Development and Validation of Cutoff Value for Reduced Muscle Mass for GLIM Criteria in Patients with Gastrointestinal and Hepatobiliary–Pancreatic Cancers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Data Collection
2.3. Muscle Mass Measurements
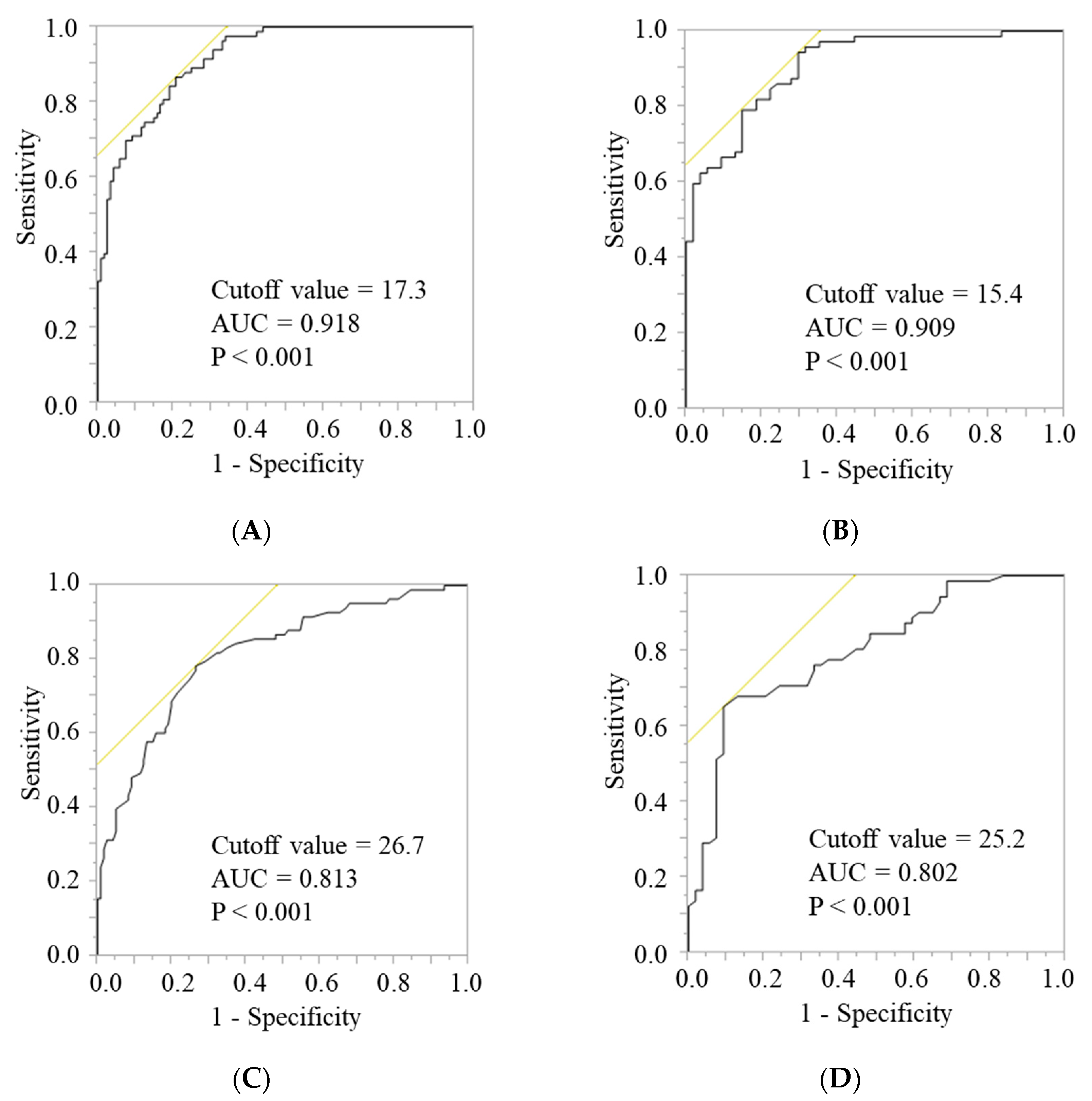
2.4. Development of Cutoff Values for the FFMI and AC
2.5. Validation of Cutoff Values for the Calculated FFMI and AC
2.6. Survival Analysis
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bozzetti, F. Rationale and indications for preoperative feeding of malnourished surgical cancer patients. Nutrition 2002, 18, 953–959. [Google Scholar] [CrossRef]
- Mosquera, C.; Koutlas, N.J.; Edwards, K.C.; Strickland, A.; Vohra, N.A.; Zervos, E.E.; Fitzgerald, T.L. Impact of malnutrition on gastrointestinal surgical patients. J. Surg. Res. 2016, 205, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Correia, M.I.; Waitzberg, D.L. The impact of malnutrition on morbidity, mortality, length of hospital stay and costs evaluated through a multivariate model analysis. Clin. Nutr. 2003, 22, 235–239. [Google Scholar] [CrossRef]
- Ho, J.W.; Wu, A.H.; Lee, M.W.; Lau, S.Y.; Lam, P.S.; Lau, W.S.; Kwok, S.S.; Kwan, R.Y.; Lam, C.F.; Tam, C.K.; et al. Malnutrition risk predicts surgical outcomes in patients undergoing gastrointestinal operations: Results of a prospective study. Clin. Nutr. 2015, 34, 679–684. [Google Scholar] [CrossRef]
- Fukuda, Y.; Yamamoto, K.; Hirao, M.; Nishikawa, K.; Maeda, S.; Haraguchi, N.; Miyake, M.; Hama, N.; Miyamoto, A.; Ikeda, M.; et al. Prevalence of malnutrition among gastric cancer patients undergoing gastrectomy and optimal preoperative nutritional support for preventing surgical site infections. Ann. Surg. Oncol. 2015, 22 (Suppl. S3), S778–S785. [Google Scholar] [CrossRef] [PubMed]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. Clin. Nutr. 2019, 38, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e2. [Google Scholar] [CrossRef]
- Ferguson, M.; Capra, S.; Bauer, J.; Banks, M. Development of a valid and reliable malnutrition screening tool for adult acute hospital patients. Nutrition 1999, 15, 458–464. [Google Scholar] [CrossRef]
- Bering, T.; Diniz, K.G.D.; Coelho, M.P.P.; Vieira, D.A.; Soares, M.M.S.; Kakehasi, A.M.; Correia, M.I.T.D.; Teixeira, R.; Queiroz, D.M.M.; Rocha, G.A.; et al. Association between pre-sarcopenia, sarcopenia, and bone mineral density in patients with chronic hepatitis C. J. Cachexia Sarcopenia Muscle 2018, 9, 255–268. [Google Scholar] [CrossRef] [Green Version]
