Preparations from Various Organs of Sea Buckthorn (Elaeagnus rhamnoides (L.) A. Nelson) as Important Regulators of Hemostasis and Their Role in the Treatment and Prevention of Cardiovascular Diseases
Abstract
1. Introduction
2. Hemostasis and Cardiovascular Diseases
3. Sea Buckthorn-Hemostasis and CVDs (In Vivo Trials)
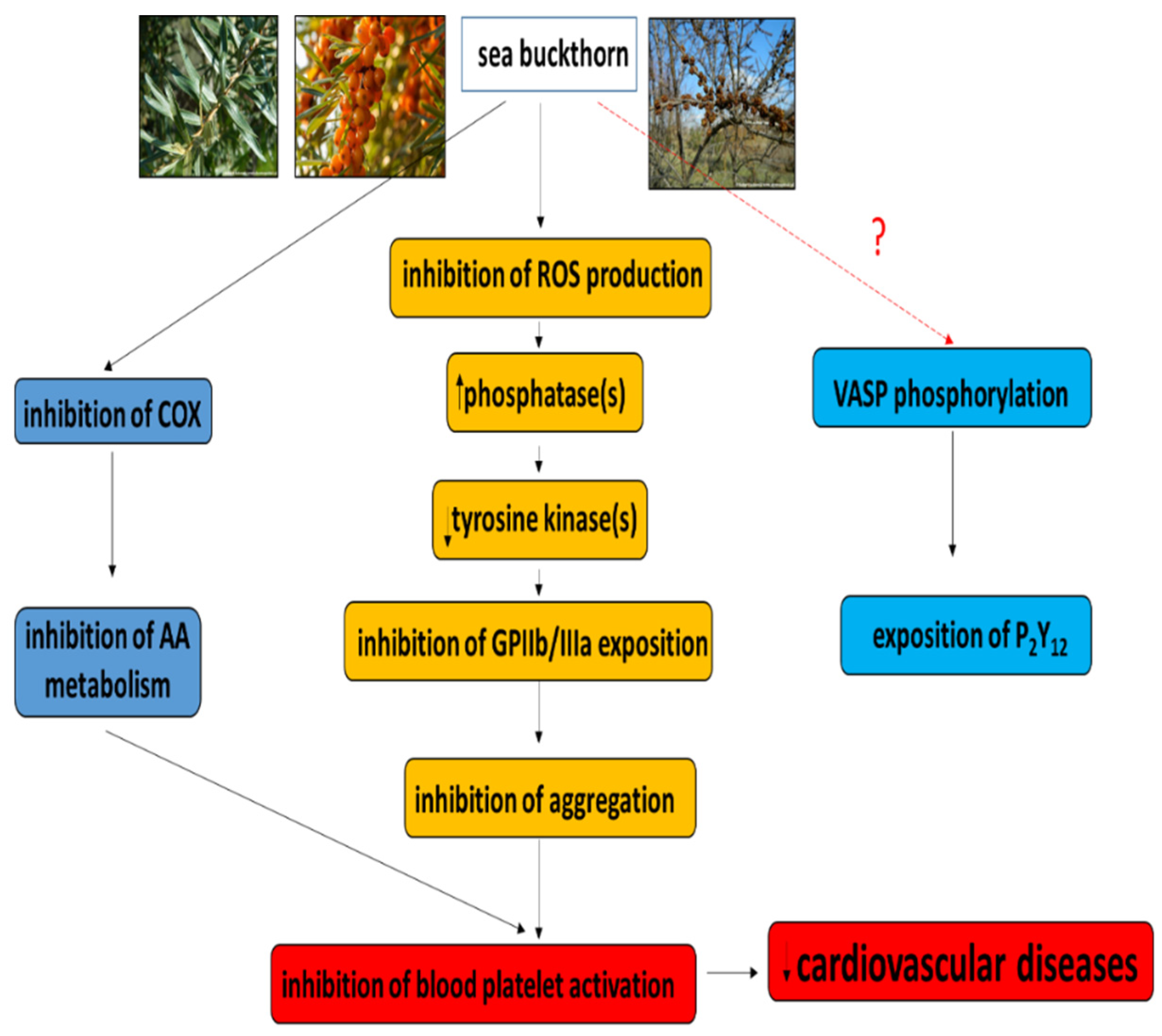
4. Sea Buckthorn and Hemostasis—In Vitro Trials
4.1. Antioxidant Potential
4.2. Sea Buckthorn Preparations and Plasma Hemostasis
4.3. Sea Buckthorn Preparations and Blood Platelets
5. Medicines and Food Products from Sea Buckthorn
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Li, G.; Zhang, J.; Liu, E.; Wang, F.H.; Qi, S.; Xiang, X.; Du, W. Effects of different sea buckthorn leaf tea processing technologies on nutrient level and fecal microflora in vitro. Food Nutr. Res. 2016, 55, 205–213. [Google Scholar]
- Malinowska, P.; Olas, B. Rokitnik—Roślina wartościowa dla zdrowia. Kosmos 2016, 2, 285–292. [Google Scholar]
- Olas, B.; Skalski, B.; Ulanowska, K. The anticancer activity of sea buckthorn [Elaeagnus rhamnoides (L.) A. Nelson]. Front. Pharmacol. 2018, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zeb, A. Important therapeutic uses of sea buckthorn (Hippopahe): A Review. J. Biol. Sci. 2004, 4, 687–693. [Google Scholar]
- Upadhyay, N.K.; Yogendra Kumar, M.S.; Gupta, A. Antioxidant, cytoprotective and antibacterial effects of sea buckthorn (Hippophae rhamnoides L.) leaves. Food Chem. Toxicol. 2010, 48, 3443–3448. [Google Scholar] [CrossRef]
- Suryakumar, G.; Gupta, A. Medicinal and therapeutic potential of sea buckthorn (Hippophae rhamnoides L.). J. Ethnopharmacol. 2011, 138, 268–278. [Google Scholar] [CrossRef]
- Christaki, E. Hippophae rhamnoides L. (sea buckthorn): A potential source of nutraceuticals. Food Public Health 2012, 2, 69–72. [Google Scholar] [CrossRef]
- Ma, X.; Yang, W.; Kallio, H.; Yang, B. Health promoting properties and sensory characteristics of phytochemicals in berries and leaves of sea buckthorn (Hippophaë rhamnoides). Crit. Rev. Food Sci. Nutr. 2021, 7, 1–19. [Google Scholar] [CrossRef]
- Ciesarová, Z.; Murkovic, M.; Cejpek, K.; Kreps, F.; Tobolková, B.; Koplík, R.; Belajová, E.; Kukurová, K.; Daško, L.; Panovská, Z.; et al. Why is sea buckthorn (Hippophae rhamnoides L.) So exceptional? A review. Food Res. Int. 2020, 133, 109170. [Google Scholar] [CrossRef]
- Skalski, B.; Kontek, B.; Olas, B.; Żuchowski, J.; Stochmal, A. Phenolic fraction and nonpolar fraction from sea buckthorn leaves and twigs: Chemical profile and biological activity. Future Med. Chem. 2018, 10, 2381–2394. [Google Scholar] [CrossRef]
- Olas, B. Sea buckthorn as a source of important bioactive compounds in cardiovascular diseases. Food Chem. Toxicol. 2016, 97, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Olas, B. The beneficial health aspects of sea buckthorn (Elaeagnus rhamnoides (L.) A. Nelson) oil. J. Ethnopharmacol. 2018, 1, 189–190. [Google Scholar]
