The Effects of Postprandial Walking on the Glucose Response after Meals with Different Characteristics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
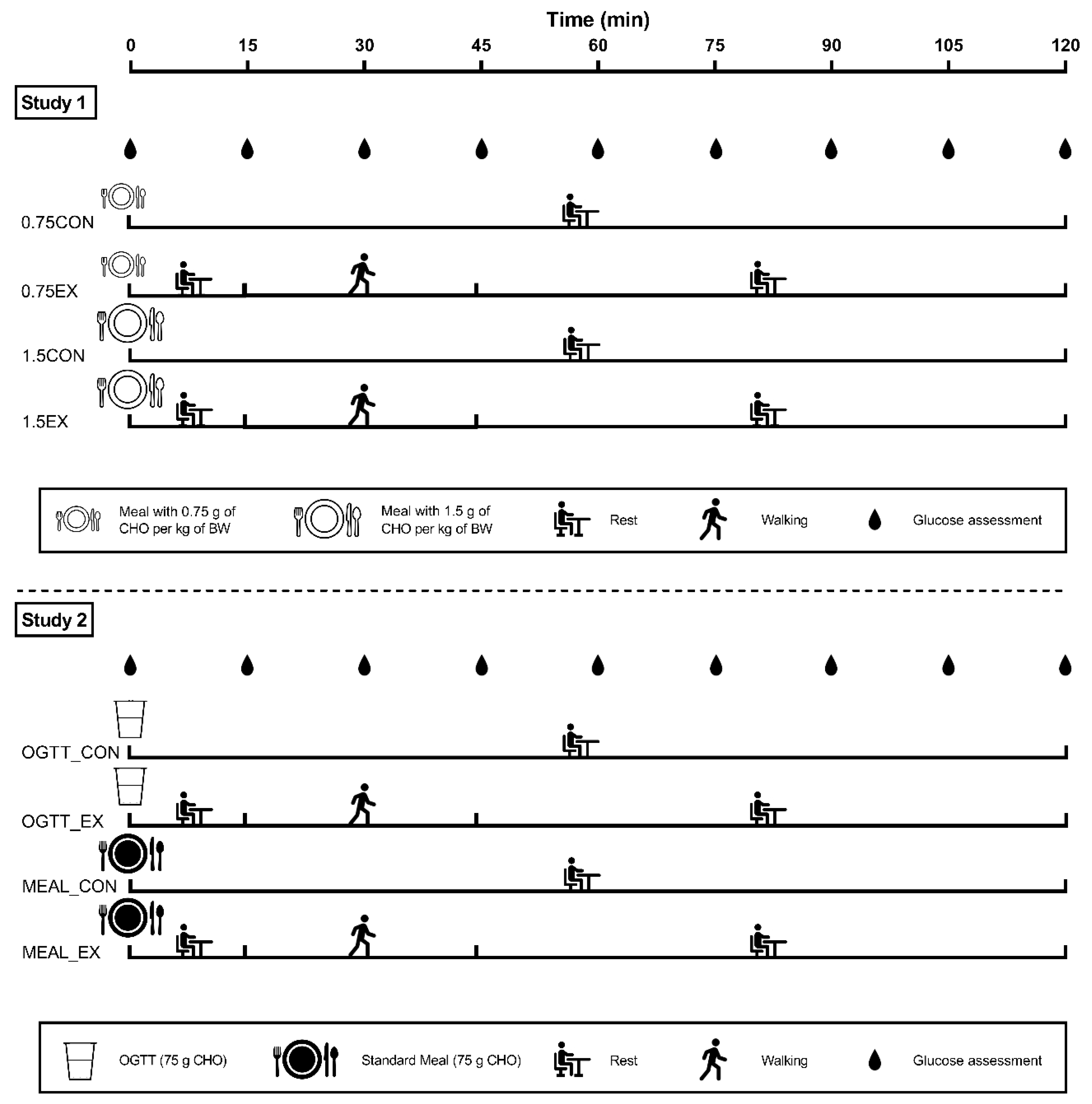
2.2. Study Overview
2.3. Familiarization
2.4. Study 1—The Effects of Postprandial Exercise on Glycemia after Consuming Mixed Meals with Different CHO Content
2.5. Study 2—The Effects of Postprandial Exercise on Glycemia after Consuming Meals with Different Macronutrient Composition
2.6. Exercise and Resting Time