- Hull, H.R.; Thornton, J.; Wang, J.; Pierson, R.N., Jr.; Kaleem, Z.; Pi-Sunyer, X.; Heymsfield, S.; Albu, J.; Fernandez, J.R.; Vanitallie, T.B.; et al. Fat-free mass index: Changes and race/ethnic differences in adulthood. Int. J. Obes. 2011, 35, 121–127. [Google Scholar] [CrossRef] [Green Version]
- Fryar, C.D.; Carroll, M.D.; Gu, Q.; Afful, J.; Ogden, C.L. Anthropometric reference data for children and adults: United states, 2015–2018. Vital Health Stat. 3 2021, 36, 1–44. [Google Scholar]
- Lee, M.M.; Jebb, S.A.; Oke, J.; Piernas, C. Reference values for skeletal muscle mass and fat mass measured by bioelectrical impedance in 390 565 UK adults. J. Cachexia Sarcopenia Muscle 2020, 11, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Jensen, B.; Moritoyo, T.; Kaufer-Horwitz, M.; Peine, S.; Norman, K.; Maisch, M.J.; Matsumoto, A.; Masui, Y.; Velázquez-González, A.; Domínguez-García, J.; et al. Ethnic differences in fat and muscle mass and their implication for interpretation of bioelectrical impedance vector analysis. Appl. Physiol. Nutr. Metab. 2019, 44, 619–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, F.J.; Liu, H.; Liu, X.L.; Jia, S.L.; Hou, L.S.; Xia, X.; Dong, B.R. Mid-upper arm circumference as an alternative screening instrument to appendicular skeletal muscle mass index for diagnosing sarcopenia. Clin. Interv. Aging 2021, 16, 1095–1104. [Google Scholar] [CrossRef]
- Hen, L.K.; Liu, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Bahyah, K.S.; Chou, M.Y.; Chen, L.Y.; Hsu, P.S.; Krairit, O.; et al. Sarcopenia in Asia: Consensus report of the Asian Working Group for Sarcopenia. J. Am. Med. Dir. Assoc. 2014, 15, 95–101. [Google Scholar] [CrossRef]
- Seino, S.; Shinkai, S.; Iijima, K.; Obuchi, S.; Fujiwara, Y.; Yoshida, H.; Kawai, H.; Nishi, M.; Murayama, H.; Taniguchi, Y.; et al. Reference values and age differences in body composition of community-dwelling older Japanese men and women: A pooled analysis of four cohort studies. PLoS ONE 2015, 10, e0131975. [Google Scholar] [CrossRef]
- Hosoya, N.; Okada, T.; Muto, Y.; Yamamori, H.; Tashiro, T.; Miwa, Y.; Moriwaki, H.; Tokai, S.; Aoyanagi, S.; Arisawa, M.; et al. Japanese anthropometric reference data 2001 (JARD 2001). Jpn. J. Nutr. Assess. 2002, 19, 1–81. (In Japanese) [Google Scholar]
- Martin-Cantero, A.; Reijnierse, E.M.; Gill, B.M.T.; Maier, A.B. Factors influencing the efficacy of nutritional interventions on muscle mass in older adults: A systematic review and meta-analysis. Nutr. Rev. 2021, 79, 315–330. [Google Scholar] [CrossRef]
- Liao, C.D.; Chen, H.C.; Huang, S.W.; Liou, T.H. The role of muscle mass gain following protein supplementation plus exercise therapy in older adults with sarcopenia and frailty risks: A systematic review and meta-regression analysis of randomized trials. Nutrients 2019, 11, 1713. [Google Scholar] [CrossRef] [Green Version]
