Gestational Age-Related Associations between Early-Life Feeding Trajectories and Growth Outcomes at Term Equivalent Age in Very Preterm Infants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. The Study Setting and Feeding Policy
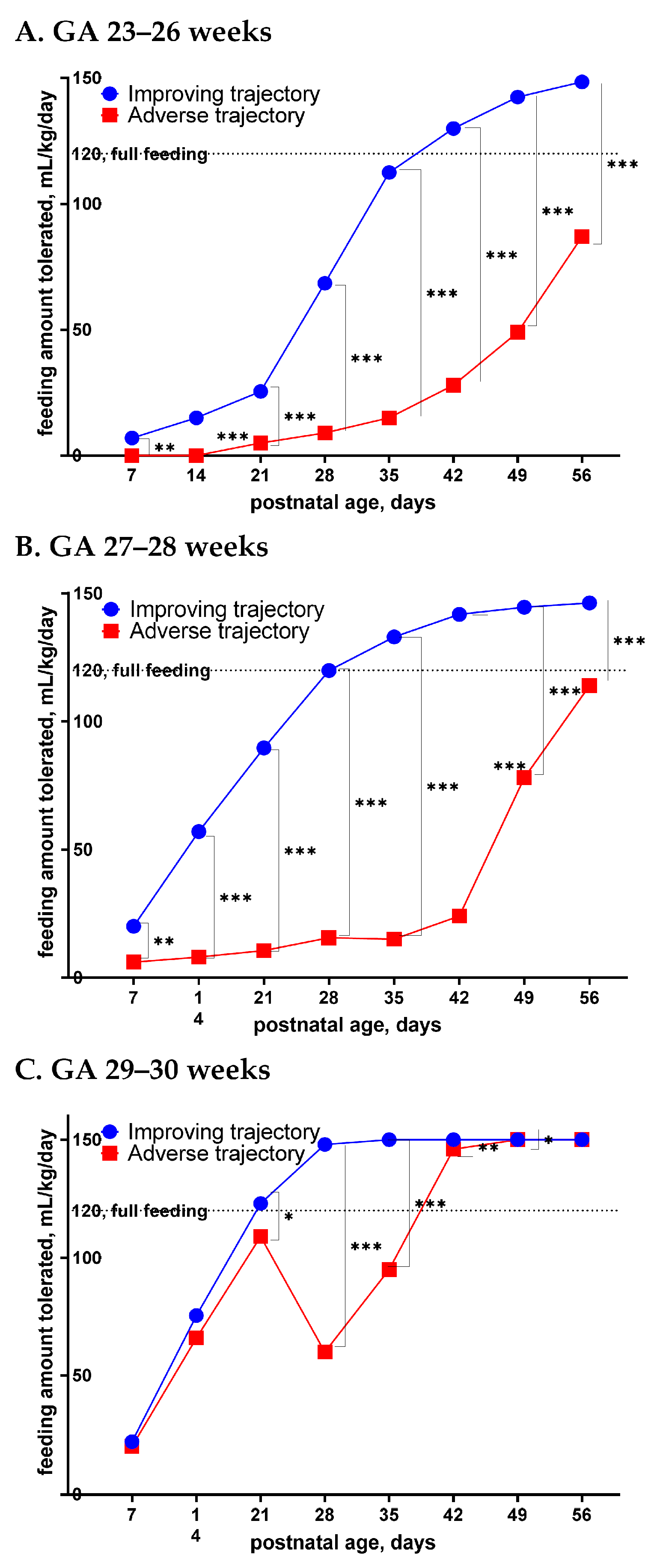
2.3. Daily Enteral Feeding Amount Calculation for Feeding Trajectory Analysis
2.4. Demographics, Risks and GI Morbidities
2.5. Primary Outcome: EUGR at TEA
2.6. Statistical Analysis
3. Results
4. Discussion
Strength and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Villar, J.; Giuliani, F.; Barros, F.; Roggero, P.; Coronado Zarco, I.A.; Rego, M.A.S.; Ochieng, R.; Gianni, M.L.; Rao, S.; Lambert, A.; et al. Monitoring the Postnatal Growth of Preterm Infants: A Paradigm Change. Pediatrics 2018, 141, e20172467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harding, J.E.; Cormack, B.E.; Alexander, T.; Alsweiler, J.M.; Bloomfield, F.H. Advances in nutrition of the newborn infant. Lancet 2017, 389, 1660–1668. [Google Scholar] [CrossRef]
- Walsh, V.; Brown, J.V.E.; Copperthwaite, B.R.; Oddie, S.J.; McGuire, W. Early full enteral feeding for preterm or low birth weight infants. Cochrane Database Syst. Rev. 2020, 12, Cd013542. [Google Scholar] [CrossRef]
- Alyahya, W.; Simpson, J.; Garcia, A.L.; Mactier, H.; Edwards, C.A. Early versus Delayed Fortification of Human Milk in Preterm Infants: A Systematic Review. Neonatology 2020, 117, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Stevens, T.P.; Shields, E.; Campbell, D.; Combs, A.; Horgan, M.; La Gamma, E.F.; Xiong, K.; Kacica, M. Statewide Initiative to Reduce Postnatal Growth Restriction among Infants <31 Weeks of Gestation. J. Pediatr. 2018, 197, 82–89.e82. [Google Scholar] [CrossRef]
- Dorling, J.; Abbott, J.; Berrington, J.; Bosiak, B.; Bowler, U.; Boyle, E.; Embleton, N.; Hewer, O.; Johnson, S.; Juszczak, E.; et al. Controlled Trial of Two Incremental Milk-Feeding Rates in Preterm Infants. N. Engl. J. Med. 2019, 381, 1434–1443. [Google Scholar] [CrossRef] [Green Version]
- Maas, C.; Franz, A.R.; von Krogh, S.; Arand, J.; Poets, C.F. Growth and morbidity of extremely preterm infants after early full enteral nutrition. Arch. Dis. Child. Fetal Neonatal Ed. 2018, 103, F79–F81. [Google Scholar] [CrossRef]
- Kwok, T.C.; Dorling, J.; Gale, C. Early enteral feeding in preterm infants. Semin. Perinatol. 2019, 43, 151159. [Google Scholar] [CrossRef]
- Travers, C.P.; Wang, T.; Salas, A.A.; Schofield, E.; Dills, M.; Laney, D.; Yee, A.; Bhatia, A.; Winter, L.; Ambalavanan, N.; et al. Higher- or Usual-Volume Feedings in Infants Born Very Preterm: A Randomized Clinical Trial. J. Pediatr. 2020, 224, 66–71.e61. [Google Scholar] [CrossRef]
