Cancer Cell Inhibiting Sea Cucumber (Holothuria leucospilota) Protein as a Novel Anti-Cancer Drug
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Sample Collection and Preparation
2.3. Cell Lines and Cell Cultures
2.4. Cellular Viability Assay
2.5. Cell Cycle Analysis
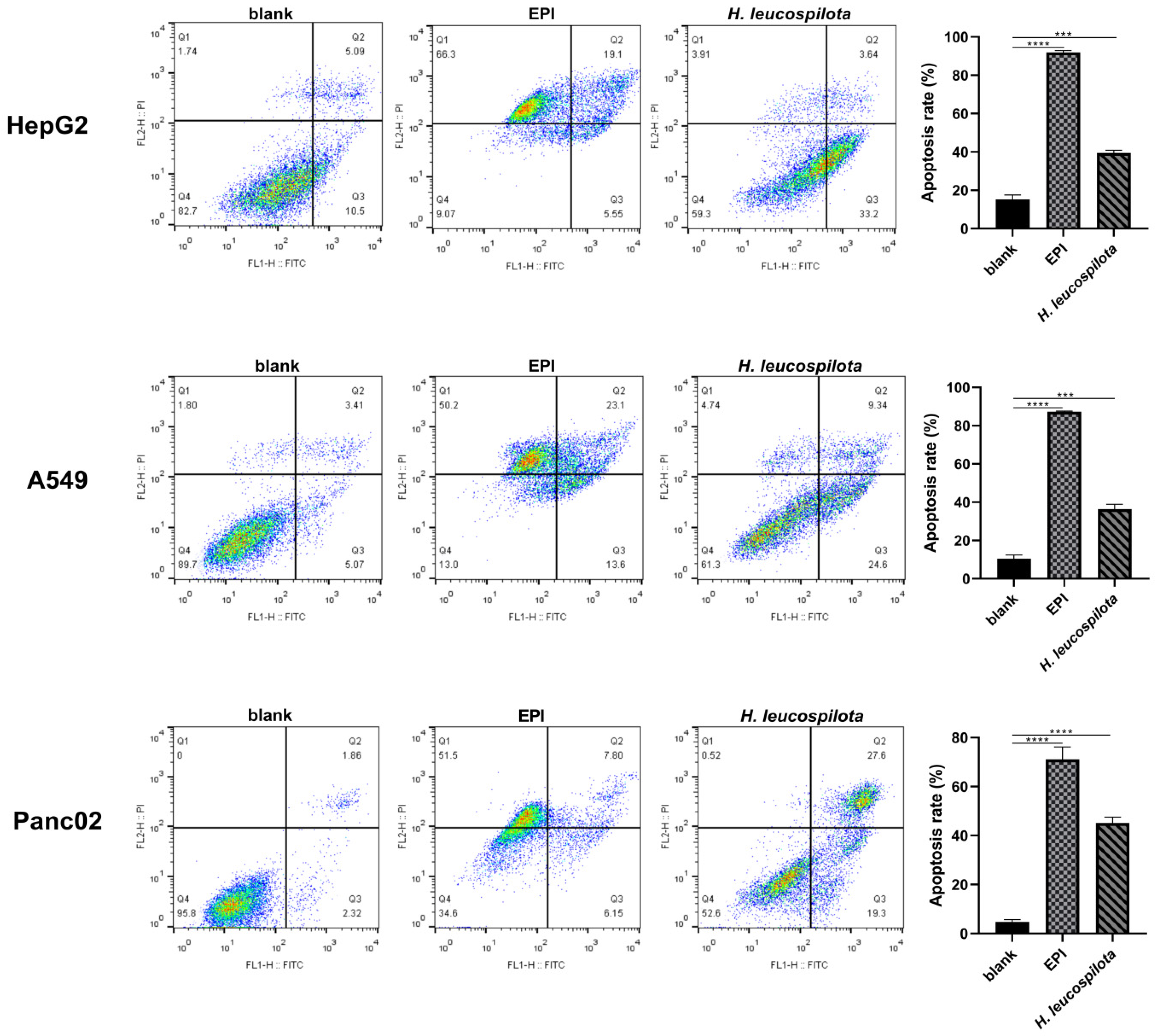
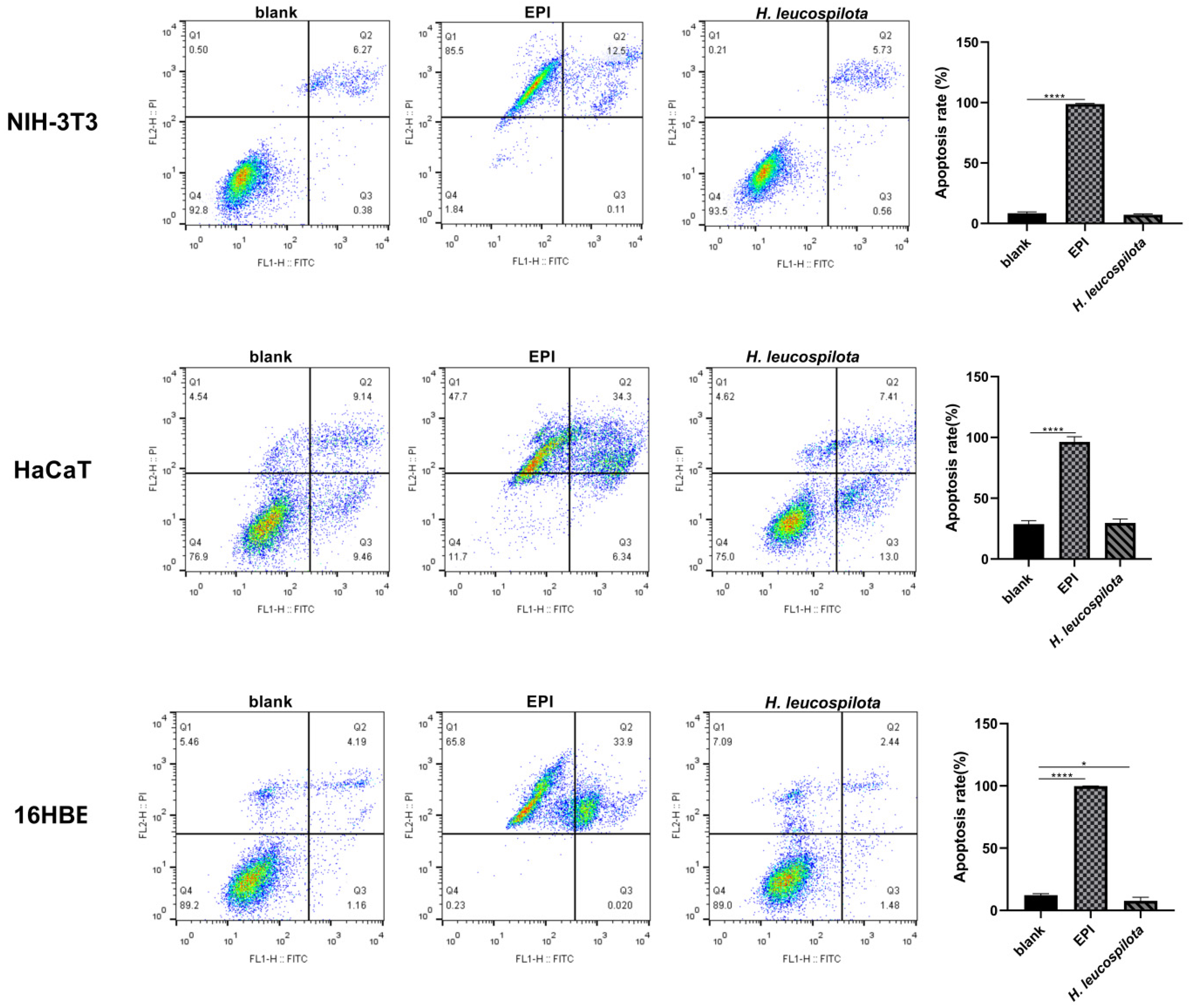
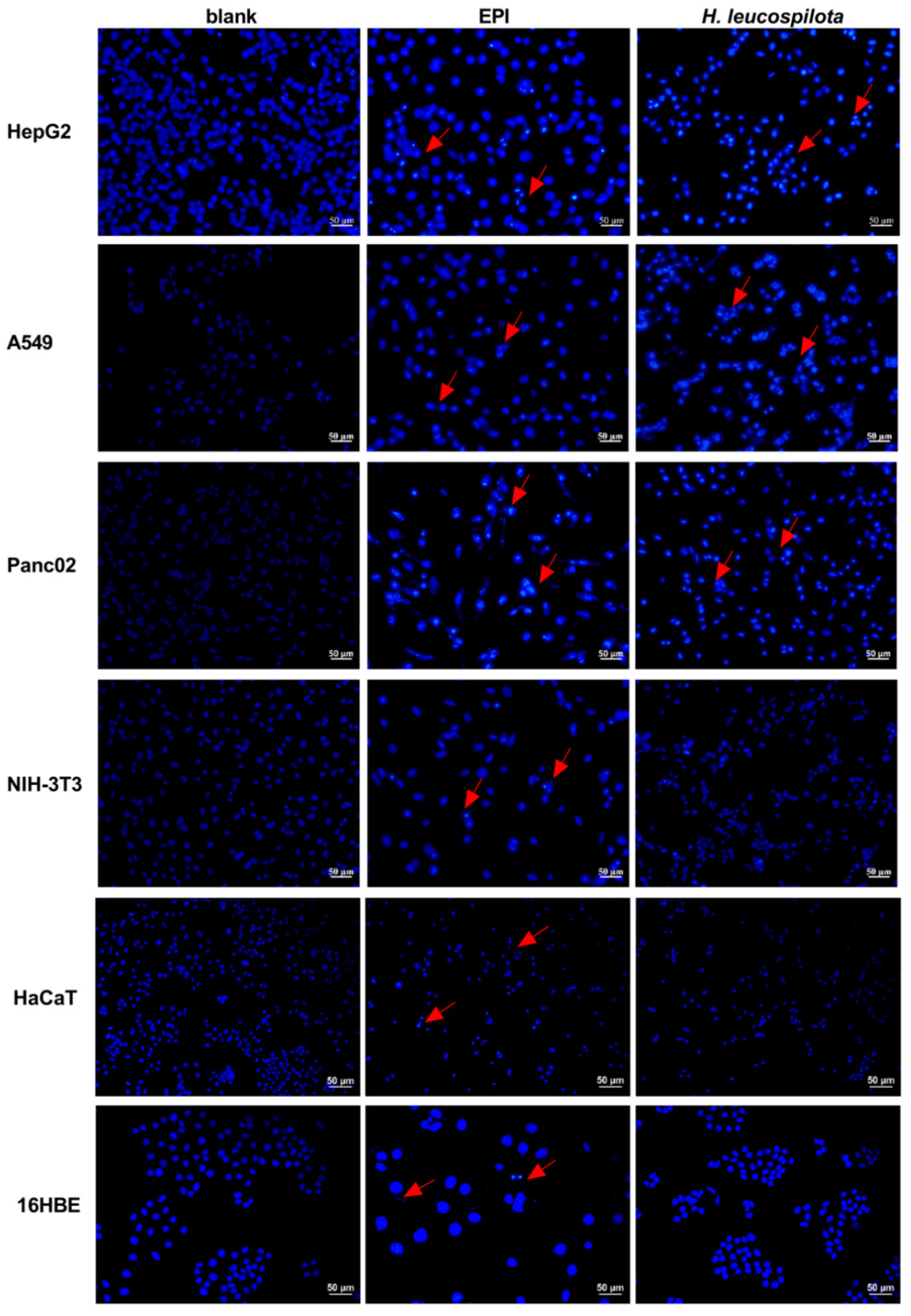
2.6. Cell Apoptosis and Morphology Assay
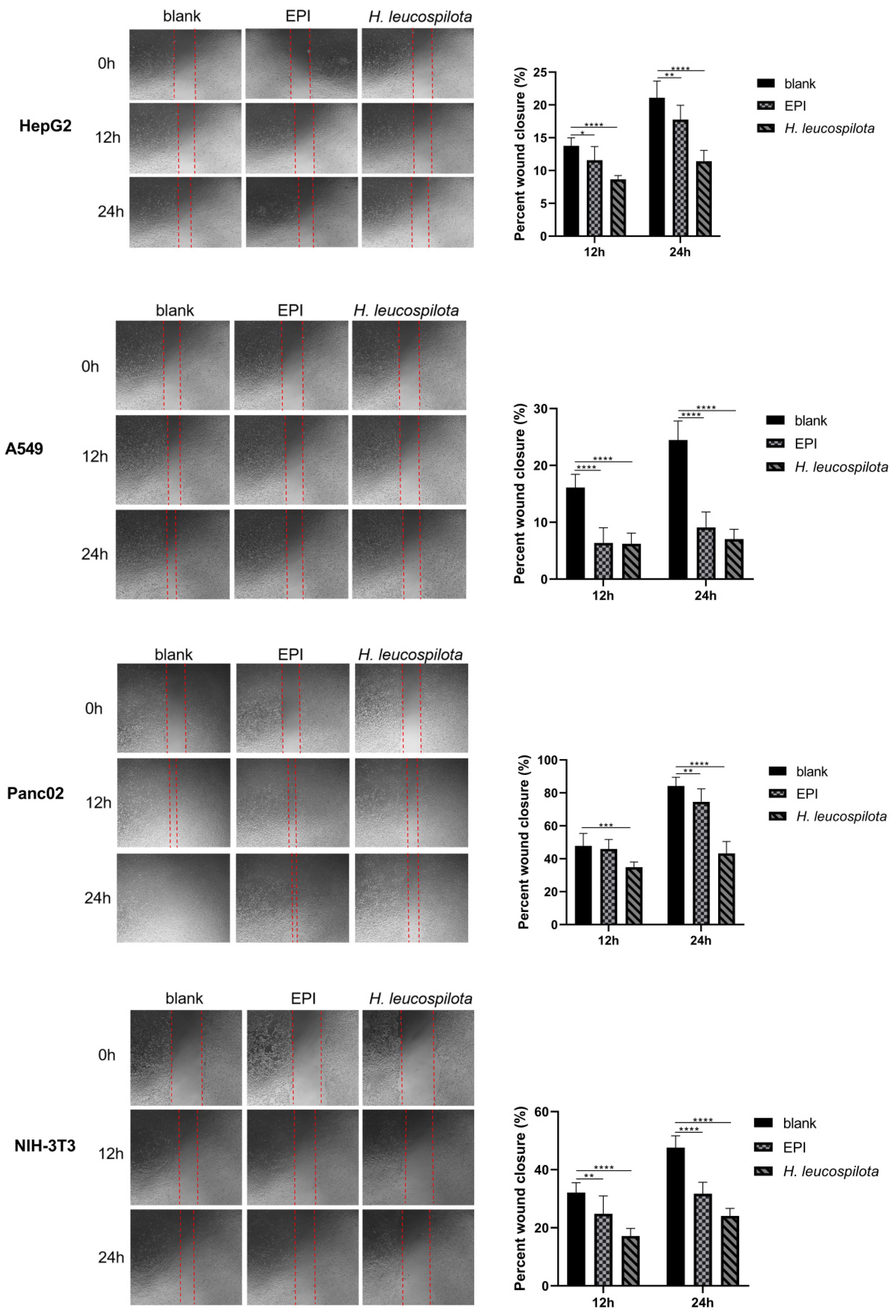
2.7. Cell Migration Assay
2.8. Statistical Analysis
3. Results
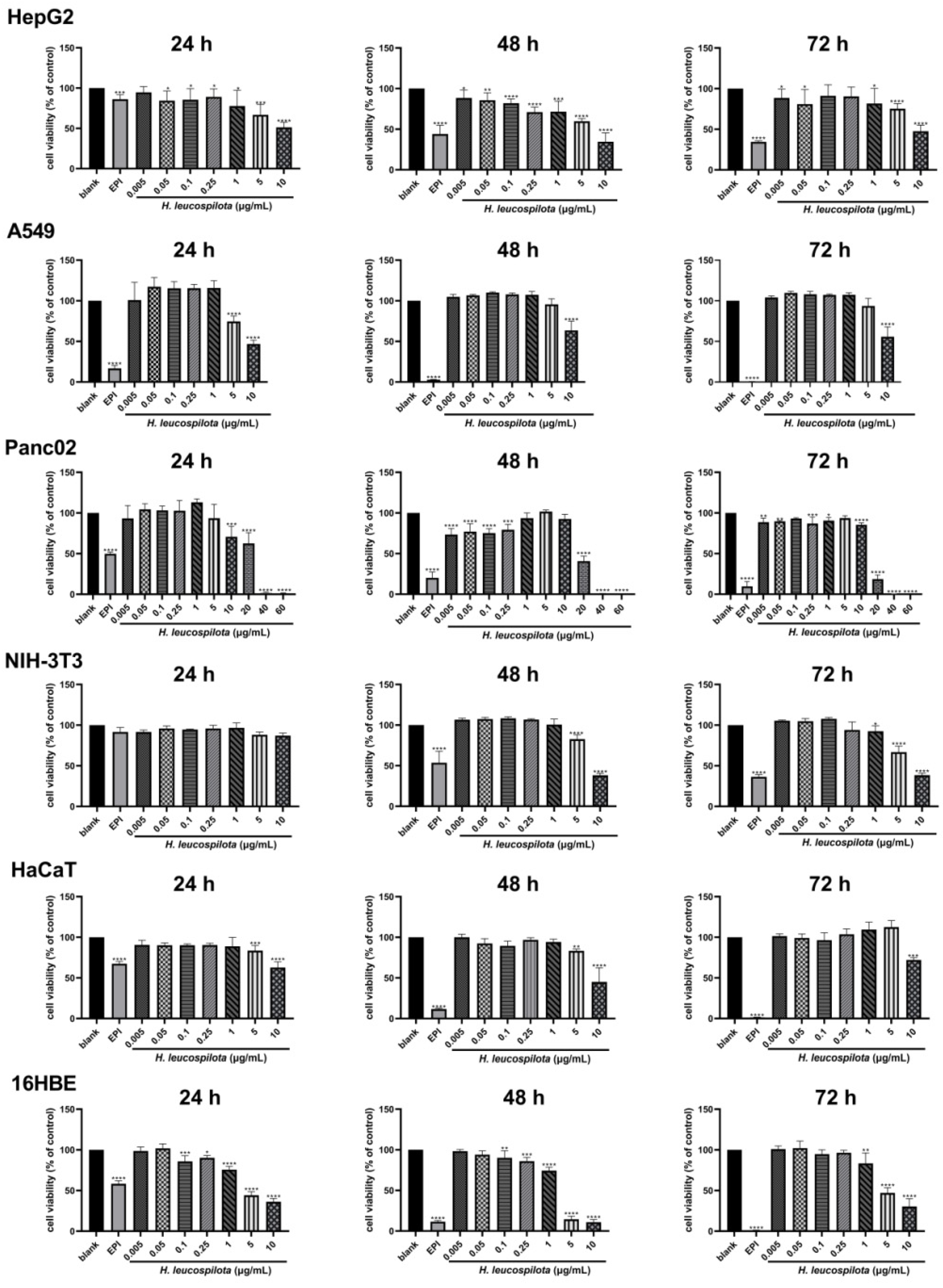
3.1. The Inhibitory Effects on Cell Viability
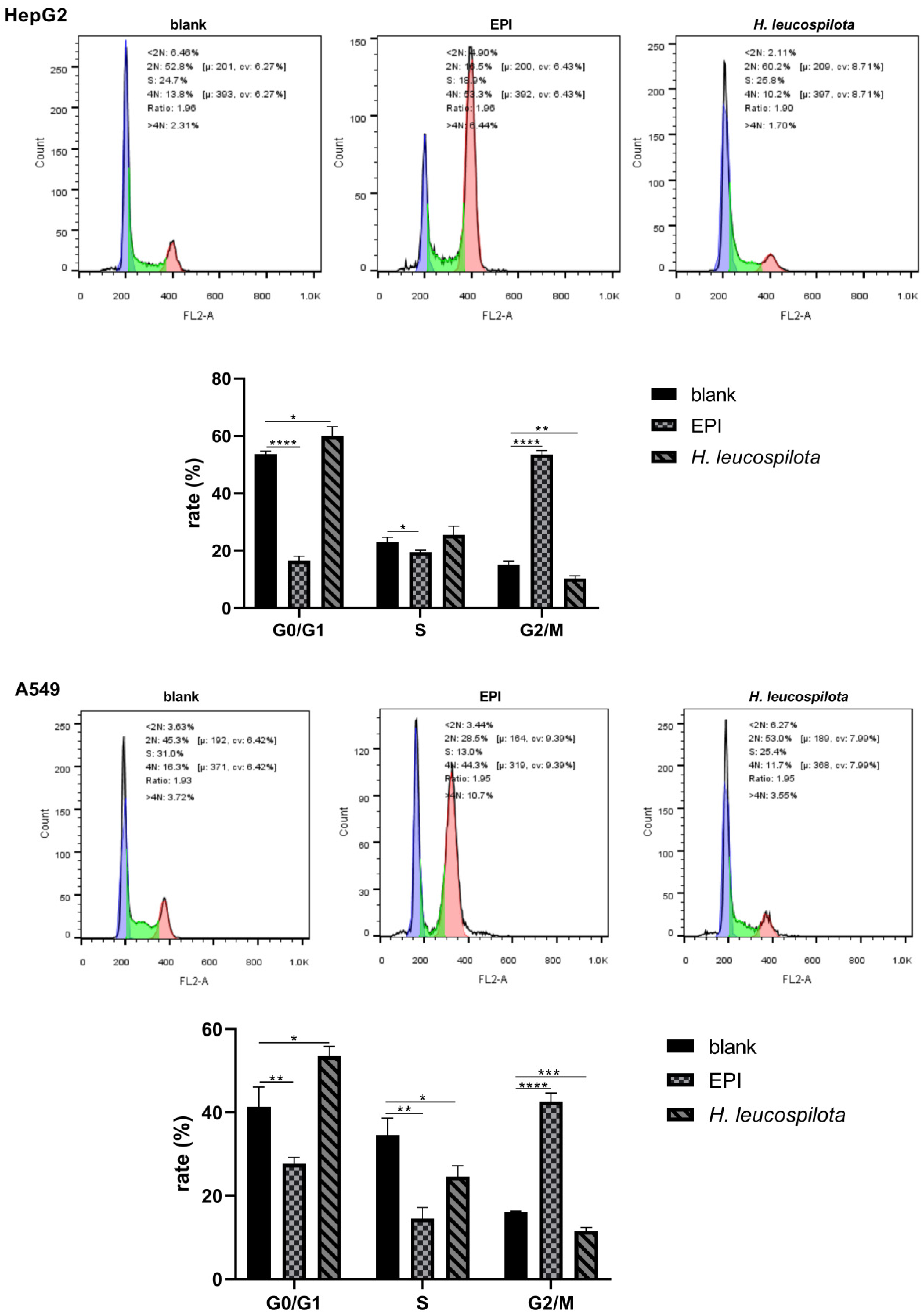
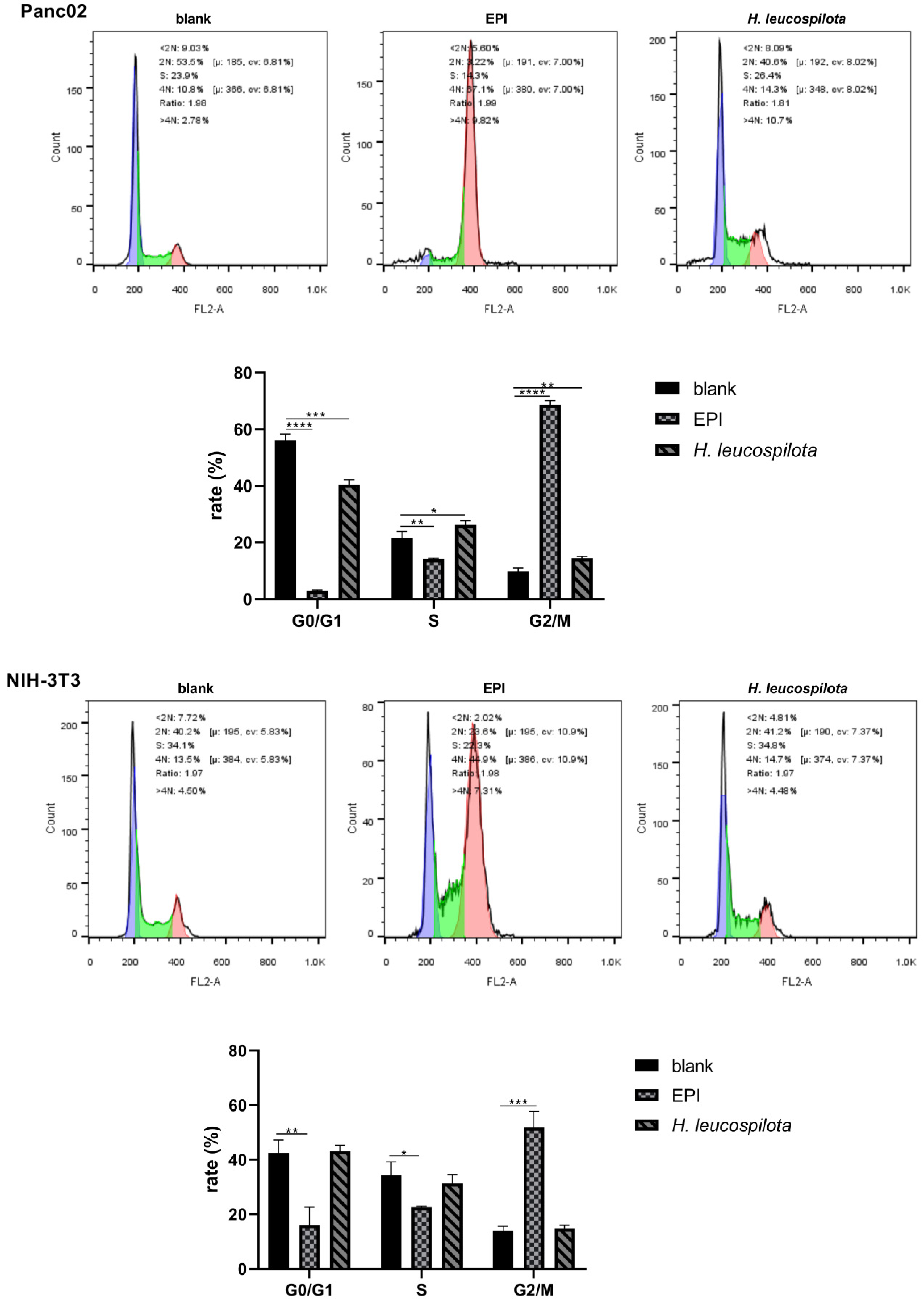
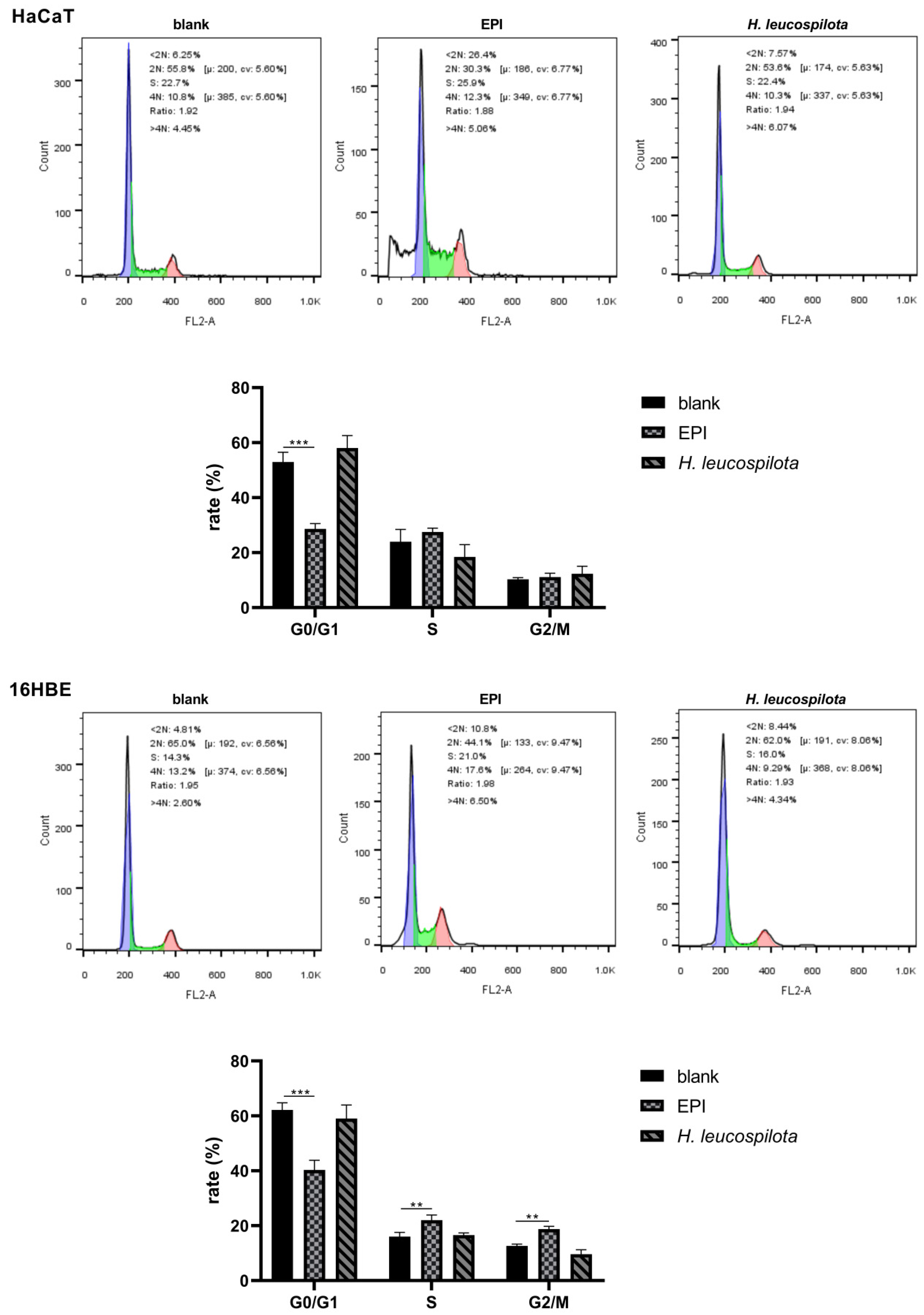
3.2. Effects of H. leucospilota Protein Treatment on the Distribution of Cell Cycle
3.3. Effects of H. leucospilota Protein Treatment on Inducing Cell Apoptosis and Nuclear Morphology Changes
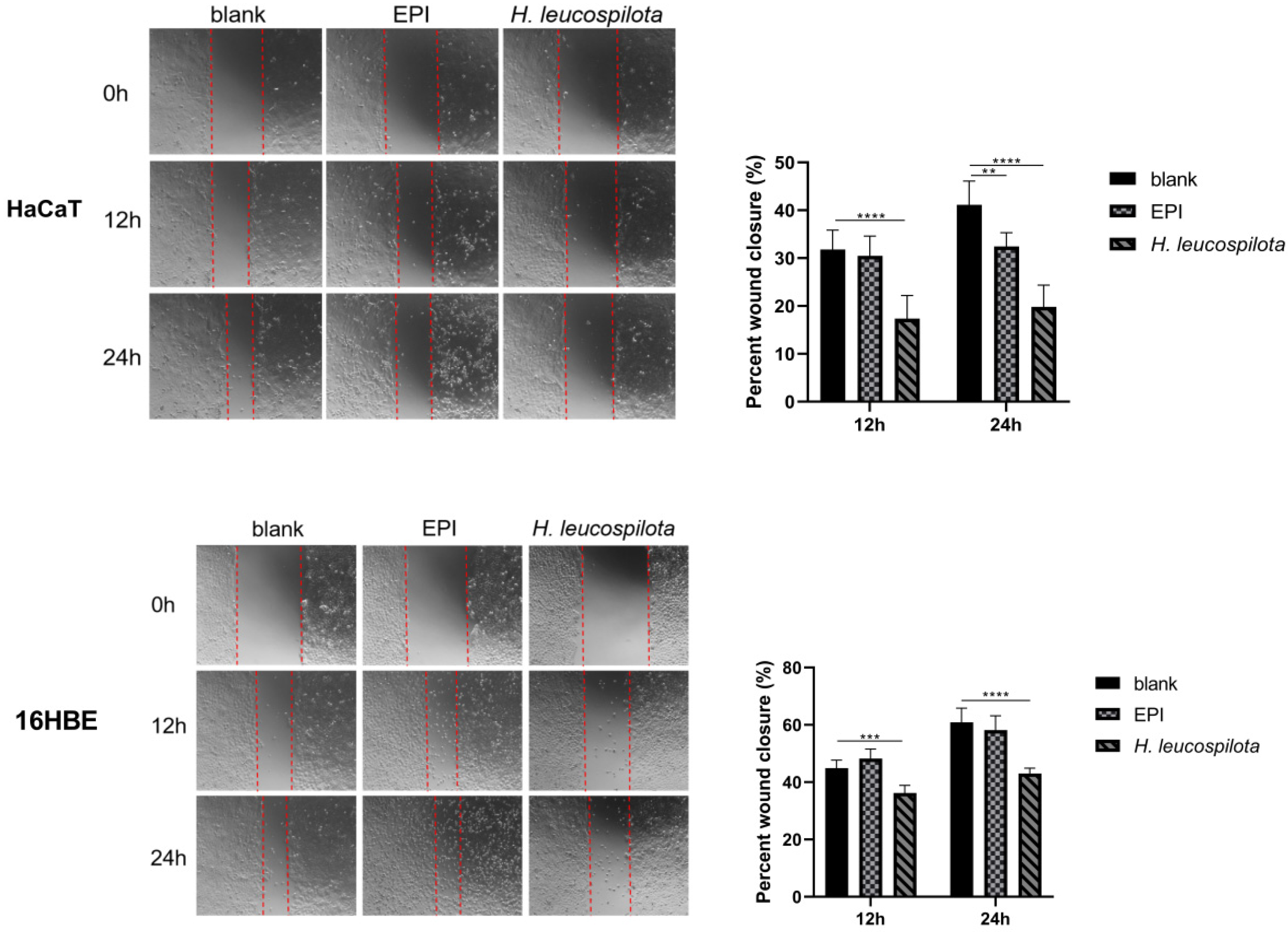
3.4. Effects of H. leucospilota Protein Treatment on Cell Migration
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Kimberly, D.M.; Hannah, E.F.; Ahmedin, J. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-M.; Hong, P.; Xu, W.W.; He, Q.-Y.; Li, B. Advances in targeted therapy for esophageal cancer. Signal Transduct. Target. Ther. 2020, 5, 229. [Google Scholar] [CrossRef]
- Hu, J.; Li, Y.; Li, H.; Shi, F.; Xie, L.; Zhao, L.; Tang, M.; Luo, X.; Jia, W.; Fan, J.; et al. Targeting Epstein-Barr virus oncoprotein LMP1-mediated high oxidative stress suppresses EBV lytic reactivation and sensitizes tumors to radiation therapy. Theranostics 2020, 10, 11921–11937. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.F.; Ying, C.R.; Kong, F.; Ying, H.; Du, C.; Huang, S.; Zhou, J.; Chen, J.; Sun, L.; Chen, X.; et al. Patterns of local-regional failure after primary intensity modulated radiotherapy for nasopharyngeal carcinoma. Radiat. Oncol. 2014, 9, 60. [Google Scholar] [CrossRef] [Green Version]
