Association between Maternal Serum 25-Hydroxyvitamin D Concentrations and the Risk of Preterm Birth in Central Sudan: A Case–Control Study
Abstract
:1. Introduction
2. Subjects and Methods
2.1. Study Setting and Design
2.2. Case and Control Definition
2.3. Processing of Blood Sample
2.4. Sample Size Calculation
2.5. Data Analysis
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Born too Soon: The Global Action Report on Preterm Birth. Australas. Med. J. 2012, 5, 598–599.
- Chawanpaiboon, S.; Vogel, J.P.; Moller, A.B.; Lumbiganon, P.; Petzold, M.; Hogan, D.; Landoulsi, S.; Jampathong, N.; Kongwattanakul, K.; Laopaiboon, M.; et al. Global, regional, and national estimates of levels of preterm birth in 2014: A systematic review and modelling analysis. Lancet Glob. Health 2019, 7, e37–e46. [Google Scholar] [CrossRef] [Green Version]
- Mondal, M.N.I.; Hossain, M.K.; Ali, M.K. Factors influencing infant and child mortality: A case study of Rajshahi District, Bangladesh. J. Hum. Ecol. 2009, 26, 31–39. [Google Scholar] [CrossRef]
- Goldenberg, R.L.; Culhane, J.F.; Iams, J.D.; Romero, R. Epidemiology and causes of preterm birth. Lancet 2008, 371, 75–84. [Google Scholar] [CrossRef]
- Zainal, H.; Dahlui, M.; Soelar, S.A.; Su, T.T. Cost of preterm birth during initial hospitalization: A care provider’s perspective. PLoS ONE 2019, 14, e0211997. [Google Scholar] [CrossRef] [Green Version]
- Aregawi, G.; Assefa, N.; Mesfin, F.; Tekulu, F.; Adhena, T.; Mulugeta, M.; Gebreayezgi, G. Preterm births and associated factors among mothers who gave birth in Axum and Adwa Town public hospitals, Northern Ethiopia, 2018. BMC Res. Notes 2019, 12, 4–9. [Google Scholar] [CrossRef] [Green Version]
- Wagura, P.M.; Wasunna, A.; Laving, A.; Wamalwa, D.; Ng’Ang’A, P. Prevalence and factors associated with preterm birth at kenyatta national hospital. BMC Pregnancy Childbirth 2018, 18, 107. [Google Scholar] [CrossRef]
- Wacker, M.; Holiack, M.F. Vitamin D-effects on skeletal and extraskeletal health and the need for supplementation. Nutrients 2013, 5, 111–148. [Google Scholar] [CrossRef] [Green Version]
- Holick, M.F. Evidence-based D-bate on health benefits of vitamin D revisited. Derm. Endocrinol. 2012, 4, 183–190. [Google Scholar] [CrossRef] [Green Version]
- Wei, S.Q.; Qi, H.P.; Luo, Z.C.; Fraser, W.D. Maternal vitamin D status and adverse pregnancy outcomes: A systematic review and meta-analysis. J. Matern. Neonatal Med. 2013, 26, 889–899. [Google Scholar] [CrossRef]
