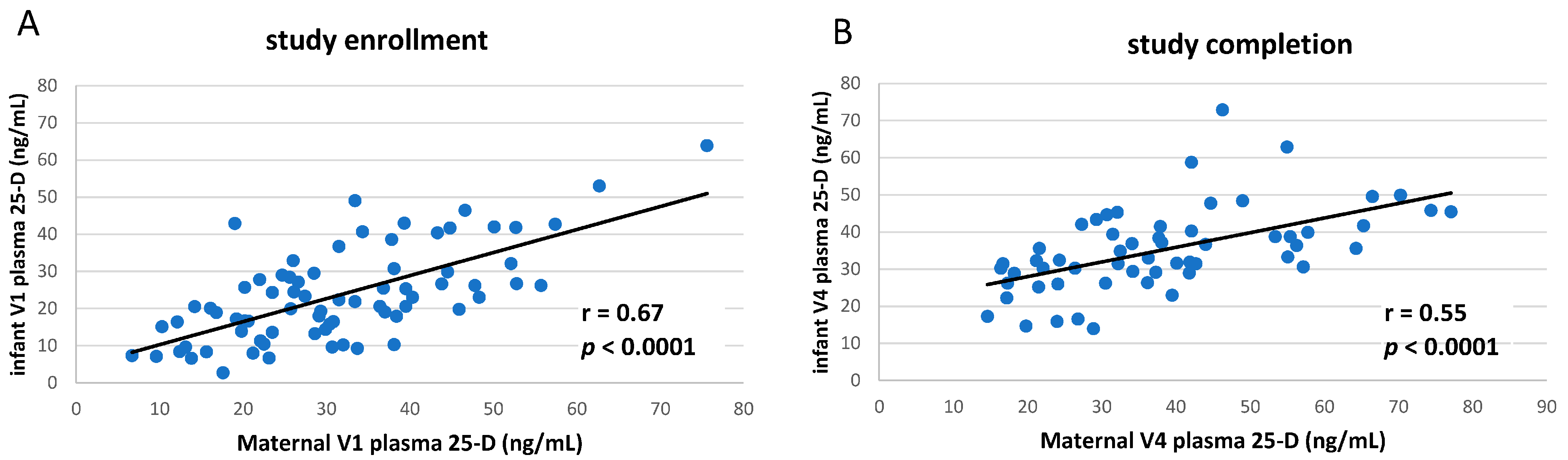
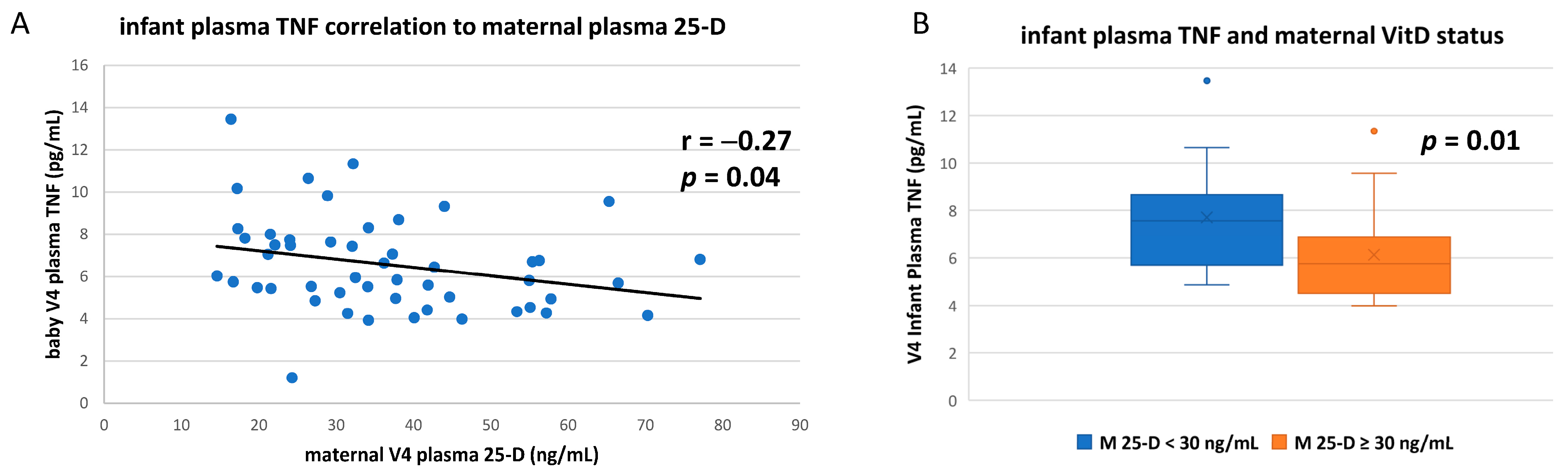
3.2. Circulating TNF Concentrations in Breastfeeding Infants Were Inversely Correlated to Maternal Vitamin D Status
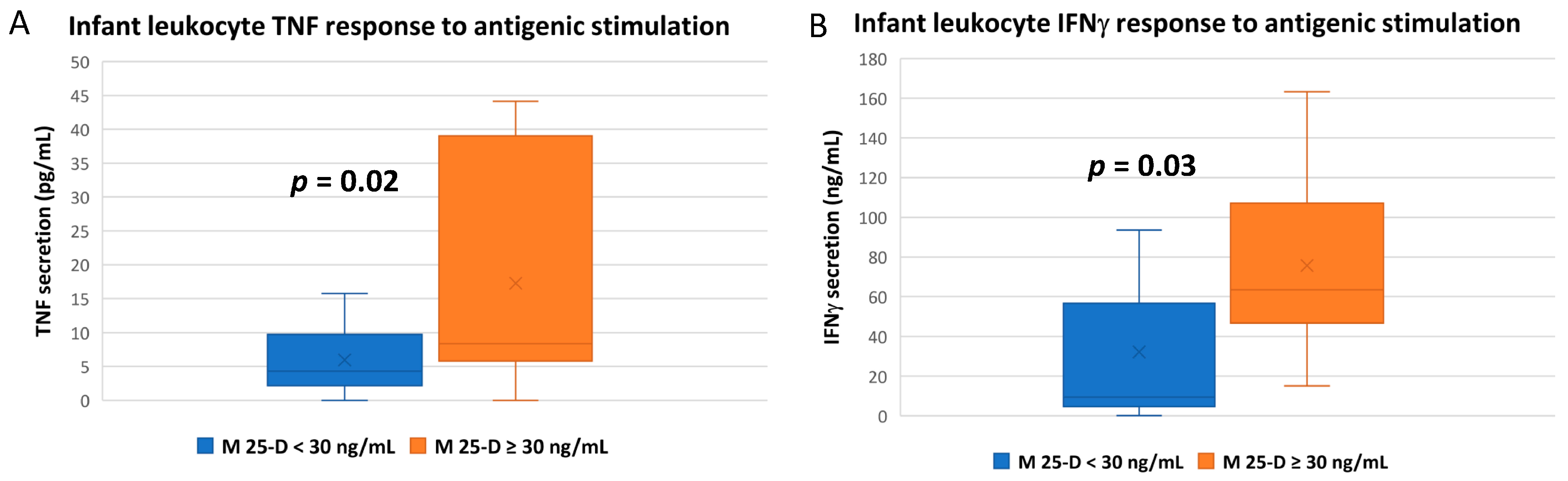
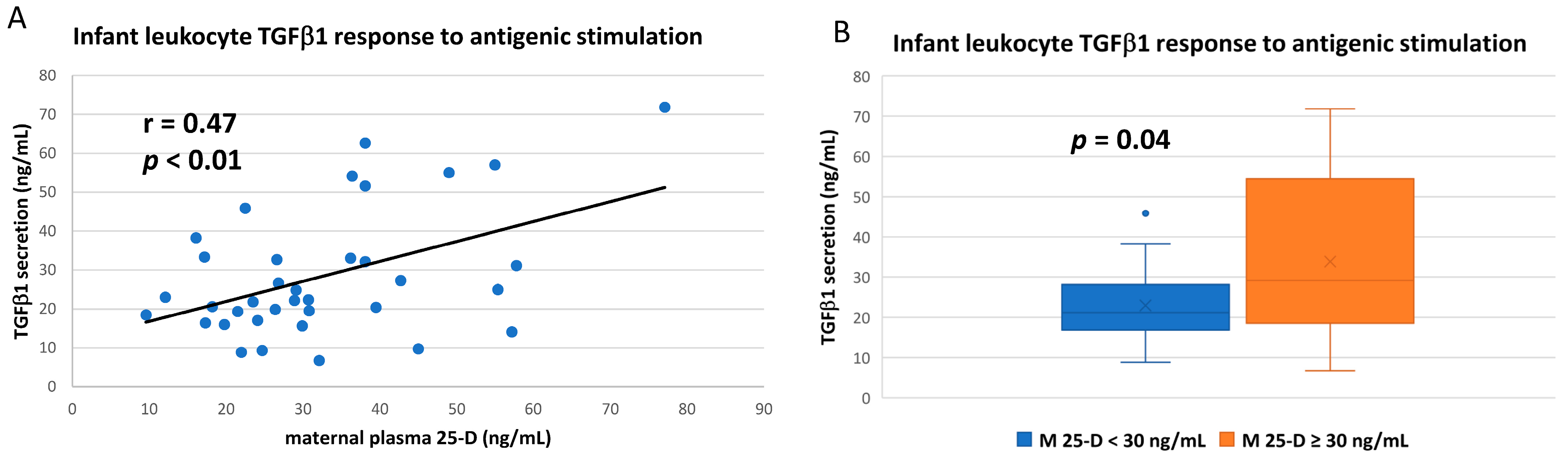
3.3. Numerous Cytokine Responses after In Vitro Antigenic Stimulation of Breastfeeding Infant Leukocytes Were Correlated to Maternal Vitamin D Status
3.4. IL-10 and IL-12 Responses after In Vitro Antigenic Stimulation of Breastfeeding Infant Leukocytes Were Correlated to Infant’s Own vitD Status
3.5. Some Antigenic Responses Had No Apparent Relationship to Either Maternal or Infant vitD Status
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wagner, C.; Taylor, S.; Hollis, B. New Insights into Vitamin D during Pregnancy, Lactation and Early Infancy, 1st ed.; Hale Publishers: Amarillo, TX, USA, 2010. [Google Scholar]
- Hollis, B.W.; Wagner, C.L. Vitamin D and pregnancy: Skeletal effects, nonskeletal effects, and birth outcomes. Calcif. Tissue Int. 2013, 92, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Pludowski, P.; Holick, M.F.; Pilz, S.; Wagner, C.L.; Hollis, B.W.; Grant, W.B.; Shoenfeld, Y.; Lerchbaum, E.; Llewellyn, D.J.; Kienreich, K.; et al. Vitamin D effects on musculoskeletal health, immunity, autoimmunity, cardiovascular disease, cancer, fertility, pregnancy, dementia and mortality—A review of recent evidence. Autoimmun. Rev. 2013, 12, 976–989. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D regulation of immune function. Vitam. Horm. 2011, 86, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J. Vitamin D cell signalling in health and disease. Biochem. Biophys. Res. Commun. 2015, 460, 53–71. [Google Scholar] [CrossRef] [PubMed]
- Al-Beltagi, M.; Rowiesha, M.; Elmashad, A.; Elrifaey, S.M.; Elhorany, H.; Koura, H.G. Vitamin D status in preterm neonates and the effects of its supplementation on respiratory distress syndrome. Pediatr. Pulmonol. 2020, 55, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Park, H.W.; Lim, G.; Park, Y.-M.; Chang, M.; Son, J.S.; Lee, R. Association between vitamin D level and bronchopulmonary dysplasia: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0235332. [Google Scholar] [CrossRef]
- Hibbs, A.M.; Wagner, C.L.; Tatsuoka, C. Vitamin D Supplementation in Young Infants and Recurrent Wheezing-Reply. JAMA 2018, 320, 1708–1709. [Google Scholar] [CrossRef] [PubMed]
- Hansdottir, S.; Monick, M.M.; Lovan, N.; Powers, L.; Gerke, A.; Hunninghake, G.W. Vitamin D decreases respiratory syncytial virus induction of NF-kappaB-linked chemokines and cytokines in airway epithelium while maintaining the antiviral state. J. Immunol. 2010, 184, 965–974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belderbos, M.E.; Houben, M.L.; Wilbrink, B.; Lentjes, E.; Bloemen, E.M.; Kimpen, J.L.; Rovers, M.; Bont, L. Cord blood vitamin D deficiency is associated with respiratory syncytial virus bronchiolitis. Pediatrics 2011, 127, e1513–e1520. [Google Scholar] [CrossRef]
- Hollams, E.M.; Hart, P.H.; Holt, B.J.; Serralha, M.; Parsons, F.; de Klerk, N.H.; Zhang, G.; Sly, P.D.; Holt, P.G. Vitamin D and atopy and asthma phenotypes in children: A longitudinal cohort study. Eur. Respir. J. 2011, 38, 1320–1327. [Google Scholar] [CrossRef]
