Vitamin C Plasma Levels Associated with Inflammatory Biomarkers, CRP and RDW: Results from the NHANES 2003–2006 Surveys
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heffernan, A.; Evans, C.; Holmes, M.; Moore, J.B. The regulation of dietary iron bioavailability by vitamin C: A systematic review and meta-analysis. Proc. Nutr. Soc. 2017, 76, E182. [Google Scholar] [CrossRef] [Green Version]
- Young, J.I.; Zuchner, S.; Wang, G. Regulation of the epigenome by vitamin C. Annu. Rev. Nutr. 2015, 35, 545–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cimmino, L.; Neel, B.G.; Aifantis, I. Vitamin C in Stem Cell Reprogramming and Cancer. Trends Cell Biol. 2018, 28, 698–708. [Google Scholar] [CrossRef] [PubMed]
- Mohajeri, M.H.; Brummer, R.J.M.; Rastall, R.A.; Weersma, R.K.; Harmsen, H.J.M.; Faas, M.; Eggersdorfer, M. The role of the microbiome for human health: From basic science to clinical applications. Eur. J. Nutr. 2018, 57 (Suppl. 1), 1–14. [Google Scholar] [CrossRef] [Green Version]
- Peterkofsky, B. Ascorbate requirement for hydroxylation and secretion of procollagen: Relationship to inhibition of collagen synthesis in scurvy. Am. J. Clin. Nutr. 1991, 54 (Suppl. 6), 1135s–1140s. [Google Scholar] [CrossRef]
- Padh, H. Vitamin C: Newer insights into its biochemical functions. Nutr. Rev. 1991, 49, 65–70. [Google Scholar] [CrossRef]
- Carr, A.R.; Rowe, S. The emerging role of vitamin C in the prevention and treatment of COVID-19. Nutrients 2020, 12, 3286. [Google Scholar] [CrossRef]
- de Melo, A.F.; Homem-de-Mello, M. High-dose intravenous vitamin C may help in cytokine storm in severe SARS-CoV-2 infection. Crit. Care 2020, 24, 500. [Google Scholar] [CrossRef]
- Scholz, S.S.; Borgstedt, R.; Ebeling, N.; Menzel, L.C.; Jansen, G.; Rehberg, S. Mortality in septic patients treated with vitamin C: A systematic meta-analysis. Crit. Care 2021, 25, 17. [Google Scholar] [CrossRef]
- Ballmer, P.E.; Reinhart, W.H.; Jordan, P.; Bühler, E.; Moser, U.K.; Gey, K. Depletion of plasma vitamin C but not of vitamin E in response to cardiac operations. J. Thorac. Cardiovasc. Surg. 1994, 108, 311–320. [Google Scholar] [CrossRef] [Green Version]
- Casanueva, E.; Ripoll, C.; Tolentino, M.; Morales, R.M.; Pfeffer, F.; Vilchis, P.; Vadillo-Ortega, F. Vitamin C supplementation to prevent premature rupture of the chorioamniotic membranes: A randomized trial. Am. J. Clin. Nutr. 2005, 81, 859–863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borrelli, E. Plasma concentrations of cytokines, their soluble receptors, and antioxidant vitamins can predict the development of multiple organ failure in patients at risk. Crit. Care Med. 1996, 24, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Crook, J.; Horgas, A.; Yoon, S.-J.; Grundmann, O.; Johnson-Mallard, V. Insufficient Vitamin C Levels among Adults in the United States: Results from the NHANES Surveys, 2003–2006. Nutrients 2021, 13, 3910. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.C.; Pullar, J.M.; Bozonet, S.M.; Vissers, M.C. Marginal ascorbate status (hypovitaminosis C) results in an attenuated response to vitamin C supplementation. Nutrients 2016, 8, 341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elste, V.; Troesch, B.; Eggersdorfer, M.; Weber, P. Emerging evidence on neutrophil motility supporting its usefulness to define vitamin C intake requirements. Nutrients 2017, 9, 