- Ogedegbe, H.O. An overview of hemostasis. Lab. Med. 2002, 33, 948–953. [Google Scholar] [CrossRef][Green Version]
- Tanaka, K.A.; Key, N.S.; Levy, J.H. Blood coagulation: Hemostasis and thrombin regulation. Anesth. Analg. 2017, 108, 1433–1446. [Google Scholar] [CrossRef]
- Majewicz, A.; Marcinkowski, J. Epidemiologia chorób układu krążenia. Dlaczego w Polsce jest tak małe zainteresowanie istniejącymi już programami profilaktycznymi? Probl. Hig. I Epidemiol. 2008, 89, 322–325. [Google Scholar]
- Nowak, P.; Olas, B.; Wachowicz, B. Stres oksydacyjny w przebiegu hemostazy. Postępy Biochem. 2010, 56, 329–347. [Google Scholar]
- Eitan, F.; Mutaz, D. Oxidative stress and platelet dysfunction. J. Thromb. Haemost. 2018, 2, 1–4. [Google Scholar]
- Ernst, E.; Resch, K.L. Fibrinogen as a cardiovascular risk factor: A meta-analysis and review of the literature. Ann. Intern. Med. 1993, 118, 956–963. [Google Scholar] [CrossRef]
- Modrzejewski, W.; Musiał, W.J. Stare i nowe czynniki ryzyka sercowo-naczyniowego—Jak zahamować epidemię miażdżycy? Część II. Forum Zaburzeń Metab. 2010, 1, 168–176. [Google Scholar]
- Smith, A.; Patterson, C.; Yarnell, J.; Rumley, A.; Ben-Shlomo, Y.; Lowe, G. Which hemostatic markers add to the predictive value of conventional risk factors for coronary heart disease and ischemic stroke? Caerphilly Study Circ. 2005, 112, 3080–3087. [Google Scholar] [CrossRef]
- Gębalska, J. Nadreaktywność osoczowego układu krzepnięcia i płytek krwi w niewydolności serca. Jak Zapobiegać I Leczyć? Borgis Postępy Nauk. Med. 2010, 1, 938–941. [Google Scholar]
- Karaźniewicz-Łada, M.; Danielak, D.; Główka, F. Leki przeciwpłytkowe nowej generacji. Farm. Współczesna 2013, 6, 1–5. [Google Scholar]
- Zuniga-Ceron, A.F.; Saavedra-Torres, J.S.; Navia-Amezquita, C.A. The role of platelet and its interaction with aspirin. J. Fac. Med. 2015, 64, 351–363. [Google Scholar] [CrossRef]
- Olas, B. The multifunctionality of berries toward blood platelet and the role of berry phenolics in cardiovascular disorders. Platelets 2017, 28, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, O. Different classes of anticoagulant drugs in clinical use. Is there a class effect? J. Hematol. Thromboembolic Dis. 2015, 3, 1–4. [Google Scholar] [CrossRef]
- Broncel, M. Zasady skutecznej i bezpiecznej terapii kwasem acetylosalicylowym. Geriatria 2019, 13, 50–62. [Google Scholar]
- Tolic, M.-T.; Jurcevic, I.L.; Krbavcic, I.P.; Markovic, K.; Vahcic, N. Phonelic content, antioxidant capacity and quality of chokeberry (Aronia melanocarpa) products. Food Technol. Biotechnol. 2015, 53, 171–179. [Google Scholar] [CrossRef]