- Maeda, K.; Ishida, Y.; Nonogaki, T.; Mori, N. Reference body mass index values and the prevalence of malnutrition according to the Global Leadership Initiative on Malnutrition criteria. Clin. Nutr. 2020, 39, 180–184. [Google Scholar] [CrossRef]
- Zhou, L.P.; Yu, D.Y.; Ma, B.W.; Shen, Z.L.; Zou, H.B.; Zhang, X.Z.; Yan, X.L.; Zhuang, C.L.; Yu, Z. Feasibility of substituting handgrip strength for muscle mass as a constituent standard in the global leadership initiative on malnutrition for diagnosing malnutrition in patients with gastrointestinal cancers. Nutrition 2021, 84, 111044. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Rodriguez, D.; Locquet, M.; Bruyère, O.; Lengelé, L.; Cavalier, E.; Reginster, J.Y.; Beaudart, C. Prediction of 5-year mortality risk by malnutrition according to the GLIM format using seven pragmatic approaches to define the criterion of loss of muscle mass. Clin. Nutr. 2021, 40, 2188–2199. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, X.; Wang, Y.; Liu, Z.; Fang, Y.; Peng, Z.; Liu, W. Body composition measurement improved performance of GLIM criteria in diagnosing malnutrition compared to PG-SGA in ambulatory cancer patients: A prospective cross-sectional study. Nutrients 2021, 13, 2744. [Google Scholar] [CrossRef] [PubMed]
- Cereda, E.; Pedrazzoli, P.; Lobascio, F.; Masi, S.; Crotti, S.; Klersy, C.; Turri, A.; Stobäus, N.; Tank, M.; Franz, K.; et al. The prognostic impact of BIA-derived fat-free mass index in patients with cancer. Clin. Nutr. 2021, 40, 3901–3907. [Google Scholar] [CrossRef] [PubMed]
- Kakavas, S.; Karayiannis, D.; Bouloubasi, Z.; Poulia, K.A.; Kompogiorgas, S.; Konstantinou, D.; Vougas, V. Global leadership initiative on malnutrition criteria predict pulmonary complications and 90-day mortality after major abdominal surgery in cancer patients. Nutrients 2020, 12, 3726. [Google Scholar] [CrossRef]
- Contreras-Bolívar, V.; Sánchez-Torralvo, F.J.; Ruiz-Vico, M.; González-Almendros, I.; Barrios, M.; Padín, S.; Alba, E.; Olveira, G. GLIM criteria using hand grip strength adequately predict six-month mortality in cancer inpatients. Nutrients 2019, 11, 2043. [Google Scholar] [CrossRef] [Green Version]
- Kootaka, Y.; Kamiya, K.; Hamazaki, N.; Nozaki, K.; Ichikawa, T.; Nakamura, T.; Yamashita, M.; Maekawa, E.; Reed, J.L.; Yamaoka-Tojo, M.; et al. The GLIM criteria for defining malnutrition can predict physical function and prognosis in patients with cardiovascular disease. Clin. Nutr. 2021, 40, 146–152. [Google Scholar] [CrossRef]
