Healthy Immunity on Preventive Medicine for Combating COVID-19
Abstract
1. Introduction
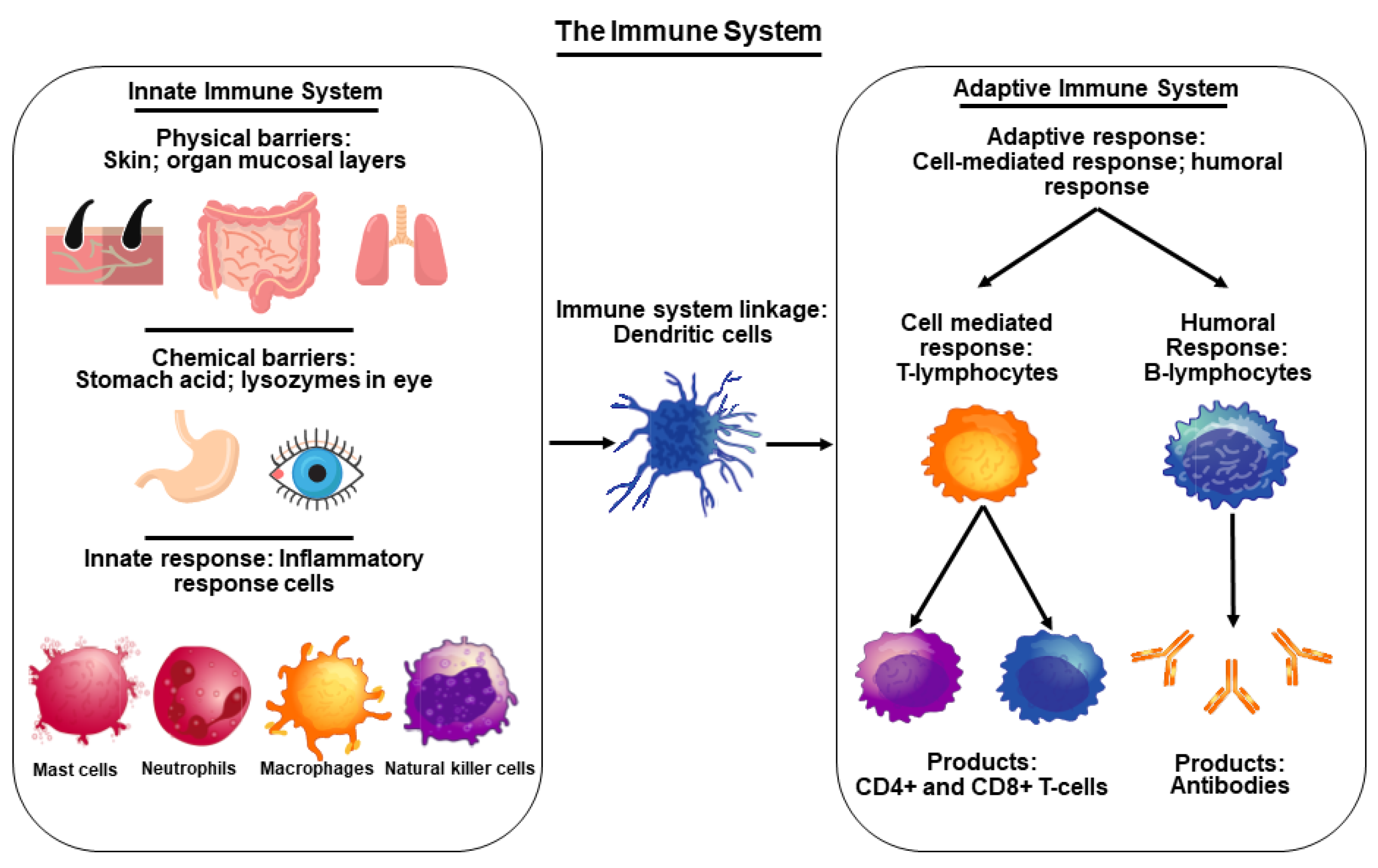
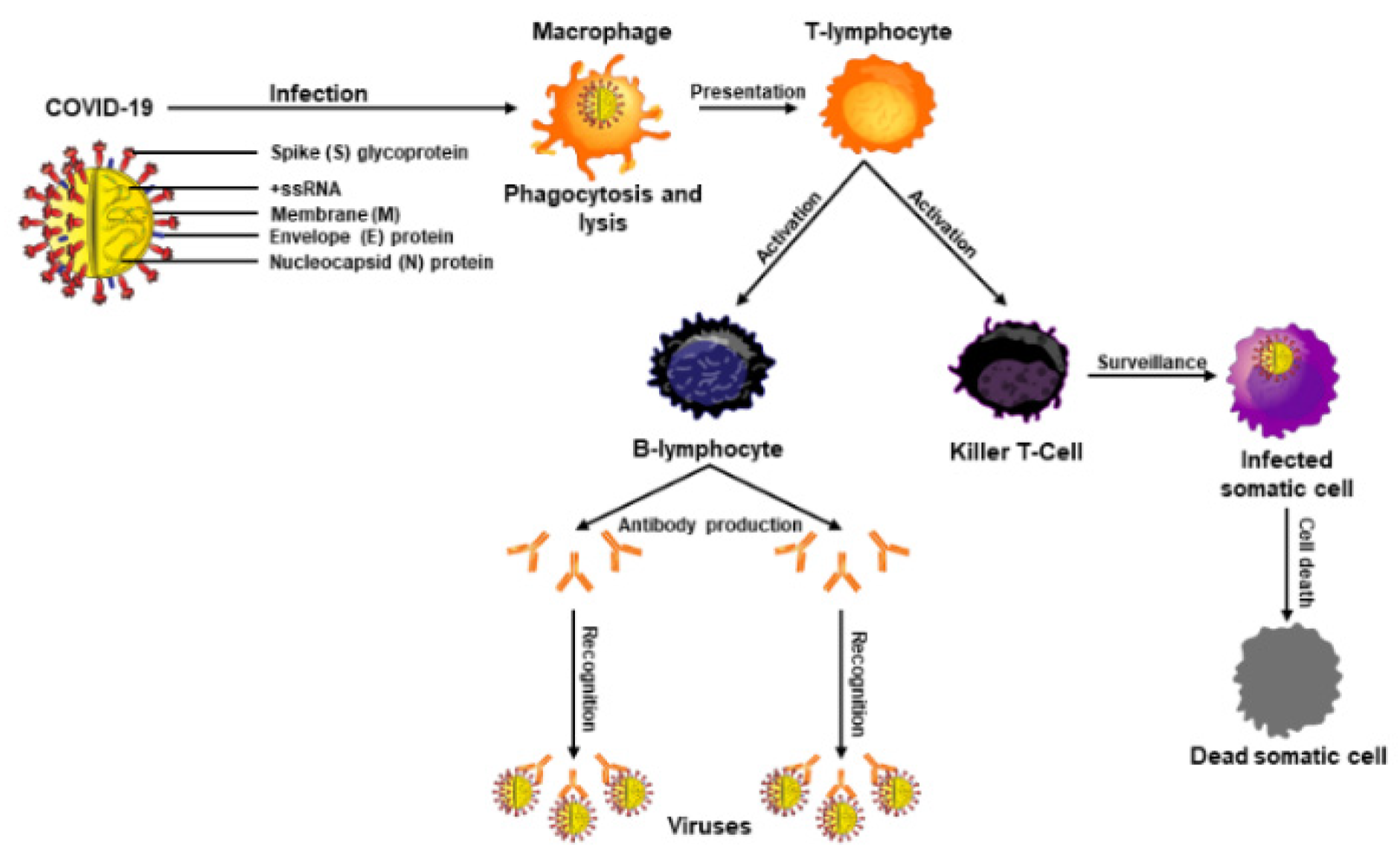
2. Functional Importance of the Immune System and Its Connection to COVID-19
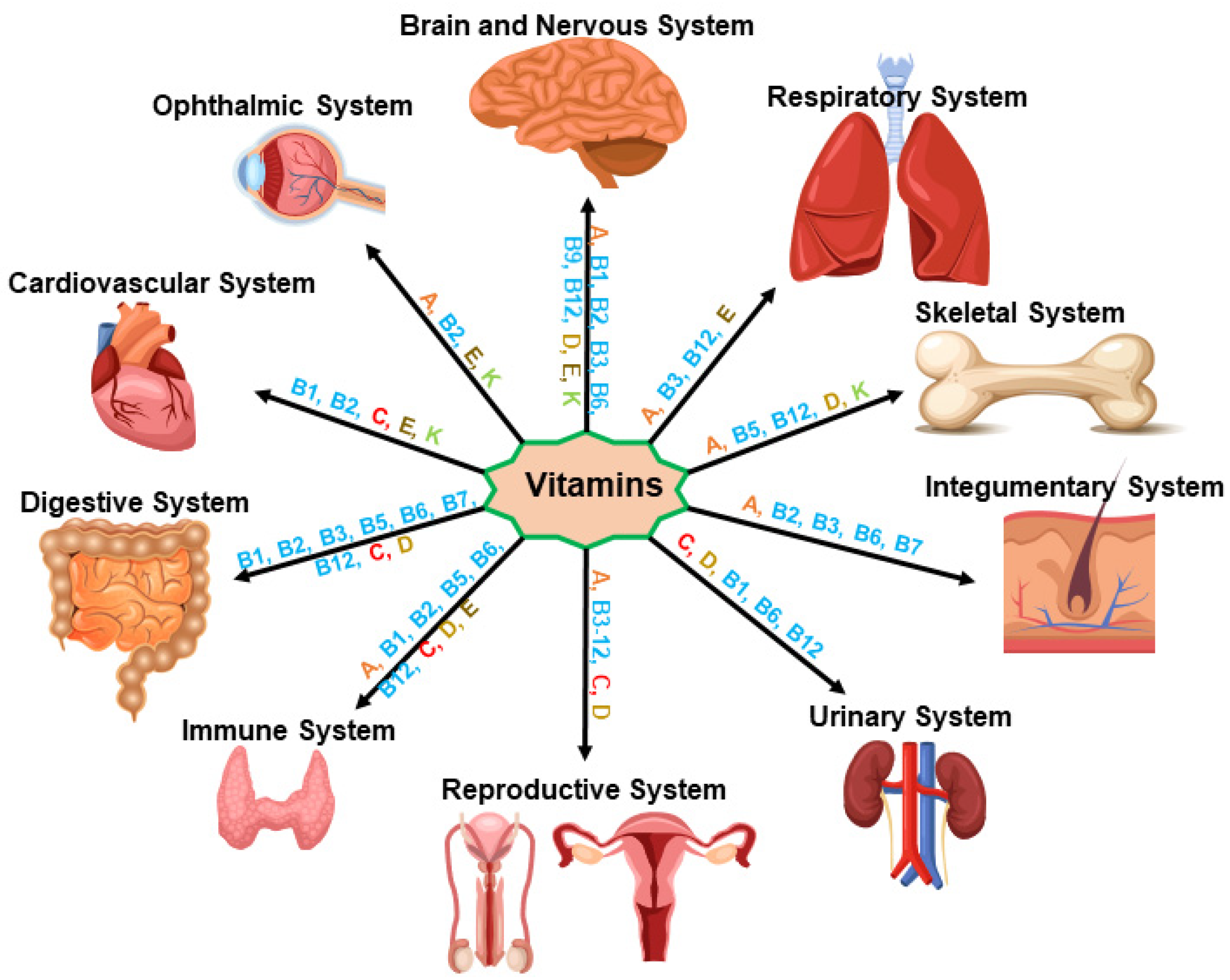
3. Vitamin and Immune Health Dynamics in Healthy Physiology
3.1. Vitamin A (Retinol)
3.2. Vitamin B
3.3. Vitamin C (Ascorbic Acid or Ascorbate)
3.4. Vitamin D (Ergocalciferol)
3.5. Vitamin E (Tocopherol)
3.6. Vitamin K (Phylloquinone)
4. COVID-19: Epidemiology, Risk Factors, and Molecular Pathogenesis
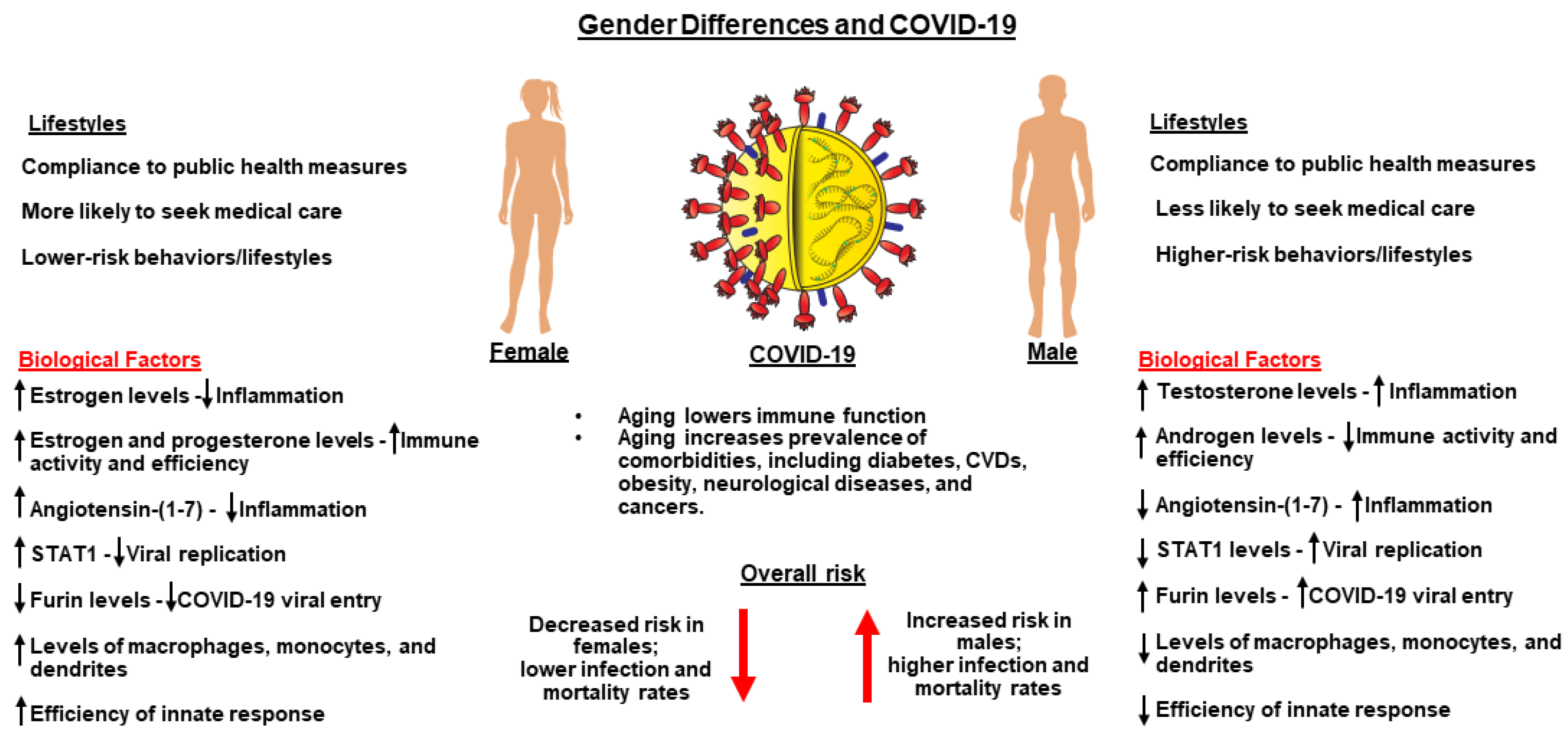
5. Sex and Gender Differences and Their Relevance to COVID-19
6. Aging, Underlying Medical Conditions, and Their Correlation to COVID-19
6.1. Obesity
6.2. Diabetes
6.3. CVDs
6.4. Cancers
6.5. Neurological Diseases
7. Therapeutic Approaches for the Prevention and Treatment of COVID-19
8. Limitations
9. Conclusions and Challenges
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Park, L.K.; Friso, S.; Choi, S.W. Nutritional influences on epigenetics and age-related disease. Proc. Nutr. Soc. 2012, 71, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Pecora, F.; Persico, F.; Argentiero, A.; Neglia, C.; Esposito, S. The role of micronutrients in support of the immune response against viral infections. Nutrients 2020, 12, 3198. [Google Scholar] [CrossRef] [PubMed]
- Manna, P.R.; Stetson, C.L.; Daugherty, C.; Shimizu, I.; Syapin, P.J.; Garrel, G.; Cohen-Tannoudji, J.; Huhtaniemi, I.; Slominski, A.T.; Pruitt, K.; et al. Up-regulation of steroid biosynthesis by retinoid signaling: Implications for aging. Mech. Ageing Dev. 2015, 150, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Manna, P.R.; Stetson, C.L.; Slominski, A.T.; Pruitt, K. Role of the steroidogenic acute regulatory protein in health and disease. Endocrine 2016, 51, 7–21. [Google Scholar] [CrossRef]
- Calder, P.C. Nutrition, immunity and COVID-19. BMJ Nutr. Prev. Health 2020, 3, 74–92. [Google Scholar] [CrossRef]
- Jayawardena, R.; Misra, A. Balanced diet is a major casualty in COVID-19. Diabetes Metab. Syndr. 2020, 14, 1085–1086. [Google Scholar] [CrossRef]
- Gorji, A.; Khaleghi Ghadiri, M. Potential roles of micronutrient deficiency and immune system dysfunction in the coronavirus disease 2019 (COVID-19) pandemic. Nutrition 2021, 82, 111047. [Google Scholar] [CrossRef]
- Liu, Y.C.; Kuo, R.L.; Shih, S.R. COVID-19: The first documented coronavirus pandemic in history. Biomed. J. 2020, 43, 328–333. [Google Scholar] [CrossRef]
- Berekaa, M.M. Insights into the COVID-19 pandemic: Origin, pathogenesis, diagnosis, and therapeutic interventions. Front. Biosci. 2021, 13, 117–139. [Google Scholar]
- Sheervalilou, R.; Shirvaliloo, M.; Dadashzadeh, N.; Shirvalilou, S.; Shahraki, O.; Pilehvar-Soltanahmadi, Y.; Ghaznavi, H.; Khoei, S.; Nazarlou, Z. COVID-19 under spotlight: A close look at the origin, transmission, diagnosis, and treatment of the 2019-nCoV disease. J. Cell Physiol. 2020, 235, 8873–8924. [Google Scholar] [CrossRef]
- Yuki, K.; Fujiogi, M.; Koutsogiannaki, S. COVID-19 pathophysiology: A review. Clin. Immunol. 2020, 215, 108427. [Google Scholar] [CrossRef] [PubMed]
- Rando, H.M.; MacLean, A.L.; Lee, A.J.; Lordan, R.; Ray, S.; Bansal, V.; Skelly, A.N.; Sell, E.; Dziak, J.J.; Shinholster, L.; et al. Pathogenesis, symptomatology, and transmission of SARS-CoV-2 through analysis of viral genomics and structure. mSystems 2021, 6, e0009521. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Wang, B.; Mao, J. The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID-19. J. Infect. 2020, 80, 607–613. [Google Scholar] [CrossRef]
- Hu, B.; Huang, S.; Yin, L. The cytokine storm and COVID-19. J. Med. Virol. 2021, 93, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Kalra, R.S.; Dhanjal, J.K.; Meena, A.S.; Kalel, V.C.; Dahiya, S.; Singh, B.; Dewanjee, S.; Kandimalla, R. COVID-19, neuropathology, and aging: SARS-CoV-2 neurological infection, mechanism, and associated complications. Front. Aging Neurosci. 2021, 13, 662786. [Google Scholar] [CrossRef]
- Mainali, S.; Darsie, M.E. Neurologic and neuroscientific evidence in aged COVID-19 patients. Front. Aging Neurosci. 2021, 13, 648662. [Google Scholar] [CrossRef] [PubMed]
- Forchette, L.; Sebastian, W.; Liu, T. A comprehensive review of COVID-19 virology, vaccines, variants, and therapeutics. Curr. Med. Sci. 2021, 41, 1037–1051. [Google Scholar] [CrossRef]
- Ahmed, M.H.; Hassan, A.; Molnar, J. The Role of Micronutrients to Support Immunity for COVID-19 Prevention. Rev. Bras. Farmacogn. 2021, 31, 361–374. [Google Scholar] [CrossRef]