- Yun, C.W.; Kim, H.J.; Lee, S.H. Therapeutic Application of Diverse Marine-derived Natural Products in Cancer Therapy. Anticancer Res. 2019, 39, 5261–5284. [Google Scholar] [CrossRef] [Green Version]
- Khalifa, S.A.M.; Elias, N.; Farag, M.A.; Chen, L.; Saeed, A.; Hegazy, M.-E.F.; Moustafa, M.S.; El-Wahed, A.A.; Al-Mousawi, S.M.; Musharraf, S.G.; et al. Marine Natural Products: A Source of Novel Anticancer Drugs. Mar. Drugs 2019, 17, 491. [Google Scholar] [CrossRef] [Green Version]
- Wali, A.F.; Majid, S.; Rasool, S.; Shehada, S.B.; Abdulkareem, S.K.; Firdous, A.; Beigh, S.; Shakeel, S.; Mushtaq, S.; Akbar, I.; et al. Natural products against cancer: Review on phytochemicals from marine sources in preventing cancer. Saudi Pharm. J. 2019, 27, 767–777. [Google Scholar] [CrossRef]
- Nigam, M.; Suleria, H.A.R.; Farzaei, M.H.; Mishra, A.P. Marine anticancer drugs and their relevant targets: A treasure from the ocean. DARU J. Pharm. Sci. 2019, 27, 491–515. [Google Scholar] [CrossRef]
- Liang, X.; Luo, D.; Luesch, H. Advances in exploring the therapeutic potential of marine natural products. Pharmacol. Res. 2019, 147, 104373. [Google Scholar] [CrossRef]
- Bowling, J.J.; Kochanowska, A.J.; Kasanah, N.; Hamann, M.T. Nature’s bounty—Drug discovery from the sea. Expert Opin. Drug Discov. 2007, 2, 1505–1522. [Google Scholar] [CrossRef] [PubMed]
- Attoub, S.; Arafat, K.; Khalaf, T.; Sulaiman, S.; Iratni, R. Frondoside A Enhances the Anti-Cancer Effects of Oxaliplatin and 5-Fluorouracil on Colon Cancer Cells. Nutrients 2018, 10, 560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yun, S.-H.; Park, E.-S.; Shin, S.-W.; Na, Y.-W.; Han, J.-Y.; Jeong, J.-S.; Shastina, V.V.; Stonik, V.; Park, J.-I.; Kwak, J.-Y. Stichoposide C Induces Apoptosis through the Generation of Ceramide in Leukemia and Colorectal Cancer Cells and Shows In Vivo Antitumor Activity. Clin. Cancer Res. 2012, 18, 5934–5948. [Google Scholar] [CrossRef] [Green Version]