503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Block, G.; Jensen, C.D.; Dalvi, T.B.; Norkus, E.P.; Hudes, M.; Crawford, P.B.; Holland, N.; Fung, E.B.; Schumacher, L.; Harmatz, P. Vitamin C treatment reduces elevated C-reactive protein. Free Radic. Biol. Med. 2009, 46, 70–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jafarnejad, S.; Boccardi, V.; Hosseini, B.; Taghizadeh, M.; Hamedifard, Z. A Meta-analysis of Randomized Control Trials: The Impact of Vitamin C Supplementation on Serum CRP and Serum hs-CRP Concentrations. Curr. Pharm. Des. 2018, 24, 3520–3528. [Google Scholar] [CrossRef] [PubMed]
- Foster, M.N.; Carr, A.C.; Antony, A.; Peng, S.; Fitzpatrick, M.G. Intravenous Vitamin C Administration Improved Blood Cell Counts and Health-Related Quality of Life of Patient with History of Relapsed Acute Myeloid Leukaemia. Antioxidants 2018, 7, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ridker, P.M. Cardiology Patient Page. C-reactive protein: A simple test to help predict risk of heart attack and stroke. Circulation 2003, 108, e81–e85. [Google Scholar] [CrossRef]
- Helal, I.; Zerelli, L.; Krid, M.; Elyounsi, F.; Ben Maiz, H.; Zouari, B.; Adelmoula, J.; Kheder, A. Comparison of C-reactive protein and high-sensitivity C-reactive protein levels in patients on hemodialysis. Saudi J. Kidney Dis. Transpl. 2012, 23, 477–483. [Google Scholar]
- Hu, L.; Li, M.; Ding, Y.; Pu, L.; Liu, J.; Xie, J.; Cabanero, M.; Li, J.; Xiang, R.; Xiong, S. Prognostic value of RDW in cancers: A systematic review and meta-analysis. Oncotarget 2017, 8, 16027–16035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Z.-D. Red blood cell distribution width: A promising index for estimating activity of autoimmune disease. J. Lab. Precis. Med. 2016, 1, 4. [Google Scholar] [CrossRef]
- Agarwal, S. Red cell distribution width, inflammatory markers and cardiorespiratory fitness: Results from the National Health and Nutrition Examination Survey. Indian Heart J. 2012, 64, 380–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, N.; Pahuja, M.; Pant, S.; Handa, A.; Agarwal, V.; Patel, N.; Dusaj, R. Red cell distribution width and risk of cardiovascular mortality: Insights from National Health and Nutrition Examination Survey (NHANES)-III. Int. J. Cardiol. 2017, 232, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Karakilcik, A.Z.; Halat, R.; Zerin, M.; Celik, H.; Nazligul, Y. Effects of vitamin C and exercise on lipid profile, platelet and erythrocyte indices in young soccer players. J. Sports Med. Phys. Fit. 2014, 54, 665–671. [Google Scholar]
- Ghosh-Jerath, S.; Devasenapathy, N.; Singh, A.; Shankar, A.; Zodpey, S. Ante natal care (ANC) utilization, dietary practices and nutritional outcomes in pregnant and recently delivered women in urban slums of Delhi, India: An exploratory cross-sectional study. Reprod. Health 2015, 12, 20. [Google Scholar] [CrossRef] [Green Version]
- CDC. National Health and Nutrition Examination Survey Data 2005–2006; Centers for Disease Control and Prevention National Center for Health Statistics: Atlanta, GA, USA, 2018.
- McQuillan, G.M.M.; McLean, J.E.; Chiappa, M.; Harris Corporation; Lukacs, S.L. National Health and Nutrition Examination Survey biospecimen program: NHANES III (1988–1994) and NHANES 1999–2014. Vital Health Stat. 2015, 170, 1–14. [Google Scholar]
- Laboratory Procedure Manual Vitamin C in Serum NHANES 2005–2006. 2007. Available online: https://wwwn.cdc.gov/nchs/data/nhanes/2005-2006/labmethods/vic_d_met.pdf (accessed on 5 February 2022).
