Body Composition Assessment in Mexican Children and Adolescents. Part 1: Comparisons between Skinfold-Thickness, Dual X-ray Absorptiometry, Air-Displacement Plethysmography, Deuterium Oxide Dilution, and Magnetic Resonance Imaging with the 4-C Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Measurements
2.2.1. Clinical Assessment
2.2.2. Anthropometry
2.2.3. Skinfold Thickness
2.2.4. Dual X-ray Absorptiometry (DXA)
2.2.5. Air-Displacement Plethysmography (ADP)
2.2.6. Deuterium Oxide Dilution (D2O)
- TBW (kg or L): total body water
- D2O dose (g): deuterium (D2O)
- Concentration (%): atom percent of supplied D2O: 99.9%
- 20: molecular weight of D2O
- 18.02: molecular weight of tap water
- APpost-dose (%): atom percent of D2O in post-dose saliva sample
- APpre-dose (%): atom percent of D2O in pre-dose saliva sample
- 10−3 (kg/g): calculation from g to kg
- 1.04: correction for proton exchange
- 0.99371 (kg/L): density of water at 36 °C
- bottle empty—an empty bottle was weighed with an Ohaus scale model PA4202C with 0.01 g precision and accuracy of two decimal places.
- bottle D2O—0.05 g of D2O per kilogram of body weight were filled into the bottle, which was weighed again.
- bottle D2O and water—50 mL of tap water were added, and again the bottle was weighed.
- bottle drunk—participants drank the D2O with tap water, and the bottle was weighed again.
2.2.7. Magnetic Resonance Imaging (MRI)
2.2.8. The 4-Compartment Model (4-C)
- FM (kg): fat mass
- BV (L): body volume measured by ADP
- TBW (L): total body water measured by D2O dilution
- DMC (g): bone mineral content measured by DXA
- Weight (kg): body weight
- FFM (kg): fat-free mass
- Weight (kg): body weight
- FM (kg): fat mass
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shamah-Levy, T.; Vielma-Orozco, E.; Heredia-Hernández, O.; Romero-Martínez, M.; Mojica-Cuevas, J.; Cuevas-Nasu, L.; Santaella-Castell, J.A.; Rivera-Dommarco, J. Encuesta Nacional de Salud y Nutrición 2018–19: Resultados Nacionales; Instituto Nacional de Salud Pública: Cuernavaca, México, 2020. [Google Scholar]
- Romero-Martínez, M.; Shamah-Levy, T.; Vielma-Orozco, E.; Heredia-Hernández, O.; Mojica-Cuevas, J.; Cuevas-Nasu, L.; Rivera-Dommarco, J. Encuesta nacional de salud y nutrición 2018-19: Metodología y perspectivas. Salud Publica Mex 2019, 61, 917–923. [Google Scholar] [CrossRef] [PubMed]
- INEGI. Available online: https://www.inegi.org.mx/sistemas/olap/registros/vitales/mortalidad/tabulados/ConsultaMortalidad.asp (accessed on 10 March 2020).
- Quetelet, A. Sur L’homme et le Développement de ses Facultés; Fayard: Paris, France, 1835. [Google Scholar]
- Eknoyan, G. Adolphe Quetelet (1796-1874)—The average man and indices of obesity. Nephrol. Dial. Transplant. 2008, 23, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Gungor, N.K. Overweight and obesity in children and adolescents. J. Clin. Res. Pediatr. Endocrinol. 2014, 6, 129–143. [Google Scholar] [CrossRef]
- Kumar, S.; Kelly, A.S. Review of childhood obesity: From epidemiology, etiology, and comorbidities to clinical assessment and treatment. Mayo Clin. Proc. 2017, 92, 251–265. [Google Scholar] [CrossRef]
- Wells, J.C. A Hattori chart analysis of body mass index in infants and children. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 325–329. [Google Scholar] [CrossRef]
- Maynard, L.M.; Wisemandle, W.; Roche, A.F.; Chumlea, W.C.; Guo, S.S.; Siervogel, R.M. Childhood body composition in relation to body mass index. Pediatrics 2001, 107, 344–350. [Google Scholar] [CrossRef]
- Lee, D.H.; Keum, N.; Hu, F.B.; Orav, E.J.; Rimm, E.B.; Willett, W.C.; Giovannucci, E.L. Predicted lean body mass, fat mass, and all cause and cause specific mortality in men: Prospective US cohort study. BMJ 2018, 362, k2575. [Google Scholar] [CrossRef]