2.7. Glycemic Assessment
2.8. Rating of Perceived Exertion
2.9. Statistical Analysis
3. Results
3.1. Study 1—The Effects of Postprandial Exercise on Glycemia after Consuming Mixed Meals with Different CHO Content
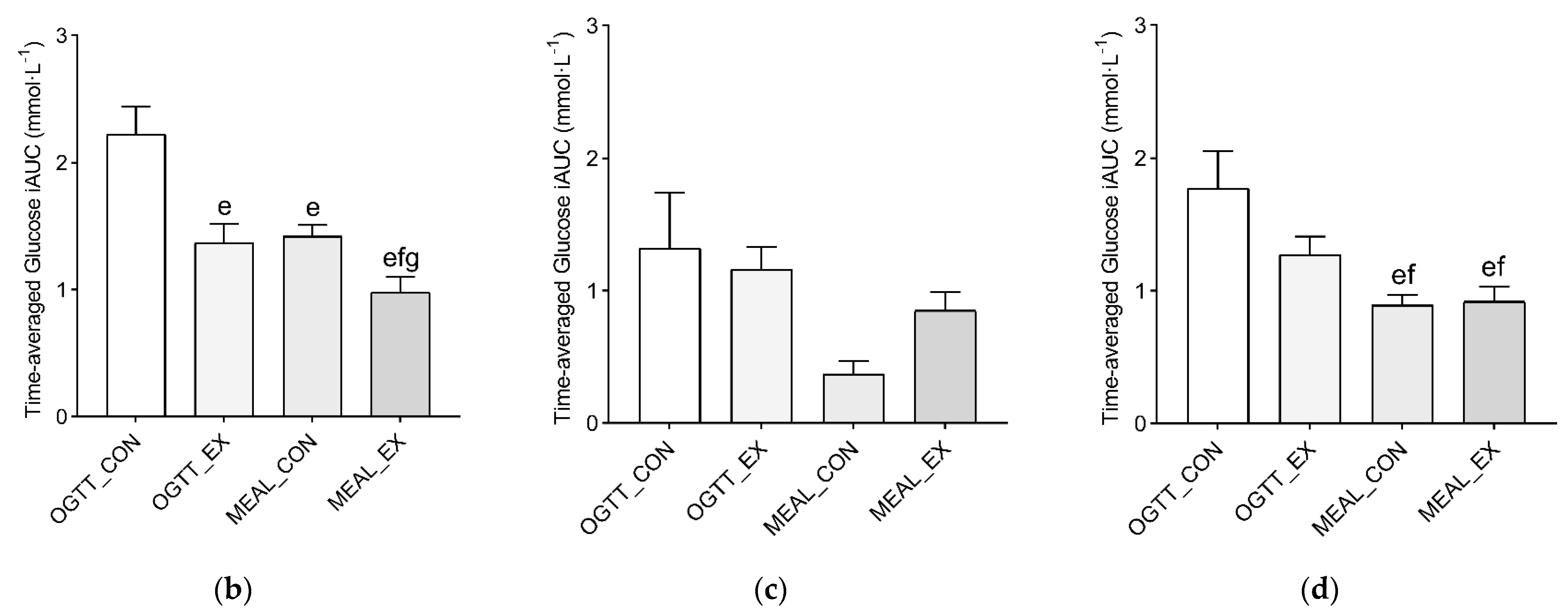
3.2. Study 2—The Effects of Postprandial Exercise on Glycemia after Consuming Meals with Different Macronutrient Composition
4. Discussion
- (i)
- Effective in reducing the glucose peak both when increasing the CHO content of a mixed meal and when consuming a CHO drink.