- Jones, A.P.; D’Vaz, N.; Meldrum, S.; Palmer, D.J.; Zhang, G.; Prescott, S.L. 25-hydroxyvitamin D3 status is associated with developing adaptive and innate immune responses in the first 6 months of life. Clin. Exp. Allergy 2015, 45, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Hollis, B.W.; Wagner, C.L.; Howard, C.R.; Ebeling, M.; Shary, J.R.; Smith, P.G.; Taylor, S.N.; Morella, K.; Lawrence, R.A.; Hulsey, T.C. Maternal Versus Infant Vitamin D Supplementation During Lactation: A Randomized Controlled Trial. Pediatrics 2015, 136, 625–634. [Google Scholar] [CrossRef] [Green Version]
- Wagner, C.L.; Hulsey, T.C.; Ebeling, M.; Shary, J.R.; Asghari, G.; Howard, C.R.; Baatz, J.E.; Newton, D.A.; Wahlquist, A.E.; Reed, S.G.; et al. Safety Aspects of a Randomized Clinical Trial of Maternal and Infant Vitamin D Supplementation by Feeding Type Through 7 Months Postpartum. Breastfeed. Med. Off. J. Acad. Breastfeed. Med. 2020, 15, 765–775. [Google Scholar] [CrossRef] [PubMed]
- Wagner, C.L.; Hulsey, T.C.; Fanning, D.; Ebeling, M.; Hollis, B.W. High-dose vitamin D3 supplementation in a cohort of breastfeeding mothers and their infants: A 6-month follow-up pilot study. Breastfeed. Med. Off. J. Acad. Breastfeed. Med. 2006, 1, 59–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hollis, B.W.; Wagner, C.L. Vitamin D requirements during lactation: High-dose maternal supplementation as therapy to prevent hypovitaminosis D for both the mother and the nursing infant. Am. J. Clin. Nutr. 2004, 80, 1752s–1758s. [Google Scholar] [CrossRef] [PubMed]
- Hollis, B.W.; Roos, B.A.; Draper, H.H.; Lambert, P.W. Vitamin D and its metabolites in human and bovine milk. J. Nutr. 1981, 111, 1240–1248. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Vitamin D metabolism, mechanism of action, and clinical applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef] [Green Version]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metabol. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [Green Version]
- Adams, J.S.; Hewison, M. Extrarenal expression of the 25-hydroxyvitamin D-1-hydroxylase. Arch. Biochem. Biophys. 2012, 523, 95–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zehnder, D.; Bland, R.; Williams, M.C.; McNinch, R.W.; Howie, A.J.; Stewart, P.M.; Hewison, M. Extrarenal expression of 25-hydroxyvitamin d(3)-1 alpha-hydroxylase. J. Clin. Endocrinol. Metabol. 2001, 86, 888–894. [Google Scholar] [CrossRef] [Green Version]
- Ramagopalan, S.V.; Heger, A.; Berlanga, A.J.; Maugeri, N.J.; Lincoln, M.R.; Burrell, A.; Handunnetthi, L.; Handel, A.E.; Disanto, G.; Orton, S.M.; et al. A ChIP-seq defined genome-wide map of vitamin D receptor binding: Associations with disease and evolution. Genome Res. 2010, 20, 1352–1360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, C.M.; Gillespie, S.L.; Thiele, D.K.; Ralph, J.L.; Ohm, J.E. Effects of Maternal Vitamin D Supplementation on the Maternal and Infant Epigenome. Breastfeed. Med. 2018, 13, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Long, M.D.; Sucheston-Campbell, L.E.; Campbell, M.J. Vitamin D receptor and RXR in the post-genomic era. J. Cell Physiol. 2015, 230, 758–766. [Google Scholar] [CrossRef] [Green Version]
- Wagner, C.L.; Eidelman, A.I. The Impact of Vitamin D on the Maternal and Infant Epigenome: The Role of Pregnancy and Breastfeeding. Breastfeed. Med. Off. J. Acad. Breastfeed. Med. 2018, 13, 305–306. [Google Scholar] [CrossRef] [PubMed]