- Costa-Urrutia, P.; Vizuet-Gámez, A.; Ramirez-Alcántara, M.; Guillen-González, M.; Medina-Contreras, O.; Valdes-Moreno, M.; Musalem-Younes, C.; Solares-Tlapechco, J.; Granados, J.; Franco-Trecu, V.; et al. Obesity measured as percent body fat, relationship with body mass index, and percentile curves for Mexican pediatric population. PLoS ONE 2019, 14, e0212792. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Ambrosi, J.; Silva, C.; Galofre, J.C.; Escalada, J.; Santos, S.; Millan, D.; Vila, N.; Ibanez, P.; Gil, M.J.; Valenti, V.; et al. Body mass index classification misses subjects with increased cardiometabolic risk factors related to elevated adiposity. Int. J. Obes. 2012, 36, 286–294. [Google Scholar] [CrossRef]
- Weber, D.R.; Moore, R.H.; Leonard, M.B.; Zemel, B.S. Fat and lean BMI reference curves in children and adolescents and their utility in identifying excess adiposity compared with BMI and percentage body fat. Am. J. Clin. Nutr. 2013, 98, 49–56. [Google Scholar] [CrossRef]
- Wells, J.C.; Coward, W.A.; Cole, T.J.; Davies, P.S. The contribution of fat and fat-free tissue to body mass index in contemporary children and the reference child. Int. J. Obes. Relat. Metab. Disord. 2002, 26, 1323–1328. [Google Scholar] [CrossRef] [PubMed]
- Gracia-Marco, L.; Moreno, L.A.; Ruiz, J.R.; Ortega, F.B.; de Moraes, A.C.; Gottrand, F.; Roccaldo, R.; Marcos, A.; Gomez-Martinez, S.; Dallongeville, J.; et al. Body composition indices and single and clustered cardiovascular disease risk factors in adolescents: Providing clinical-based cut-points. Prog. Cardiovasc. Dis. 2016, 58, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.C. Body composition in childhood: Effects of normal growth and disease. Proc. Nutr. Soc. 2003, 62, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, P.B.; Alder, N.; Bachlet, A.M.; Grant, H.; Juszczak, E.; Henry, J.; Vernon-Roberts, A.; Warner, J.; Wells, J. Gastrostomy feeding in cerebral palsy: Too much of a good thing? Dev. Med. Child. Neurol 2006, 48, 877–882. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.C.; Mok, Q.; Johnson, A.W.; Lanigan, J.A.; Fewtrell, M.S. Energy requirements and body composition in stable pediatric intensive care patients receiving ventilatory support. Food Nutr. Bull. 2002, 23, 95–98. [Google Scholar] [CrossRef]
- McCormack, S.E.; Cousminer, D.L.; Chesi, A.; Mitchell, J.A.; Roy, S.M.; Kalkwarf, H.J.; Lappe, J.M.; Gilsanz, V.; Oberfield, S.E.; Shepherd, J.A.; et al. Association between linear growth and bone accrual in a diverse cohort of children and adolescents. JAMA Pediatr. 2017, 171, e171769. [Google Scholar] [CrossRef]
- Wells, J.C.; Williams, J.E.; Chomtho, S.; Darch, T.; Grijalva-Eternod, C.; Kennedy, K.; Haroun, D.; Wilson, C.; Cole, T.J.; Fewtrell, M.S. Body-composition reference data for simple and reference techniques and a 4-component model: A new UK reference child. Am. J. Clin. Nutr. 2012, 96, 1316–1326. [Google Scholar] [CrossRef]
- Wells, J.C.; Fewtrell, M.S. Measuring body composition. Arch. Dis. Child. 2006, 91, 612–617. [Google Scholar] [CrossRef]
- Lopez-Gonzalez, D.; Wells, J.C.; Cortina-Borja, M.; Fewtrell, M.; Partida-Gaytan, A.; Clark, P. Reference values for bone mineral density in healthy Mexican children and adolescents. Bone 2021, 142, 115734. [Google Scholar] [CrossRef]
- Hulley, S.B.; Cummings, S.R.; Browner, W.S.; Grady, D.; Hearst, N.; Newman, T.B. Designing Clinical Research: An Epidemiologic Approach, 4th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013; p. 79. [Google Scholar]
- Marshall, W.A.; Tanner, J.M. Variations in pattern of pubertal changes in girls. Arch. Dis. Child. 1969, 44, 291–303. [Google Scholar] [CrossRef]
- Marshall, W.A.; Tanner, J.M. Variations in the pattern of pubertal changes in boys. Arch. Dis. Child. 1970, 45, 13–23. [Google Scholar] [CrossRef] [PubMed]
- De Jesus, J.M. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: Summary report. Pediatrics 2011, 128 (Suppl. 5), S213–S256. [Google Scholar] [CrossRef]
- WHO. Waist Circumference and Waist-Hip Ratio: Report of a WHO Expert Consultation. Available online: https://apps.who.int/iris/handle/10665/44583 (accessed on 26 July 2016).