- Parus, A. Przeciwutleniające i farmakologiczne właściwości kwasów fenolowych. Postępy Fitoter. 2013, 1, 48–53. [Google Scholar]
- Balsam, P.; Grabowski, M. Analiza właściwości przeciwpłytkowych wystandaryzowanego ekstraktu z pomidorów. Farmakoter. Chorób Układu Krążenia 2014, 11, 1–6. [Google Scholar]
- Luo, X.; Du, C.; Cheng, H.; Chen, J.; Lin, C. Study in the anticoagulant or procoagulant activities of type II phenolic acid derivatives. Molecules 2017, 22, 2047. [Google Scholar] [CrossRef]
- Johansson, A.K.; Korte, H.; Yang, B.; Stanley, J.C.; Kallio, H.P. Sea buckthorn berry oil inhibits platelet aggregation. J. Nutr. Biochem. 2000, 10, 491–495. [Google Scholar] [CrossRef]
- Basu, M.; Prasad, R.; Jayamurthy, P.; Pal, K.; Arumughan, C.; Sawhney, R.C. Anti-atherogenic effects of seabuckthorn (Hippophaea rhamnoides) seed oil. Phytomedicine 2007, 11, 770–777. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Zhao, J.; Zhang, W. Antihypertensive effect of total flavonoids extracted from seed residues of Hippophae rhamnoides L. in sucrose-fed rats. J. Ethnopharmacol. 2008, 117, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Koyama, T.; Taka, A.; Togashi, H. Effects of a herbal medicine, Hippophae rhamnoides, on cardiovascular functions and coronary microvessels in the spontaneously hypertensive stroke-prone rat. Clin. Hemorheol Microcircul 2009, 41, 17–26. [Google Scholar] [CrossRef]
- Lee, H.I.; Kim, M.S.; Lee, K.M.; Park, S.K.; Seo, K.I.; Kim, H.J.; Kim, M.J.; Choi, M.S.; Lee, M.K. Anti-visceral obesity and antioxidant effects of powdered sea buckthorn (Hippophae rhamnoides L.) leaf tea in diet-induced obese mice. Food Chem. Toxicol. 2011, 49, 2370–2376. [Google Scholar] [CrossRef]
- Pichiah, B.P.T.; Moon, H.-J.; Park, J.-E.; Moon, Y.-J.; Cha, Y.-S. Ethanolic extract of seabuckthorn (Hippophae rhamnoides L.) prevents high-fat diet-induced obesity in mice through down-regulation of adipogenic and lipogenic gene expression. Nutr. Res. 2012, 11, 856–864. [Google Scholar] [CrossRef]
- Suchal, K.; Bhatia, J.; Malik, S.; Malhotra, R.K.; Gamad, N.; Goyal, S.; Nag, T.C.; Arya, D.S.; Ojha, S. Sea buckthorn Pulp Oil Protects against Myocardial Ischemia-Reperfusion Injury in Rats through Activation of Akt/eNOS. Front. Pharmacol. 2016, 7, 155. [Google Scholar] [CrossRef]
- Eccleston, C.; Yang, B.; Tahvonen, R.; Kallio, H. Effect of an antioxidant-rich juice (sea buckthorn) on risk factors for coronary disease in humans. J. Nutr. Biochem. 2002, 13, 346–354. [Google Scholar] [CrossRef]
- Xu, Y.-J.; Kaur, M.; Dhillon, R.S.; Tappia, P.S.; Dhalla, N.S. Health benefits of sea buckthorn for the prevention of cardiovascular diseases. J. Funct. Foods 2011, 3, 2–12. [Google Scholar] [CrossRef]
- Negi, B.; Kaur, R.; Dey, G. Protective effects of a novel sea buckthorn wine on oxidative stress and hypercholesterolemia. Food Funct. 2013, 4, 240–248. [Google Scholar] [CrossRef]