- McCreary, E.K.; Angus, D.C. Efficacy of Remdesivir in COVID-19. JAMA 2020, 324, 1041–1042. [Google Scholar] [CrossRef]
- Wang, M.; Cao, R.; Zhang, L.; Yang, X.; Liu, J.; Xu, M.; Shi, Z.; Hu, Z.; Zhong, W.; Xiao, G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020, 30, 269–271. [Google Scholar] [CrossRef]
- O’Brien, M.P.; Forleo-Neto, E.; Musser, B.J.; Isa, F.; Chan, K.C.; Sarkar, N.; Bar, K.J.; Barnabas, R.V.; Barouch, D.H.; Cohen, M.S.; et al. COVID-19 Phase 3 Prevention Trial, T. Subcutaneous REGEN-COV antibody combination to prevent Covid-19. N. Engl. J. Med. 2021, 385, 1184–1195. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.S.; Nirula, A.; Mulligan, M.J.; Novak, R.M.; Marovich, M.; Yen, C.; Stemer, A.; Mayer, S.M.; Wohl, D.; Brengle, B.; et al. Effect of bamlanivimab vs placebo on incidence of COVID-19 among residents and staff of skilled nursing and assisted living facilities: A randomized clinical trial. JAMA 2021, 326, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, J.S.; Bhatti, G.K.; Khullar, N.; Reddy, A.P.; Reddy, P.H. Therapeutic Strategies in the Development of Anti-viral Drugs and Vaccines Against SARS-CoV-2 Infection. Mol. Neurobiol. 2020, 57, 4856–4877. [Google Scholar] [CrossRef] [PubMed]
- Kandimalla, R.; John, A.; Abburi, C.; Vallamkondu, J.; Reddy, P.H. Current status of multiple drug molecules, and vaccines: An update in SARS-CoV-2 therapeutics. Mol. Neurobiol. 2020, 57, 4106–4116. [Google Scholar] [CrossRef]
- Alavi Darazam, I.; Shokouhi, S.; Mardani, M.; Pourhoseingholi, M.A.; Rabiei, M.M.; Hatami, F.; Shabani, M.; Moradi, O.; Gharehbagh, F.J.; Irvani, S.S.N.; et al. Umifenovir in hospitalized moderate to severe COVID-19 patients: A randomized clinical trial. Int. Immunopharmacol. 2021, 99, 107969. [Google Scholar] [CrossRef]
- Chaplin, D.D. Overview of the immune response. J. Allergy Clin. Immunol. 2010, 125, S3–S23. [Google Scholar] [CrossRef]
- Niyonsaba, F.; Kiatsurayanon, C.; Chieosilapatham, P.; Ogawa, H. Friends or Foes? Host defense (antimicrobial) peptides and proteins in human skin diseases. Exp. Dermatol. 2017, 26, 989–998. [Google Scholar] [CrossRef]
- Idborg, H.; Oke, V. Cytokines as biomarkers in systemiclupus erythematosus: Value for diagnosis and drug therapy. Int. J. Mol. Sci. 2021, 22, 11327. [Google Scholar] [CrossRef]
- Schmid-Wendtner, M.H.; Korting, H.C. The pH of the skin surface and its impact on the barrier function. Skin Pharmacol. Physiol. 2006, 19, 296–302. [Google Scholar] [CrossRef]
- Nguyen, A.V.; Soulika, A.M. The dynamics of the skin’s immune system. Int. J. Mol. Sci. 2019, 20, 1811. [Google Scholar] [CrossRef]
- Liew, P.X.; Kubes, P. The neutrophil’s role during health and disease. Physiol. Rev. 2019, 99, 1223–1248. [Google Scholar] [CrossRef] [PubMed]
- Hoebe, K.; Janssen, E.; Beutler, B. The interface between innate and adaptive immunity. Nat. Immunol. 2004, 5, 971–974. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, F.A.; Oettgen, H.C. Adaptive immunity. J. Allergy Clin. Immunol. 2010, 125, S33–S40. [Google Scholar] [CrossRef] [PubMed]
- Dong, C. Cytokine regulation and function in T cells. Annu. Rev. Immunol. 2021, 39, 51–76. [Google Scholar] [CrossRef] [PubMed]
- Seifert, M.; Kuppers, R. Human memory B cells. Leukemia 2016, 30, 2283–2292. [Google Scholar] [CrossRef] [PubMed]
- Hillion, S.; Arleevskaya, M.I.; Blanco, P.; Bordron, A.; Brooks, W.H.; Cesbron, J.Y.; Kaveri, S.; Vivier, E.; Renaudineau, Y. The innate part of the adaptive immune system. Clin. Rev. Allergy Immunol. 2020, 58, 151–154. [Google Scholar] [CrossRef]
- Ganji, R.; Reddy, P.H. Impact of COVID-19 on mitochondrial-based immunity in aging and age-related diseases. Front. Aging Neurosci. 2020, 12, 614650. [Google Scholar] [CrossRef]
- Brodin, P. Immune determinants of COVID-19 disease presentation and severity. Nat. Med. 2021, 27, 28–33. [Google Scholar] [CrossRef]
- Kaufmann, S.H.E. Immunology’s coming of age. Front. Immunol. 2019, 10, 684. [Google Scholar] [CrossRef]
- Zabetakis, I.; Lordan, R.; Norton, C.; Tsoupras, A. COVID-19: The inflammation link and the role of nutrition in potential mitigation. Nutrients 2020, 12, 1466. [Google Scholar] [CrossRef]
- Galmes, S.; Serra, F.; Palou, A. Current state of evidence: Influence of nutritional and nutrigenetic factors on immunity in the COVID-19 pandemic framework. Nutrients 2020, 12, 2738. [Google Scholar] [CrossRef] [PubMed]
- Holder, K.; Reddy, P.H. The COVID-19 effect on the immune system and mitochondrial dynamics in diabetes, obesity, and dementia. Neuroscientist 2021, 27, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B.; Lahore, H.; McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Aliano, J.L.; Bhattoa, H.P. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients 2020, 12, 988. [Google Scholar] [CrossRef] [PubMed]