- Izquierdo Renau, M.; Aldecoa-Bilbao, V.; Balcells Esponera, C.; Del Rey Hurtado de Mendoza, B.; Iriondo Sanz, M.; Iglesias-Platas, I. Applying Methods for Postnatal Growth Assessment in the Clinical Setting: Evaluation in a Longitudinal Cohort of Very Preterm Infants. Nutrients 2019, 11, 2772. [Google Scholar] [CrossRef] [Green Version]
- Martini, S.; Aceti, A.; Galletti, S.; Beghetti, I.; Faldella, G.; Corvaglia, L. To Feed or Not to Feed: A Critical Overview of Enteral Feeding Management and Gastrointestinal Complications in Preterm Neonates with a Patent Ductus Arteriosus. Nutrients 2019, 12, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zozaya, C.; Avila-Alvarez, A.; Arruza, L.; Garcia-Munoz Rodrigo, F.; Fernandez-Perez, C.; Castro, A.; Cuesta, M.T.; Vacas, B.; Couce, M.L.; Vento Torres, M.; et al. The Effect of Morbidity and Sex on Postnatal Growth of Very Preterm Infants: A Multicenter Cohort Study. Neonatology 2019, 115, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Clyman, R.I.; Liebowitz, M.; Kaempf, J.; Erdeve, O.; Bulbul, A.; Håkansson, S.; Lindqvist, J.; Farooqi, A.; Katheria, A.; Sauberan, J.; et al. PDA-TOLERATE Trial: An Exploratory Randomized Controlled Trial of Treatment of Moderate-to-Large Patent Ductus Arteriosus at 1 Week of Age. J. Pediatr. 2019, 205, 41–48.e46. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekharan, P.; Lakshminrusimha, S.; Chowdhury, D.; Van Meurs, K.; Keszler, M.; Kirpalani, H.; Das, A.; Walsh, M.C.; McGowan, E.C.; Higgins, R.D. Early Hypoxic Respiratory Failure in Extreme Prematurity: Mortality and Neurodevelopmental Outcomes. Pediatrics 2020, 146, e20193318. [Google Scholar] [CrossRef] [PubMed]
- Buddington, R.K.; Yakimkova, T.; Adebiyi, A.; Chizhikov, V.V.; Iskusnykh, I.Y.; Buddington, K.K. Organ Growth and Intestinal Functions of Preterm Pigs Fed Low and High Protein Formulas with or without Supplemental Leucine or Hydroxymethylbutyrate as Growth Promoters. Front. Nutr. 2021, 8, 687703. [Google Scholar] [CrossRef]
- Wendel, K.; Pfeiffer, H.C.V.; Fugelseth, D.M.; Nestaas, E.; Domellöf, M.; Skålhegg, B.S.; Elgstøen, K.B.P.; Rootwelt, H.; Pettersen, R.D.; Pripp, A.H.; et al. Effects of nutrition therapy on growth, inflammation and metabolism in immature infants: A study protocol of a double-blind randomized controlled trial (ImNuT). BMC Pediatr. 2021, 21, 19. [Google Scholar] [CrossRef]