- Sayegh, M.; Miglio, C.; Ray, S. Potential cardiovascular implications of Sea Buckthorn berry consumption in humans. J. Food Sci. Nutr. 2014, 65, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Zhang, J.; Zhao, A.; Zhang, Y.; Wang, P. Effects of sea buckthorn puree on risk factors of cardiovascular disease in hypercholesterolemia population: A double-blind, randomized, placebo-controlled trial. Anim. Biotechnol. 2020, 2, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, X.; Tian, Y.; Wei, Y.; Deng, Y.; Wu, Y.; Tao, C. Flavone of Hippophae (H-flavone) lowers atherosclerotic risk factors via upregulation the adipokine C1q/tumor necrosis factor-related protein 6 (CTRP6) in macrophages. Biosci. Biotechnol. Biochem. 2019, 83, 2000–2007. [Google Scholar] [CrossRef] [PubMed]
- Skalski, B.; Kontek, B.; Lis, B.; Olas, B.; Grabarczyk, Ł.; Stochmal, A.; Żuchowski, J. Biological properties of Elaeagnus rhamnoides (L.) A. Nelson twig and leaf extracts. BMC Complement. Altern. Med. 2019, 19, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Żuchowski, J.; Skalski, B.; Juszczak, M.; Woźniak, K.; Stochmal, A.; Olas, B. LC/MS Analysis of saponin fraction from the leaves of Elaeagnus rhamnoides (L.) A. Nelson and its biological properties in different in vitro models. Molecules 2021, 25, 3004. [Google Scholar] [CrossRef]
- Olas, B.; Kontek, B.; Malinowska, P.; Żuchowksi, J.; Stochmal, A. Hippophae rhamnoides L. fruits reduce the oxidative stress in human blood platelets and plasma. Oxid. Med. Cell. Longev. 2016, 1, 1–8. [Google Scholar] [CrossRef]
- Skalski, B.; Lis, B.; Pecio, Ł.; Kontek, B.; Olas, B.; Żuchowski, J.; Stochmal, A. Isorhamnetin and its new derivatives isolated from sea buckthorn berries prevent H2O2/Fe—Induced oxidative stress and changes in hemostasis. Food Chem. Toxicol. 2019, 125, 614–620. [Google Scholar] [CrossRef]
- Olas, B.; Wachowicz, B. Role of reactive nitrogen species in blood platelet functions. Platelets 2007, 18, 555–565. [Google Scholar] [CrossRef]
- Skalski, B.; Kontek, B.; Rolnik, A.; Olas, B.; Stochmal, A.; Żuchowski, J. Anti-platelet properties of phenolic extracts from the leaves and twig of Elaeagnus rhamnoides (L.) A. Nelson. Molecules 2019, 24, 3620. [Google Scholar] [CrossRef]
- Skalski, B.; Stochmal, A.; Żuchowski, J.; Grabarczyk, Ł.; Olas, B. Response of blood platelets to phenolic fraction and non-polar fraction from the leaves and twigs of Elaeagnus rhamnoides (L.) A. Nelson in vitro. Biomed. Pharmacother. 2020, 124, 1–12. [Google Scholar] [CrossRef]
- Olas, B.; Kontek, B.; Szczsna, M.; Grabarczyk, L.; Stochmal, A.; Zuchowski, J. Inhibition of blood platelet adhesion by phenolics’ rich fraction of Hippophae rhamnoides L. fruits. J. Physiol. Pharmacol. 2017, 68, 223–229. [Google Scholar] [PubMed]