- Schmidt, A.F.; Finan, C. Linear regression and the normality assumption. J. Clin. Epidemiol. 2018, 98, 146–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, R.S.; Aiello, A.E.; Mensah, F.K.; Gasser, C.E.; Rueb, K.; Cordell, B.; Juonala, M.; Wake, M.; Burgner, D.P. Socioeconomic status in childhood and C reactive protein in adulthood: A systematic review and meta-analysis. J. Epidemiol. Community Health 2017, 71, 817–826. [Google Scholar] [CrossRef] [Green Version]
- Stepanikova, I.; Bateman, L.B.; Oates, G.R. Systemic Inflammation in Midlife: Race, Socioeconomic Status, and Perceived Discrimination. Am. J. Prev. Med. 2017, 52, S63–S76. [Google Scholar] [CrossRef] [Green Version]
- Bergmans, R.S.; Palta, M.; Robert, S.A.; Berger, L.M.; Ehrenthal, D.B.; Malecki, K.M. Associations between Food Security Status and Dietary Inflammatory Potential within Lower-Income Adults from the United States National Health and Nutrition Examination Survey, Cycles 2007 to 2014. J. Acad. Nutr. Diet. 2018, 118, 994–1005. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.; Willis, J.; Gearry, R.B.; Hughes, A.; Lawley, B.; Skidmore, P.; Frampton, C.; Fleming, E.; Anderson, A.; Jones, L.; et al. SunGold Kiwifruit Supplementation of Individuals with Prediabetes Alters Gut Microbiota and Improves Vitamin C Status, Anthropometric and Clinical Markers. Nutrients 2018, 10, 895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Giudice, M.; Gangestad, S.W. Rethinking IL-6 and CRP: Why they are more than inflammatory biomarkers, and why it matters. Brain Behav. Immun. 