Efficacy of Vitamin D Supplements in Treatment of Acute Respiratory Infection: A Meta-Analysis for Randomized Controlled Trials
Abstract
:1. Introduction
2. Methods and Materials
2.1. Literature Search Strategy
2.2. Selection Criteria
2.3. Selection of Relevant Studies
2.4. Assessment of Methodological Quality
2.5. Main and Subgroup Analyses
2.6. Statistical Analysis
3. Results
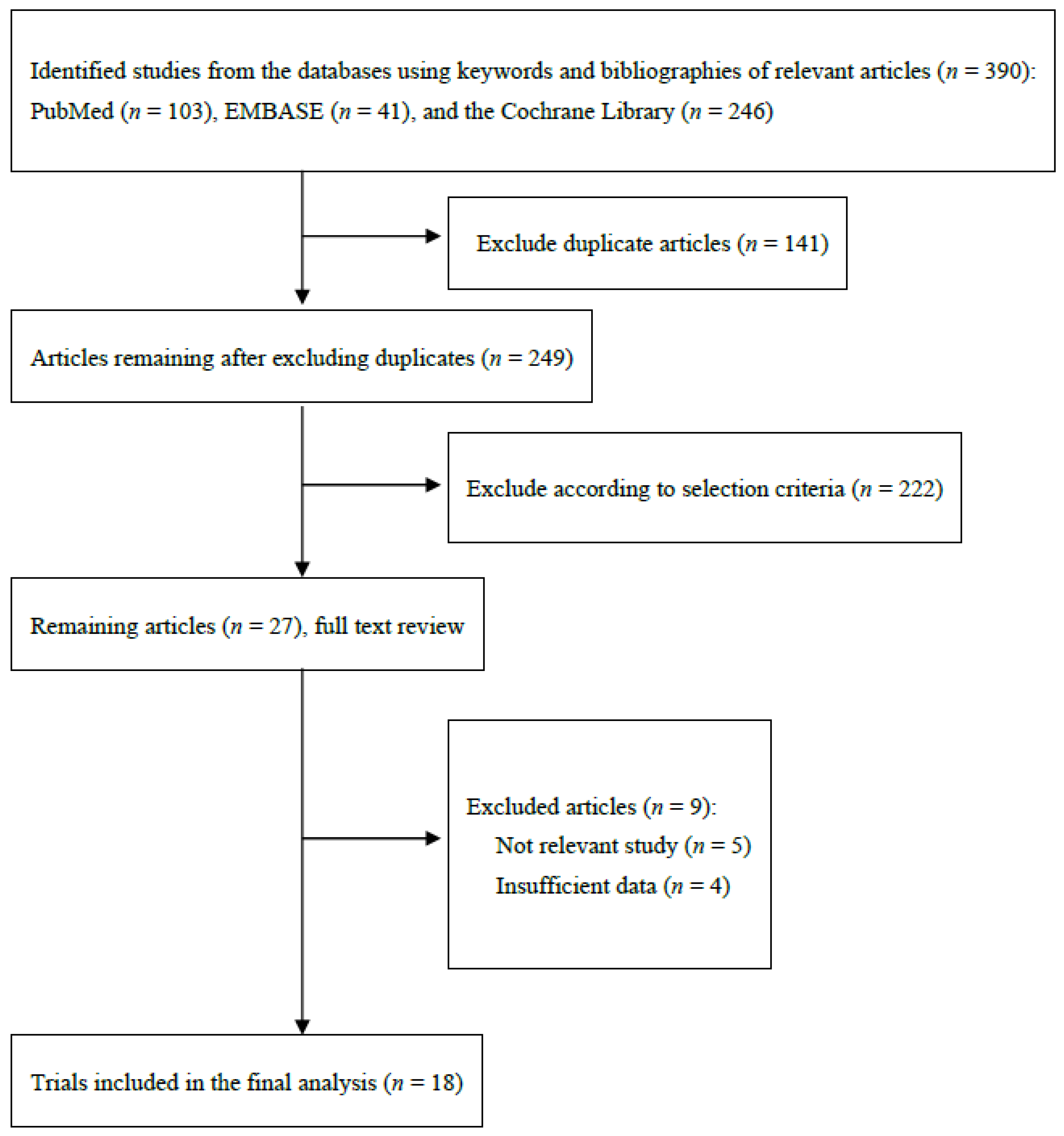
3.1. Identification of Relevant Studies
3.2. General Characteristics of Trials
3.3. Main Findings
3.4. Assessment of Methodological Quality of Studies
3.5. Subgroup Meta-Analysis by Various Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Troeger, C.; Forouzanfar, M.; Rao, P.C.; Khalil, I.; Brown, A.; Swartz, S.; Fullman, N.; Mosser, J.; Thompson, R.L.; Reiner Jr, R.C.; et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory tract infections in 195 countries: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect. Dis. 2017, 17, 1133–1161. [Google Scholar] [CrossRef] [Green Version]
- Simoes, E.A.F.; Cherian, T.; Chow, J.; Shahid-Salles, S.A.; Laxminarayan, R.; John, T.J. Acute Respiratory Infections in Children. In Disease Control Priorities in Developing Countries; Jamison, D.T., Breman, J.G., Measham, A.R., Alleyne, G., Claeson, M., Evans, D.B., Jha, P., Mills, A., Musgrove, P., Eds.; Oxford University Press: New York, NY, USA, 2006. [Google Scholar]
- Dasaraju, P.V.; Liu, C. Infections of the Respiratory System. In Medical Microbiology; Baron, S., Ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996. [Google Scholar]
- Thomas, M.; Bomar, P.A. Upper Respiratory Tract Infection. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK532961/ (accessed on 1 December 2021).