- Sakihama, Y.; Cohen, M.F.; Grace, S.C.; Yamasaki, H. Plant phenolic antioxidant and prooxidant activities: Phenolic-induced oxidative damage mediated by metals in plants. Toxicology 2002, 177, 67–80. [Google Scholar] [CrossRef]
- Stadler, N.; Lindner, R.A.; Davies, M.J. Direct detection and quantification of transition metal ions in human atherosclerotic plaques: Evidence for the presence of elevated levels of iron and copper. Arter. Thromb. Vasc. Biol. 2004, 24, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Procházková, D.; Boušová, I.; Wilhelmová, N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Bakir, T.; Sonmezoglu, I.; Imer, F.; Apak, R. Antioxidant/prooxidant effects of α-tocopherol, quercetin and isoramnetin on linoleic acid peroxidation induced by Cu(II) and H2O2. Int. J. Food Sci. Nutr. 2014, 65, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Skalski, B.; Rywaniak, J.; Szustka, A.; Żuchowski, J.; Stochmal, A.; Olas, B. Anti-platelet properties of phenolic and nonpolar fractions isolated from various organs of Elaeagnus rhamnoides (L.) A. Int. J. Mol. Sci. 2021, 22, 3282. [Google Scholar] [CrossRef]
- Juszczak, M.; Kluska, M.; Skalski, B.; Żuchowski, J.; Stochmal, A.; Olas, B.; Woźniak, K. Multidirectional effects of saponin fraction isolated from the leaves of sea buckthorn Elaeagnus rhamnoides (L.) A. Nelson. Biomed. Pharmacother. 2021, 137, 1–13. [Google Scholar] [CrossRef]
- Choi, J.H.; Kim, K.J.; Kim, S. Comparative effect of quercetin and quercetin-3-O-βd-glucoside on fibrin polymers, blood clots, and in rodent models. J. Biochem. Mol. Toxicol. 2016, 30, 548–558. [Google Scholar] [CrossRef]
- Cheng, J.; Kondo, K.; Suzuki, Y.; Ikeda, Y.; Meng, X.; Umermura, K. Inhibitory effects of total flavones of Hippophae Rhamnoides L on thrombosis in mouse femoral artery and in vitro platelet aggregation. Life Sci. 2003, 72, 2263–2271. [Google Scholar] [CrossRef]
- Chong, M.F.F.; Macdonald, R.; Lovegrove, J.A. Fruit polyphenols and CDV risk: A review of human intervention studies. Br. J. Nutr. 2010, 104, 28–39. [Google Scholar] [CrossRef]
- Singh, I.P.; Ahmad, F.; Gore, D.D.; Tikoo, K.; Bansal, A.; Jachak, S.M.; Jena, G. Therapeutic potential of seabuckthorn: A patent review (2000–2018). Expert Opin. Ther. Pat. 2019, 29, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Tulsawani, R. Ninety day repeated gavage administration of Hipphophae rhamnoides extract in rats. Food Chem. Toxicol. 2010, 48, 2483–2489. [Google Scholar] [CrossRef] [PubMed]
- The Chinese Pharmacopoeia 2010. In Pharmacopoeia of the People’s Republic of China; Chinese Edition; China Medical Science and Technology Press: Beijing, China, 2010.
- Wang, K.; Xu, Z.; Liao, X. Bioactive compounds, health benefits and functional food products of sea buckthorn: A review. Crit. Rev. Food Sci. Nutr. 2021, 1, 1–22. [Google Scholar] [CrossRef]