- Raverdeau, M.; Mills, K.H. Modulation of T cell and innate immune responses by retinoic Acid. J. Immunol. 2014, 192, 2953–2958. [Google Scholar] [CrossRef] [PubMed]
- Manna, P.R.; Slominski, A.T.; King, S.R.; Stetson, C.L.; Stocco, D.M. Synergistic activation of steroidogenic acute regulatory protein expression and steroid biosynthesis by retinoids: Involvement of cAMP/PKA signaling. Endocrinology 2014, 155, 576–591. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, G.Y.; Han, S.N. Direct-to-consumer genetic testing in Korea: Current status and significance in clinical nutrition. Clin. Nutr. Res. 2021, 10, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Chu, H.; Chan, J.F.; Ye, Z.W.; Wen, L.; Yan, B.; Lai, P.M.; Tee, K.M.; Huang, J.; Chen, D.; et al. SREBP-dependent lipidomic reprogramming as a broad-spectrum antiviral target. Nat. Commun. 2019, 10, 120. [Google Scholar] [CrossRef]
- Eckle, S.B.; Corbett, A.J.; Keller, A.N.; Chen, Z.; Godfrey, D.I.; Liu, L.; Mak, J.Y.; Fairlie, D.P.; Rossjohn, J.; McCluskey, J. Recognition of vitamin B precursors and byproducts by mucosal associated invariant T cells. J. Biol. Chem. 2015, 290, 30204–30211. [Google Scholar] [CrossRef]
- Peterson, C.T.; Rodionov, D.A.; Osterman, A.L.; Peterson, S.N. B Vitamins and Their Role in Immune Regulation and Cancer. Nutrients 2020, 12, 3380. [Google Scholar] [CrossRef]
- BourBour, F.; Mirzaei Dahka, S.; Gholamalizadeh, M.; Akbari, M.E.; Shadnoush, M.; Haghighi, M.; Taghvaye-Masoumi, H.; Ashoori, N.; Doaei, S. Nutrients in prevention, treatment, and management of viral infections; special focus on Coronavirus. Arch. Physiol. Biochem. 2020, 1–10. [Google Scholar] [CrossRef]
- Shakeri, H.; Azimian, A.; Ghasemzadeh-Moghaddam, H.; Safdari, M.; Haresabadi, M.; Daneshmand, T.; Namdar Ahmadabad, H. Evaluation of the relationship between serum levels of zinc, vitamin B12, vitamin D, and clinical outcomes in patients with COVID-19. J. Med. Virol. 2022, 94, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.C.; Maggini, S. Vitamin C and immune function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef] [PubMed]
- Bae, M.; Kim, H. Mini-Review on the roles of vitamin C, vitamin D, and selenium in the immune system against COVID-19. Molecules 2020, 25, 5346. [Google Scholar] [CrossRef]
- Zdrenghea, M.T.; Makrinioti, H.; Bagacean, C.; Bush, A.; Johnston, S.L.; Stanciu, L.A. Vitamin D modulation of innate immune responses to respiratory viral infections. Rev. Med. Virol. 2017, 27, e1909. [Google Scholar] [CrossRef]
- Luciani, F.; Caroleo, M.C.; Cannataro, R.; Mirra, D.; D’Agostino, B.; Gallelli, L.; Cione, E. Immunological response to SARS-CoV-2 is sustained by vitamin D: A case presentation of one-year follow-up. Reports 2021, 4, 18. [Google Scholar] [CrossRef]
- Panfili, F.M.; Roversi, M.; D’Argenio, P.; Rossi, P.; Cappa, M.; Fintini, D. Possible role of vitamin D in Covid-19 infection in pediatric population. J. Endocrinol. Investig. 2021, 44, 27–35. [Google Scholar] [CrossRef]
- Taha, R.; Abureesh, S.; Alghamdi, S.; Hassan, R.Y.; Cheikh, M.M.; Bagabir, R.A.; Almoallim, H.; Abdulkhaliq, A. The relationship between vitamin D and infections including COVID-19: Any hopes? Int. J. Gen. Med. 2021, 14, 3849–3870. [Google Scholar] [CrossRef] [PubMed]
- Gallelli, L.; Mannino, G.C.; Luciani, F.; de Sire, A.; Mancuso, E.; Gangemi, P.; Cosco, L.; Monea, G.; Averta, C.; Minchella, P.; et al. Vitamin D serum levels in subjects tested for SARS-CoV-2: What are the differences among acute, healed, and negative COVID-19 patients? A multicenter real-practice study. Nutrients 2021, 13, 3932. [Google Scholar] [CrossRef]
- Bouillon, R.; Manousaki, D.; Rosen, C.; Trajanoska, K.; Rivadeneira, F.; Richards, J.B. The health effects of vitamin D supplementation: Evidence from human studies. Nat. Rev. Endocrinol. 2022, 18, 96–110. [Google Scholar] [CrossRef]
- Mohd Zaffarin, A.S.; Ng, S.F.; Ng, M.H.; Hassan, H.; Alias, E. Pharmacology and pharmacokinetics of vitamin E: Nanoformulations to enhance bioavailability. Int. J. Nanomed. 2020, 15, 9961–9974. [Google Scholar] [CrossRef]
- Muller, D.P. Vitamin E and neurological function. Mol. Nutr. Food Res. 2010, 54, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Galli, F.; Azzi, A.; Birringer, M.; Cook-Mills, J.M.; Eggersdorfer, M.; Frank, J.; Cruciani, G.; Lorkowski, S.; Ozer, N.K. Vitamin E: Emerging aspects and new directions. Free Radic. Biol. Med. 2017, 102, 16–36. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.Y.; Han, S.N. The Role of vitamin E in immunity. Nutrients 2018, 10, 1614. [Google Scholar] [CrossRef] [PubMed]