2018, 70, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Higgins, M.R.; Izadi, A.; Kaviani, M. Antioxidants and Exercise Performance: With a Focus on Vitamin E and C Supplementation. Int. J. Environ. Res. Public Health 2020, 17, 8452. [Google Scholar] [CrossRef]
- Yimcharoen, M.; Kittikunnathum, S.; Suknikorn, C.; Nak-On, W.; Yeethong, P.; Anthony, T.G.; Bunpo, P. Effects of ascorbic acid supplementation on oxidative stress markers in healthy women following a single bout of exercise. J. Int. Soc. Sports Nutr. 2019, 16, 2. [Google Scholar] [CrossRef] [Green Version]
- Laborde, D.; Martin, W.; Swinnen, J.; Vos, R. COVID-19 risks to global food security. Science 2020, 369, 500–502. [Google Scholar] [CrossRef]
- Gabay, C. Interleukin-6 and chronic inflammation. Arthritis Res. Ther. 2006, 8, S3. [Google Scholar] [CrossRef] [Green Version]
| Characteristics | n | Weighted n (%) | Mean (SD) | Range |
|---|---|---|---|---|
| Gender | ||||
| Male | 3699 | 48.7% ± 0.7% | ||
| Female | 3908 | 51.3% ± 0.7% | ||
| Age | ||||
| Young Adult 20–39 | 2751 | 37.5% ± 0.7% | ||
| Middle Adult 40–59 | 2295 | 40.1% ± 0.7% | ||
| Late Adult ≥ 60 | 2561 | 22.4% ± 0.5% | ||
| Race/Ethnicity | ||||