- Beveridge, T.; Li, T.S.C.; Oomah, B.D.; Smith, A. Sea Buckthorn Products: Manufacture and Compositions. J. Agric. Food Chem. 1999, 47, 3480–3488. [Google Scholar] [CrossRef] [PubMed]
- Cui, B.; Jia, L. Traditional Chinese Medicine Composition of Total Flavonoids of Sea-Buckthorn and Linoleic Acid and Preparation Method of Composition. Chinese Patent CN103505484A, 2014. [Google Scholar]
- Cui, B.; Jia, L. Traditional Chinese Medicine Composition for Treating Cardiovascular Diseases and Preparation Method Thereof. Chinese Patent CN103505451A, 2014. [Google Scholar]
- Cui, B.; Jia, L. Blood Lipid Reducing Composition Containing Sea Buckthorn Oil and Phytosterol and Preparation Method of Composition. Chinese Patent CN103505483A, 2014. [Google Scholar]
- Early, T.; Xu, D.; Hu, C.; Zhao, C.; Mei, J. Method for Extracting Alkaloid from Seabuckthorn Seeds and Application of Active Component of Alkaloid to Angiocardiopathy Prevention. Chinese Patent CN101612176A, 2009. [Google Scholar]
- Ou, L.; Wang, W. Seabuckthorn Leaf Extract Preparation and Preparation Method Thereof. Chinese Patent CN102058631A, 2011. [Google Scholar]

| Sea Buckthorn Product | Dose/Days | Subjects | Activity of Sea Buckthorn Product | References |
|---|---|---|---|---|
| Juice | 300 mL/day (56 days) | 30 Healthy people (male non-smokers, aged 18–55 years) | Increase in HDL concentration | [38] |
| Pulp and seed oil | Ten 500 mg capsules/day (42 days) | Healthy people (aged 20–59 years, body mass index 19.6–26.5 kg/m2) | Inhibition of blood platelet aggregation | [31] |
| Fruit puree | 30 g/day (90 days) | Healthy people (males aged 50–70 years old or postmenopausal females) | Increase in HDL concentration | [42] |
| Seed oil | 5 mL/day (60 days) | Adult New Zealand white rabbits (2.50–1.0 kg bodyweight) | Decrease in total cholesterol | [32] |
| Dry fruits (powder) | 0.7 g/kg/day (60 days) | Stroke-prone rats | Decrease in total plasma cholesterol, triglyceride, heart rate, and blood pressure | [34] |
| Pulp oil | 5, 10 and 20 mL/kg/day (30 days) | Wistar rats (males, albino) | Protecting against myocardial ischemia-reperfusion injury | [37] |
| Total flavones extracted from seed residues | 150 mg/kg/day (42 days) | Chronic sucrose-fed rats | Antihypertensive action | [33] |
| Tea from leaves | 1 or 5%/day (42 days) | Obese mice | Anti-visceral obesity potential | [35] |
| Ethanolic extract of leaves | 500 or 1000 mg/kg | C57BL/6J mice (male) | Anti-obesity potential | [36] |
| Flavone (obtained from powdered leaves and whole fruits) | 75 mg/kg/day (42 or 84 days) | The apoE deficient mouse | Inhibition of macrophage foaming, inflammation, and vascular plaque formation | [43] |
| Element of Hemostasis | Sea Buckthorn | Reference | ||
|---|---|---|---|---|
| Fruits | Leaves | Twigs | ||