- Heikkinen, T.; Ruuskanen., O. Upper Respiratory Tract Infection. In Encyclopedia of Respiratory Medicine; Laurent, G.J., Shapiro, S.D., Eds.; Academic Press: Oxford, UK, 2006; pp. 385–388. [Google Scholar]
- Vos, T.; Lim, S.S.; Abbafati, C.; Abbas, K.M.; Abbasi, M.; Abbasifard, M.; Abbasi-Kangevari, M.; Abbastabar, H.; Abd-Allah, F.; Abdelalim, A.; et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Woodhead, M.; Blasi, F.; Ewig, S.; Garau, J.; Huchon, G.; Ieven, M.; Ortqvist, A.; Schaberg, T.; Torres, A.; van der Heijden, G.; et al. Guidelines for the management of adult lower respiratory tract infections—Full version. Clin. Microbiol. Infect. 2011, 17, E1–E59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasper, D.L.; Fauci, A.S.; Hauser, S.L.; Longo, D.L.; Jameson, J.L.; Loscalzo, J. Harrison’s Principles of Internal Medicine; McGraw Hill Education Medical: New York, NY, USA, 2015. [Google Scholar]
- Beard, J.A.; Bearden, A.; Striker, R. Vitamin D and the anti-viral state. J. Clin. Virol. 2011, 50, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Vitamin D and immune function: Understanding common pathways. Curr. Osteoporos. Rep. 2009, 7, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Telcian, A.G.; Zdrenghea, M.T.; Edwards, M.R.; Laza-Stanca, V.; Mallia, P.; Johnston, S.L.; Stanciu, L.A. Vitamin D increases the antiviral activity of bronchial epithelial cells in vitro. Antiviral. Res. 2017, 137, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, K.; Wessner, B.; Laggner, U.; Ploder, M.; Tamandl, D.; Friedl, J.; Zügel, U.; Steinmeyer, A.; Pollak, A.; Roth, E.; et al. Vitamin D3 down-regulates monocyte TLR expression and triggers hyporesponsiveness to pathogen-associated molecular patterns. Eur. J. Immunol. 2006, 36, 361–370. [Google Scholar] [CrossRef]
- Hayashi, H.; Okamatsu, M.; Ogasawara, H.; Tsugawa, N.; Isoda, N.; Matsuno, K.; Sakoda, Y. Oral Supplementation of the Vitamin D Metabolite 25(OH)D3 Against Influenza Virus Infection in Mice. Nutrients. 2020, 12, 2000. [Google Scholar] [CrossRef]
- Nursyam, E.W.; Amin, Z.; Rumende, C.M. The effect of vitamin D as supplementary treatment in patients with moderately advanced pulmonary tuberculous lesion. Acta Med. Indones. 2006, 38, 3–5. [Google Scholar]
- Wejse, C.; Gomes, V.F.; Rabna, P.; Gustafson, P.; Aaby, P.; Lisse, I.M.; Andersen, P.L.; Glerup, H.; Sodemann, M. Vitamin D as supplementary treatment for tuberculosis: A double-blind, randomized, placebo-controlled trial. Am. J. Respir. Crit. Care Med. 2009, 179, 843–850. [Google Scholar] [CrossRef]
- Martineau, A.R.; Timms, P.M.; Bothamley, G.H.; Hanifa, Y.; Islam, K.; Claxton, A.P.; Packe, G.E.; Moore-Gillon, J.C.; Darmalingam, M.; Davidson, R.N.; et al. High-dose vitamin D(3) during intensive-phase antimicrobial treatment of pulmonary tuberculosis: A double-blind randomised controlled trial. Lancet 2011, 377, 242–250. [Google Scholar] [CrossRef] [Green Version]