| Mexican American | 1516 | 7.6% ± 0.2% | ||
| Other Hispanic | 230 | 3.4% ± 0.3% | ||
| Non-Hispanic White | 4305 | 73.6% ± 0.5% | ||
| Non-Hispanic Black | 1536 | 10.5% ± 0.3% | ||
| Other | 290 | 4.9% ± 0.3% | ||
| Family PIR 1 | ||||
| High (0–1.5) | 5206 | 63.9% ± 0.5% | ||
| Medium (1.51–4.5) | 1614 | 22.6% ± 0.5% | ||
| Low (>4.51) | 787 | 13.5% ± 0.5% | ||
| Smoking Status | ||||
| Yes | 3392 | 29.4% ± 0.6% | ||
| No | 5610 | 70.6% ± 0.6% | ||
| Food Insecure | ||||
| Yes | 1449 | 14.1% ± 0.4% | ||
| No | 6158 | 85.9% ± 0.4% | ||
| BMI 2 | 7607 | 28.7 (6.44) | 13.4–76.1 | |
| Vitamin C 3 | 7607 | 54.4 (28.6) | 0.6–274.2 | |
| CRP 4 | 7607 | 0.48 (0.92) | 0.01–25.4 | |
| RDW 5 | 7607 | 12.9 (1.2) | 10.7–26.9 |
| Vitamin C Plasma Level | Bonferonni Post hoc Test p | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Deficiency Group I (n = 467) | Hypo-vitminosis Group II (n = 722) | Inadequate Group III (n = 1991) | Adequate Group IV (n = 2567) | Saturating Group V (n = 1960) | F | p | I vs. II | I vs. III | I vs. IV | I vs. V | II vs. III | II vs. IV | II vs. V | III vs. IV | III vs. V | IV vs. V | |
| Marker | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | ||||||||||||
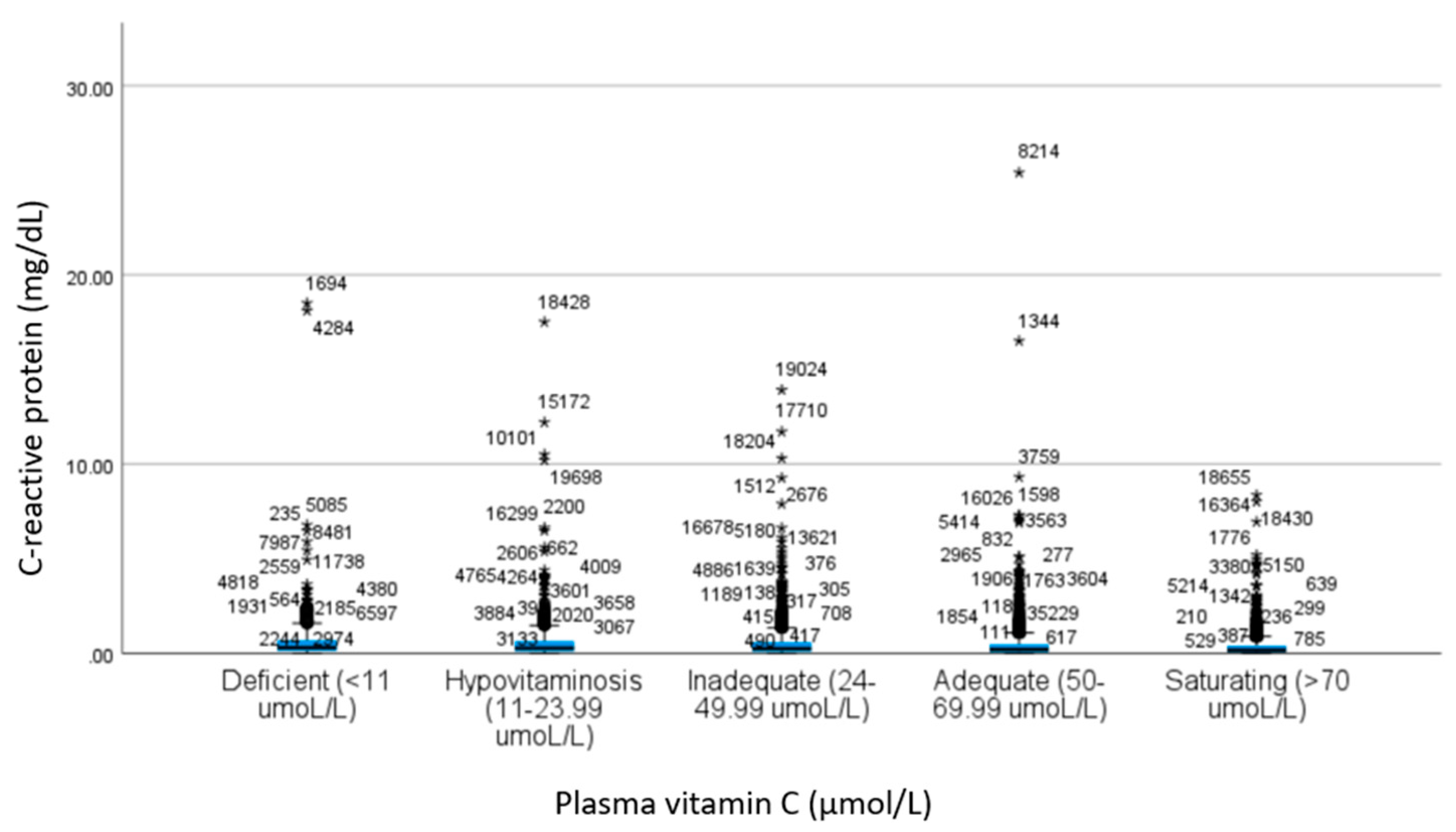
| CRP a | 0.67 ± 1.44 | 0.61 ± 1.22 | 0.53 ± 0.88 | 0.45 ± 0.91 | 0.37 ± 0.60 | 19.4 | <0.001 c | 1.00 | 0.28 | <0.001 | <0.001 | 0.42 | <0.001 | <0.001 | 0.03 | <0.001 | 0.03 |