- Chizhikov, D.; Buddington, R.K.; Iskusnykh, I.Y. Effects of Phosphatidylserine Source of Docosahexaenoic Acid on Cerebellar Development in Preterm Pigs. Brain Sci. 2020, 10, 475. [Google Scholar] [CrossRef]
- Buddington, R.K.; Chizhikov, V.V.; Iskusnykh, I.Y.; Sable, H.J.; Sable, J.J.; Holloway, Z.R.; Blumenfeld Katzir, T.; van der Merwe, M.; Yakimkova, T.; Buddington, K.K.; et al. A Phosphatidylserine Source of Docosahexanoic Acid Improves Neurodevelopment and Survival of Preterm Pigs. Nutrients 2018, 10, 637. [Google Scholar] [CrossRef] [Green Version]
- Genolini, C.; Ecochard, R.; Benghezal, M.; Driss, T.; Andrieu, S.; Subtil, F. kmlShape: An Efficient Method to Cluster Longitudinal Data (Time-Series) According to Their Shapes. PLoS ONE 2016, 11, e0150738. [Google Scholar] [CrossRef]
- MacBean, V.; Lunt, A.; Drysdale, S.B.; Yarzi, M.N.; Rafferty, G.F.; Greenough, A. Predicting healthcare outcomes in prematurely born infants using cluster analysis. Pediatr. Pulmonol. 2018, 53, 1067–1072. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.C.; Chen, Y.J.; Huang, C.C.; Shieh, C.C. Concentrated Preterm Formula as a Liquid Human Milk Fortifier at Initiation Stage in Extremely Low Birth Weight Preterm Infants: Short Term and 2-year Follow-up Outcomes. Nutrients 2020, 12, 2229. [Google Scholar] [CrossRef] [PubMed]
- Salas, A.A.; Cuna, A.; Bhat, R.; McGwin, G., Jr.; Carlo, W.A.; Ambalavanan, N. A randomised trial of re-feeding gastric residuals in preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 2015, 100, F224–F228. [Google Scholar] [CrossRef] [PubMed]
- Glackin, S.J.; O’Sullivan, A.; George, S.; Semberova, J.; Miletin, J. High flow nasal cannula versus NCPAP, duration to full oral feeds in preterm infants: A randomised controlled trial. Arch. Dis. Child. Fetal Neonatal Ed. 2017, 102, F329–F332. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.C.; Doyle, L.W.; Bear, M.J.; Inder, T.E. Alterations in neurobehavior at term reflect differing perinatal exposures in very preterm infants. Pediatrics 2006, 118, 2461–2471. [Google Scholar] [CrossRef]
- Fenton, T.R.; Nasser, R.; Eliasziw, M.; Kim, J.H.; Bilan, D.; Sauve, R. Validating the weight gain of preterm infants between the reference growth curve of the fetus and the term infant. BMC Pediatr. 2013, 13, 92. [Google Scholar] [CrossRef] [Green Version]
- Fenton, T.R.; Cormack, B.; Goldberg, D.; Nasser, R.; Alshaikh, B.; Eliasziw, M.; Hay, W.W.; Hoyos, A.; Anderson, D.; Bloomfield, F.; et al. “Extrauterine growth restriction” and “postnatal growth failure” are misnomers for preterm infants. J. Perinatol. 2020, 40, 704–714. [Google Scholar] [CrossRef]