- Choudhary, N.; Gupta, P. Vitamin D supplementation for severe pneumonia--a randomized controlled trial. Indian Pediatr. 2012, 49, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Ralph, A.P.; Waramori, G.; Pontororing, G.J.; Kenangalem, E.; Wiguna, A.; Tjitra, E.; Sandjaja; Lolong, D.B.; Yeo, T.W.; Chatfield, M.D.; et al. L-arginine and vitamin D adjunctive therapies in pulmonary tuberculosis: A randomised, double-blind, placebo-controlled trial. PLoS ONE 2013, 8, e70032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salahuddin, N.; Ali, F.; Hasan, Z.; Rao, N.; Aqeel, M.; Mahmood, F. Vitamin D accelerates clinical recovery from tuberculosis: Results of the SUCCINCT Study (Supplementary Cholecalciferol in recovery from tuberculosis). A randomized, placebo-controlled, clinical trial of vitamin D supplementation in patients with pulmonary tuberculosis’. BMC Infect. Dis. 2013, 13, 22. [Google Scholar] [CrossRef] [Green Version]
- Daley, P.; Jagannathan, V.; John, K.R.; Sarojini, J.; Latha, A.; Vieth, R.; Suzana, S.; Jeyaseelan, L.; Christopher, D.J.; Smieja, M.; et al. Adjunctive vitamin D for treatment of active tuberculosis in India: A randomised, double-blind, placebo-controlled trial. Lancet Infect. Dis. 2015, 15, 528–534. [Google Scholar] [CrossRef]
- Mily, A.; Rekha, R.S.; Kamal, S.M.; Arifuzzaman, A.S.; Rahim, Z.; Khan, L.; Haq, M.A.; Zaman, K.; Bergman, P.; Brighenti, S.; et al. Significant Effects of Oral Phenylbutyrate and Vitamin D3 Adjunctive Therapy in Pulmonary Tuberculosis: A Randomized Controlled Trial. PLoS ONE 2015, 10, e0138340. [Google Scholar] [CrossRef]
- Tukvadze, N.; Sanikidze, E.; Kipiani, M.; Hebbar, G.; Easley, K.A.; Shenvi, N.; Kempker, R.R.; Frediani, J.K.; Mirtskhulava, V.; Alvarez, J.A.; et al. High-dose vitamin D3 in adults with pulmonary tuberculosis: A double-blind randomized controlled trial. Am. J. Clin. Nutr. 2015, 102, 1059–1069. [Google Scholar] [CrossRef] [Green Version]
- Gupta, P.; Dewan, P.; Shah, D.; Sharma, N.; Bedi, N.; Kaur, I.R.; Kumar Bansal, A.; Madhu, S.V. Vitamin D Supplementation for Treatment and Prevention of Pneumonia in Under-five Children: A Randomized Double-blind Placebo Controlled Trial. Indian Pediatr. 2016, 53, 967–976. [Google Scholar] [CrossRef]
- Ganmaa, D.; Munkhzul, B.; Fawzi, W.; Spiegelman, D.; Willett, W.C.; Bayasgalan, P.; Oyun-Erdene, S.; Jolliffe, D.A.; Xenakis, T.; Bromage, S.; et al. High-Dose Vitamin D3 during Tuberculosis Treatment in Mongolia. A Randomized Controlled Trial. Am. J. Respir. Crit. Care. Med. 2017, 196, 628–637. [Google Scholar] [CrossRef]
- Somnath, S.H.; Biswal, N.; Chandrasekaran, V.; Jagadisan, B.; Bobby, Z. Therapeutic effect of vitamin D in acute lower respiratory infection: A randomized controlled trial. Clin. Nutr. ESPEN 2017, 20, 24–28. [Google Scholar] [CrossRef]
- Miroliaee, A.E.; Salamzadeh, J.; Shokouhi, S.; Sahraei, Z. The study of vitamin D administration effect on CRP and Interleukin-6 as prognostic biomarkers of ventilator associated pneumonia. J. Crit. Care. 2018, 44, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Slow, S.; Epton, M.; Storer, M.; Thiessen, R.; Lim, S.; Wong, J.; Chin, P.; Tovaranonte, P.; Pearson, J.; Chambers, S.T.; et al. Effect of adjunctive single high-dose vitamin D3 on outcome of community-acquired pneumonia in hospitalised adults: The VIDCAPS randomised controlled trial. Sci. Rep. 2018, 8, 13829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasanain, A.F.A.; Zayed, A.A.H.; Abd-Ellatief, R.B.; Nafee, A.M.A. Efficacy and safety of cholecalciferol-augmented anti-tuberculosis therapy for treatment of naïve patients with pulmonary tuberculosis: A randomized, controlled, clinical study. Indian. J. Tuberc. 2019, 66, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Entrenas Castillo, M.; Entrenas Costa, L.M.; Vaquero Barrios, J.M.; Alcalá Díaz, J.F.; López Miranda, J.; Bouillon, R.; Quesada Gomez, J.M. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study. J. Steroid. Biochem. Mol. Biol. 2020, 203, 105751. [Google Scholar] [CrossRef] [PubMed]