- Marfell-Jones, M.; Olds, T.; Stewart, A.; Crater, J.E.L. International Standards for Anthropometric Assessment. Potchefstroom, South. Africa: International Society for the Advancement of Kinanthropometry—ISAK; International Society for the Advancement of Kinanthropometry: Potchefstroom, South Africa, 2006. [Google Scholar]
- De Waard, F. Body mass index. J. Chronic. Dis. 1978, 31, 129. [Google Scholar] [CrossRef]
- WHO. Obesity: Preventing and Managing the Global Epidemic. Report of a WHO Consultation; Series 894; WHO Consultation on Obesity & World Health Organization: Geneva, Switzerland, 2000; pp. 1–253. [Google Scholar]
- Slaughter, M.H.; Lohman, T.G.; Boileau, R.A.; Horswill, C.A.; Stillman, R.J.; Van Loan, M.D.; Bemben, D.A. Skinfold equations for estimation of body fatness in children and youth. Hum. Biol. 1988, 60, 709–723. [Google Scholar] [PubMed]
- Chomtho, S.; Fewtrell, M.S.; Jaffe, A.; Williams, J.E.; Wells, J.C. Evaluation of arm anthropometry for assessing pediatric body composition: Evidence from healthy and sick children. Pediatr. Res. 2006, 59, 860–865. [Google Scholar] [CrossRef]
- Shepherd, J.A.; Baim, S.; Bilezikian, J.P.; Schousboe, J.T. Executive summary of the 2013 international society for clinical densitometry position development conference on body composition. J. Clin. Densitom. 2013, 16, 489–495. [Google Scholar] [CrossRef]
- McCrory, M.A.; Gomez, T.D.; Bernauer, E.M.; Mole, P.A. Evaluation of a new air displacement plethysmograph for measuring human body composition. Med. Sci. Sports Exerc. 1995, 27, 1686–1691. [Google Scholar] [CrossRef]
- Fields, D.A.; Allison, D.B. Air-displacement plethysmography pediatric option in 2-6 years old using the four-compartment model as a criterion method. Obesity 2012, 20, 1732–1737. [Google Scholar] [CrossRef]
- Fields, D.A.; Goran, M.I. Body composition techniques and the four-compartment model in children. J. Appl. Physiol. 2000, 89, 613–620. [Google Scholar] [CrossRef]
- Buchholz, A.C.; McGillivray, C.F.; Pencharz, P.B. Differences in resting metabolic rate between paraplegic and able-bodied subjects are explained by differences in body composition. Am. J. Clin. Nutr. 2003, 77, 371–378. [Google Scholar] [CrossRef]
- Fuller, N.J.; Jebb, S.A.; Laskey, M.A.; Coward, W.A.; Elia, M. Four-component model for the assessment of body composition in humans: Comparison with alternative methods, and evaluation of the density and hydration of fat-free mass. Clin. Sci. 1992, 82, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Bland, J.M.; Altman, D.G. Measuring agreement in method comparison studies. Stat. Methods Med. Res. 1999, 8, 135–160. [Google Scholar] [CrossRef] [PubMed]
- Sopher, A.B.; Thornton, J.C.; Wang, J.; Pierson, R.N., Jr.; Heymsfield, S.B.; Horlick, M. Measurement of percentage of body fat in 411 children and adolescents: A comparison of dual-energy X-ray absorptiometry with a four-compartment model. Pediatrics 2004, 113, 1285–1290. [Google Scholar] [CrossRef] [PubMed]