- Miliku, K.; Vinkhuyzen, A.; Blanken, L.M.E.; McGrath, J.J.; Eyles, D.W.; Burne, T.H.; Hofman, A.; Tiemeier, H.; Steegers, E.A.P.; Gaillard, R.; et al. Maternal vitamin D concentrations during pregnancy, fetal growth patterns, and risks of adverse birth outcomes. Am. J. Clin. Nutr. 2016, 103, 1514–1522. [Google Scholar] [CrossRef] [PubMed]
- María Pérez-Castillo, Í.; Rivero-Blanco, T.; Alejandra León-Ríos, X.; Expósito-Ruiz, M.; Setefilla López-Criado, M.; Aguilar-Cordero, M.J. Associations of Vitamin D Deficiency, Parathyroid hormone, Calcium, and Phosphorus with Perinatal Adverse Outcomes. A Prospective Cohort Study. Nutrients 2020, 12, 3279. [Google Scholar] [CrossRef] [PubMed]
- Fondjo, L.A.; Tashie, W.; Owiredu, W.K.B.A.; Adu-Gyamfi, E.A.; Seidu, L. High prevalence of vitamin D deficiency among normotensive and hypertensive pregnant women in Ghana. BMC Pregnancy Childbirth 2021, 21, 331. [Google Scholar] [CrossRef] [PubMed]
- Oluwole, A.A.; Okunade, K.S.; Okojie, O.E. Maternal serum vitamin D levels and preterm delivery among low-risk parturients in Lagos, Nigeria. Int. J. Gynecol. Obstet. 2019, 144, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Jao, J.; Freimanis, L.; Mussi-Pinhata, M.M.; Cohen, R.A.; Monteiro, J.P.; Cruz, M.L.; Branch, A.; Sperling, R.S.; Siberry, G.K. Severe Vitamin D Deficiency in Human Immunodeficiency Virus-Infected Pregnant Women is Associated with Preterm Birth. Am. J. Perinatol. 2017, 34, 486–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, L.L.; Lu, F.G.; Yang, S.H.; Xu, H.L.; Luo, B.A. Does maternal Vitamin D deficiency increase the risk of preterm birth: A meta-analysis of observational studies. Nutrients 2016, 8, 301. [Google Scholar] [CrossRef]
- Lian, R.H.; Qi, P.A.; Yuan, T.; Yan, P.J.; Qiu, W.W.; Wei, Y.; Hu, Y.G.; Yang, K.H.; Yi, B. Systematic review and meta-analysis of vitamin D deficiency in different pregnancy on preterm birth: Deficiency in middle pregnancy might be at risk. Medicine 2021, 100, e26303. [Google Scholar] [CrossRef]
- Pérez-López, F.R.; Pasupuleti, V.; Mezones-Holguin, E.; Benites-Zapata, V.A.; Thota, P.; Deshpande, A.; Hernandez, A.V. Effect of vitamin D supplementation during pregnancy on maternal and neonatal outcomes: A systematic review and meta-analysis of randomized controlled trials. Fertil. Steril. 2015, 103, 1278–1288.e4. [Google Scholar] [CrossRef]
- Yu, L.; Guo, Y.; Ke, H.J.; He, Y.-S.; Che, D.; Wu, J.L. Vitamin D status in pregnant women in southern China and risk of preterm birth: A large-scale retrospective cohort study. Med. Sci. Monit. 2019, 25, 7755–7762. [Google Scholar] [CrossRef]