- Labib, J.R.; Ibrahem, S.K.; Ismail, M.M.; Fatah, S.A.M.A.E.; Sedrak, A.S.; Attia, M.A.S.; El-Hanafi, H.M.; Kamel, M.H. Vitamin D supplementation and improvement of pneumonic children at a tertiary pediatric hospital in Egypt: A randomized controlled trial. Medicine 2021, 100, e25011. [Google Scholar] [CrossRef]
- Murai, I.H.; Fernandes, A.L.; Sales, L.P.; Pinto, A.J.; Goessler, K.F.; Duran, C.S.C.; Silva, C.B.R.; Franco, A.S.; Macedo, M.B.; Dalmolin, H.H.H.; et al. Effect of a Single High Dose of Vitamin D3 on Hospital Length of Stay in Patients With Moderate to Severe COVID-19: A Randomized Clinical Trial. JAMA 2021, 325, 1053–1060. [Google Scholar] [CrossRef]
- Wu, H.X.; Xiong, X.F.; Zhu, M.; Wei, J.; Zhuo, K.Q.; Cheng, D.Y. Effects of vitamin D supplementation on the outcomes of patients with pulmonary tuberculosis: A systematic review and meta-analysis. BMC Pulm. Med. 2018, 18, 108. [Google Scholar] [CrossRef]
- Jolliffe, D.A.; Ganmaa, D.; Wejse, C.; Raqib, R.; Haq, M.A.; Salahuddin, N.; Daley, P.K.; Ralph, A.P.; Ziegler, T.R.; Martineau, A.R. Adjunctive vitamin D in tuberculosis treatment: Meta-analysis of individual participant data. Eur. Respir. J. 2019, 53, 1802003. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Chen, C.; Yang, J. Effectiveness of vitamin D supplementation on the outcome of pulmonary tuberculosis treatment in adults: A meta-analysis of randomized controlled trials. Chin. Med. J. 2019, 132, 2950–2959. [Google Scholar] [CrossRef]
- Tang, X.Z.; Huang, T.; Ma, Y.; Fu, X.Y.; Qian, K.; Yi, Y.L.; Wu, G.H. Meta-analysis of efficacy and safety of vitamin D supplementation in the treatment of pulmonary tuberculosis. Zhonghua Yi Xue Za Zhi 2020, 100, 2525–2531. [Google Scholar]
- Mu, S.Y.; Zou, Y.X.; Zhai, J.; Yao, G.H. Efficacy and safety of vitamin D as adjuvant therapy for childhood pneumonia: A Meta analysis. Zhongguo Dang Dai Er Ke Za Zhi 2020, 22, 124–129. [Google Scholar] [PubMed]
- Das, R.R.; Singh, M.; Naik, S.S. Vitamin D as an adjunct to antibiotics for the treatment of acute childhood pneumonia. Cochrane Database Syst. Rev. 2018, 7, CD011597. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Lu, Y.; Wan, M.; Xu, D.; Yang, X.; Yang, L.; Wang, S.; Sun, G. Efficacy of High-Dose Vitamin D Supplementation as an Adjuvant Treatment on Pneumonia: Systematic Review and a Meta-Analysis of Randomized Controlled Studies. Nutr. Clin. Pract. 2021, 36, 368–384. [Google Scholar] [CrossRef] [PubMed]
- Hayford, F.E.A.; Dolman, R.C.; Blaauw, R.; Nienaber, A.; Smuts, C.M.; Malan, L.; Ricci, C. The effects of anti-inflammatory agents as host-directed adjunct treatment of tuberculosis in humans: A systematic review and meta-analysis. Respir. Res. 2020, 21, 223. [Google Scholar] [CrossRef] [PubMed]
- Cabrera Andrade, B.K.; Garcia-Perdomo, H.A. Effectiveness of micronutrients supplement in patients with active tuberculosis on treatment: Systematic review/Meta-analysis. Complement. Ther. Med. 2020, 48, 102268. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.; Saxena, D.; Mavalankar, D. Vitamin D supplementation, COVID-19 and disease severity: A meta-analysis. QJM 2021, 114, 175–181. [Google Scholar] [CrossRef]