- Shioi, A.; Morioka, T.; Shoji, T.; Emoto, M. The inhibitory roles of vitamin K in progression of vascular calcification. Nutrients 2020, 12, 583. [Google Scholar] [CrossRef] [PubMed]
- Anastasi, E.; Ialongo, C.; Labriola, R.; Ferraguti, G.; Lucarelli, M.; Angeloni, A. Vitamin K deficiency and covid-19. Scand. J. Clin. Lab. Investig. 2020, 80, 525–527. [Google Scholar] [CrossRef] [PubMed]
- Vogrig, A.; Gigli, G.L.; Bna, C.; Morassi, M. Stroke in patients with COVID-19: Clinical and neuroimaging characteristics. Neurosci. Lett. 2021, 743, 135564. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Kumar, M.; Bedi, O.; Gupta, M.; Kumar, S.; Jaiswal, G.; Rahi, V.; Yedke, N.G.; Bijalwan, A.; Sharma, S.; et al. Role of vitamins and minerals as immunity boosters in COVID-19. Inflammopharmacology 2021, 29, 1001–1016. [Google Scholar] [CrossRef] [PubMed]
- Domingo, J.L.; Marques, M.; Rovira, J. Influence of airborne transmission of SARS-CoV-2 on COVID-19 pandemic. A review. Environ. Res. 2020, 188, 109861. [Google Scholar] [CrossRef]
- Xu, L.; Mao, Y.; Chen, G. Risk factors for 2019 novel coronavirus disease (COVID-19) patients progressing to critical illness: A systematic review and meta-analysis. Aging 2020, 12, 12410–12421. [Google Scholar] [CrossRef]
- Rong, Y.; Wang, F.; Liu, J.; Zhou, Y.; Li, X.; Liang, X.; Zhang, D.; Zeng, H.; Wang, J.; Shi, Y. Clinical characteristics and risk factors of mild-to-moderate COVID-19 patients with false-negative SARS-CoV-2 nucleic acid. J. Med. Virol. 2021, 93, 448–455. [Google Scholar] [CrossRef]
- Zella, D.; Giovanetti, M.; Benedetti, F.; Unali, F.; Spoto, S.; Guarino, M.; Angeletti, S.; Ciccozzi, M. The variants question: What is the problem? J. Med. Virol. 2021, 93, 6479–6485. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhang, L.; Du, H.; Zhang, J.; Li, Y.Y.; Qu, J.; Zhang, W.; Wang, Y.; Bao, S.; Li, Y.; et al. Chinese pediatric novel coronavirus study, SARS-CoV-2 infection in children. N. Engl. J. Med. 2020, 382, 1663–1665. [Google Scholar] [CrossRef] [PubMed]
- Wolff, D.; Nee, S.; Hickey, N.S.; Marschollek, M. Risk factors for Covid-19 severity and fatality: A structured literature review. Infection 2021, 49, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Boopathi, S.; Poma, A.B.; Kolandaivel, P. Novel 2019 coronavirus structure, mechanism of action, antiviral drug promises and rule out against its treatment. J. Biomol. Struct. Dyn. 2021, 39, 3409–3418. [Google Scholar] [CrossRef]
- Mir, T.; Almas, T.; Kaur, J.; Faisaluddin, M.; Song, D.; Ullah, W.; Mamtani, S.; Rauf, H.; Yadav, S.; Latchana, S.; et al. Coronavirus disease 2019 (COVID-19): Multisystem review of pathophysiology. Ann. Med. Surg. 2021, 69, 102745. [Google Scholar] [CrossRef]
- Wehbe, Z.; Hammoud, S.H.; Yassine, H.M.; Fardoun, M.; El-Yazbi, A.F.; Eid, A.H. Molecular and biological mechanisms underlying gender differences in COVID-19 severity and mortality. Front. Immunol. 2021, 12, 659339. [Google Scholar] [CrossRef]
- Heidari, S.; Palmer-Ross, A.; Goodman, T. A systematic review of the sex and gender reporting in COVID-19 clinical trials. Vaccines 2021, 9, 1322. [Google Scholar] [CrossRef]
- Jin, J.M.; Bai, P.; He, W.; Wu, F.; Liu, X.F.; Han, D.M.; Liu, S.; Yang, J.K. Gender Differences in patients with COVID-19: Focus on severity and mortality. Front. Public Health 2020, 8, 152. [Google Scholar] [CrossRef]
- Akbari, A.; Fathabadi, A.; Razmi, M.; Zarifian, A.; Amiri, M.; Ghodsi, A.; Vafadar Moradi, E. Characteristics, risk factors, and outcomes associated with readmission in COVID-19 patients: A systematic review and meta-analysis. Am. J. Emerg. Med. 2021, 52, 166–173. [Google Scholar] [CrossRef]
- Gebhard, C.; Regitz-Zagrosek, V.; Neuhauser, H.K.; Morgan, R.; Klein, S.L. Impact of sex and gender on COVID-19 outcomes in Europe. Biol. Sex Differ. 2020, 11, 29. [Google Scholar] [CrossRef]
- Peckham, H.; de Gruijter, N.M.; Raine, C.; Radziszewska, A.; Ciurtin, C.; Wedderburn, L.R.; Rosser, E.C.; Webb, K.; Deakin, C.T. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat. Commun. 2020, 11, 6317. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.D.; Ding, M.; Dong, X.; Zhang, J.J.; Kursat Azkur, A.; Azkur, D.; Gan, H.; Sun, Y.L.; Fu, W.; Li, W.; et al. Risk factors for severe and critically ill COVID-19 patients: A review. Allergy 2021, 76, 428–455. [Google Scholar] [CrossRef] [PubMed]
- Ya’qoub, L.; Elgendy, I.Y.; Pepine, C.J. Sex and gender differences in COVID-19: More to be learned! Am. Heart J. Plus 2021, 3, 100011. [Google Scholar] [CrossRef] [PubMed]