- Ampofo, E.; Später, T.; Nalbach, L.; Menger, M.D.; Laschke, M.W. The Marine-Derived Triterpenoid Frondoside A Inhibits Thrombus Formation. Mar. Drugs 2020, 18, 111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dyshlovoy, S.A.; Madanchi, R.; Hauschild, J.; Otte, K.; Alsdorf, W.H.; Schumacher, U.; Kalinin, V.I.; Silchenko, A.S.; Avilov, S.A.; Honecker, F.; et al. The marine triterpene glycoside frondoside A induces p53-independent apoptosis and inhibits autophagy in urothelial carcinoma cells. BMC Cancer 2017, 17, 23. [Google Scholar] [CrossRef] [Green Version]
- Dyshlovoy, S.A.; Menchinskaya, E.S.; Venz, S.; Rast, S.; Amann, K.; Hauschild, J.; von Amsberg, G.; Otte, K.; Kalinin, V.I.; Silchenko, A.S.; et al. The marine triterpene glycoside frondoside A exhibits activity in vitro and in vivo in prostate cancer. Int. J. Cancer 2016, 138, 2450–2465. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Zhu, X.; Jin, Q.; Sui, A.; Li, J.; Shen, L. Effects of Holothurian Glycosaminoglycan on the Sensitivity of Lung Cancer to Chemotherapy. Integr. Cancer Ther. 2020, 19, 1534735420911430. [Google Scholar] [CrossRef]
- Song, J.; Li, T.; Cheng, X.; Ji, X.; Gao, D.; Du, M.; Jiang, N.; Liu, X.; Mao, X. Sea cucumber peptides exert anti-inflammatory activity through suppressing NF-kappaB and MAPK and inducing HO-1 in RAW264.7 macrophages. Food Funct. 2016, 7, 2773–2779. [Google Scholar] [CrossRef]
- Tian, F.; Zhang, X.; Tong, Y.; Yi, Y.; Zhang, S.; Liping, L.; Sun, P.; Lin, L.; Ding, J. PE, a new sulfated saponin from sea cucumber, Exhibits anti-angiogenic and anti-tumor activities in vitro and in vivo. Cancer Biol. Ther. 2005, 4, 874–882. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Cai, C.; Chang, Y.; Zhang, F.; Linhardt, R.J.; Xue, C.; Li, G.; Yu, G. A novel structural fucosylated chondroitin sulfate from Holothuria Mexicana and its effects on growth factors binding and anticoagulation. Carbohydr. Polym. 2017, 181, 1160–1168. [Google Scholar] [CrossRef]