- Rawat, D.; Roy, A.; Maitra, S.; Shankar, V.; Khanna, P.; Baidya, D.K. Vitamin D supplementation and COVID-19 treatment: A systematic review and meta-analysis. Diabetes. Metab. Syndr. 2021, 15, 102189. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Savovic, J.; Page, M.J.; Elbers, R.G.; Sterne, J.A.C. Chapter 8: Assessing risk of bias in a randomized trial. In Cochrane Handbook for Systematic Reviews of Interventions; Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; John Wiley & Sons: Chichester, UK, 2019. [Google Scholar]
- Jadad, A.R.; Moore, R.A.; Carroll, D.; Jenkinson, C.; Reynolds, D.J.; Gavaghan, D.J.; McQuay, H.J. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control. Clin. Trials. 1996, 17, 1–12. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Choi, H.J. New Insight into the Action of Vitamin D. Korean. J. Fam. Med. 2011, 32, 89–96. [Google Scholar]
- Wang, T.T.; Nestel, F.P.; Bourdeau, V.; Nagai, Y.; Wang, Q.; Liao, J.; Tavera-Mendoza, L.; Lin, R.; Hanrahan, J.W.; Mader, S.; et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J. Immunol. 2004, 173, 2909–2912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bikle, D. Nonclassic actions of vitamin D. J. Clin. Endocrinol. Metab. 2009, 94, 26–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Sims, G.P.; Chen, X.X.; Gu, Y.Y.; Chen, S.; Lipsky, P.E. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J. Immunol. 2007, 179, 1634–1647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemire, J.M.; Archer, D.C.; Beck, L.; Spiegelberg, H.L. Immunosuppressive actions of 1,25-dihydroxyvitamin D3: Preferential inhibition of Th1 functions. J. Nutr. 1995, 125, 1704–1708. [Google Scholar]
- van Etten, E.; Mathieu, C. Immunoregulation by 1,25-dihydroxyvitamin D3: Basic concepts. J. Steroid. Biochem. Mol. Biol. 2005, 97, 93–101. [Google Scholar] [CrossRef]
- Zheng, S.; Yang, J.; Hu, X.; Li, M.; Wang, Q.; Dancer, R.C.A.; Parekh, D.; Gao-Smith, F.; Thickett, D.R.; Jin, S. Vitamin D attenuates lung injury via stimulating epithelial repair, reducing epithelial cell apoptosis and inhibits TGF-β induced epithelial to mesenchymal transition. Biochem. Pharmacol. 2020, 177, 113955. [Google Scholar] [CrossRef]

| Study | Country | Study Design | Participants (Average Age, y; Women, %) | Supplementation Period (Follow-Up Period) | Intervention vs. Control | Main Outcome Measures | No. of Participants in Remission/No. of Participants | ||
|---|---|---|---|---|---|---|---|---|---|
| Supplement Group | Placebo Group | ||||||||
| 1 | 2006, Nursyam et al. [14] | Indonesia | RDBPCT | 67 patients with moderately advanced pulmonary TB (31; 41) | 6 w (6 w) | Vitamin D (0.25 mg/d) vs. placebo (PO) | Sputum conversion | 34/34 | 25/33 |
| 2 | 2009, Wejse et al. [15] | Guinea-Bissau | RDBPCT | 365 patients with TB (37; 40) | 8 m (12 m) | Vitamin D (100,000 IU 3 times) vs. placebo (PO) | Survival rate | 157/187 | 154/178 |
| 3 | 2011, Martineau et al. [16] | UK | RDBPCT | 108 patients with pulmonary TB (30; 22) | 56 d (56 d) | Vitamin D (100,000 IU/2 w 4 times) vs. placebo (PO) | Sputum conversion | 41/52 | 45/56 |
| 4 | 2012, Choudhary et al. [17] | India | RDBPCT | 200 patients with severe pneumonia (14 months; 40) | 5 d (5 d) | Vitamin D (1000 IU or 2000 IU/d) vs. placebo (PO) | Discharged within 120 h | 58/100 | 65/100 |
| 5 | 2013, Ralph et al. [18] | Indonesia | RDBPCT | 155 patients with pulmonary TB (28; 35) | 2 m (1 m) | Vitamin D (50,000 IU/m) vs. placebo (PO) | Sputum conversion | 44/75 | 52/80 |