- Gately, P.J.; Radley, D.; Cooke, C.B.; Carroll, S.; Oldroyd, B.; Truscott, J.G.; Coward, W.A.; Wright, A. Comparison of body composition methods in overweight and obese children. J. Appl. Physiol. 2003, 95, 2039–2046. [Google Scholar] [CrossRef][Green Version]
- Heard-Lipsmeyer, M.E.; Hull, H.; Sims, C.R.; Cleves, M.A.; Andres, A. Evaluating body composition in infancy and childhood: A comparison between 4C, QMR, DXA, and ADP. Pediatr. Obes. 2020, 15, e12617. [Google Scholar] [CrossRef]
- Ong, C.; Lee, J.H.; Puthucheary, Z.A. Muscle weakness and wasting in pediatric critical illness. Pediatric Med. 2021, 4, 13. [Google Scholar] [CrossRef]
- Mok, E.; Beghin, L.; Gachon, P.; Daubrosse, C.; Fontan, J.E.; Cuisset, J.M.; Gottrand, F.; Hankard, R. Estimating body composition in children with Duchenne muscular dystrophy: Comparison of bioelectrical impedance analysis and skinfold-thickness measurement. Am. J. Clin. Nutr. 2006, 83, 65–69. [Google Scholar] [CrossRef][Green Version]
- Shepherd, J.A.; Ng, B.K.; Sommer, M.J.; Heymsfield, S.B. Body composition by DXA. Bone 2017, 104, 101–105. [Google Scholar] [CrossRef]
- Field, A.E.; Laird, N.; Steinberg, E.; Fallon, E.; Semega-Janneh, M.; Yanovski, J.A. Which metric of relative weight best captures body fatness in children? Obes. Res. 2003, 11, 1345–1352. [Google Scholar] [CrossRef]
- Gutin, B.; Litaker, M.; Islam, S.; Manos, T.; Smith, C.; Treiber, F. Body-composition measurement in 9-11-y-old children by dual-energy X-ray absorptiometry, skinfold-thickness measurements, and bioimpedance analysis. Am. J. Clin. Nutr. 1996, 63, 287–292. [Google Scholar] [CrossRef]
- Roemmich, J.N.; Clark, P.A.; Weltman, A.; Rogol, A.D. Alterations in growth and body composition during puberty. I. Comparing multicompartment body composition models. J. Appl. Physiol. 1997, 83, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Lazzer, S.; Boirie, Y.; Meyer, M.; Vermorel, M. Evaluation of two foot-to-foot bioelectrical impedance analysers to assess body composition in overweight and obese adolescents. Br. J. Nutr. 2003, 90, 987–992. [Google Scholar] [CrossRef]
- Rodriguez, G.; Moreno, L.A.; Blay, M.G.; Blay, V.A.; Fleta, J.; Sarria, A.; Bueno, M.; Group, A.V.-Z.S. Body fat measurement in adolescents: Comparison of skinfold thickness equations with dual-energy X-ray absorptiometry. Eur. J. Clin. Nutr. 2005, 59, 1158–1166. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, E.A.; Strauss, B.J.; Walker, S.P.; Permezel, M. Determination of maternal body composition in pregnancy and its relevance to perinatal outcomes. Obstet. Gynecol. Surv. 2004, 59, 731–742, quiz 745-736. [Google Scholar] [CrossRef]
- Borga, M.; West, J.; Bell, J.D.; Harvey, N.C.; Romu, T.; Heymsfield, S.B.; Dahlqvist Leinhard, O. Advanced body composition assessment: From body mass index to body composition profiling. J. Investig. Med. 2018, 66, 1–9. [Google Scholar] [CrossRef] [PubMed]

| Children 4–10 Years | Adolescents 11–18 Years | |||
|---|---|---|---|---|
| Female n = 63 | Male n = 54 | Female n = 92 | Male n = 79 | |
| Age (years) | 8.1 ± 1.8 | 8.4 ± 1.7 | 14.5 ± 2.1 | 14.8 ± 2.0 |