- Sambandam, Y.; Reddy, S.V.; Mulligan, J.L.; Voelkel-Johnson, C.; Wagner, C.L. Vitamin D Modulation of TRAIL Expression in Human Milk and Mammary Epithelial Cells. Sci. Rep. 2017, 7, 4362. [Google Scholar] [CrossRef] [Green Version]
- Specker, B.L.; Tsang, R.C.; Hollis, B.W. Effect of race and diet on human-milk vitamin D and 25-hydroxyvitamin D. Am. J. Dis. Child. 1985, 139, 1134–1137. [Google Scholar] [CrossRef]
- Wagner, C.L.; Taylor, S.N.; Hollis, B.W. Does vitamin D make the world go ‘round’? Breastfeed. Med. Off. J. Acad. Breastfeed. Med. 2008, 3, 239–250. [Google Scholar] [CrossRef] [Green Version]
- Wagner, C.L.; Taylor, S.N.; Johnson, D. Host factors in amniotic fluid and breast milk that contribute to gut maturation. Clin. Rev. Allergy Immunol. 2008, 34, 191–204. [Google Scholar] [CrossRef]
- Hollis, B.W.; Kamerud, J.Q.; Selvaag, S.R.; Lorenz, J.D.; Napoli, J.L. Determination of vitamin D status by radioimmunoassay with an 125I-labeled tracer. Clin. Chem. 1993, 39, 529–533. [Google Scholar] [CrossRef]
- Ai, W.; Li, H.; Song, N.; Li, L.; Chen, H. Optimal method to stimulate cytokine production and its use in immunotoxicity assessment. Int. J. Environ. Res. Public Health 2013, 10, 3834–3842. [Google Scholar] [CrossRef] [Green Version]
- Damsgaard, C.T.; Lauritzen, L.; Calder, P.C.; Kjaer, T.M.; Frøkiaer, H. Whole-blood culture is a valid low-cost method to measure monocytic cytokines—A comparison of cytokine production in cultures of human whole-blood, mononuclear cells and monocytes. J. Immunol. Methods 2009, 340, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Thurm, C.W.; Halsey, J.F. Measurement of cytokine production using whole blood. Curr. Protoc. Immunol. 2005, 66, 7–18. [Google Scholar] [CrossRef]
- Roberts, A.; Sporn, M. The transforming growth factor-βs. In Peptide Growth Factors and Their Receptors I; Springer: New York, NY, USA, 1991; pp. 419–472. [Google Scholar]
- Amegah, A.K.; Klevor, M.K.; Wagner, C.L. Maternal vitamin D insufficiency and risk of adverse pregnancy and birth outcomes: A systematic review and meta-analysis of longitudinal studies. PLoS ONE 2017, 12, e0173605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bodnar, L.M.; Simhan, H.N. Vitamin D may be a link to black-white disparities in adverse birth outcomes. Obstetr. Gynecol. Surv. 2010, 65, 273–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cetinkaya, M.; Cekmez, F.; Buyukkale, G.; Erener-Ercan, T.; Demir, F.; Tunc, T.; Aydin, F.N.; Aydemir, G. Lower vitamin D levels are associated with increased risk of early-onset neonatal sepsis in term infants. J. Perinatol. Off. J. Calif. Perinat. Assoc. 2015, 35, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Dawodu, A.; Wagner, C.L. Prevention of vitamin D deficiency in mothers and infants worldwide—A paradigm shift. Paediatr. Int. Child. Health 2012, 32, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Hewison, M.; Adams, J.S. Vitamin D insufficiency and skeletal development in utero. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2010, 25, 11–13. [Google Scholar] [CrossRef] [PubMed]
- Hollis, B.W.; Johnson, D.; Hulsey, T.C.; Ebeling, M.; Wagner, C.L. Vitamin D supplementation during pregnancy: Double-blind, randomized clinical trial of safety and effectiveness. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2011, 26, 2341–2357. [Google Scholar] [CrossRef] [Green Version]