| 6 | 2013, Salahuddin et al. [19] | Pakistan | RDBPCT | 259 patients with pulmonary TB (28; 46) | 2 m (3 m) | Vitamin D (600,000 IU/m) vs. placebo (IM) | Sputum conversion | 108/132 | 103/127 |
| 7 | 2015, Daley et al. [20] | India | RDBPCT | 198 patients with pulmonary TB (42; 23) | 6 w (6 m) | Vitamin D (2.5 mg /2 w) vs. placebo (PO) | Sputum conversion | 87/99 | 82/99 |
| 8 | 2015, Mily et al. [21] | Bangladesh | RDBPCT | 126 patients with pulmonary TB (27; 38) | 1 m (1 m) | Vitamin D (5000 IU/d) vs. placebo (PO) | Sputum conversion | 38/62 | 27/64 |
| 9 | 2015, Tukvadze et al. [22] | Georgia | RDBPCT | 192 patients with pulmonary TB (33; 36) | 4 m (4 m) | Vitamin D (50,000 IU 3 times/w for 8 w, 50,000 IU/2 w for additional 8 w) vs. placebo (PO) | Sputum conversion | 85/97 | 84/95 |
| 10 | 2016, Gupta et al. [23] | India | RDBPCT | 309 patients with pneumonia (12 months; 30) | once (about 30 h) | Vitamin D (100,000 IU once at enrollment) vs. placebo (PO) | Time to resolution of severe pneumonia | 133/153 | 120/156 |
| 11 | 2017, Ganmaa et al. [24] | Mongolia | RDBPCT | 352 patients with pulmonary TB (33; 67) | 2 m (2 m) | Vitamin D (140,000 IU/2 w) vs. placebo (PO) | Sputum conversion | 152/174 | 153/178 |
| 12 | 2017, Somnath et al. [25] | India | OLRCT | 154 patients with acute lower respiratory infection (13 months; 32) | once (4.5–9 d) | Vitamin D (100,000 IU once) vs. placebo (PO) | No need for PICU transfer | 72/78 | 69/76 |
| 13 | 2018, Miroliaee et al. [26] | Iran | RDBPCT | 46 patients with ventilator-associated pneumonia (57; 43) | once (1 m) | Vitamin D (300,000 IU once) vs. placebo (IM) | Survival rate | 19/24 | 11/22 |
| 14 | 2018, Slow et al. [27] | New Zealand | RDBPCT | 117 patients with community-acquired pneumonia (62; 37) | once (6 w) | Vitamin D (200,000 IU once) vs. placebo (PO) | Complete resolution of chest radiograph infiltrate | 30/60 | 27/57 |
| 15 | 2019, Hasanain et al. [28] | Egypt | OLRCT | 496 patients with TB (32; 56) | 4 m (4 m) | Vitamin D (600 IU/d) vs. placebo (PO) | Negative sputum culture | 194/249 | 153/247 |
| 16 | 2020, Entrenas Castillo et al. [29] | Spain | OLRCT | 76 patients with COVID-19 (53; 41) | n.a. (n.a.) | Vitamin D (32,000 IU at admission, 16,000 IU on day 3 and day 7, and 16,000 IU/w) vs. placebo (PO) | Not requiring ICU admission | 49/50 | 13/26 |
| 17 | 2021, Labib et al. [30] | Egypt | RDBPCT | 191 patients with pneumonia (2; 29) | once (n.a.) | Vitamin D (100,000 IU once) vs. placebo (PO) | Survival rate | 70/93 | 66/98 |
| 18 | 2021, Murai et al. [31] | Brazil | RDBPCT | 337 patients with COVID-19 (56; 43) | once (about 7 d) | Vitamin D (200,000 IU once) vs. placebo (PO) | Survival rate | 110/119 | 112/118 |
| Study | Random Sequence Generation | Allocation Concealment | Blinding of Participants and Personnel | Blinding of Outcome Assessment | Incomplete Outcome Data | Selective Reporting | Other Bias | No. of Low Risk of Bias |
|---|---|---|---|---|---|---|---|---|
| 2006, Nursyam et al. [14] | Unclear | Unclear | Low | Unclear | Unclear | Low | Low | 3 |
| 2009, Wejse et al. [15] | Low | Low | Low | Low | High | Low | Low | 6 |
| 2011, Martineau et al. [16] | Low | Low | Low | Low | High | Low | Low | 6 |
| 2012, Choudhary et al. [17] | Low | Low | Low | Unclear | Unclear | Low | Low | 5 |