- Vivanti, A.J.; Monier, I.; Salakos, E.; Elie, C.; Tsatsaris, V.; Senat, M.V.; Jani, J.; Jouannic, J.M.; Winer, N.; Zeitlin, J.; et al. Vitamin D and pregnancy outcomes: Overall results of the FEPED study. J. Gynecol. Obstet. Hum. Reprod. 2020, 49, 101883. [Google Scholar] [CrossRef]
- Monier, I.; Baptiste, A.; Tsatsaris, V.; Senat, M.V.; Jani, J.; Jouannic, J.M.; Winer, N.; Elie, C.; Souberbielle, J.C.; Zeitlin, J.; et al. First Trimester Maternal Vitamin D Status and Risks of Preterm Birth and Small-For-Gestational Age. Nutrients 2019, 11, 3042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahamar, A.; Andemel, N.; Swihart, B.; Sidibe, Y.; Gaoussou, S.; Barry, A.; Traore, M.; Attaher, O.; Dembele, A.B.; Diarra, B.S.; et al. Malaria Infection Is Common and Associated with Perinatal Mortality and Preterm Delivery Despite Widespread Use of Chemoprevention in Mali: An Observational Study 2010 to 2014. Clin. Infect. Dis. 2021, 73, 1355–1361. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, S.Z.; Saad, A.A.; Mohieldein, A.H.; Nasr, A.M.; Adam, I. Serum level of 25-hydroxyvitamin D and obesity among early pregnant women. J. Obstet. Gynaecol. Res. 2019, 45, 2338–2342. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahiem, S.K.; Ahmed, A.B.A.; Sharif, M.E.; Adam, I. Association between maternal serum 25-hydroxyvitamin D concentrations and the risk of pre-eclampsia in central Sudan: A case-control study. Trans. R. Soc. Trop. Med. Hyg. 2021, trab163. [Google Scholar] [CrossRef]
- Zhou, S.S.; Tao, Y.H.; Huang, K.; Zhu, B.B.; Tao, F.B. Vitamin D and risk of preterm birth: Up-to-date meta-analysis of randomized controlled trials and observational studies. J. Obstet. Gynaecol. Res. 2017, 43, 247–256. [Google Scholar] [CrossRef] [Green Version]
- Aguilar-Cordero, M.J.; Lasserrot-Cuadrado, A.; Mur-Villar, N.; León-Ríos, X.A.; Rivero-Blanco, T.; Pérez-Castillo, I.M. Vitamin D, preeclampsia and prematurity: A systematic review and meta-analysis of observational and interventional studies. Midwifery 2020, 87, 102707. [Google Scholar] [CrossRef]
- de Souza, E.A.; Pisani, L.P. The relationship among vitamin D, TLR4 pathway and preeclampsia. Mol. Biol. Rep. 2020, 47, 6259–6267. [Google Scholar] [CrossRef]
- Smith, T.A.; Kirkpatrick, D.R.; Kovilam, O.; Agrawal, D.K. Immunomodulatory role of Vitamin D in the pathogenesis of preeclampsia. Expert Rev. Clin. Immunol. 2015, 11, 1055–1063. [Google Scholar] [CrossRef]
- Xu, J.; Jia, X.; Gu, Y.; Lewis, D.F.; Gu, X.; Wang, Y. Vitamin D reduces oxidative stress-induced procaspase-3/ROCK1 activation and mp release by placental trophoblasts. J. Clin. Endocrinol. Metab. 2017, 102, 2100–2110. [Google Scholar] [CrossRef] [Green Version]