- Hollis, B.W.; Wagner, C.L. Vitamin D supplementation during pregnancy: Improvements in birth outcomes and complications through direct genomic alteration. Mol. Cell. Endocrinol. 2017, 453, 113–130. [Google Scholar] [CrossRef]
- Quaresima, P.; Angeletti, M.; Luziatelli, D.; Luziatelli, S.; Venturella, R.; Di Carlo, C.; Bernardo, S. Pregnancy associated transient osteoporosis of the hip (PR-TOH): A non-obstetric indication to caesarean section. A case report with literature review. Eur. J. Obstetr. Gynecol. Reprod. Biol. 2021, 262, 28–35. [Google Scholar] [CrossRef]
- Wagner, C.L.; Taylor, S.N.; Dawodu, A.; Johnson, D.D.; Hollis, B.W. Vitamin D and its role during pregnancy in attaining optimal health of mother and fetus. Nutrients 2012, 4, 208–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassiotou, F.; Geddes, D.T. Immune cell-mediated protection of the mammary gland and the infant during breastfeeding. Adv. Nutr. 2015, 6, 267–275. [Google Scholar] [CrossRef] [Green Version]
- Hewison, M.; Wagner, C.L.; Hollis, B.W. Vitamin D Supplementation in Pregnancy and Lactation and Infant Growth. N. Engl. J. Med. 2018, 379, 1880–1881. [Google Scholar] [CrossRef]
- Pfeffer, P.E.; Hawrylowicz, C.M. Vitamin D in Asthma: Mechanisms of Action and Considerations for Clinical Trials. Chest 2018, 153, 1229–1239. [Google Scholar] [CrossRef] [Green Version]
- Roth, D.E.; Morris, S.K.; Zlotkin, S.; Gernand, A.D.; Ahmed, T.; Shanta, S.S.; Papp, E.; Korsiak, J.; Shi, J.; Islam, M.M.; et al. Vitamin D Supplementation in Pregnancy and Lactation and Infant Growth. N. Engl. J. Med. 2018, 379, 535–546. [Google Scholar] [CrossRef]
- Khatiwada, A.; Wolf, B.J.; Mulligan, J.K.; Shary, J.R.; Hewison, M.; Baatz, J.E.; Newton, D.A.; Hawrylowicz, C.; Hollis, B.W.; Wagner, C.L. Effects of vitamin D supplementation on circulating concentrations of growth factors and immune-mediators in healthy women during pregnancy. Pediatr. Res. 2020, 89, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Wagner, C.L.; Baatz, J.E.; Newton, D.; Hollis, B.W. Analytical considerations and general diagnostic and therapeutic ramifications of milk hormones during lactation. Best Pract. Res. Clin. Endocrinol. Metabol. 2018, 32, 5–16. [Google Scholar] [CrossRef]
- Newton, D.A.; Baatz, J.E.; Kindy, M.S.; Gattoni-Celli, S.; Shary, J.R.; Hollis, B.W.; Wagner, C.L. Vitamin D binding protein polymorphisms significantly impact vitamin D status in children. Pediatr. Res. 2019, 86, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Wagner, C.L.; Taylor, S.N.; Johnson, D.D.; Hollis, B.W. The role of vitamin D in pregnancy and lactation: Emerging concepts. Womens Health 2012, 8, 323–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sedger, L.M.; McDermott, M.F. TNF and TNF-receptors: From mediators of cell death and inflammation to therapeutic giants—past, present and future. Cytokine Growth Factor Rev. 2014, 25, 453–472. [Google Scholar] [CrossRef] [Green Version]
- Cawthorn, W.P.; Sethi, J.K. TNF-alpha and adipocyte biology. FEBS Lett. 2008, 582, 117–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sethi, J.K.; Hotamisligil, G.S. Metabolic Messengers: Tumour necrosis factor. Nat. Metabol. 2021, 3, 1302–1312. [Google Scholar] [CrossRef]