- Sanchez-Rodriguez, D.; Locquet, M.; Reginster, J.Y.; Cavalier, E.; Bruyère, O.; Beaudart, C. Mortality in malnourished older adults diagnosed by ESPEN and GLIM criteria in the SarcoPhAge study. J. Cachexia Sarcopenia Muscle 2020, 11, 1200–1211. [Google Scholar] [CrossRef]
- Brito, J.E.; Burgel, C.F.; Lima, J.; Chites, V.S.; Saragiotto, C.B.; Rabito, E.I.; Silva, F.M. GLIM criteria for malnutrition diagnosis of hospitalized patients presents satisfactory criterion validity: A prospective cohort study. Clin. Nutr. 2021, 40, 4366–4372. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, M.; Zhang, Q.; Zhang, K.P.; Guo, Z.Q.; Xu, H.X.; Yuan, K.T.; Yu, M.; Braga, M.; Cederholm, T.; et al. The GLIM criteria as an effective tool for nutrition assessment and survival prediction in older adult cancer patients. Clin. Nutr. 2021, 40, 1223–1232. [Google Scholar] [CrossRef]
- Hirose, S.; Matsue, Y.; Kamiya, K.; Kagiyama, N.; Hiki, M.; Dotare, T.; Sunayama, T.; Konishi, M.; Saito, H.; Saito, K.; et al. Prevalence and prognostic implications of malnutrition as defined by GLIM criteria in elderly patients with heart failure. Clin. Nutr. 2021, 40, 4334–4340. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.D.; Wu, G.F.; Luo, X.; Song, H.N.; Wang, W.B.; Liu, N.X.; Yu, Z.; Dong, Q.T.; Chen, X.L.; Yan, J.Y. Value of muscle quality, strength and gait speed in supporting the predictive power of GLIM-defined malnutrition for postoperative outcomes in overweight patients with gastric cancer. Clin. Nutr. 2021, 40, 4201–4208. [Google Scholar] [CrossRef] [PubMed]
- Steffee, W.P. Malnutrition in hospitalized patients. JAMA 1980, 244, 2630–2635. [Google Scholar] [CrossRef] [PubMed]

| All | Development | Validation | p-Value | |
|---|---|---|---|---|
| n = 660 | n = 330 | n = 330 | ||
| Age (years) | 70 (63–76) | 70 (64–76) | 70 (63–76) | 0.572 |
| Sex (n, %) | 0.936 | |||
| Men | 407 (61.7) | 204 (61.8) | 203 (61.5) | |
| Women | 253 (38.3) | 126 (38.2) | 127 (38.5) | |
| Cancer site (n, %) | 0.686 | |||
| Colorectal | 259 (39.2) | 137 (41.5) | 122 (37.0) | |
| Stomach | 188 (28.5) | 86 (26.1) | 102 (30.9) | |
| Liver | 81 (12.3) | 41 (12.4) | 40 (12.1) | |
| Bile duct | 59 (8.9) | 30 (9.1) | 29 (8.8) | |
| Pancreas | 73 (11.1) | 36 (10.9) | 37 (11.2) | |
| Stage (n, %) | 0.169 | |||
| I | 206 (31.2) | 106 (32.1) | 100 (30.3) | |
| II | 236 (35.8) | 116 (35.2) | 120 (36.4) | |
| III | 156 (23.6) | 70 (21.2) | 86 (26.1) | |
| IV | 62 (9.4) | 38 (11.5) | 24 (7.3) | |
| Surgical approach (n, %) | 0.876 | |||
| Laparotomy | 296 (44.9) | 147 (44.6) | 149 (45.2) | |
| Laparoscopic surgery | 364 (55.2) | 183 (55.5) | 181 (54.9) | |