| 2013, Ralph et al. [18] | Low | Low | Low | Low | High | Low | Low | 6 |
| 2013, Salahuddin et al. [19] | Low | Unclear | Low | Low | Low | Low | Low | 6 |
| 2015, Daley et al. [20] | Low | Low | Low | Low | High | Low | Low | 6 |
| 2015, Mily et al. [21] | Low | Low | Low | Low | High | Low | Low | 6 |
| 2015, Tukvadze et al. [22] | Low | Low | Low | Low | Low | Low | Low | 7 |
| 2016, Gupta et al. [23] | Low | Low | Low | Low | Low | Low | Low | 7 |
| 2017, Ganmaa et al. [24] | Low | Low | Low | Low | Low | Low | Low | 7 |
| 2017, Somnath et al. [25] | Unclear | High | High | High | Low | Low | Low | 3 |
| 2018, Miroliaee et al. [26] | Low | Unclear | Unclear | Low | Low | Low | Low | 5 |
| 2018, Slow et al. [27] | Low | Low | Low | Low | High | Low | Low | 6 |
| 2019, Hasanain et al. [28] | Low | High | High | High | Low | Low | Low | 4 |
| 2020, Entrenas Castillo et al. [29] | Low | High | High | High | High | Low | Low | 3 |
| 2021, Labib et al. [30] | Low | Low | Low | Low | Low | High | Low | 6 |
| 2021, Murai et al. [31] | Low | Low | Low | Low | Low | Low | Low | 7 |
| Source | Randomization | Description of Randomization Methods | Double-Blind | Using Identical Placebo | Follow-Up Reporting | Total Score | |
|---|---|---|---|---|---|---|---|
| 1 | 2006, Nursyam et al. [14] | 1 | 0 | 1 | 1 | 0 | 3 |
| 2 | 2009, Wejse et al. [15] | 1 | 1 | 1 | 1 | 1 | 5 |
| 3 | 2011, Martineau et al. [16] | 1 | 1 | 1 | 1 | 1 | 5 |
| 4 | 2012, Choudhary et al. [17] | 1 | 1 | 1 | 1 | 0 | 4 |
| 5 | 2013, Ralph et al. [18] | 1 | 1 | 1 | 1 | 1 | 5 |
| 6 | 2013, Salahuddin et al. [19] | 1 | 1 | 1 | 0 | 1 | 4 |
| 7 | 2015, Daley et al. [20] | 1 | 1 | 1 | 1 | 1 | 5 |
| 8 | 2015, Mily et al. [21] | 1 | 1 | 1 | 1 | 1 | 5 |
| 9 | 2015, Tukvadze et al. [22] | 1 | 1 | 1 | 1 | 1 | 5 |
| 10 | 2016, Gupta et al. [23] | 1 | 1 | 1 | 1 | 1 | 5 |
| 11 | 2017, Ganmaa et al. [24] | 1 | 1 | 1 | 1 | 1 | 5 |
| 12 | 2017, Somnath et al. [25] | 1 | 1 | 0 | 0 | 1 | 3 |
| 13 | 2018, Miroliaee et al. [26] | 1 | 1 | 1 | 1 | 1 | 5 |
| 14 | 2018, Slow et al. [27] | 1 | 1 | 1 | 1 | 1 | 5 |
| 15 | 2019, Hasanain et al. [28] | 1 | 1 | 0 | 0 | 1 | 3 |
| 16 | 2020, Entrenas Castillo et al. [29] | 1 | 1 | 0 | 0 | 1 | 3 |
| 17 | 2021, Labib et al. [30] | 1 | 1 | 1 | 1 | 1 | 5 |
| 18 | 2021, Murai et al. [31] | 1 | 1 | 1 | 1 | 1 | 5 |
| Certainty Assessment | No. of Patients | Effect | Certainty | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | Vitamin D | Placebo | RR (95% CI) | RD (95% CI) | |
| Outcome: Treatment efficacy (sputum conversion, survival rate, therapeutic success, resolution of chest radiograph infiltrate, and hospital discharge) | |||||||||||
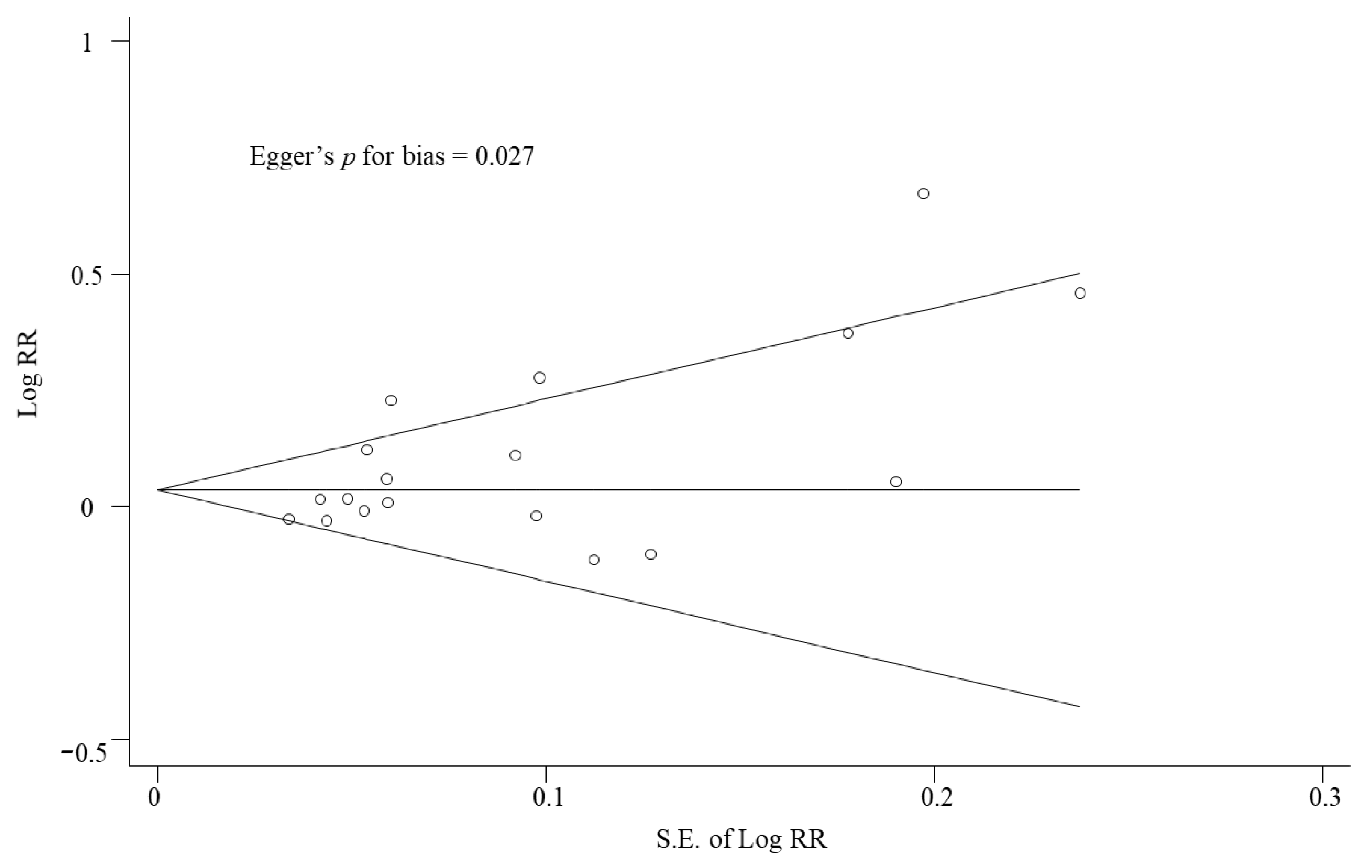
| 18 | Randomized controlled trials | Serious a | Serious b | Serious c | Not serious d | Publication bias strongly suspected e | 1481/1838 (80.6%) | 1361/1810 (75.2%) | RR 1.07 (1.01 to 1.13) | 53 more per 1000 (from 8 more to 98 more) | ⨁◯◯◯ Very low |
| Factor | No. of Trials | Summary RR (95% CI) | Heterogeneity, I2 |
|---|---|---|---|
| All * | 18 [14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31] | 1.07 (1.01–1.13) | 66.9% |
| Dosage | |||
| Low dose (total dose < 350,000 IU) | 12 [15,17,18,21,23,25,26,27,28,29,30,31] | 1.09 (1.00–1.20) | 76.3% |
| High dose (total dose ≥ 350,000 IU) | 6 [14,16,19,20,22,24] | 1.03 (0.98–1.10) | 29.2% |
| Assessment of treatment efficacy | |||