- Davanzo, R.; Zauli, G.; Monasta, L.; Vecchi Brumatti, L.; Abate, M.V.; Ventura, G.; Rimondi, E.; Secchiero, P.; Demarini, S. Human colostrum and breast milk contain high levels of TNF-related apoptosis-inducing ligand (TRAIL). J. Hum. Lact. Off. J. Int. Lact. Consult. Assoc. 2013, 29, 23–25. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Leung, D.Y.M.; Richers, B.N.; Liu, Y.; Remigio, L.K.; Riches, D.W.; Goleva, E. Vitamin D Inhibits Monocyte/Macrophage Proinflammatory Cytokine Production by Targeting MAPK Phosphatase-1. J. Immunol. 2012, 188, 2127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivashkiv, L.B. IFNγ: Signalling, epigenetics and roles in immunity, metabolism, disease and cancer immunotherapy. Nat. Rev. Immunol. 2018, 18, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Ragab, D.; Soliman, D.; Samaha, D.; Yassin, A. Vitamin D status and its modulatory effect on interferon gamma and interleukin-10 production by peripheral blood mononuclear cells in culture. Cytokine 2016, 85, 5–10. [Google Scholar] [CrossRef]
- Švajger, U.; Rožman, P.J. Synergistic Effects of Interferon-γ and Vitamin D3 Signaling in Induction of ILT-3highPDL-1high Tolerogenic Dendritic Cells. Front. Immunol. 2019, 10, 2627. [Google Scholar] [CrossRef]
- Bao, K.; Reinhardt, R.L. The differential expression of IL-4 and IL-13 and its impact on type-2 immunity. Cytokine 2015, 75, 25–37. [Google Scholar] [CrossRef] [Green Version]
- Samanta, S. Vitamin D and immunomodulation in the skin: A useful affirmative nexus. Explor. Immunol. 2021, 1, 90–111. [Google Scholar] [CrossRef]
- Umar, M.; Sastry, K.S.; Al Ali, F.; Al-Khulaifi, M.; Wang, E.; Chouchane, A.I. Vitamin D and the Pathophysiology of Inflammatory Skin Diseases. Skin Pharmacol. Physiol. 2018, 31, 74–86. [Google Scholar] [CrossRef]
- Bartram, U.; Speer, C.P. The role of transforming growth factor beta in lung development and disease. Chest 2004, 125, 754–765. [Google Scholar] [CrossRef] [PubMed]
- Isik, S.; Ozuguz, U.; Ates Tutuncu, Y.; Erden, G.; Berker, D.; Acar, K.; Aydin, Y.; Akbaba, G.; Helvaci, N.; Guler, S. Serum transforming growth factor-beta levels in patients with vitamin D deficiency. Eur. J. Intern. Med. 2012, 23, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, M.; Vieira, P.; O’Garra, A. Biology and therapeutic potential of interleukin-10. J. Exp. Med. 2019, 217, e20190418. [Google Scholar] [CrossRef] [Green Version]
- Heine, G.; Niesner, U.; Chang, H.D.; Steinmeyer, A.; Zügel, U.; Zuberbier, T.; Radbruch, A.; Worm, M. 1,25-dihydroxyvitamin D(3) promotes IL-10 production in human B cells. Eur. J. Immunol. 2008, 38, 2210–2218. [Google Scholar] [CrossRef] [PubMed]
- Wöbke, T.K.; Sorg, B.L.; Steinhilber, D. Vitamin D in inflammatory diseases. Front. Physiol. 2014, 5, 69–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahangar-Parvin, R.; Mohammadi-Kordkhayli, M.; Azizi, S.V.; Nemati, M.; Khorramdelazad, H.; Taghipour, Z.; Hassan, Z.; Moazzeni, S.M.; Jafarzadeh, A. The Modulatory Effects of Vitamin D on the Expression of IL-12 and TGF-β in the Spinal Cord and Serum of Mice with Experimental Autoimmune Encephalomyelitis. Iran. J. Pathol. 2018, 13, 10–22. [Google Scholar] [PubMed]
- Abboud, M.; Rybchyn, M.S.; Rizk, R.; Fraser, D.R.; Mason, R.S. Sunlight exposure is just one of the factors which influence vitamin D status. Photochem. Photobiol. Sci. Off. J. Eur. Photochem. Assoc. Eur. Soc. Photobiol. 2017, 16, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Hollis, B.W.; Wagner, C.L.; Drezner, M.K.; Binkley, N.C. Circulating vitamin D3 and 25-hydroxyvitamin D in humans: An important tool to define adequate nutritional vitamin D status. J. Steroid Biochem. Mol. Biol. 2007, 103, 631–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagner, C.L.; Hollis, B.W. The Implications of Vitamin D Status During Pregnancy on Mother and her Developing Child. Front. Endocrinol. 2018, 9, 500. [Google Scholar] [CrossRef] [Green Version]