| Inhibition of blood platelet adhesion to collagen and fibrinogen (using washed human blood platelets) | + | + | + | [50,51] |
| Inhibition of blood platelet aggregation stimulated by thrombin (using washed human blood platelets) | ? | - | - | [50,51] |
| Inhibition of blood platelet aggregation stimulated by ADP (using human platelet rich-plasma) | - | - | + | [50,51] |
| Inhibition of blood platelet aggregation stimulated by collagen (using human platelet rich-plasma) | ? | - | - | [50,51,59] |
| Inhibition of eicosanoid synthesis (using human washed blood platelets) | + | + | + | [10,46] |
| Antioxidant activity (using human washed blood platelets and plasma) | + | + | + | [10,45,46] |
| Reduction of GPIIb/IIIa exposition (using human whole blood) | + | + | + | [56] |
| Reduction of thrombus formation (using human whole blood) | + | + | + | [56,57] |
| Number and Name of Patent | Chemical Content | Dose/Days | Subjects |
|---|---|---|---|
| A Chinese patent (CN103505484A) | Flavonoids, hydroxypropyl, and linoleic acid | 20 mg daily | Patients with coronary heart disease; angina pectoris |
| A Chinese patent (CN103505451A) | Isorhamnetin, quercetin, and kaempferol | 20 mg daily | Patients with coronary heart disease; angina pectoris |
| A Chinese patent (CN103505483A) | Fatty acids, phytosterol | - | Patients with hyperlipidemic |
| A Chinese patent (CN101612176A) | Tryptamine derivatives | - | Ischemic myocardial cells, in vitro |
| A Chinese patent (CN102058631A) | Flavonoids | 1, 2, 4 g/kg bw for 14 days | Sprague Dawley rat model |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olas, B.; Skalski, B. Preparations from Various Organs of Sea Buckthorn (Elaeagnus rhamnoides (L.) A. Nelson) as Important Regulators of Hemostasis and Their Role in the Treatment and Prevention of Cardiovascular Diseases. Nutrients 2022, 14, 991. https://doi.org/10.3390/nu14050991
Olas B, Skalski B. Preparations from Various Organs of Sea Buckthorn (Elaeagnus rhamnoides (L.) A. Nelson) as Important Regulators of Hemostasis and Their Role in the Treatment and Prevention of Cardiovascular Diseases. Nutrients. 2022; 14(5):991. https://doi.org/10.3390/nu14050991
Chicago/Turabian StyleOlas, Beata, and Bartosz Skalski. 2022. "Preparations from Various Organs of Sea Buckthorn (Elaeagnus rhamnoides (L.) A. Nelson) as Important Regulators of Hemostasis and Their Role in the Treatment and Prevention of Cardiovascular Diseases" Nutrients 14, no. 5: 991. https://doi.org/10.3390/nu14050991
APA StyleOlas, B., & Skalski, B. (2022). Preparations from Various Organs of Sea Buckthorn (Elaeagnus rhamnoides (L.) A. Nelson) as Important Regulators of Hemostasis and Their Role in the Treatment and Prevention of Cardiovascular Diseases. Nutrients, 14(5), 991. https://doi.org/10.3390/nu14050991