- La, M.-P.; Li, C.; Li, L.; Sun, P.; Tang, H.; Liu, B.-S.; Gong, W.; Han, H.; Yi, Y.-H.; Zhang, W. New Bioactive Sulfated Alkenes from the Sea Cucumber Apostichopus japonicus. Chem. Biodivers. 2012, 9, 1166–1171. [Google Scholar] [CrossRef] [PubMed]
- Olivera-Castillo, L.; Grant, G.; Kantún-Moreno, N.; Barrera-Pérez, H.A.; Montero, J.; Olvera-Novoa, M.A.; Carrillo-Cocom, L.M.; Acevedo, J.J.; Puerto-Castillo, C.; Solís, V.M.; et al. A Glycosaminoglycan-Rich Fraction from Sea Cucumber Isostichopus badionotus Has Potent Anti-Inflammatory Properties In Vitro and In Vivo. Nutrients 2020, 12, 1698. [Google Scholar] [CrossRef] [PubMed]
- Kareh, M.; El Nahas, R.; Al-Aaraj, L.; Al-Ghadban, S.; Naser Al Deen, N.; Saliba, N.; El-Sabban, M.; Talhouk, R. Anti-proliferative and anti-inflammatory activities of the sea cucumber Holothuria polii aqueous extract. SAGE Open Med. 2018, 6, 2050312118809541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, X.-Q.; Zhang, L.-Y.; Ding, L.; Shi, H.-H.; Xue, C.-H.; Zhang, T.-T.; Wang, Y.-M. Synergistic effect of sea cucumber saponins and EPA-enriched phospholipids on insulin resistance in high-fat diet-induced obese mice. Food Funct. 2019, 10, 3955–3964. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Y.; Hao, J.; Zhao, X.; Lang, Y.; Fan, F.; Cai, C.; Li, G.; Zhang, L.; Yu, G. In Vivo Anti-Cancer Mechanism of Low-Molecular-Weight Fucosylated Chondroitin Sulfate (LFCS) from Sea Cucumber Cucumaria frondosa. Molecules 2016, 21, 625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Q.; Xue, Y.; Wang, J.F.; Li, H.; Long, T.T.; Li, Z.; Wang, Y.-M.; Dong, P.; Xue, C.H. In vitro and in vivo anti-tumour activities of echinoside A and ds-echinoside A from Pear-sonothuria graeffei. J. Sci. Food Agric. 