- Grant, W.B. Adequate vitamin D during pregnancy reduces the risk of premature birth by reducing placental colonization by bacterial vaginosis species. MBio 2011, 2, e00022-11. [Google Scholar] [CrossRef] [Green Version]
- Liu, N.Q.; Hewison, M. Vitamin D, the placenta and pregnancy. Arch. Biochem. Biophys. 2012, 523, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.Q.; Kaplan, A.T.; Lagishetty, V.; Ouyang, Y.B.; Ouyang, Y.; Simmons, C.F.; Equils, O.; Hewison, M. Vitamin D and the regulation of placental inflammation. J. Immunol. 2011, 186, 5968–5974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, N.; Kaplan, A.T.; Low, J.; Nguyen, L.; Liu, G.Y.; Equils, O.; Hewison, M. Vitamin D induces innate antibacterial responses in human trophoblasts via an intracrine pathway. Biol. Reprod. 2009, 80, 398–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, K.N.; Nguyen, L.; Chan, J.; Innes, B.A.; Bulmer, J.N.; Kilby, M.D.; Hewison, M. Effects of 25-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 on cytokine production by human decidual cells. Biol. Reprod. 2006, 75, 816–822. [Google Scholar] [CrossRef] [Green Version]
- Dunlop, A.L.; Taylor, R.N.; Tangpricha, V.; Fortunato, S.; Menon, R. Maternal vitamin D, folate, and polyunsaturated fatty acid status and bacterial vaginosis during pregnancy. Infect. Dis. Obstet. Gynecol. 2011, 2011, 216217. [Google Scholar] [CrossRef] [Green Version]
- Fichorova, R.N.; Onderdonk, A.B.; Yamamoto, H.; Delaney, M.L.; DuBois, A.M.; Allred, E.; Levitonc, A. Maternal microbe-specific modulation of inflammatory response in extremely low-gestational-age newborns. MBio 2011, 2, e00280-10. [Google Scholar] [CrossRef] [Green Version]
- Gaffer, A.A.; Rayis, D.A.; Elhussein, O.G.; Adam, I. Vitamin D status in Sudanese pregnant women: A cross-sectional study. Trans. R. Soc. Trop. Med. Hyg. 2019, 113, 569–571. [Google Scholar] [CrossRef] [PubMed]
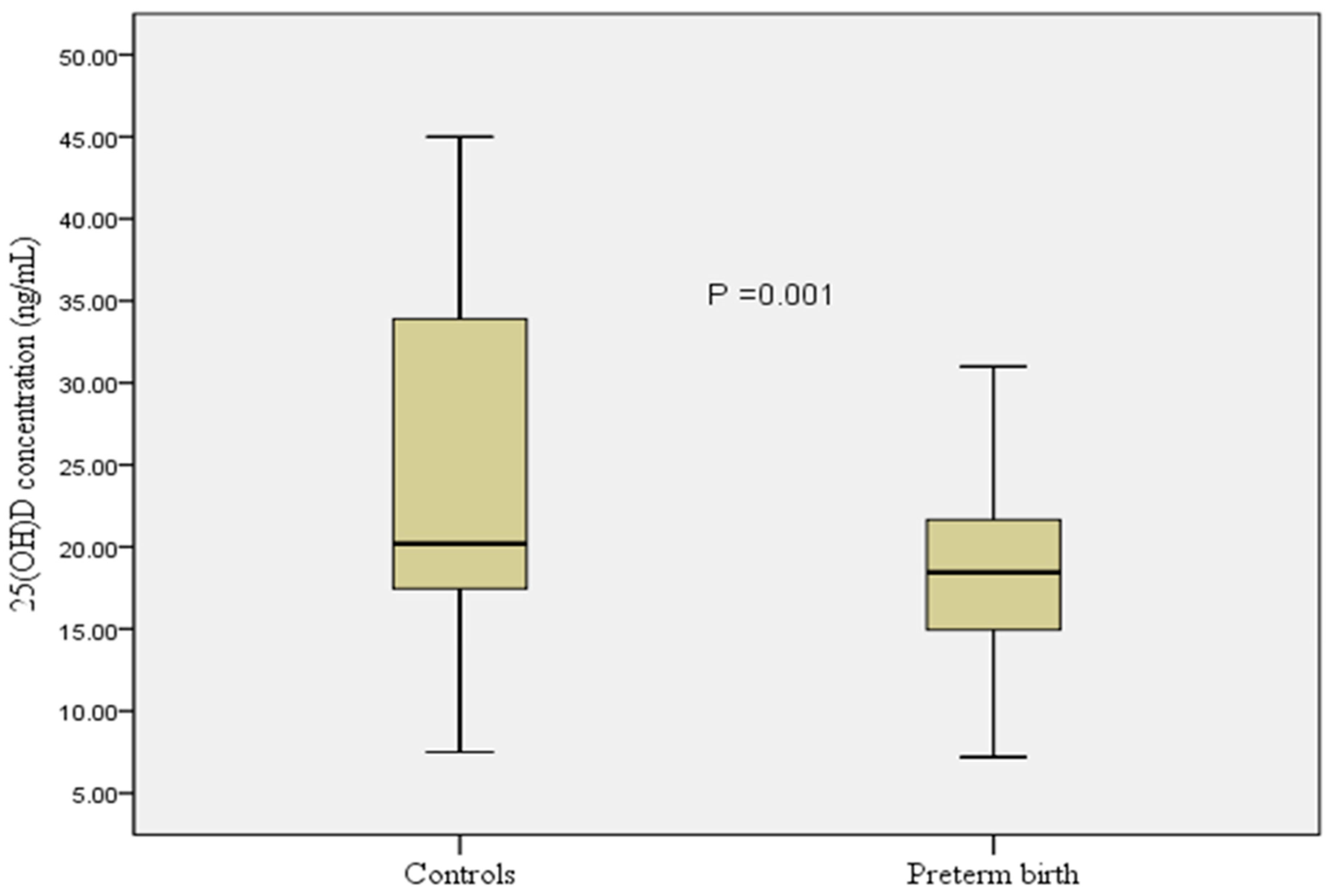
| Variables | Preterm Birth (n = 60) | Controls (n = 60) | OR (95% CI) | p-Value |
|---|---|---|---|---|
| Median (Interquartile Range) | ||||
| Age (years) | 28.5 (9.5) | 28.5 (4.7) | 0.98 (0.92–1.05) | 0.732 |
| Parity | 2.0 (3.0) | 3.0 (3.0) | 0.80 (0.64–0.99) | 0.047 |
| BMI (kg/m2) | 25.4 (6.1) | 26.1 (5.0) | 0.96 (0.89–1.04) | 0.414 |
| Haemoglobin level (g/dl) | 11.7 (1.7) | 11.8 (1.5) | 1.01(0.80–1.26) | 0.953 |
| 25(OH)D concentration (ng/mL) | 18.4 (7.3) | 20.2 (16.5) | 0.92 (0.87–0.96) | 0.001 |
| Frequency (proportion) | ||||
| Education level | ||||
| Secondary or higher | 15 (25.0) | 22 (36.7) | Reference | |