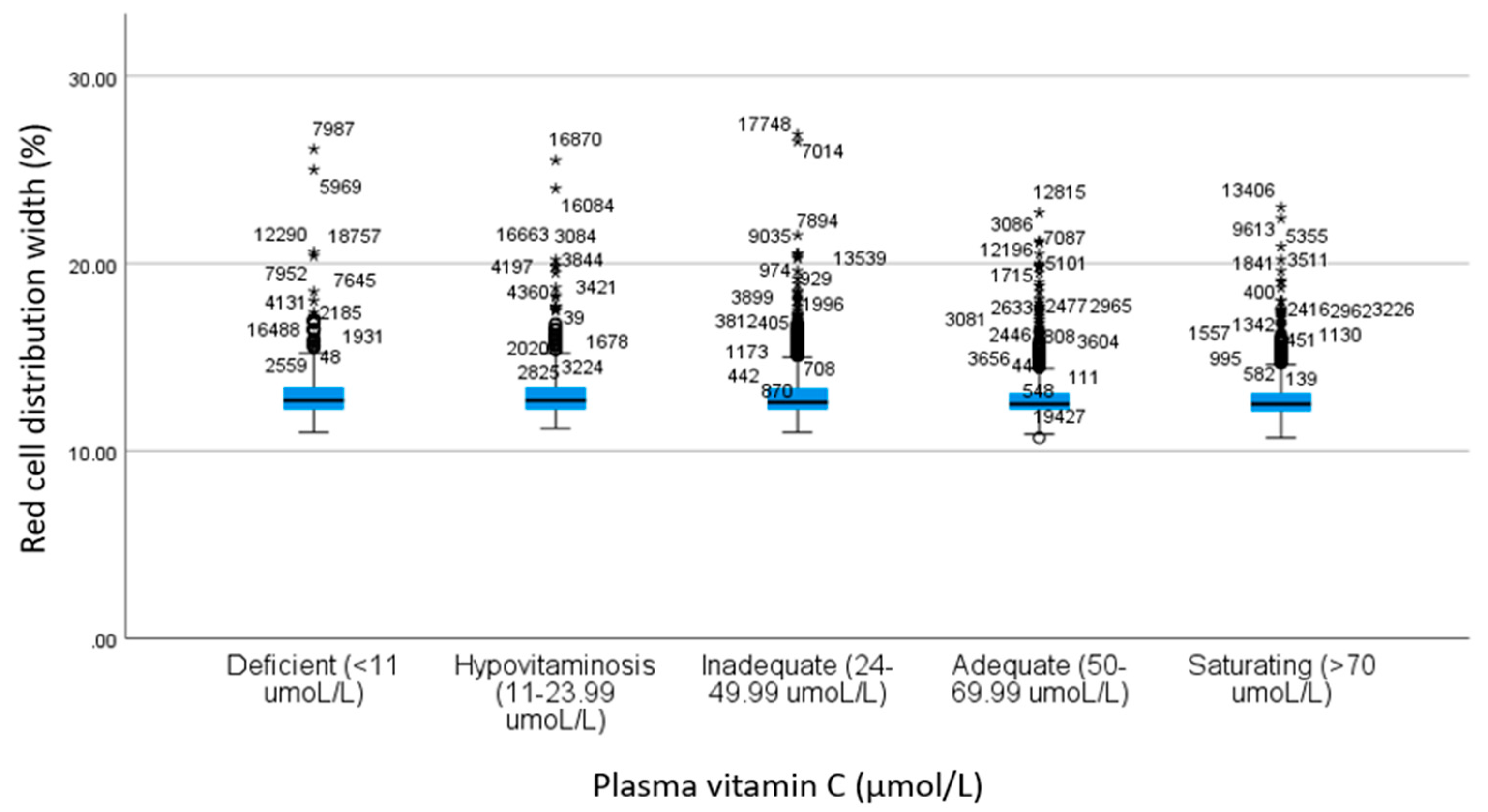
| RDW b | 13.0 ± 1.49 | 13.0 ± 1.35 | 12.8 ± 1.25 | 12.8 ± 1.11 | 12.8 ± 1.09 | 11.2 | <0.001 c | 1.00 | 0.96 | 0.001 | <0.001 | 1.00 | 0.001 | <0.001 | 0.002 | <0.001 | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crook, J.M.; Horgas, A.L.; Yoon, S.L.; Grundmann, O.; Johnson-Mallard, V. Vitamin C Plasma Levels Associated with Inflammatory Biomarkers, CRP and RDW: Results from the NHANES 2003–2006 Surveys. Nutrients 2022, 14, 1254. https://doi.org/10.3390/nu14061254
Crook JM, Horgas AL, Yoon SL, Grundmann O, Johnson-Mallard V. Vitamin C Plasma Levels Associated with Inflammatory Biomarkers, CRP and RDW: Results from the NHANES 2003–2006 Surveys. Nutrients. 2022; 14(6):1254. https://doi.org/10.3390/nu14061254
Chicago/Turabian StyleCrook, Jennifer Marie, Ann L. Horgas, Saunjoo L. Yoon, Oliver Grundmann, and Versie Johnson-Mallard. 2022. "Vitamin C Plasma Levels Associated with Inflammatory Biomarkers, CRP and RDW: Results from the NHANES 2003–2006 Surveys" Nutrients 14, no. 6: 1254. https://doi.org/10.3390/nu14061254
APA StyleCrook, J. M., Horgas, A. L., Yoon, S. L., Grundmann, O., & Johnson-Mallard, V. (2022). Vitamin C Plasma Levels Associated with Inflammatory Biomarkers, CRP and RDW: Results from the NHANES 2003–2006 Surveys. Nutrients, 14(6), 1254. https://doi.org/10.3390/nu14061254