- van der Aar, A.M.G.; Sibiryak, D.S.; Bakdash, G.; van Capel, T.M.M.; van der Kleij, H.P.M.; Opstelten, D.-J.E.; Teunissen, M.B.M.; Kapsenberg, M.L.; de Jong, E.C. Vitamin D3 targets epidermal and dermal dendritic cells for induction of distinct regulatory T cells. J. Allergy Clin. Immunol. 2011, 127, 1532–1540.e1537. [Google Scholar] [CrossRef]
- Urry, Z.; Chambers, E.S.; Xystrakis, E.; Dimeloe, S.; Richards, D.F.; Gabrysova, L.; Christensen, J.; Gupta, A.; Saglani, S.; Bush, A.; et al. The role of 1alpha,25-dihydroxyvitamin D3 and cytokines in the promotion of distinct Foxp3+ and IL-10+ CD4+ T cells. Eur. J. Immunol. 2012, 42, 2697–2708. [Google Scholar] [CrossRef] [Green Version]
- Fisher, S.A.; Rahimzadeh, M.; Brierley, C.; Gration, B.; Doree, C.; Kimber, C.E.; Plaza Cajide, A.; Lamikanra, A.A.; Roberts, D.J. The role of vitamin D in increasing circulating T regulatory cell numbers and modulating T regulatory cell phenotypes in patients with inflammatory disease or in healthy volunteers: A systematic review. PLoS ONE 2019, 14, e0222313. [Google Scholar] [CrossRef] [Green Version]
- Cacho, N.T.; Lawrence, R.M. Innate Immunity and Breast Milk. Front. Immunol. 2017, 8, 584. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Lawlor, N.T.; Newburg, D.S. Human Milk Components Modulate Toll-Like Receptor-Mediated Inflammation. Adv. Nutr. 2016, 7, 102–111. [Google Scholar] [CrossRef] [Green Version]
- Goldman, A.S. Evolution of the mammary gland defense system and the ontogeny of the immune system. J. Mammary Gland Biol. Neoplasia 2002, 7, 277–289. [Google Scholar] [CrossRef]
- Bikle, D. Vitamin D: Production, Metabolism, and Mechanisms of Action. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., Dungan, K., Grossman, A., Hershman, J.M., Kaltsas, G., Koch, C., Kopp, P., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Constans, J.; Gouaillard, C.; Bouissou, C.; Dugoujon, J.M. Polymorphism of the vitamin D binding protein (DBP) among primates: An evolutionary analysis. Am. J. Phys. Anthropol. 1987, 73, 365–377. [Google Scholar] [CrossRef]
- Jablonski, N.G.; Chaplin, G. The evolution of human skin coloration. J. Hum. Evol. 2000, 39, 57–106. [Google Scholar] [CrossRef] [Green Version]
- Kamboh, M.I.; Ferrell, R.E. Ethnic variation in vitamin D-binding protein (GC): A review of isoelectric focusing studies in human populations. Hum. Genet. 1986, 72, 281–293. [Google Scholar] [CrossRef]
- Oftedal, O.T. The mammary gland and its origin during synapsid evolution. J. Mammary Gland Biol. Neoplasia 2002, 7, 225–252. [Google Scholar] [CrossRef]
- Vorbach, C.; Capecchi, M.R.; Penninger, J.M. Evolution of the mammary gland from the innate immune system? BioEssays News Rev. Mol. Cell. Dev. Biol. 2006, 28, 606–616. [Google Scholar] [CrossRef]
- Alsaweed, M.; Hartmann, P.E.; Geddes, D.T.; Kakulas, F. MicroRNAs in Breastmilk and the Lactating Breast: Potential Immunoprotectors and Developmental Regulators for the Infant and the Mother. Int. J. Environ. Res. Public Health 2015, 12, 13981–14020. [Google Scholar] [CrossRef] [Green Version]