2012, 92, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wu, G.; Jiang, Y.; Li, B.; Feng, C.; Ge, Y.; Le, H.; Jiang, L.; Liu, H.; Shi, Y.; et al. Sea Cucumber Peptides Improved the Mitochondrial Capacity of Mice: A Potential Mechanism to Enhance Gluconeogenesis and Fat Catabolism during Exercise for Improved Antifatigue Property. Oxidative Med. Cell. Longev. 2020, 2020, 4604387. [Google Scholar] [CrossRef]
- Zheng, J.-L.; Wang, S.-S.; Shen, K.-P.; Chen, L.; Peng, X.; Chen, J.-F.; An, H.-M.; Hu, B. Ursolic acid induces apoptosis and anoikis in colorectal carcinoma RKO cells. BMC Complement. Med. Ther. 2021, 21, 52. [Google Scholar] [CrossRef]
- Cao, X.; Hou, J.; An, Q.; Assaraf, Y.G.; Wang, X. Towards the overcoming of anticancer drug resistance mediated by p53 mutations. Drug Resist. Updat. 2019, 49, 100671. [Google Scholar] [CrossRef]
- Yu, Q. Restoring p53-mediated apoptosis in cancer cells: New opportunities for cancer therapy. Drug Resist. Updat. 2006, 9, 19–25. [Google Scholar] [CrossRef]
- Ueda, K.; Cardarelli, C.; Gottesman, M.M.; Pastan, I. Expression of a full-length cDNA for the human “MDR1” gene confers resistance to colchicine, doxorubicin, and vinblastine. Proc. Natl. Acad. Sci. USA 1987, 84, 3004–3008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Q.; Cao, H.; Qi, X.; Li, H.; Ye, P.; Wang, Z.; Wang, D.; Sun, M.; Guo, H.C.Q. Research Progress in Reversal of Tumor Multi-drug Resistance via Natural Products. Anti-Cancer Agents Med. Chem. 2017, 17, 1466–1476. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Singh, J.V.; Bhagat, K.; Gulati, H.K.; Sanduja, M.; Kumar, N.; Kinarivala, N.; Sharma, S. Rational approaches, design strategies, structure activity relationship and mechanistic insights for therapeutic coumarin hybrids. Bioorg. Med. Chem. 2019, 27, 3477–3510. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Singh, A.; Sharma, S.; Sharma, R.; Singh, J.; Kinarivala, N.; Kunal, N.; Liou, J. P Tailored Quinolines Demonstrate Flexibility to Exert Antitumor Effects through Varied Mechanisms-A Me-dicinal Perspective. Anticancer Agents Med Chem. 2021, 21, 288–315. [Google Scholar] [CrossRef]
- Long, S.; Sousa, M.E.; Kijjoa, A.; Pinto, M.M.M. Marine Natural Products as Models to Circumvent Multidrug Resistance. Molecules 2016, 21, 892. [Google Scholar] [CrossRef] [Green Version]
- Fan, M.; Nath, A.K.; Tang, Y.; Choi, Y.-J.; Debnath, T.; Choi, E.-J.; Kim, E.-K. Investigation of the Anti-Prostate Cancer Properties of Marine-Derived Compounds. Mar. Drugs 2018, 16, 160. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, N.H.; Ta, Q.T.H.; Pham, Q.T.; Luong, T.N.H.; Phung, V.T.; Duong, T.-H.; Vo, V.G. Anticancer Activity of Novel Plant Extracts and Compounds from Adenosma bracteosum (Bonati) in Human Lung and Liver Cancer Cells. Molecules 2020, 25, 2912. [Google Scholar] [CrossRef]
- He, L.-X.; Zhang, Z.-F.; Sun, B.; Chen, Q.-H.; Liu, R.; Ren, J.-W.; Wang, J.-B.; Li, Y. Sea cucumber (Codonopsis pilosula) oligopeptides: Immunomodulatory effects based on stimulating Th cells, cytokine secretion and antibody production. Food Funct. 2016, 7, 1208–1216. [Google Scholar] [CrossRef]
- Wargasetia, T.L. Widodo Mechanisms of cancer cell killing by sea cucumber-derived compounds. Investig. New Drugs 2017, 35, 820–826. [Google Scholar] [CrossRef] [Green Version]
- Al Marzouqi, N.; Iratni, R.; Nemmar, A.; Arafat, K.; Al Sultan, M.A.; Yasin, J.; Collin, P.; Mester, J.; Adrian, T.E.; Attoub, S. Frondoside A inhibits human breast cancer cell survival, migration, invasion and the growth of breast tumor xenografts. Eur. J. Pharmacol. 2011, 668, 25–34. [Google Scholar] [CrossRef]