| Sputum conversion | 8 [14,16,18,19,20,21,22,24] | 1.04 (0.97–1.11) | 41.5% |
| Increased survival rate | 4 [15,26,30,31] | 1.02 (0.92–1.13) | 60.1% |
| Therapeutic success | 4 [17,23,27,28] | 1.11 (0.97–1.27) | 60.7% |
| No need for ICU | 2 [25,29] | 1.39 (0.60–3.22) | 94.4% |
| Type of disease | |||
| Pulmonary TB | 10 [14,15,16,18,19,20,21,22,24,28] | 1.06 (0.99–1.14) | 65.2% |
| Pneumonia | 6 [17,23,25,26,27,30] | 1.07 (0.98–1.17) | 35.0% |
| COVID-19 | 2 [29,31] | 1.36 (0.54–3.43) | 95.5% |
| Age | |||
| ≥15 years ** | 14 [14,15,16,18,19,20,21,22,24,26,27,28,29,31] | 1.08 (1.00–1.16) | 72.8% |
| <5 years | 4 [17,23,25,30] | 1.05 (0.97–1.15) | 37.7% |
| Route of administration | |||
| Oral ** | 16 [14,15,16,17,18,20,21,22,23,24,25,27,28,29,30,31] | 1.07 (1.00–1.13) | 68.6% |
| Injection | 2 [19,26] | 1.20 (0.77–1.86) | 72.4% |
| Type of continent | |||
| Asia | 10 [14,17,18,19,20,21,23,24,25,26] | 1.06 (0.99–1.14) | 50.6% |
| Europe | 3 [16,22,29] | 1.17 (0.86–1.59) | 85.6% |
| Africa | 3 [15,28,30] | 1.11 (0.92–1.33) | 85.8% |
| Oceania | 1 [27] | 1.06 (0.73–1.53) | n.a. |
| South America | 1 [31] | 0.97 (0.91–1.04) | n.a. |
| Study design | |||
| RDBPCT | 15 [14,15,16,17,18,19,20,21,22,23,24,26,27,30,31] | 1.03 (0.99–1.09) | 45.3% |
| OLRCT | 3 [25,28,29] | 1.28 (0.96–1.71) | 90.7% |
| Methodological quality | |||
| High-quality (Risk of Bias ≥ 6) | 12 [15,16,18,19,20,21,22,23,24,27,30,31] | 1.02 (0.98–1.06) | 24.0% |
| Low-quality (Risk of Bias < 6) * | 6 [14,17,25,26,28,29] | 1.22 (1.02–1.42) | 81.1% |
| High-quality (Jadad score = 5) | 11 [15,16,18,20,21,23,24,26,27,30,31] | 1.04 (0.98–1.10) | 46.0% |
| Low-quality (Jadad score ≤ 4) | 7 [14,17,19,22,25,28,29] | 1.11 (0.99–1.26) | 79.8% |
| Duration of treatment | |||
| <12 weeks | 11 [14,16,17,18,21,23,24,26,27,30,31] | 1.06 (0.98–1.15) | 57.6% |
| ≥12 weeks | 5 [15,19,20,22,28] | 1.05 (0.96–1.15) | 73.7% |
| Not mentioned | 2 [25,29] | 1.39 (0.60–3.22) | 94.4% |
| Supply source for supplements | |||
| Pharmaceutical industry | 6 [15,16,20,23,25,31] | 1.02 (0.97–1.07) | 33.8% |
| No pharmaceutical industry | 2 [18,21] | 1.13 (0.70–1.80) | 79.3% |
| Not mentioned * | 10 [14,17,19,22,24,26,27,28,29,30] | 1.12 (1.01–1.24) | 72.5% |
| No. of participants in each trial | |||
| <200 | 11 [14,16,18,21,22,25,26,27,29,30,31] | 1.10 (1.00–1.22) | 71.8% |
| ≥200 | 7 [15,17,19,20,23,24,28] | 1.05 (0.98–1.13) | 66.2% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, H.; Myung, S.-K.; Cho, H.-E. Efficacy of Vitamin D Supplements in Treatment of Acute Respiratory Infection: A Meta-Analysis for Randomized Controlled Trials. Nutrients 2022, 14, 1144. https://doi.org/10.3390/nu14061144
Cho H, Myung S-K, Cho H-E. Efficacy of Vitamin D Supplements in Treatment of Acute Respiratory Infection: A Meta-Analysis for Randomized Controlled Trials. Nutrients. 2022; 14(6):1144. https://doi.org/10.3390/nu14061144
Chicago/Turabian StyleCho, Herim, Seung-Kwon Myung, and Hae-Eun Cho. 2022. "Efficacy of Vitamin D Supplements in Treatment of Acute Respiratory Infection: A Meta-Analysis for Randomized Controlled Trials" Nutrients 14, no. 6: 1144. https://doi.org/10.3390/nu14061144
APA StyleCho, H., Myung, S.-K., & Cho, H.-E. (2022). Efficacy of Vitamin D Supplements in Treatment of Acute Respiratory Infection: A Meta-Analysis for Randomized Controlled Trials. Nutrients, 14(6), 1144. https://doi.org/10.3390/nu14061144