- Galasso, V.; Pons, V.; Profeta, P.; Becher, M.; Brouard, S.; Foucault, M. Gender differences in COVID-19 attitudes and behavior: Panel evidence from eight countries. Proc. Natl. Acad. Sci. USA 2020, 117, 27285–27291. [Google Scholar] [CrossRef]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef]
- Webb, K.; Peckham, H.; Radziszewska, A.; Menon, M.; Oliveri, P.; Simpson, F.; Deakin, C.T.; Lee, S.; Ciurtin, C.; Butler, G.; et al. Sex and pubertal dfferences in the Type 1 interferon pathway associate with both X chromosome number and serum sex hormone concentration. Front. Immunol. 2018, 9, 3167. [Google Scholar] [CrossRef]
- Villa, A.; Rizzi, N.; Vegeto, E.; Ciana, P.; Maggi, A. Estrogen accelerates the resolution of inflammation in macrophagic cells. Sci. Rep. 2015, 5, 15224. [Google Scholar] [CrossRef]
- Sullivan, J.C.; Rodriguez-Miguelez, P.; Zimmerman, M.A.; Harris, R.A. Differences in angiotensin (1–7) between men and women. Am. J. Physiol.-Heart Circ. Physiol. 2015, 308, H1171–H1176. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Chinn, J.; De Ferrante, M.; Kirby, K.A.; Hohmann, S.F.; Amin, A. Male gender is a predictor of higher mortality in hospitalized adults with COVID-19. PLoS ONE 2021, 16, e0254066. [Google Scholar] [CrossRef]
- Chen, Y.; Klein, S.L.; Garibaldi, B.T.; Li, H.; Wu, C.; Osevala, N.M.; Li, T.; Margolick, J.B.; Pawelec, G.; Leng, S.X. Aging in COVID-19: Vulnerability, immunity and intervention. Ageing Res. Rev. 2021, 65, 101205. [Google Scholar] [CrossRef]
- Lewis, A.; Duch, R. Gender differences in perceived risk of COVID-19. Soc. Sci. Q. 2021, 102, 2124–2133. [Google Scholar] [CrossRef] [PubMed]
- Batrinos, M.L. The aging of the endocrine hypothalamus and its dependent endocrine glands. Hormones 2012, 11, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Barzilai, N.; Huffman, D.M.; Muzumdar, R.H.; Bartke, A. The critical role of metabolic pathways in aging. Diabetes 2012, 61, 1315–1322. [Google Scholar] [CrossRef] [PubMed]
- Ortuno-Sahagun, D.; Pallas, M.; Rojas-Mayorquin, A.E. Oxidative stress in aging: Advances in proteomic approaches. Oxid. Med. Cell. Longev. 2014, 2014, 573208. [Google Scholar] [CrossRef]
- Slominski, A.; Zbytek, B.; Nikolakis, G.; Manna, P.R.; Skobowiat, C.; Zmijewski, M.; Li, W.; Janjetovic, Z.; Postlethwaite, A.; Zouboulis, C.C.; et al. Steroidogenesis in the skin: Implications for local immune functions. J. Steroid Biochem. Mol. Biol. 2013, 137, 107–123. [Google Scholar] [CrossRef]
- Manna, P.R.; Sennoune, S.R.; Martinez-Zaguilan, R.; Slominski, A.T.; Pruitt, K. Regulation of retinoid mediated cholesterol efflux involves liver X receptor activation in mouse macrophages. Biochem. Biophys. Res. Commun. 2015, 464, 312–317. [Google Scholar] [CrossRef]
- Dewanjee, S.; Vallamkondu, J.; Kalra, R.S.; Puvvada, N.; Kandimalla, R.; Reddy, P.H. Emerging COVID-19 neurological manifestations: Present outlook and potential neurological challenges in COVID-19 pandemic. Mol. Neurobiol. 2021, 58, 4694–4715. [Google Scholar] [CrossRef]
- Lian, J.; Yue, Y.; Yu, W.; Zhang, Y. Immunosenescence: A key player in cancer development. J. Hematol. Oncol. 2020, 13, 151. [Google Scholar] [CrossRef]
- Cunha, L.L.; Perazzio, S.F.; Azzi, J.; Cravedi, P.; Riella, L.V. Remodeling of the Immune Response With Aging: Immunosenescence and Its Potential Impact on COVID-19 Immune Response. Front. Immunol. 2020, 11, 1748. [Google Scholar] [CrossRef]
- Babbar, M.; Basu, S.; Yang, B.; Croteau, D.L.; Bohr, V.A. Mitophagy and DNA damage signaling in human aging. Mech. Ageing Dev. 2020, 186, 111207. [Google Scholar] [CrossRef]
- Rodrigues Siqueira, I.; Fochesatto, C.; da Silva Torres, I.L.; Dalmaz, C.; Alexandre Netto, C. Aging affects oxidative state in hippocampus, hypothalamus and adrenal glands of Wistar rats. Life Sci. 2005, 78, 271–278. [Google Scholar] [CrossRef]
- Beattie, M.C.; Chen, H.; Fan, J.; Papadopoulos, V.; Miller, P.; Zirkin, B.R. Aging and luteinizing hormone effects on reactive oxygen species production and DNA damage in rat Leydig cells. Biol. Reprod. 2013, 88, 100. [Google Scholar] [CrossRef] [PubMed]
- De Frel, D.L.; Atsma, D.E.; Pijl, H.; Seidell, J.C.; Leenen, P.J.M.; Dik, W.A.; van Rossum, E.F.C. The impact of obesity and lifestyle on the immune system and susceptibility to infections such as COVID-19. Front. Nutr. 2020, 7, 597600. [Google Scholar] [CrossRef] [PubMed]