- (ii)
- Less effective in improving the total glycemic response two hours after the meal when the CHO content of a mixed meal is relatively high.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ceriello, A.; Genovese, S. Atherogenicity of postprandial hyperglycemia and lipotoxicity. Rev. Endocr. Metab. Disord. 2016, 17, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Ceriello, A.; Esposito, K.; Piconi, L.; Ihnat, M.A.; Thorpe, J.E.; Testa, R.; Boemi, M.; Giugliano, D. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes 2008, 57, 1349–1354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceriello, A.; Taboga, C.; Tonutti, L.; Quagliaro, L.; Piconi, L.; Bais, B.; Da Ros, R.; Motz, E. Evidence for an independent and cumulative effect of postprandial hypertriglyceridemia and hyperglycemia on endothelial dysfunction and oxidative stress generation: Effects of short- and long-term simvastatin treatment. Circulation 2002, 106, 1211–1218. [Google Scholar] [CrossRef] [Green Version]
- Esposito, K.; Nappo, F.; Marfella, R.; Giugliano, G.; Giugliano, F.; Ciotola, M.; Quagliaro, L.; Ceriello, A.; Giugliano, D. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: Role of oxidative stress. Circulation 2002, 106, 2067–2072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coutinho, M.; Gerstein, H.C.; Wang, Y.; Yusuf, S. The relationship between glucose and incident cardiovascular events. A metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes Care 1999, 22, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Gillen, J.B.; Estafanos, S.; Govette, A. Exercise-nutrient interactions for improved postprandial glycemic control and insulin sensitivity. Appl. Physiol. Nutr. Metab. 2021, 46, 856–865. [Google Scholar] [CrossRef]
- Chacko, E. Exercising tactically for taming postmeal glucose surges. Scientifica 2016, 2016, 4045717. [Google Scholar] [CrossRef]
- Bellini, A.; Nicolò, A.; Bazzucchi, I.; Sacchetti, M. Effects of different exercise strategies to improve postprandial glycemia in healthy individuals. Med. Sci. Sports Exerc. 2021, 53, 1334–1344. [Google Scholar] [CrossRef]
- Aqeel, M.; Forster, A.; Richards, E.A.; Hennessy, E.; McGowan, B.; Bhadra, A.; Guo, J.; Gelfand, S.; Delp, E.; Eicher-Miller, H.A. The effect of timing of exercise and eating on postprandial response in adults: A systematic review. Nutrients 2020, 12, 221. [Google Scholar] [CrossRef] [Green Version]
- Solomon, T.P.J.; Tarry, E.; Hudson, C.O.; Fitt, A.I.; Laye, M.J. Immediate post-breakfast physical activity improves interstitial postprandial glycemia: A comparison of different activity-meal timings. Pflugers Arch. Eur. J. Physiol. 2020, 472, 271–280. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, A.; Venn, B. The timing of activity after eating affects the glycaemic response of healthy adults: A randomised controlled trial. Nutrients 2018, 10, 1743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haxhi, J.; Scotto di Palumbo, A.; Sacchetti, M. Exercising for metabolic control: Is timing important. Ann. Nutr. Metab. 2013, 62, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Kanaley, J.A.; Colberg, S.R.; Corcoran, M.H.; Malin, S.K.; Rodriguez, N.R.; Crespo, C.J.; Kirwan, J.P.; Zierath, J.R. Exercise/physical activity in individuals with type 2 diabetes: A consensus statement from the American College of Sports Medicine. Med. Sci. Sports Exerc. 2022, 54, 353–368. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wongpipit, W.; Sun, F.; Sheridan, S.; Huang, W.Y.J.; Sit, C.H.P.; Wong, S.H.S. Walking initiated 20 minutes before the time of individual postprandial glucose peak reduces the glucose response in young men with overweight or obesity: A randomized crossover study. J. Nutr. 2021, 151, 866–875. [Google Scholar] [CrossRef]