| Weight (kg) | 26.1 ± 8.1 | 28.6 ± 8.1 | 51.2 ± 12.0 | 53.9 ± 11.6 |
| Weight z-score | −0.21 ± 0.97 | 0.13 ± 1.19 | - | - |
| Height (cm) | 123.5 ± 14.7 | 127.9 ± 11.1 | 155.1 ± 7.3 | 163.4 ± 10.3 |
| Height z-score | 0.11 ± 1.07 | 0.53 ± 1.14 | −0.44 ± 0.92 | −0.25 ± 0.97 |
| BMI (kg/m2) | 17.1 ± 6.0 | 17.2 ± 2.6 | 21.1 ± 3.9 | 20.0 ± 3.1 |
| BMI z-score | 0.11 ± 1.07 | 0.53 ± 1.14 | 0.32 ± 1.12 | −0.05 ± 1.19 |
| Waist circumference (cm) | 57.2 ± 8.0 | 60.4 ± 7.6 | 71.3 ± 7.8 | 72.1 ± 8.8 |
| Tanner | ||||
| 1 | 49 (78%) | 53 (98%) | 1 (1%) | 4 (5%) |
| 2 | 9 (14%) | 1 (2%) | 4 (4%) | 6 (8%) |
| 3 | 5 (8%) | 0 | 29 (32%) | 36 (46%) |
| 4 | 0 | 0 | 45 (49%) | 31 (39%) |
| 5 | 0 | 0 | 13 (14%) | 2 (3%) |
| BMI category | ||||
| Healthy weight | 49 (78%) | 40 (74%) | 65 (71%) | 62 (78%) |
| Overweight | 10 (16%) | 9 (17%) | 20 (22%) | 10 (13%) |
| Obesity | 3 (5%) | 5 (9%) | 6 (7%) | 4 (5%) |
| Low weight | 1 (2%) | 0 | 1 (1%) | 3 (4%) |
| Body composition variables | ||||
| SF-sum (mm) | 21.2 ± 8.6 | 22.0 ± 9.4 | 32.3 ± 12.7 | 22.1 ± 10.9 |
| SF FM (%) | 18.1 ± 5.3 | 17.1 ± 6.9 | 24.8 ± 7.7 | 17.2 ± 8.0 |
| SF-FM (kg) | 5.0 ± 3.3 | 5.5 ± 3.4 | 13.5 ± 7.2 | 9.7 ± 6.0 |
| SF-FFM (kg) | 21.1 ± 5.2 | 23.3 ± 5.1 | 37.9 ± 6.5 | 44.4 ± 8.9 |
| D2O TBW (kg) | 15.01 ± 3.7 | 16.9 ± 3.6 | 26.6 ± 5.0 | 31.9 ± 7.2 |
| D2O FM (%) | 19.7 ± 10.3 | 18.4 ± 9.7 | 28.2 ± 10.1 | 19.3 ± 10.0 |
| D2O FM (kg) | 5.4 ± 4.5 | 5.5 ± 4.4 | 15.2 ± 8.1 | 10.3 ± 6.4 |
| D2O FFM (kg) | 20.5 ± 5.1 | 22.9 ± 4.9 | 36.1 ± 6.7 | 43.3 ± 9.8 |
| DXA BMC (kg) | 0.9 ± 0.2 | 1.0 ± 0.2 | 1.9 ± 0.4 | 2.1 ± 0.5 |
| DXA FM (%) | 29.6 ± 6.2 | 28.6 ± 6.9 | 33.6 ± 6.1 | 23.3 ± 7.9 |
| DXA FM (kg) | 8.2 ± 4.2 | 8.3 ± 4.2 | 17.5 ± 6.7 | 12.5 ± 5.8 |
| DXA FFM (kg) | 17.8 ± 4.4 | 20.1 ± 4.3 | 33.7 ± 5.8 | 40.8 ± 9.9 |
| DXA LM (kg) | 16.9 ± 4.2 | 19.1 ± 4.1 | 31.8 ± 5.5 | 39.2 ± 8.4 |
| ADP BV (L) | 24.9 ± 8.3 | 20.1 ± 9.3 | 49.1 ± 13.1 | 50.8 ± 11.3 |
| ADP TGV (L) | 1.2 ± 0.4 | 1.3 ± 0.3 | 2.3 ± 0.4 | 2.8 ± 0.6 |
| ADP FM (%) | 14.1 ± 8.6 | 17.0 ± 8.7 | 22.6 ± 7.9 | 15.2 ± 8.8 |
| ADP FM (kg) | 4.1 ± 4.0 | 5.2 ± 4.2 | 12.2 ± 6.9 | 8.3 ± 5.9 |
| ADP FFM (kg) | 23.0 ± 9.8 | 22.9 ± 5.4 | 39.2 ± 8.3 | 45.3 ± 9.1 |
| 4-C FM (%) | 19.4 ± 8.7 | 19.1 ± 9.0 | 26.8 ± 8.4 | 17.4 ± 8.9 |
| 4-C FM (kg) | 5.5 ± 4.2 | 5.8 ± 4.3 | 14.4 ± 7.3 | 9.3 ± 5.8 |
| 4-C FFM (kg) | 20.6 ± 4.7 | 22.7 ± 4.9 | 36.8 ± 6.4 | 44.4 ± 9.8 |
| Hydration (%) | 72.9 ± 5.5 | 73.9 ± 4.4 | 71.7 ± 5.0 | 71.6 ± 5.2 |
| MRI subsample (n = 52) | ||||
| MRI FM (%) | 31.0 ± 7.4 | 25.9 ± 6.2 | 31.5 ± 5.9 | 23.6 ± 6.0 |
| MRI FM (kg) | 8.8 ± 5.5 | 7.0 ± 4.2 | 16.4 ± 4.8 | 12.6 ± 4.8 |
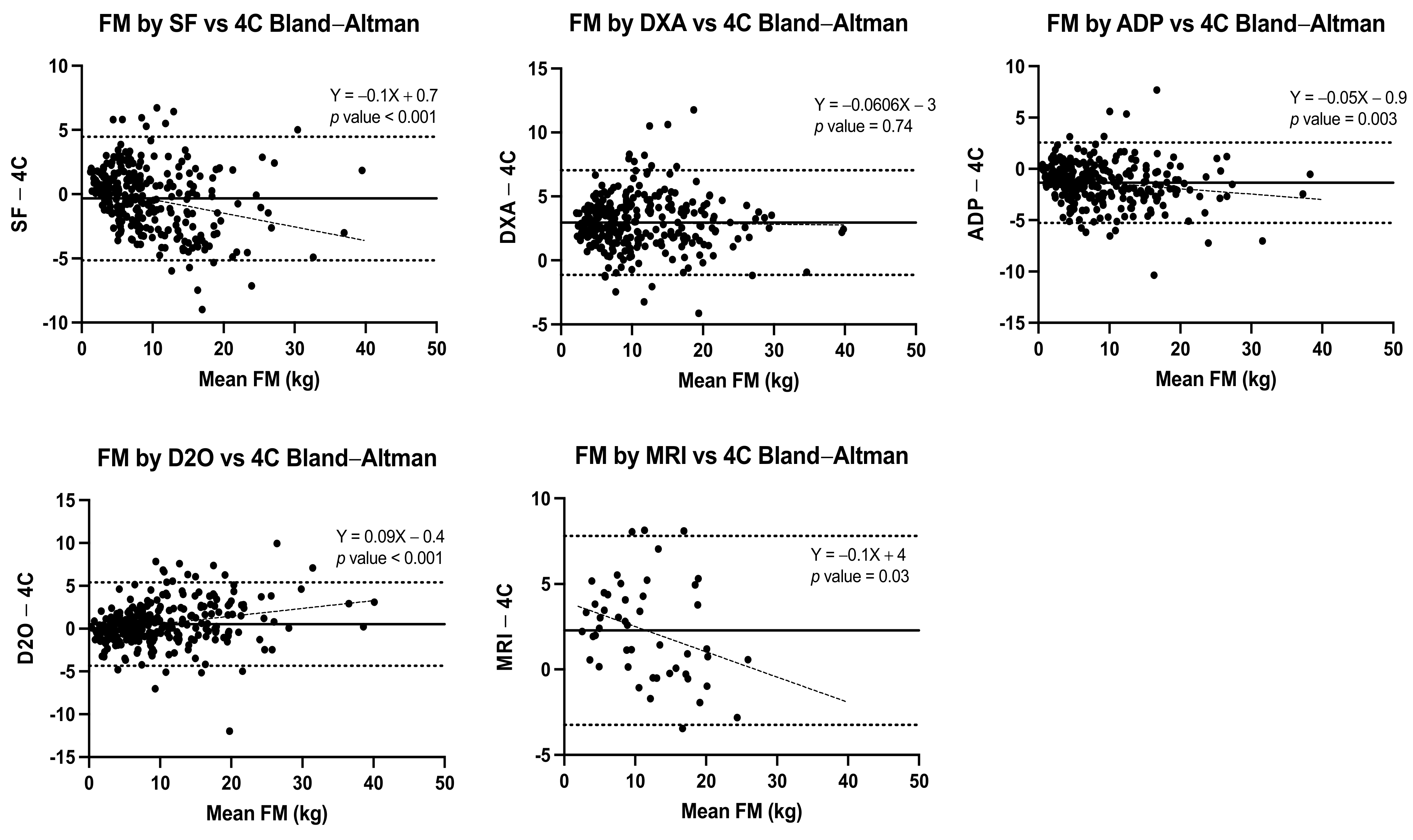
| Method | Pearson’s R, (95% CI) | Regression Equation | Lin’s Concordance Coefficient (95% CI) | Bland–Altman Difference Mean ± SD | BA LOA | Regression Analysis from Bland–Altman Differences | p Value |
|---|---|---|---|---|---|---|---|
| Total sample | |||||||
| SF (n = 288) | 0.93 (0.92–0.95) | Y = 0.84 (0.80 to 0.88) X + 1.2 (0.72 to 1.6) | 0.93 (0.91–0.94) | −0.34 ± 2.5 | (−5.2 to 4.5) | Y = −0.1X + 0.7 | p < 0.001 |