- Petrakis, D.; Margina, D.; Tsarouhas, K.; Tekos, F.; Stan, M.; Nikitovic, D.; Kouretas, D.; Spandidos, D.A.; Tsatsakis, A. Obesity a risk factor for increased COVID19 prevalence, severity and lethality. Mol. Med. Rep. 2020, 22, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Kane, H.; Lynch, L. Innate immune control of adipose tissue homeostasis. Trends Immunol. 2019, 40, 857–872. [Google Scholar] [CrossRef]
- Dixon, A.E.; Peters, U. The effect of obesity on lung function. Expert Rev. Respir. Med. 2018, 12, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Feldman, E.L.; Savelieff, M.G.; Hayek, S.S.; Pennathur, S.; Kretzler, M.; Pop-Busui, R. COVID-19 and diabetes: A collision and collusion of two diseases. Diabetes 2020, 69, 2549–2565. [Google Scholar] [CrossRef]
- Muniyappa, R.; Wilkins, K.J. Diabetes, obesity, and risk prediction of severe COVID-19. J. Clin. Endocrinol. Metab. 2020, 105, e3812–e3814. [Google Scholar] [CrossRef]
- Lim, S.; Bae, J.H.; Kwon, H.S.; Nauck, M.A. COVID-19 and diabetes mellitus: From pathophysiology to clinical management. Nat. Rev. Endocrinol. 2021, 17, 11–30. [Google Scholar] [CrossRef]
- Pal, R.; Bhansali, A. COVID-19, diabetes mellitus and ACE2: The conundrum. Diabetes Res. Clin. Pract. 2020, 162, 108132. [Google Scholar] [CrossRef]
- Sabri, S.; Bourron, O.; Phan, F.; Nguyen, L.S. Interactions between diabetes and COVID-19: A narrative review. World J. Diabetes 2021, 12, 1674–1692. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, S.K.; Venkatratnam, P.; Mahendra, J.; Devarajan, N. Increased mortality of COVID-19 infected diabetes patients: Role of furin proteases. Int. J. Obes. 2020, 44, 2486–2488. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, R.P.; Lee, J.M.; Greaves, D.R. Mechanisms of disease: Macrophage-derived foam cells emerging as therapeutic targets in atherosclerosis. Nat. Clin. Pract. Cardiovasc. Med. 2005, 2, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Li, P.; Ye, J. Lipid homeostasis and the formation of macrophage-derived foam cells in atherosclerosis. Protein Cell 2012, 3, 173–181. [Google Scholar] [CrossRef]
- Ning, Y.; Bai, Q.; Lu, H.; Li, X.; Pandak, W.M.; Zhao, F.; Chen, S.; Ren, S.; Yin, L. Overexpression of mitochondrial cholesterol delivery protein, StAR, decreases intracellular lipids and inflammatory factors secretion in macrophages. Atherosclerosis 2009, 204, 114–120. [Google Scholar] [CrossRef][Green Version]
- Taylor, J.M.; Borthwick, F.; Bartholomew, C.; Graham, A. Overexpression of steroidogenic acute regulatory protein increases macrophage cholesterol efflux to apolipoprotein AI. Cardiovasc. Res. 2010, 86, 526–534. [Google Scholar] [CrossRef]
- Voloshyna, I.; Reiss, A.B. The ABC transporters in lipid flux and atherosclerosis. Prog Lipid Res. 2011, 50, 213–224. [Google Scholar] [CrossRef]
- Manna, P.R. Retinoid regulated macrophage cholesterol efflux involves the steroidogenic acute regulatory protein. Data Brief 2016, 7, 940–945. [Google Scholar] [CrossRef][Green Version]
- Chen, Q.; Xu, L.; Zhu, W.; Ge, J. Cardiovascular manifestations in severe and critical patients with COVID-19. Clin. Cardiol. 2020, 43, 1054. [Google Scholar] [CrossRef]
- Clerkin, K.J.; Fried, J.A.; Raikhelkar, J.; Sayer, G.; Griffin, J.M.; Masoumi, A.; Jain, S.S.; Burkhoff, D.; Kumaraiah, D.; Rabbani, L.; et al. COVID-19 and Cardiovascular Disease. Circulation 2020, 141, 1648–1655. [Google Scholar] [CrossRef]
- Manna, P.R.; Molehin, D.; Ahmed, A.U. Dysregulation of aromatase in breast, endometrial, and ovarian cancers: An overview of therapeutic strategies. Prog. Mol. Biol. Transl. Sci. 2016, 144, 487–537. [Google Scholar] [PubMed]
- Manna, P.R.; Ahmed, A.U.; Yang, S.; Narasimhan, M.; Cohen-Tannoudji, J.; Slominski, A.T.; Pruitt, K. Genomic profiling of the steroidogenic acute regulatory Protein in breast cancer: In silico assessments and a mechanistic perspective. Cancers 2019, 11, 623. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Guan, W.; Chen, R.; Wang, W.; Li, J.; Xu, K.; Li, C.; Ai, Q.; Lu, W.; Liang, H.; et al. Cancer patients in SARS-CoV-2 infection: A nationwide analysis in China. Lancet Oncol. 2020, 21, 335–337. [Google Scholar] [CrossRef]
- Dai, M.; Liu, D.; Liu, M.; Zhou, F.; Li, G.; Chen, Z.; Zhang, Z.; You, H.; Wu, M.; Zheng, Q.; et al. Patients with cancer appear more vulnerable to SARS-CoV-2: A multicenter study during the COVID-19 outbreak. Cancer Discov. 2020, 10, 783–791. [Google Scholar] [PubMed]
- Dunn, G.P.; Bruce, A.T.; Ikeda, H.; Old, L.J.; Schreiber, R.D. Cancer immunoediting: From immunosurveillance to tumor escape. Nat. Immunol. 2002, 3, 991–998. [Google Scholar] [CrossRef]
- Han, H.J.; Nwagwu, C.; Anyim, O.; Ekweremadu, C.; Kim, S. COVID-19 and cancer: From basic mechanisms to vaccine development using nanotechnology. Int. Immunopharmacol. 2021, 90, 107247. [Google Scholar] [CrossRef]