- Shambrook, P.; Kingsley, M.I.; Wundersitz, D.W.; Xanthos, P.D.; Wyckelsma, V.L.; Gordon, B.A. Glucose response to exercise in the post-prandial period is independent of exercise intensity. Scand. J. Med. Sci. Sports 2018, 28, 939–946. [Google Scholar] [CrossRef]
- Yoko, N.; Hiroshi, Y.; Ying, J. Type and timing of exercise during lunch breaks for suppressing postprandial increases in blood glucose levels in workers. J. Occup. Health 2021, 63, e12199. [Google Scholar] [CrossRef]
- Colberg, S.R.; Zarrabi, L.; Bennington, L.; Nakave, A.; Thomas Somma, C.; Swain, D.P.; Sechrist, S.R. Postprandial walking is better for lowering the glycemic effect of dinner than pre-dinner exercise in type 2 diabetic individuals. J. Am. Med. Dir. Assoc. 2009, 10, 394–397. [Google Scholar] [CrossRef]
- Teo, S.Y.M.; Kanaley, J.A.; Guelfi, K.J.; Cook, S.B.; Hebert, J.J.; Forrest, M.R.L.; Fairchild, T.J. Exercise timing in type 2 diabetes mellitus: A systematic review. Med. Sci. Sports Exerc. 2018, 50, 2387–2397. [Google Scholar] [CrossRef]
- Sacchetti, M.; Haxhi, J.; Sgrò, P.; Scotto di Palumbo, A.; Nicolò, A.; Bellini, A.; Bazzucchi, I.; di Luigi, L. Effects of exercise before and/or after a mixed lunch on postprandial metabolic responses in healthy male individuals. Eur. J. Nutr. 2021, 60, 3437–3447. [Google Scholar] [CrossRef]
- Meier, J.J.; Baller, B.; Menge, B.A.; Gallwitz, B.; Schmidt, W.E.; Nauck, M.A. Excess glycaemic excursions after an oral glucose tolerance test compared with a mixed meal challenge and self-measured home glucose profiles: Is the OGTT a valid predictor of postprandial hyperglycaemia and vice versa? Diabetes Obes. Metab. 2009, 11, 213–222. [Google Scholar] [CrossRef]
- Galgani, J.; Aguirre, C.; Díaz, E. Acute effect of meal glycemic index and glycemic load on blood glucose and insulin responses in humans. Nutr. J. 2006, 5, 22. [Google Scholar] [CrossRef] [Green Version]
- Alssema, M.; Schindhelm, R.K.; Rijkelijkhuizen, J.M.; Kostense, P.J.; Teerlink, T.; Nijpels, G.; Heine, R.J.; Dekker, J.M. Meal composition affects insulin secretion in women with type 2 diabetes: A comparison with healthy controls. The hoorn prandial study. Eur. J. Clin. Nutr. 2009, 63, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Gentilcore, D.; Chaikomin, R.; Jones, K.L.; Russo, A.; Feinle-Bisset, C.; Wishart, J.M.; Rayner, C.K.; Horowitz, M. Effects of fat on gastric emptying of and the glycemic, insulin, and incretin responses to a carbohydrate meal in type 2 diabetes. J. Clin. Endocrinol. Metab. 2006, 91, 2062–2067. [Google Scholar] [CrossRef] [PubMed]
- King, D.G.; Walker, M.; Campbell, M.D.; Breen, L.; Stevenson, E.J.; West, D.J. A small dose of whey protein co-ingested with mixed-macronutrient breakfast and lunch meals improves postprandial glycemia and suppresses appetite in men with type 2 diabetes: A randomized controlled trial. Am. J. Clin. Nutr. 2018, 107, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Matthan, N.R.; Ausman, L.M.; Lichtenstein, A.H. Effect of prior meal macronutrient composition on postprandial glycemic responses and glycemic index and glycemic load value determinations. Am. J. Clin. Nutr. 2017, 106, 1246–1256. [Google Scholar] [CrossRef] [Green Version]