| Primary or lower | 45 (75.0) | 38 (63.7) | 1.73 (0.79–3.81) | 0.168 |
| Antenatal care | ||||
| ≥2 visits | 51 (85.0) | 59 (98.3) | Reference | |
| ˂2 visits | 9 (15.0) | 1 (1.7) | 4.07 (1.55–10.54) | 0.004 |
| Employment | ||||
| Housewives | 55 (91.7) | 54 (90.0) | Reference | |
| Employed | 5 (8.3) | 6 (10.0) | 0.81 (0.23–2.84) | 0.752 |
| History of miscarriage | ||||
| No | 49 (81.7) | 57 (95.0) | Reference | |
| Yes | 11 (18.3) | 3 (5.0) | 4.26 (1.12–16.16) | 0.033 |
| Vitamin D deficiency | ||||
| No | 18 (30.0) | 31 (51.7) | Reference | 0.017 |
| Yes | 42 (70.0) | 29 (48.3) | 2.49 (1.17–5.27) | |
| Non-Adjusted | Adjusted | |||
|---|---|---|---|---|
| Variables | OR (95% CI) | p-Value | aOR (95% CI) | p-Value |
| Parity | 0.79 (0.61–1.01) | 0.064 | ||
| 25-hydroxyvitamin D concentration (ng/mL) * | 0.92 (0.87–0.97) | 0.006 | 0.92 (0.87–0.97) | 0.006 |
| Education level | ||||
| Secondary or higher | Reference | |||
| Primary or lower | 2.21 (0.89–5.43) | 0.084 | ||
| Antenatal care | ||||
| ≥2 visits | Reference | Reference | ||
| ˂2 visits | 4.78 (1.69–13.49) | 0.003 | 4.78 (1.69–13.49) | 0.003 |
| History of miscarriage | ||||
| No | Reference | Reference | ||
| Yes | 4.86 (1.14–20.62) | 0.032 | 4.86 (1.14–20.62) | 0.032 |
| Vitamin D deficiency * | ||||
| No | Reference | Reference | ||
| Yes | 2.84 (1.21–6.64) | 0.016 | 2.69 (1.17–6.23) | 0.012 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelrahiem, S.K.; Sharif, M.E.; ALhabardi, N.; Al-Wutayd, O.; Adam, I. Association between Maternal Serum 25-Hydroxyvitamin D Concentrations and the Risk of Preterm Birth in Central Sudan: A Case–Control Study. Nutrients 2022, 14, 891. https://doi.org/10.3390/nu14040891
Abdelrahiem SK, Sharif ME, ALhabardi N, Al-Wutayd O, Adam I. Association between Maternal Serum 25-Hydroxyvitamin D Concentrations and the Risk of Preterm Birth in Central Sudan: A Case–Control Study. Nutrients. 2022; 14(4):891. https://doi.org/10.3390/nu14040891
Chicago/Turabian StyleAbdelrahiem, Somia K., Manal E. Sharif, Nadiah ALhabardi, Osama Al-Wutayd, and Ishag Adam. 2022. "Association between Maternal Serum 25-Hydroxyvitamin D Concentrations and the Risk of Preterm Birth in Central Sudan: A Case–Control Study" Nutrients 14, no. 4: 891. https://doi.org/10.3390/nu14040891
APA StyleAbdelrahiem, S. K., Sharif, M. E., ALhabardi, N., Al-Wutayd, O., & Adam, I. (2022). Association between Maternal Serum 25-Hydroxyvitamin D Concentrations and the Risk of Preterm Birth in Central Sudan: A Case–Control Study. Nutrients, 14(4), 891. https://doi.org/10.3390/nu14040891