- Du Plessis, M.; Fourie, C.; Riedemann, J.; de Villiers, W.J.S.; Engelbrecht, A.M. Cancer and Covid-19: Collectively catastrophic. Cytokine Growth Factor Rev. 2021, 2021, 1–12. [Google Scholar] [CrossRef]
- Sarfati, D.; Koczwara, B.; Jackson, C. The impact of comorbidity on cancer and its treatment. CA Cancer J. Clin. 2016, 66, 337–350. [Google Scholar] [CrossRef]
- Gosain, R.; Abdou, Y.; Singh, A.; Rana, N.; Puzanov, I.; Ernstoff, M.S. COVID-19 and Cancer: A Comprehensive Review. Curr. Oncol. Rep. 2020, 22, 53. [Google Scholar] [CrossRef]
- Gottschalk, G.; Knox, K.; Roy, A. ACE2: At the crossroad of COVID-19 and lung cancer. Gene Rep. 2021, 23, 101077. [Google Scholar] [CrossRef]
- Stewart, C.A.; Gay, C.M.; Ramkumar, K.; Cargill, K.R.; Cardnell, R.J.; Nilsson, M.B.; Heeke, S.; Park, E.M.; Kundu, S.T.; Diao, L.; et al. Lung cancer models reveal severe acute respiratory syndrome coronavirus 2-induced epithelial-to-mesenchymal transition contributes to coronavirus disease 2019 pathophysiology. J. Thorac. Oncol. 2021, 16, 1821–1839. [Google Scholar] [CrossRef] [PubMed]
- Pugazhenthi, S.; Qin, L.; Reddy, P.H. Common neurodegenerative pathways in obesity, diabetes, and Alzheimer’s disease. Biochim. Biophys. Acta Mol. Basis. Dis. 2017, 1863, 1037–1045. [Google Scholar] [CrossRef] [PubMed]
- Fotuhi, M.; Mian, A.; Meysami, S.; Raji, C.A. Neurobiology of COVID-19. J. Alzheimers Dis. 2020, 76, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Mandik, F.; Vos, M. Neurodegenerative disorders: Spotlight on sphingolipids. Int. J. Mol. Sci. 2021, 22, 11998. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Rafiq, A.; Shabaneh, O.; Gittner, L.S.; Reddy, P.H. Current issues in chronic diseases: A focus on dementia and hypertension in rural west Texans. J. Alzheimers Dis. 2019, 72, S59–S69. [Google Scholar] [CrossRef]
- Reddy, P.H.; Yin, X.; Manczak, M.; Kumar, S.; Pradeepkiran, J.A.; Vijayan, M.; Reddy, A.P. Mutant APP and amyloid beta-induced defective autophagy, mitophagy, mitochondrial structural and functional changes and synaptic damage in hippocampal neurons from Alzheimer’s disease. Hum. Mol. Genet. 2018, 27, 2502–2516. [Google Scholar] [CrossRef]
- Amakiri, N.; Kubosumi, A.; Tran, J.; Reddy, P.H. Amyloid beta and microRNAs in Alzheimer’s disease. Front. Neurosci. 2019, 13, 430. [Google Scholar] [CrossRef]
- Lim, K.H.; Yang, S.; Kim, S.H.; Joo, J.Y. Elevation of ACE2 as a SARS-CoV-2 entry receptor gene expression in Alzheimer’s disease. J. Infect. 2020, 81, e33–e34. [Google Scholar] [CrossRef]
- Chang, R.; Liu, X.; Li, S.; Li, X.J. Transgenic animal models for study of the pathogenesis of Huntington’s disease and therapy. Drug Des. Devel. Ther. 2015, 9, 2179–2188. [Google Scholar]
- Sawant, N.; Morton, H.; Kshirsagar, S.; Reddy, A.P.; Reddy, P.H. Mitochondrial abnormalities and synaptic damage in huntington’s disease: A focus on defective Mitophagy and mitochondria-targeted therapeutics. Mol. Neurobiol. 2021, 58, 6350–6377. [Google Scholar] [CrossRef]
- Picca, A.; Guerra, F.; Calvani, R.; Romano, R.; Coelho-Junior, H.J.; Bucci, C.; Marzetti, E. Mitochondrial dysfunction, protein misfolding and neuroinflammation in Parkinson’s disease: Roads to biomarker discovery. Biomolecules 2021, 11, 1508. [Google Scholar] [CrossRef] [PubMed]
- Helmich, R.C.; Bloem, B.R. The impact of the COVID-19 pandemic on Parkinson’s disease: Hidden sorrows and emerging opportunities. J. Parkinsons Dis. 2020, 10, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Gombart, A.F.; Pierre, A.; Maggini, S. A Review of micronutrients and the immune system-working in harmony to reduce the risk of infection. Nutrients 2020, 12, 236. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, M.K.; Nims, R.W.; de Szalay, S.; Rubino, J.R. Soap, water, and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): An ancient handwashing strategy for preventing dissemination of a novel virus. PeerJ. 2021, 9, e12041. [Google Scholar] [CrossRef]
- Roncati, L.; Vadala, M.; Corazzari, V.; Palmieri, B. COVID-19 vaccine and boosted immunity: Nothing ad interim to do? Vaccine 2020, 38, 7581–7584. [Google Scholar] [CrossRef]
- Scurr, M.J.; Zelek, W.M.; Lippiatt, G.; Somerville, M.; Burnell, S.E.A.; Capitani, L.; Davies, K.; Lawton, H.; Tozer, T.; Rees, T.; et al. Whole blood-based measurement of SARS-CoV-2-specific T cells reveals asymptomatic infection and vaccine immunogenicity in healthy subjects and patients with solid organ cancers. Immunology 2021, 2021, 1–10. [Google Scholar] [CrossRef]