| DXA (n = 288) | 0.95 (0.94–0.96) | Y = 0.95 (0.91to 0.98)X + 3.4 (3.0 to 3.9) | 0.87 (0.85–0.89) | 2.95 ± 2.1 | (−1.1 to 7.0) | Y = −0.006X + 3 | p = 0.74 |
| ADP (n = 288) | 0.96 (0.95–0.97) | Y = 0.91 (0.88 to 0.94)X − 0.48 (−0.86to −0.10) | 0.94 (0.92–0.95) | −1.35 ± 2.0 | (−5.3 to 2.6) | Y = −0.05X − 0.9 | p = 0.003 |
| D2O (n = 288) | 0.94 (0.93–0.96) | Y = 1.0 (0.99 to 1.1)X + 0.23 (−0.27 to 0.72) | 0.94 (0.92–0.95) | 0.52 ± 2.5 | (−4.3 to 5.4) | Y = 0.09X − 0.4 | p < 0.001 |
| MRI (n = 49) | 0.91 (0.84–0.95) | Y = 0.79 (0.68to0.89)X + 4.5 (3.2to 5.8) | 0.4 (0.17–0.59) | 2.28 ± 2.8 | (−3.2 to 7.8) | Y = −0.1X + 4 | p = 0.03 |
| Female children | |||||||
| SF (n = 63) | 0.93 (0.89–0.96) | Y = −0.22 (−0.92 to 0.48) + 1.6 (1.04 to 1.3)X | 0.9 (0.86–0.94) | −0.49 ± 1.6 | (−3.6 to 2.6) | Y = −0.2X + 0.7 | p < 0.001 |
| DXA (n = 63) | 0.93 (0.89–0.96) | Y = −2.3 (−2.99 to −1.61) + 0.95 (0.88 to 1.03)X | 0.79 (0.71–0.85) | 2.73 ± 1.3 | (0.3 to 5.2) | Y = 0.004X + 3 | p = 0.91 |
| ADP (n = 63) | 0.96 (0.93–0.97) | Y = 1.4 (0.86 to 1.93) + 0.99 (0.9 to 1.08)X | 0.89 (0.83–0.93) | −1.37 ± 1.5 | (−4.3 to 1.5) | Y = −0.05X − 1 | p = 0.26 |
| D2O (n = 63) | 0.94 (0.90–0.96) | Y = 0.91 (0.25 to 1.56) + 0.85 (0.76 to 0.95)X | 0.94 (0.90–0.96) | 0.15 ± 1.6 | (−3.0 to 3.3) | Y = 0.06X − 0.2 | p = 0.19 |
| MRI (n = 7) | 0.95 (0.69–0.99) | Y = −2.16 (−5.75 to–1.43) + 0.93 (0.57 to 1.28)X | 0.76 (0.35–0.92) | 2.79 ± 1.7 | (−0.6 to 6.2) | Y = 0.02X + 3 | p = 0.88 |
| Male children | |||||||
| SF (n = 54) | 0.93 (0.89–0.96) | Y = 0.19 (−0.53 to 0.92) + 1.07 (0.9 to 1.19)X | 0.91 (0.85–0.94) | −0.3 ± 1.6 | (−3.5 to 2.9) | Y = −0.2X + 1 | p < 0.001 |
| DXA (n = 54) | 0.95 (0.91–0.97) | Y = −2.22 (−3.02 to–1.43) + 0.96 (0.87 to 1.04)X | 0.8 (0.71–0.86) | 2.59 ± 1.4 | (−0.1 to 5.3) | Y = −0.009X + 3 | p = 0.84 |
| ADP (n = 54) | 0.96 (0.93–0.98) | Y = 0.61 (0.09 to 1.14) + 0.98 (0.9 to 1.06)X | 0.95 (0.92–0.97) | −0.57 ± 1.2 | (−2.9 to 1.8) | Y = −0.01X − 0.5 | p = 0.74 |
| D2O (n = 54) | 0.96 (0.93–0.98) | Y = 0.77 (0.23 to 1.31) + 0.91 (0.83 to 0.99) X | 0.96 (0.93–0.97) | −0.17 ± 1.2 | (−2.6 to 2.3) | Y = 0.03X − 0.3 | p = 0.53 |
| MRI (n = 6) | 0.94 (0.77–0.99) | Y = −2.14 (−4.41to 0.13) + 1.05 (0.73 to 1.37)X | 0.92 (0.56–0.99) | −1.82 ± 1.5 | (−1.2 to 4.8) | Y = −0.1X + 3 | p = 0.54 |
| Female adolescents | |||||||
| SF (n = 92) | 0.93 (0.90–0.95) | Y = 1.98 (0.59 to 3.4) + 0.92 (0.83 to 1.01)X | 0.91 (0.88–0.94) | −1.2 ± 2.7 | (−6.4 to 4.0) | Y = −0.09X + 0.01 | p = 0.03 |
| DXA (n = 92) | 0.96 (0.94–0.97) | Y = −3.79 (−4.97 to−2.61) + 1.04 (0.98 to 1.1)X | 0.87 (0.83–0.91) | 3.13 ± 2.0 | (−0.9 to 7.1) | Y = −0.08X + 4 | p = 0.01 |
| ADP (n = 92) | 0.96 (0.94–0.98) | Y = 1.89 (1.05 to 2.73) + 1.02 (0.96 to 1.08)X | 0.92 (0.89–0.95) | −2.10 ± 2.0 | (−6.0 to 1.8) | Y = −0.06X − 1 | p = 0.06 |
| D2O (n = 92) | 0.95 (0.93–0.97) | Y = 1.38 (0.38 to 2.37) + 0.86 (0.8 to 0.92)X | 0.94 (0.91–0.96) | 0.79 ± 2.6 | (−4.3 to 5.8) | Y = 0.1X − 0.9 | p < 0.001 |