- Beckett, E.L.; Yates, Z.; Veysey, M.; Duesing, K.; Lucock, M. The role of vitamins and minerals in modulating the expression of microRNA. Nutr. Res. Rev. 2014, 27, 94–106. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.Y.; Yi, D.Y. Components of human breast milk: From macronutrient to microbiome and microRNA. Clin. Exp. Pediatr. 2020, 63, 301–309. [Google Scholar] [CrossRef] [Green Version]
- Admyre, C.; Johansson, S.M.; Qazi, K.R.; Filen, J.J.; Lahesmaa, R.; Norman, M.; Neve, E.P.; Scheynius, A.; Gabrielsson, S. Exosomes with immune modulatory features are present in human breast milk. J. Immunol. 2007, 179, 1969–1978. [Google Scholar] [CrossRef]
- Alsaweed, M.; Lai, C.T.; Hartmann, P.E.; Geddes, D.T.; Kakulas, F. Human Milk Cells Contain Numerous miRNAs that May Change with Milk Removal and Regulate Multiple Physiological Processes. Int. J. Mol. Sci. 2016, 17, 956. [Google Scholar] [CrossRef] [Green Version]
- Alsaweed, M.; Lai, C.T.; Hartmann, P.E.; Geddes, D.T.; Kakulas, F. Human Milk Cells and Lipids Conserve Numerous Known and Novel miRNAs, Some of Which Are Differentially Expressed during Lactation. PLoS ONE 2016, 11, e0152610. [Google Scholar] [CrossRef] [Green Version]
- Alsaweed, M.; Lai, C.T.; Hartmann, P.E.; Geddes, D.T.; Kakulas, F. Human milk miRNAs primarily originate from the mammary gland resulting in unique miRNA profiles of fractionated milk. Sci. Rep. 2016, 6, 20680. [Google Scholar] [CrossRef]
- Armogida, S.A.; Yannaras, N.M.; Melton, A.L.; Srivastava, M.D. Identification and quantification of innate immune system mediators in human breast milk. Allergy Asthma Proc. 2004, 25, 297–304. [Google Scholar]
- Chen, C.Z.; Schaffert, S.; Fragoso, R.; Loh, C. Regulation of immune responses and tolerance: The microRNA perspective. Immunol. Rev. 2013, 253, 112–128. [Google Scholar] [CrossRef] [Green Version]
- Field, C.J. The immunological components of human milk and their effect on immune development in infants. J. Nutr. 2005, 135, 1–4. [Google Scholar] [CrossRef]
- Garcia-Segura, L.; Perez-Andrade, M.; Miranda-Rios, J. The emerging role of MicroRNAs in the regulation of gene expression by nutrients. J. Nutrigenet. Nutrigenom. 2013, 6, 16–31. [Google Scholar] [CrossRef]
- Giangreco, A.A.; Nonn, L. The sum of many small changes: MicroRNAs are specifically and potentially globally altered by vitamin D3 metabolites. J. Steroid Biochem. Mol. Biol. 2013, 136, 86–93. [Google Scholar] [CrossRef] [Green Version]
- Kosaka, N.; Izumi, H.; Sekine, K.; Ochiya, T. microRNA as a new immune-regulatory agent in breast milk. Silence 2010, 1, 7. [Google Scholar] [CrossRef] [Green Version]
- Lasser, C.; Alikhani, V.S.; Ekstrom, K.; Eldh, M.; Paredes, P.T.; Bossios, A.; Sjostrand, M.; Gabrielsson, S.; Lotvall, J.; Valadi, H. Human saliva, plasma and breast milk exosomes contain RNA: Uptake by macrophages. J. Transl. Med. 2011, 9, 9. [Google Scholar] [CrossRef] [Green Version]
- Na, R.S.; Ee, G.-X.; Sun, W.; Sun, X.W.; Qiu, X.Y.; Chen, L.P.; Huang, Y.F. Expressional analysis of immune-related miRNAs in breast milk. Genet. Mol. Res GMR 2015, 14, 11371–11376. [Google Scholar] [CrossRef]
- White, J.H. Vitamin D metabolism and signaling in the immune system. Rev. Endocr. Metab. Disord. 2012, 13, 21–29. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, M.; Wang, X.; Li, Q.; Wang, T.; Zhu, Q.; Zhou, X.; Gao, X.; Li, X. Immune-related microRNAs are abundant in breast milk exosomes. Int. J. Biol. Sci. 2012, 8, 118–123. [Google Scholar] [CrossRef]