- Kim, Y.J.; Shin, S.H.; Cho, H.; Shin, S.H.; Kim, S.H.; Song, I.G.; Kim, E.K.; Kim, H.S. Extrauterine growth restriction in extremely preterm infants based on the Intergrowth-21st Project Preterm Postnatal Follow-up Study growth charts and the Fenton growth charts. Eur. J. Pediatr. 2020, 180, 817–824. [Google Scholar] [CrossRef]
- Pampanini, V.; Boiani, A.; De Marchis, C.; Giacomozzi, C.; Navas, R.; Agostino, R.; Dini, F.; Ghirri, P.; Cianfarani, S. Preterm infants with severe extrauterine growth retardation (EUGR) are at high risk of growth impairment during childhood. Eur. J. Pediatr. 2015, 174, 33–41. [Google Scholar] [CrossRef]
- De Rose, D.U.; Cota, F.; Gallini, F.; Bottoni, A.; Fabrizio, G.C.; Ricci, D.; Romeo, D.M.; Mercuri, E.; Vento, G.; Maggio, L. Extra-uterine growth restriction in preterm infants: Neurodevelopmental outcomes according to different definitions. Eur. J. Paediatr. Neurol. 2021, 33, 135–145. [Google Scholar] [CrossRef]
- Martinez-Jimenez, M.D.; Gomez-Garcia, F.J.; Gil-Campos, M.; Perez-Navero, J.L. Comorbidities in childhood associated with extrauterine growth restriction in preterm infants: A scoping review. Eur. J. Pediatr. 2020, 179, 1255–1265. [Google Scholar] [CrossRef]
- Clark, R.H.; Thomas, P.; Peabody, J. Extrauterine growth restriction remains a serious problem in prematurely born neonates. Pediatrics 2003, 111, 986–990. [Google Scholar] [CrossRef] [PubMed]
- Fabrizio, V.; Shabanova, V.; Taylor, S.N. Factors in Early Feeding Practices That May Influence Growth and the Challenges that Arise in Growth Outcomes Research. Nutrients 2020, 12, 1939. [Google Scholar] [CrossRef] [PubMed]
- Maiocco, G.; Migliaretti, G.; Cresi, F.; Peila, C.; Deantoni, S.; Trapani, B.; Giuliani, F.; Bertino, E.; Coscia, A. Evaluation of Extrauterine Head Growth from 14–21 days to Discharge with Longitudinal Intergrowth-21st Charts: A New Approach to Identify Very Preterm Infants at Risk of Long-Term Neurodevelopmental Impairment. Front. Pediatr. 2020, 8, 572930. [Google Scholar] [CrossRef] [PubMed]
- Cresi, F.; Maggiora, E.; Borgione, S.M.; Spada, E.; Coscia, A.; Bertino, E.; Meneghin, F.; Corvaglia, L.T.; Ventura, M.L.; Lista, G. Enteral Nutrition Tolerance And REspiratory Support (ENTARES) Study in preterm infants: Study protocol for a randomized controlled trial. Trials 2019, 20, 67. [Google Scholar] [CrossRef] [Green Version]
- Bozzetti, V.; De Angelis, C.; Tagliabue, P.E. Nutritional approach to preterm infants on noninvasive ventilation: An update. Nutrition 2017, 37, 14–17. [Google Scholar] [CrossRef]