| MRI (n = 17) | 0.86 (0.65–0.95) | Y = −1.83 (−7.5 to 3.83) + 1.03 (0.7 to 1.36)X | 0.75 (0.53–0.88) | 1.37 ± 2.9 | (−4.3 to 7.0) | Y = −0.2X + 4 | p = 0.2 |
| Male adolescents | |||||||
| SF (n = 79) | 0.88 (0.82–0.92) | Y = 1.16 (−0.001 to 2.32) + 0.86 (0.76 to 0.96)X | 0.84 (0.77–0.90) | 0.74 ± 2.9 | (−4.8 to 6.3) | Y = −0.1X + 2 | p = 0.09 |
| DXA (n = 79) | 0.89 (0.83–0.93) | Y = −1.56 (−3.01 to 0.12) + 0.87 (0.77 to 0.97)X | 0.77 (0.68–0.83) | 3.16 ± 2.9 | (−2.5 to 8.8) | Y = 0.02X + 3 | p = 0.68 |
| ADP (n = 79) | 0.92 (0.88–0.95) | Y = 1.89 (0.97 to 2.8) + 0.89 (0.8 to 0.98)X | 0.87 (0.81–0.92) | −1.00 ± 2.5 | (−5.9 to 3.9) | Y = 0.04X − 1 | p = 0.49 |
| D2O (n = 79) | 0.86 (0.78–0.91) | Y = 1.13 (−0.09 to 2.35) + 0.8 (0.7 to 0.9)X | 0.84 (0.76–0.89) | 0.98 ± 3.3 | (−5.6 to 7.5) | Y = 0.08X + 0.2 | p = 0.24 |
| MRI (n = 19) | 0.86 (0.65–0.95) | Y = −3.3 (−7.98 to 1.38) + 1.02 (0.67 to 1.37)X | 0.6 (0.28–0.80) | 3.04 ± 3.2 | (−3.3 to 9.4) | Y = −0.X + 6 | p = 0.14 |
| Method | Pearson’s R, (95% CI) | Regression Equation | Lin’s Concordance Coefficient (95% CI) | Bland–Altman Difference Mean ± SD | BA LOA | Regression Analysis from Bland–Altman Differences | p Value |
|---|---|---|---|---|---|---|---|
| Total sample | |||||||
| SF | 0.98 (0.97–0.98) | Y = 0.95X + 2.1 | 0.98 (0.97–0.98) | 0.31 ± 2.4 | (−4.5 to 5.1) | Y = −0.03X + 1 | p = 0.006 |
| DXA | 0.98 (0.98–0.99) | Y = 0.94X − 1.1 | 0.95 (0.94–0.96) | −2.97 ± 2.2 | (−7.2 to 1.3) | Y = −0.04X − 2 | p < 0.001 |
| ADP | 0.98 (0.98–0.99) | Y = 0.98X + 1.8 | 0.98 (0.97–0.98) | 1.28 ± 2.2 | (−3.1 to 5.7) | Y = −0.0005X + 1 | p = 0.96 |
| D2O | 0.98 (0.97–0.98) | Y = 0.96X + 0.83 | 0.98 (0.97–0.98) | −0.53 ± 2.5 | (−5.4 to 4.3) | Y = −0.02X + 0.2 | p = 0.08 |
| MRI | 0.97 (0.94–0.99) | Y = 0.92X + 0.80 | 0.97 (0.95–0.98) | −2.28 ± 2.8 | (−7.2 to 3.2) | Y = −0.04X − 0.9 | p = 0.25 |
| Female children | |||||||
| SF | 0.95 (0.93–0.97) | Y = 1.1X − 0.93 | 0.94 (0.91–0.96) | 0.46 ± 1.6 | (−2.7 to 3.6) | Y = 0.1X − 2 | p = 0.004 |
| DXA | 0.96 (0.93–0.97) | Y = 0.90X − 0.77 | 0.8 (0.72–0.86) | −2.84 ± 1.3 | (−5.5 to −0.2) | Y = −0.07X − 2 | p = 0.08 |
| ADP | 0.94 (0.91–0.97) | Y = 1.0X + 0.35 | 0.91 (0.86–0.94) | 1.28 ± 2.2 | (−3.0 to 5.6) | Y = 0.004X + 1 | p = 0.74 |
| D2O | 0.95 (0.92–0.97) | Y = 1.0X − 0.62 | 0.95 (0.91–0.97) | −0.12 ± 1.6 | (−3.3 to 3.0) | Y = 0.08X − 2 | p = 0.06 |
| MRI | 0.91 (0.48–0.99) | Y = 0.91X − 0.95 | 0.81 (0.36–0.95) | −2.79 ± 1.7 | (−6.2 to 0.63) | Y = 0.009X − 3 | p = 0.96 |
| Male children | |||||||
| SF | 0.95 (0.92–0.97) | Y = 1.0X − 0.54 | 0.95 (0.91–0.97) | 0.22 ± 1.6 | (−2.9 to 3.3) | Y = 0.09X − 2 | p = 0.03 |
| DXA | 0.96 (0.94–0.98) | Y = 0.86X + 0.61 | 0.82 (0.75–0.88) | −2.7 ± 1.3 | (−5.2 to −0.04) | Y = −0.1X − 0.3 | p = 0.004 |
| ADP | 0.96 (0.93–0.98) | Y = 0.94X + 1.7 | 0.95 (0.92–0.97) | 0.42 ± 1.36 | (−2.2 to 3.1) | Y = −0.007X + 0.6 | p = 0.85 |
| D2O | 0.97 (0.94–0.98) | Y = 0.97X + 1.0 | 0.97 (0.94–0.98) | 0.21 ± 1.2 | (−2.2 to 2.5) | Y = 0.005X + 0.08 | p = 0.87 |
| MRI | 0.96 (0.66–0.99) | Y = 1.0X − 2.0 | 0.73 (0.26–0.92) | −2.82 ± 1.5 | (−4.8 to 1.2) | Y = 0.05X − 3.0 | p = 0.73 |