- Kandimalla, R.; Chakraborty, P.; Vallamkondu, J.; Chaudhary, A.; Samanta, S.; Reddy, P.H.; De Feo, V.; Dewanjee, S. Counting on COVID-19 vaccine: Insights into the current strategies, progress and future challenges. Biomedicines 2021, 9, 1740. [Google Scholar] [CrossRef]
- Singh, A.; Khillan, R.; Mishra, Y.; Khurana, S. The safety profile of COVID-19 vaccinations in the United States. Am. J. Infect. Control 2022, 50, 15–19. [Google Scholar] [CrossRef]
- Ferreira, A.O.; Polonini, H.C.; Dijkers, E.C.F. Postulated adjuvant therapeutic strategies for COVID-19. J. Pers. Med. 2020, 10, 80. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, X.; Ni, L.; Di, X.; Ma, B.; Niu, S.; Liu, C.; Reiter, R.J. COVID-19: Melatonin as a potential adjuvant treatment. Life Sci. 2020, 250, 117583. [Google Scholar] [CrossRef]
- Fernandez-Lazaro, D.; Fernandez-Lazaro, C.I.; Mielgo-Ayuso, J.; Adams, D.P.; Garcia Hernandez, J.L.; Gonzalez-Bernal, J.; Gonzalez-Gross, M. Glycophosphopeptical AM3 food supplement: A potential adjuvant in the treatment and vaccination of SARS-CoV-2. Front. Immunol. 2021, 12, 698672. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Yang, H.; Ji, W.; Wu, W.; Chen, S.; Zhang, W.; Duan, G. Virology, epidemiology, pathogenesis, and control of COVID-19. Viruses 2020, 12, 372. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Saurabh, M.K.; Maharshi, V.; Saikia, D. A narrative review of antiviral drugs used for COVID-19 pharmacotherapy. J. Pharm. Bioallied Sci. 2021, 13, 163–171. [Google Scholar] [PubMed]
- Wahid, B.; Amir, A.; Ameen, A.; Idrees, M. Current status of therapeutic approaches and vaccines for SARS-CoV-2. Future Microbiol. 2021, 16, 1319–1326. [Google Scholar] [CrossRef]
- Cao, B.; Wang, Y.; Wen, D.; Liu, W.; Wang, J.; Fan, G.; Ruan, L.; Song, B.; Cai, Y.; Wei, M.; et al. A trial of lopinavir-ritonavir in adults hospitalized with severe COVID-19. N. Engl. J. Med. 2020, 382, 1787–1799. [Google Scholar] [CrossRef]
- Whitley, R. Molnupiravir—A step toward orally bioavailable therapies for Covid-19. N. Engl. J. Med. 2021, 386, 592–593. [Google Scholar] [CrossRef]
- Pourkarim, F.; Pourtaghi-Anvarian, S.; Rezaee, H. Molnupiravir: A new candidate for COVID-19 treatment. Pharmacol. Res. Perspect. 2022, 10, e00909. [Google Scholar] [CrossRef]
- Mahase, E. COVID-19: Pfizer’s paxlovid is 89% effective in patients at risk of serious illness, company reports. BMJ 2021, 375, n2713. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, L. In the age of Omicron variant: Paxlovid raises new hopes of COVID-19 recovery. J. Med. Virol. 2021, 2021, 1–2. [Google Scholar] [CrossRef]
- Bohn, M.K.; Hall, A.; Sepiashvili, L.; Jung, B.; Steele, S.; Adeli, K. Pathophysiology of COVID-19: Mechanisms underlying disease severity and progression. Physiology 2020, 35, 288–301. [Google Scholar] [CrossRef]
- Elekhnawy, E.; Kamar, A.A.; Sonbol, F. Present and future treatment strategies for coronavirus disease 2019. Futur. J. Pharm. Sci. 2021, 7, 84. [Google Scholar] [CrossRef] [PubMed]

| Risk Factors | Risk Levels | ||
|---|---|---|---|
| Low | Medium | High | |
| Women | + | ||
| Men | ++ | ||
| Ages (0–24 years) | + | ||
| Ages (25–64 years) | + | ||
| Ages (65+ years) | +++ | ||
| Hotel stays (<2 nights) | + | ||
| Visiting museums or libraries | + | + | |
| Public playground | + | ||
| Attending dinner parties | + | + | |
| Shopping at malls | + | ||
| School/daycare | + | + | |
| Indoor in-person jobs | + | ||
| Haircut/salon visit | + | ||
| Wedding | + | ||
| Restaurant eating | + | ||
| Working out at gyms | + | ||
| Attending sporting events | + | ||
| Drinking at bars | + | ||
| Attending concerts | ++ | ||
| Public pool | + | ||
| Visiting friends/relatives | + | ||
| Going to movie/theater | + | ||
| Parties | ++ | ||
| Travels (bus/train/plain) | + | ||
| Public transport | ++ | ||
| Attending in-person classes | + | + | |
| Cruise travel | ++ | ||
| Hospital visit | + | ||
| Graduation parties | ++ | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manna, P.R.; Gray, Z.C.; Reddy, P.H. Healthy Immunity on Preventive Medicine for Combating COVID-19. Nutrients 2022, 14, 1004. https://doi.org/10.3390/nu14051004
Manna PR, Gray ZC, Reddy PH. Healthy Immunity on Preventive Medicine for Combating COVID-19. Nutrients. 2022; 14(5):1004. https://doi.org/10.3390/nu14051004
Chicago/Turabian StyleManna, Pulak R., Zackery C. Gray, and P. Hemachandra Reddy. 2022. "Healthy Immunity on Preventive Medicine for Combating COVID-19" Nutrients 14, no. 5: 1004. https://doi.org/10.3390/nu14051004
APA StyleManna, P. R., Gray, Z. C., & Reddy, P. H. (2022). Healthy Immunity on Preventive Medicine for Combating COVID-19. Nutrients, 14(5), 1004. https://doi.org/10.3390/nu14051004