- Malkar, M.B.; Gardner, W.; Welty, S.E.; Jadcherla, S.R. Antecedent Predictors of Feeding Outcomes in Premature Infants with Protracted Mechanical Ventilation. J. Pediatr. Gastroenterol. Nutr. 2015, 61, 591–595. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists. Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin Summary, Number 222. Obstet. Gynecol. 2020, 135, e237–e260. [Google Scholar] [CrossRef]
- Fenton, T.R.; Kim, J.H. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013, 13, 59. [Google Scholar] [CrossRef] [Green Version]
- Sweet, D.G.; Carnielli, V.; Greisen, G.; Hallman, M.; Ozek, E.; te Pas, A.; Plavka, R.; Roehr, C.C.; Saugstad, O.D.; Simeoni, U.; et al. European Consensus Guidelines on the Management of Respiratory Distress Syndrome—2019 Update. Neonatology 2019, 115, 432–450. [Google Scholar] [CrossRef] [Green Version]
- Gillam-Krakauer, M.; Reese, J. Diagnosis and Management of Patent Ductus Arteriosus. Neoreviews 2018, 19, e394–e402. [Google Scholar] [CrossRef]
- Neu, J.; Walker, W.A. Necrotizing enterocolitis. N. Engl. J. Med. 2011, 364, 255–264. [Google Scholar] [CrossRef] [Green Version]
- Valdez Sandoval, P.; Hernandez Rosales, P.; Quinones Hernandez, D.G.; Chavana Naranjo, E.A.; Garcia Navarro, V. Intraventricular hemorrhage and posthemorrhagic hydrocephalus in preterm infants: Diagnosis, classification, and treatment options. Childs Nerv. Syst. 2019, 35, 917–927. [Google Scholar] [CrossRef]
- Deng, W.; Pleasure, J.; Pleasure, D. Progress in periventricular leukomalacia. Arch. Neurol. 2008, 65, 1291–1295. [Google Scholar] [CrossRef] [Green Version]
- Jobe, A.H.; Bancalari, E. Bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 2001, 163, 1723–1729. [Google Scholar] [CrossRef]
- American Academy of Pediatrics. Section on Ophthalmology. Screening examination of premature infants for retinopathy of prematurity. Pediatrics 2001, 108, 809–811. [Google Scholar] [CrossRef] [Green Version]

| Preterm Group | GA 23–26 Weeks | GA 27–28 Weeks | GA 29–30 Weeks | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Feeding Trajectories | Improving | Adverse | p | Improving | Adverse | p | Improving | Adverse | p |
| Case number | 94 | 89 | 173 | 42 | 188 | 39 | |||
| Demographics | |||||||||
| Gestational age, mean (SD), weeks | 25.2 (1.0) | 24.7 (1.0) | 0.001 | 27.6 (0.5) | 27.4 (0.5) | 0.022 | 29.6 (0.5) | 29.4 (0.5) | 0.061 |
| Multiple gestation, n (%) | 31 (33) | 24 (27) | 0.468 | 45 (26) | 18 (43) | 0.050 | 59 (31) | 6 (15) | 0.069 |
| Preeclampsia, n (%) | 14 (15) | 15 (17) | 0.873 | 32 (18) | 8 (19) | 1.000 | 48 (26) | 18 (46) | 0.017 |