| Preoperative therapy (n, %) | 73 (11.1) | 35 (10.6) | 38 (11.5) | 0.710 |
| Adjuvant chemotherapy (n, %) | 249 (37.7) | 127 (38.5) | 122 (37.0) | 0.688 |
| Height (cm) | 160.0 (152.0–167.0) | 160.0 (152.0–167.1) | 160.0 (151.8–167.0) | 0.649 |
| Body weight (kg) | 56.8 (49.0–64.4) | 57.2 (49.7–65.6) | 56.3 (48.7–63.7) | 0.305 |
| BMI (kg/m2) | 22.3 (20.3–24.2) | 22.4 (20.5–24.4) | 22.2 (20.1–24.0) | 0.289 |
| SMI (kg/m2) | 6.6 (5.7–7.4) | 6.7 (5.7–7.3) | 6.5 (5.7–7.4) | 0.595 |
| Low SMI * (n, %) | 312 (47.3) | 155 (47.0) | 157 (47.6) | 0.876 |
| FFMI (kg/m2) | 16.4 (15.1–17.9) | 16.6 (15.1–17.9) | 16.3 (15.2–18.0) | 0.712 |
| AC (cm) | 26.6 (24.6–28.6) | 26.6 (24.6–28.6) | 26.7 (24.8–28.4) | 0.999 |
| FFMI ** | AC † | |
|---|---|---|
| Sensitivity (%) | 79.0 | 65.0 |
| Specificity (%) | 90.8 | 72.3 |
| PPV (%) | 88.6 | 68.0 |
| NPV (%) | 82.6 | 69.4 |
| Accuracy (%) | 85.2 | 68.8 |
| GLIM Using FFMI ** | GLIM Using AC † | |
|---|---|---|
| Sensitivity (%) | 93.2 | 91.9 |
| Specificity (%) | 100.0 | 99.2 |
| PPV (%) | 100.0 | 97.1 |
| NPV (%) | 98.1 | 97.7 |
| Accuracy (%) | 98.5 | 97.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takimoto, M.; Yasui-Yamada, S.; Nasu, N.; Kagiya, N.; Aotani, N.; Kurokawa, Y.; Tani-Suzuki, Y.; Kashihara, H.; Saito, Y.; Nishi, M.; et al. Development and Validation of Cutoff Value for Reduced Muscle Mass for GLIM Criteria in Patients with Gastrointestinal and Hepatobiliary–Pancreatic Cancers. Nutrients 2022, 14, 943. https://doi.org/10.3390/nu14050943
Takimoto M, Yasui-Yamada S, Nasu N, Kagiya N, Aotani N, Kurokawa Y, Tani-Suzuki Y, Kashihara H, Saito Y, Nishi M, et al. Development and Validation of Cutoff Value for Reduced Muscle Mass for GLIM Criteria in Patients with Gastrointestinal and Hepatobiliary–Pancreatic Cancers. Nutrients. 2022; 14(5):943. https://doi.org/10.3390/nu14050943
Chicago/Turabian StyleTakimoto, Mami, Sonoko Yasui-Yamada, Nanami Nasu, Natsumi Kagiya, Nozomi Aotani, Yumiko Kurokawa, Yoshiko Tani-Suzuki, Hideya Kashihara, Yu Saito, Masaaki Nishi, and et al. 2022. "Development and Validation of Cutoff Value for Reduced Muscle Mass for GLIM Criteria in Patients with Gastrointestinal and Hepatobiliary–Pancreatic Cancers" Nutrients 14, no. 5: 943. https://doi.org/10.3390/nu14050943
APA StyleTakimoto, M., Yasui-Yamada, S., Nasu, N., Kagiya, N., Aotani, N., Kurokawa, Y., Tani-Suzuki, Y., Kashihara, H., Saito, Y., Nishi, M., Shimada, M., & Hamada, Y. (2022). Development and Validation of Cutoff Value for Reduced Muscle Mass for GLIM Criteria in Patients with Gastrointestinal and Hepatobiliary–Pancreatic Cancers. Nutrients, 14(5), 943. https://doi.org/10.3390/nu14050943