- Parr, E.; Devlin, B.; Callahan, M.; Radford, B.; Blankenship, J.; Dunstan, D.; Hawley, J. Effects of providing high-fat versus high-carbohydrate meals on daily and postprandial physical activity and glucose patterns: A randomised controlled trial. Nutrients 2018, 10, 557. [Google Scholar] [CrossRef] [Green Version]
- Brodovicz, K.G.; Girman, C.J.; Simonis-Bik, A.M.C.; Rijkelijkhuizen, J.M.; Zelis, M.; Bunck, M.C.; Mari, A.; Nijpels, G.; Eekhoff, E.M.W.; Dekker, J.M. Postprandial metabolic responses to mixed versus liquid meal tests in healthy men and men with type 2 diabetes. Diabetes Res. Clin. Pract. 2011, 94, 449–455. [Google Scholar] [CrossRef] [Green Version]
- Lupoli, R.; Pisano, F.; Capaldo, B. Postprandial glucose control in type 1 diabetes: Importance of the gastric emptying rate. Nutrients 2019, 11, 1559. [Google Scholar] [CrossRef] [Green Version]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.-P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef]
- King, D.S.; Baldus, P.J.; Sharp, R.L.; Kesl, L.D.; Feltmeyer, T.L.; Riddle, M.S. Time course for exercise-induced alterations in insulin action and glucose tolerance in middle-aged people. J. Appl. Physiol. 1995, 78, 17–22. [Google Scholar] [CrossRef]
- Rowe, D.A.; Welk, G.J.; Heil, D.P.; Mahar, M.T.; Kemble, C.D.; Calabró, M.A.; Camenisch, K. Stride rate recommendations for moderate-intensity walking. Med. Sci. Sports Exerc. 2011, 43, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Tudor-Locke, C.; Aguiar, E.J.; Han, H.; Ducharme, S.W.; Schuna, J.M.; Barreira, T.V.; Moore, C.C.; Busa, M.A.; Lim, J.; Sirard, J.R.; et al. Walking cadence (steps/min) and intensity in 21–40-year-olds: CADENCE-adults. Int. J. Behav. Nutr. Phys. Act. 2019, 16, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narang, B.J.; Atkinson, G.; Gonzalez, J.T.; Betts, J.A. A tool to explore discrete-time data: The time series response analyser. Int. J. Sport Nutr. Exerc. Metab. 2020, 30, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1988; ISBN 978-0-8058-0283-2. [Google Scholar]
- Monnier, L.; Colette, C.; Owens, D.R. Glycemic variability: The third component of the dysglycemia in diabetes. Is it important? How to measure it? J. Diabetes Sci. Technol. 2008, 2, 1094–1100. [Google Scholar] [CrossRef]
- Haxhi, J.; Leto, G.; di Palumbo, A.S.; Sbriccoli, P.; Guidetti, L.; Fantini, C.; Buzzetti, R.; Caporossi, D.; Di Luigi, L.; Sacchetti, M. Exercise at lunchtime: Effect on glycemic control and oxidative stress in middle-aged men with type 2 diabetes. Eur. J. Appl. Physiol. 2016, 116, 573–582. [Google Scholar] [CrossRef]
- Holst, J.J.; Gribble, F.; Horowitz, M.; Rayner, C.K. Roles of the gut in glucose homeostasis. Diabetes Care 2016, 39, 884–892. [Google Scholar] [CrossRef] [Green Version]
- Loh, R.; Stamatakis, E.; Folkerts, D.; Allgrove, J.E.; Moir, H.J. Effects of interrupting prolonged sitting with physical activity breaks on blood glucose, insulin and triacylglycerol measures: A systematic review and meta-analysis. Sports Med. 2020, 50, 295–330. [Google Scholar] [CrossRef] [Green Version]
- Saunders, T.J.; Atkinson, H.F.; Burr, J.; MacEwen, B.; Skeaff, C.M.; Peddie, M.C. The acute metabolic and vascular impact of interrupting prolonged sitting: A systematic review and meta-analysis. Sports Med. 2018, 48, 2347–2366. [Google Scholar] [CrossRef]