| 5 min Apgar score <7, n (%) | 35 (38) | 35 (40) | 0.887 | 23 (13) | 14 (33) | 0.005 | 18 (10) | 2 (5) | 0.540 |
| Respiratory/hemodynamic morbidities | |||||||||
| RDS requiring surfactant therapy, n (%) | 47 (50) | 48 (54) | 0.701 | 54 (31) | 14 (33) | 0.936 | 25 (13) | 7 (18) | 0.612 |
| Hypotension requiring vasopressors, n (%) | 66 (70) | 76 (85) | 0.022 | 59 (34) | 29 (69) | <0.001 | 55 (29) | 11 (28) | 1.000 |
| cPVL, n (%) | 5 (5) | 9 (10) | 0.347 | 4 (2) | 4 (10) | 0.049 | 4 (2) | 1 (3) | 1.000 |
| hs-PDA requiring surgery, n (%) | 24 (26) | 38 (43) | 0.022 | 6 (3) | 7 (17) | 0.005 | 3 (2) | 0 (0) | 1.000 |
| Duration of IMV, median (Q1–Q3), days | 3 (0–14) | 13 (5–27) | <0.001 | 0 (0–2) | 2 (0–9) | <0.001 | 0 (0–0) | 0 (0–0) | 0.111 |
| Late-onset sepsis, n (%) | 15 (16) | 40 (45) | <0.001 | 5 (3) | 7 (17) | 0.003 | 9 (5) | 3 (8) | 0.438 |
| GI morbidities | |||||||||
| NEC stage 1, n (%) | 10 (11) | 12 (14) | 0.716 | 15 (9) | 9 (21) | 0.028 | 6 (3) | 19 (49) | <0.001 |
| Severe NEC, n (%) | 6 (6) | 14 (16) | 0.074 | 4 (2) | 9 (21) | <0.001 | 1 (1) | 7 (18) | <0.001 |
| Non-NEC events requiring surgery, n (%) | 4 (4) | 15 (17) | 0.011 | 1 (1) | 7 (17) | <0.001 | 2 (1) | 1 (3) | 0.434 |
| Severe BPD, n (%) | 47 (51) | 64 (72) | 0.005 | 31 (18) | 13 (31) | 0.096 | 6 (3%) | 3 (8) | 0.187 |
| Severe ROP, n (%) | 21 (22) | 27 (30) | 0.289 | 7 (4) | 5 (12) | 0.061 | 2 (1%) | 1 (3) | 0.434 |
| Preterm Group | GA 23–26 weeks | GA 27–28 weeks | GA 29–30 weeks | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| Demographics | ||||||
| Gestational age | --- | 0.817 | --- | 0.222 | --- | --- |
| Multiple gestation | --- | --- | --- | 0.579 | --- | 0.307 |
| Preeclampsia | --- | --- | --- | --- | 3.53 (1.25–9.99) | 0.013 |
| 5 min Apgar score <7 | --- | --- | 2.60 (0.90–7.49) | 0.077 | --- | --- |
| Respiratory/hemodynamic/ morbidities | ||||||
| Late-onset sepsis | 3.43 (1.64–7.19) | 0.001 | 13.92 (3.51–55.26) | <0.001 | --- | --- |
| Hypotension requiring vasopressors | --- | 0.692 | 2.17 (0.85–5.54) | 0.103 | --- | --- |
| cPVL | --- | --- | 11.93 (2.12–67.06) | 0.005 | --- | --- |
| hs-PDA requiring surgery | --- | 0.311 | 6.75 (1.41–32.46) | 0.017 | --- | --- |
| Duration of IMV | 1.03 (1.01–1.06) | 0.007 | --- | 0.218 | 1.22 (1.07–1.39) | 0.004 |
| GI morbidities | ||||||
| NEC stage I | --- | --- | 5.92 (1.84–19.08) | 0.003 | 55.50 (17.19–179.3) | <0.001 |
| Severe NEC | 2.18 (0.73–6.50) | 0.163 | 26.20 (6.12–112.2) | <0.001 | 117.9 (12.2–1137) | <0.001 |