- Melnik, B.C.; Schmitz, G. MicroRNAs: Milk’s epigenetic regulators. Best Pract. Res. Clin. Endocrinol. Metabol. 2017, 31, 427–442. [Google Scholar] [CrossRef]
- Civardi, E.; Garofoli, F.; Tzialla, C.; Paolillo, P.; Bollani, L.; Stronati, M. Microorganisms in human milk: Lights and shadows. J. Matern.-Fetal Neonatal Med. 2013, 26 (Suppl. 2), 30–34. [Google Scholar] [CrossRef]
- Hosea Blewett, H.J.; Cicalo, M.C.; Holland, C.D.; Field, C.J. The immunological components of human milk. Adv. Food Nutr. Res. 2008, 54, 45–80. [Google Scholar] [CrossRef]



| Vit D Status 1 | Mothers at Enrollment | Infants at Enrollment 2 | Mothers at Completion 3 | Infants at Completion 3 |
|---|---|---|---|---|
| Deficiency | 17 | 34 | 7 | 5 |
| Insufficiency | 19 | 21 | 12 | 12 |
| Sufficiency | 38 | 19 | 39 | 41 |
| Total | 74 | 74 | 58 | 58 |
| Cytokine 1 | Enrollment 2 | Completion 2 | p-Value 3 | 95% CI 4 |
|---|---|---|---|---|
| IL-1β | 0.41 ± 0.6 | 1.63 ± 3.5 | 0.004 | 0.41, 2.04 |
| IL-2 | 0.48 ± 0.42 | 0.64 ± 0.68 | 0.14 | −0.05, 0.36 |
| IL-4 | 0.09 ± 0.09 | 0.10 ± 0.12 | 0.63 | −0.02, 0.04 |
| IL-6 | 1.09 ± 1.17 | 1.86 ± 2.36 | 0.03 | 0.09, 1.44 |
| IL-8 | 19.3 ± 11.7 | 15.8 ± 6.6 | 0.01 | −6.2, −0.81 |
| IL-10 | 1.05 ± 1.22 | 1.33 ± 1.1 | 0.19 | −0.14, 0.7 |
| IL-12 | 0.34 ± 0.3 | 0.46 ± 0.35 | 0.04 | 0.01, 0.24 |
| IL-13 | 1.69 ± 1.7 | 1.66 ± 2.4 | 0.94 | −0.78, 0.73 |
| IFNγ | 12.1 ± 10.9 | 24.8 ± 29.6 | 0.004 | 4.3, 21.1 |
| TNF | 5.4 ± 1.9 | 6.6 ± 2.7 | 0.003 | 0.44, 1.97 |
| TGFβ1 | 64,012 ± 37,882 | 51,741 ± 29,456 | 0.008 | −21,132, −3410 |
| Cytokine 1 | Control Blood Mean Conc. 2 (pg/mL) 3 | Stimulated Blood Mean Conc. 2 (pg/mL) 4 | p-Value 5 | Fold-Increase 6 |
|---|---|---|---|---|
| IL-1β | 17.4 ± 45.5 | 6249 ± 10174 | 0.0006 | 359 |
| IL-2 | 3.1 ± 4.5 | 98,980 ± 104,223 | <0.0001 | 31,929 |
| IL-4 | 0.53 ± 0.67 | 44.4 ± 27.2 | <0.0001 | 83.8 |
| IL-6 | 247 ± 780 | 44,362 ± 49,062 | <0.0001 | 180 |
| IL-8 | 3720 ± 4502 | 83,729 ± 33,745 | <0.0001 | 23 |
| IL-10 | 3.0 ± 4.6 | 232 ± 180 | <0.0001 | 77 |
| IL-12 | 1.4 ± 1.7 | 49.5 ± 48.2 | <0.0001 | 35 |
| IL-13 | 20.3 ± 25.1 | 451 ± 230 | <0.0001 | 22 |
| IFNγ | 32.0 ± 23.2 | 58,820 ± 43,560 | <0.0001 | 1838 |
| TNF | 41.0 ± 88.8 | 20,516 ± 29,638 | <0.0001 | 500 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Newton, D.A.; Baatz, J.E.; Chetta, K.E.; Walker, P.W.; Washington, R.O.; Shary, J.R.; Wagner, C.L. Maternal Vitamin D Status Correlates to Leukocyte Antigenic Responses in Breastfeeding Infants. Nutrients 2022, 14, 1266. https://doi.org/10.3390/nu14061266
Newton DA, Baatz JE, Chetta KE, Walker PW, Washington RO, Shary JR, Wagner CL. Maternal Vitamin D Status Correlates to Leukocyte Antigenic Responses in Breastfeeding Infants. Nutrients. 2022; 14(6):1266. https://doi.org/10.3390/nu14061266
Chicago/Turabian StyleNewton, Danforth A., John E. Baatz, Katherine E. Chetta, Preston W. Walker, Reneé O. Washington, Judy R. Shary, and Carol L. Wagner. 2022. "Maternal Vitamin D Status Correlates to Leukocyte Antigenic Responses in Breastfeeding Infants" Nutrients 14, no. 6: 1266. https://doi.org/10.3390/nu14061266
APA StyleNewton, D. A., Baatz, J. E., Chetta, K. E., Walker, P. W., Washington, R. O., Shary, J. R., & Wagner, C. L. (2022). Maternal Vitamin D Status Correlates to Leukocyte Antigenic Responses in Breastfeeding Infants. Nutrients, 14(6), 1266. https://doi.org/10.3390/nu14061266