- Shambrook, P.; Kingsley, M.I.; Taylor, N.F.; Wundersitz, D.W.; Wundersitz, C.E.; Paton, C.D.; Gordon, B.A. A comparison of acute glycaemic responses to accumulated or single bout walking exercise in apparently healthy, insufficiently active adults. J. Sci. Med. Sport 2020, 23, 902–907. [Google Scholar] [CrossRef]
- Zhang, X.; Zheng, C.; Ho, R.S.T.; Miyashita, M.; Wong, S.H.S. The effects of accumulated versus continuous exercise on postprandial glycemia, insulin, and triglycerides in adults with or without diabetes: A systematic review and meta-analysis. Sports Med. Open 2022, 8, 14. [Google Scholar] [CrossRef]
- Morrison, D.J.; Kowalski, G.M.; Grespan, E.; Mari, A.; Bruce, C.R.; Wadley, G.D. Measurement of postprandial glucose fluxes in response to acute and chronic endurance exercise in healthy humans. Am. J. Physiol. Endocrinol. Metab. 2018, 314, E503–E511. [Google Scholar] [CrossRef] [PubMed]
- Schiavon, M.; Hinshaw, L.; Mallad, A.; Man, C.D.; Sparacino, G.; Johnson, M.; Carter, R.; Basu, R.; Kudva, Y.; Cobelli, C.; et al. Postprandial glucose fluxes and insulin sensitivity during exercise: A study in healthy individuals. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E557–E566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sylow, L.; Kleinert, M.; Richter, E.A.; Jensen, T.E. Exercise-stimulated glucose uptake—Regulation and implications for glycaemic control. Nat. Rev. Endocrinol. 2017, 13, 133–148. [Google Scholar] [CrossRef] [PubMed]


| Study 1 | Study 2 | |
|---|---|---|
| Sample size (M/F) | 10 (5/5) | 11 (5/6) |
| Age (years) | 25 ± 3 | 25 ± 2 |
| Weight (kg) | 66 ± 9 | 68 ± 10 |
| Height (m) | 1.70 ± 0.09 | 1.74 ± 0.13 |
| BMI (kg/m2) | 22.9 ± 2.6 | 22.5 ± 2.3 |
| Study 1 | Study 2 | |||
|---|---|---|---|---|
| Meal 1 | Meal 2 | Meal 1 | Meal 2 | |
| Energy intake (kcal) | 276.20 ± 29.97 | 551.73 ± 62.57 | 297.00 ± 0.00 | 421.84 ± 0.00 |
| CHO (g) | 50.57 ± 5.76 | 100.77 ± 11.34 | 75.00 ± 0.00 | 75.00 ± 0.00 |
| Protein (g) | 8.57 ± 8.51 | 17.22 ± 4.32 | 0 ± 0.00 | 14.50 ± 0.00 |
| Fat (g) | 4.12 ± 1.23 | 8.28 ± 2.46 | 0 ± 0.00 | 6.78 ± 0.00 |
| CHO (%) | 73.79 ± 6.17 | 73.66 ± 6.05 | 100.00 ± 0.00 | 71.38 ± 0.00 |
| Protein (%) | 12.38 ± 12.37 | 12.45 ± 12.40 | 0 ± 0.00 | 13.82 ± 0.00 |
| Fat (%) | 13.35 ± 3.48 | 13.42 ± 3.42 | 0 ± 0.00 | 14.51 ± 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellini, A.; Nicolò, A.; Bazzucchi, I.; Sacchetti, M. The Effects of Postprandial Walking on the Glucose Response after Meals with Different Characteristics. Nutrients 2022, 14, 1080. https://doi.org/10.3390/nu14051080
Bellini A, Nicolò A, Bazzucchi I, Sacchetti M. The Effects of Postprandial Walking on the Glucose Response after Meals with Different Characteristics. Nutrients. 2022; 14(5):1080. https://doi.org/10.3390/nu14051080
Chicago/Turabian StyleBellini, Alessio, Andrea Nicolò, Ilenia Bazzucchi, and Massimo Sacchetti. 2022. "The Effects of Postprandial Walking on the Glucose Response after Meals with Different Characteristics" Nutrients 14, no. 5: 1080. https://doi.org/10.3390/nu14051080
APA StyleBellini, A., Nicolò, A., Bazzucchi, I., & Sacchetti, M. (2022). The Effects of Postprandial Walking on the Glucose Response after Meals with Different Characteristics. Nutrients, 14(5), 1080. https://doi.org/10.3390/nu14051080