| Female adolescents | |||||||
| SF | 0.90 (0.85–0.93) | Y = 0.89X + 5.1 | 0.89 (0.83–0.92) | 1.14 ± 2.6 | (−4.0 to 6.3) | Y = −0.01X + 1 | p = 0.79 |
| DXA | 0.95 (0.92–0.97) | Y = 0.87X + 1.6 | 0.83 (0.77–0.87) | −3.27 ± 2.0 | (−7.2 to 0.7) | Y = −0.09X − 0.02 | p = 0.009 |
| ADP | 0.94 (0.91–0.96) | Y = 0.91X + 5.5 | 0.89 (0.85–0.93) | 1.28 ± 2.3 | (−3.2 to 5.8) | Y = −0.001X + 1 | p = 0.005 |
| D2O | 0.93 (0.89–0.95) | Y = 0.98X − 0.29 | 0.92 (0.88–0.95) | −0.86 ± 2.5 | (−5.8 to 4.0) | Y = 0.06X − 3 | p = 0.14 |
| MRI | 0.88 (0.70–0.96) | Y = 1.0X − 1.4 | 0.86 (0.66–0.94) | 2.79 ± 1.7 | (−0.6 to 6.2) | Y = 0.02X + 3 | p = 0.88 |
| Male adolescents | |||||||
| SF | 0.96 (0.94–0.97) | Y = 0.84X + 6.1 | 0.95 (0.92–0.97) | −0.74 ± 2.8 | (−6.2 to 4.7) | Y = −0.1X + 5 | p < 0.001 |
| DXA | 0.95 (0.93–0.97) | Y = 0.85X + 3.7 | 0.90 (0.85–0.93) | −3.0 ± 3.1 | (−9.0 to 3.0) | Y = −0.1X + 2 | p = 0.001 |
| ADP | 0.95 (0.93–0.97) | Y = 0.88X + 6.2 | 0.94 (0.90–0.96) | 1.03 ± 2.7 | (−4.3 to 6.3) | Y = −0.07X + 4 | p = 0.04 |
| D2O | 0.94 (0.91–0.96) | Y = 0.93X + 2.3 | 0.95 (0.92–0.97) | −0.80 ± 3.3 | (−7.3 to 5.7) | Y = −0.009X − 0.4 | p = 0.82 |
| MRI | 0.96 (0.90–0.99) | Y = 0.84X + 3.9 | 0.89 (0.65–0.97) | −3.04 ± 3.2 | (−9.4 to 3.3) | Y = −0.1X + 3 | p = 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopez-Gonzalez, D.; Wells, J.C.K.; Parra-Carriedo, A.; Bilbao, G.; Mendez, M.; Clark, P. Body Composition Assessment in Mexican Children and Adolescents. Part 1: Comparisons between Skinfold-Thickness, Dual X-ray Absorptiometry, Air-Displacement Plethysmography, Deuterium Oxide Dilution, and Magnetic Resonance Imaging with the 4-C Model. Nutrients 2022, 14, 1073. https://doi.org/10.3390/nu14051073
Lopez-Gonzalez D, Wells JCK, Parra-Carriedo A, Bilbao G, Mendez M, Clark P. Body Composition Assessment in Mexican Children and Adolescents. Part 1: Comparisons between Skinfold-Thickness, Dual X-ray Absorptiometry, Air-Displacement Plethysmography, Deuterium Oxide Dilution, and Magnetic Resonance Imaging with the 4-C Model. Nutrients. 2022; 14(5):1073. https://doi.org/10.3390/nu14051073
Chicago/Turabian StyleLopez-Gonzalez, Desiree, Jonathan C. K. Wells, Alicia Parra-Carriedo, Gladys Bilbao, Martín Mendez, and Patricia Clark. 2022. "Body Composition Assessment in Mexican Children and Adolescents. Part 1: Comparisons between Skinfold-Thickness, Dual X-ray Absorptiometry, Air-Displacement Plethysmography, Deuterium Oxide Dilution, and Magnetic Resonance Imaging with the 4-C Model" Nutrients 14, no. 5: 1073. https://doi.org/10.3390/nu14051073
APA StyleLopez-Gonzalez, D., Wells, J. C. K., Parra-Carriedo, A., Bilbao, G., Mendez, M., & Clark, P. (2022). Body Composition Assessment in Mexican Children and Adolescents. Part 1: Comparisons between Skinfold-Thickness, Dual X-ray Absorptiometry, Air-Displacement Plethysmography, Deuterium Oxide Dilution, and Magnetic Resonance Imaging with the 4-C Model. Nutrients, 14(5), 1073. https://doi.org/10.3390/nu14051073