| Non-NEC events requiring GI surgery | 5.21 (1.57–17.34) | 0.007 | 37.01 (3.69–371.0) | 0.002 | --- | --- |
| Preterm Group | GA 23–26 Weeks | GA 27–28 Weeks | GA 29–30 Weeks | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Feeding Trajectory | Improving | Adverse | p | Improving | Adverse | p | Improving | Adverse | p |
| Enrolled numbers, n | 94 | 89 | 173 | 42 | 188 | 39 | |||
| Body weight | |||||||||
| ∆z, mean ± SD | −0.91 ± 1.20 | −2.10 ± 0.96 | <0.001 | −0.23 ± 0.96 | −1.24 ± 1.15 | <0.001 | 0.12 ± 0.92 | −0.44 ± 0.90 | <0.001 |
| EUGR, n (%) | 46 (50) | 77 (88) | <0.001 | 36 (21) | 24 (57) | <0.001 | 20 (11) | 11 (30) | 0.007 |
| Severe EUGR, n (%) | 14 (15) | 46 (52) | <0.001 | 5 (3) | 10 (24) | <0.001 | 2 (1) | 2 (5) | 0.135 |
| Head circumference | |||||||||
| ∆z, mean ± SD | −1.08 ± 1.30 | −2.00 ± 1.34 | <0.001 | −0.24 ± 1.04 | −1.23 ± 1.21 | <0.001 | 0.15 ± 0.97 | −0.19 ± 1.01 | 0.054 |
| EUGR, n (%) | 47 (51) | 73 (84) | <0.001 | 36 (21) | 24 (59) | <0.001 | 20 (11) | 7 (19) | 0.271 |
| Term Equivalent Age | ||||||
|---|---|---|---|---|---|---|
| ∆z of Body Weight < −1 | ∆z of Head Circumference < −1 | |||||
| Preterm Group | aOR | 95% CI | p | aOR | 95% CI | p |
| GA 23–26 weeks | 7.31 | 3.34–15.99 | <0.001 | 3.88 | 1.80–8.34 | 0.001 |
| GA 27–28 weeks | 3.13 | 1.35–7.22 | 0.008 | 3.33 | 1.41–7.85 | 0.006 |
| GA 29–30 weeks | 4.02 | 1.58–10.22 | 0.004 | 1.38 | 0.45–4.18 | 0.572 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-C.; Chu, C.-H.; Chen, Y.-J.; Chen, R.-B.; Huang, C.-C. Gestational Age-Related Associations between Early-Life Feeding Trajectories and Growth Outcomes at Term Equivalent Age in Very Preterm Infants. Nutrients 2022, 14, 1032. https://doi.org/10.3390/nu14051032
Lin Y-C, Chu C-H, Chen Y-J, Chen R-B, Huang C-C. Gestational Age-Related Associations between Early-Life Feeding Trajectories and Growth Outcomes at Term Equivalent Age in Very Preterm Infants. Nutrients. 2022; 14(5):1032. https://doi.org/10.3390/nu14051032
Chicago/Turabian StyleLin, Yung-Chieh, Chi-Hsiang Chu, Yen-Ju Chen, Ray-Bing Chen, and Chao-Ching Huang. 2022. "Gestational Age-Related Associations between Early-Life Feeding Trajectories and Growth Outcomes at Term Equivalent Age in Very Preterm Infants" Nutrients 14, no. 5: 1032. https://doi.org/10.3390/nu14051032
APA StyleLin, Y.-C., Chu, C.-H., Chen, Y.-J., Chen, R.-B., & Huang, C.-C. (2022). Gestational Age-Related Associations between Early-Life Feeding Trajectories and Growth Outcomes at Term Equivalent Age in Very Preterm Infants. Nutrients, 14(5), 1032. https://doi.org/10.3390/nu14051032