- Jing, H.; Lee, S.Y. NF-kappaB in cellular senescence and cancer treatment. Mol Cells. 2014, 37, 189–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siddiqui, W.A.; Ahad, A.; Ahsan, H. The mystery of BCL2 family: Bcl-2 proteins and apoptosis: An update. Arch. Toxicol. 2015, 89, 289–317. [Google Scholar] [CrossRef] [PubMed]
- Patil, M.; Pabla, N.; Dong, Z. Checkpoint kinase 1 in DNA damage response and cell cycle regulation. Cell. Mol. Life Sci. 2013, 70, 4009–4021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrade-Tomaz, M.; De Souza, I.; Rocha, C.R.R.; Gomes, L.R. The Role of Chaperone-Mediated Autophagy in Cell Cycle Control and Its Implications in Cancer. Cells 2020, 9, 2140. [Google Scholar] [CrossRef] [PubMed]
- Leal-Esteban, L.C.; Fajas, L. Cell cycle regulators in cancer cell metabolism. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165715. [Google Scholar] [CrossRef] [PubMed]
- Montalto, F.I.; De Amicis, F. Cyclin D1 in Cancer: A Molecular Connection for Cell Cycle Control, Adhesion and Invasion in Tumor and Stroma. Cells 2020, 9, 2648. [Google Scholar] [CrossRef]
- Cui, C.; Cui, N.; Wang, P.; Song, S.; Liang, H.; Ji, A. Neuroprotective effect of sulfated polysaccharide isolated from sea cucumber Stichopus ja-ponicus on 6-OHDA-induced death in SH-SY5Y through inhibition of MAPK and NF-kappaB and activation of PI3K/Akt signaling pathways. Biochem. Biophys. Res. Commun. 2016, 470, 375–383. [Google Scholar] [CrossRef]
- Wang, S.; Chen, M.; Yin, Y.; Storey, K.B. MiR-200-3p Is Potentially Involved in Cell Cycle Arrest by Regulating Cyclin A during Aestivation in Apostichopus japonicus. Cells 2019, 8, 843. [Google Scholar] [CrossRef] [Green Version]
- Wei, W.; Fan, X.-M.; Jia, S.-H.; Zhang, X.-P.; Zhang, Z.; Zhang, X.-J.; Zhang, J.-X.; Zhang, Y.-W. Sea Cucumber Intestinal Peptide Induces the Apoptosis of MCF-7 Cells by Inhibiting PI3K/AKT Pathway. Front. Nutr. 2021, 8, 3692. [Google Scholar] [CrossRef]
- Wargasetia, T.L.; Ratnawati, H.; Widodo, N.; Widyananda, M.H. Bioinformatics Study of Sea Cucumber Peptides as Antibreast Cancer Through Inhibiting the Activity of Overexpressed Protein (EGFR, PI3K, AKT1, and CDK4). Cancer Inform. 2021, 20, 11769351211031864. [Google Scholar] [CrossRef]
- Mao, J.; Zhang, Z.; Chen, Y.; Wu, T.; Fersht, V.; Jin, Y.; Meng, J.; Zhang, M. Sea cucumber peptides inhibit the malignancy of NSCLC by regulating miR-378a-5p targeted TUSC2. Food Funct. 2021, 12, 12362–12371. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Mishra, A.; Boschetti, C.; Lin, J.; Liu, Y.; Huang, H.; Kaminski, C.F.; Huang, Z.; Tunnacliffe, A.; Schierle, G.S.K. Sea Cucumber-Derived Peptides Alleviate Oxidative Stress in Neuroblastoma Cells and Improve Survival in C. elegans Exposed to Neurotoxic Paraquat. Oxidative Med. Cell. Longev. 2021, 2021, 8842926. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ru, R.; Guo, Y.; Mao, J.; Yu, Z.; Huang, W.; Cao, X.; Hu, H.; Meng, M.; Yuan, L. Cancer Cell Inhibiting Sea Cucumber (Holothuria leucospilota) Protein as a Novel Anti-Cancer Drug. Nutrients 2022, 14, 786. https://doi.org/10.3390/nu14040786
Ru R, Guo Y, Mao J, Yu Z, Huang W, Cao X, Hu H, Meng M, Yuan L. Cancer Cell Inhibiting Sea Cucumber (Holothuria leucospilota) Protein as a Novel Anti-Cancer Drug. Nutrients. 2022; 14(4):786. https://doi.org/10.3390/nu14040786
Chicago/Turabian StyleRu, Ruizhen, Yanzheng Guo, Juanxuan Mao, Zonghe Yu, Wen Huang, Xudong Cao, Huijian Hu, Minjie Meng, and Lihong Yuan. 2022. "Cancer Cell Inhibiting Sea Cucumber (Holothuria leucospilota) Protein as a Novel Anti-Cancer Drug" Nutrients 14, no. 4: 786. https://doi.org/10.3390/nu14040786
APA StyleRu, R., Guo, Y., Mao, J., Yu, Z., Huang, W., Cao, X., Hu, H., Meng, M., & Yuan, L. (2022). Cancer Cell Inhibiting Sea Cucumber (Holothuria leucospilota) Protein as a Novel Anti-Cancer Drug. Nutrients, 14(4), 786. https://doi.org/10.3390/nu14040786






