Dietary Omega-3 Polyunsaturated Fatty-Acid Supplementation Upregulates Protective Cellular Pathways in Patients with Type 2 Diabetes Exhibiting Improvement in Painful Diabetic Neuropathy
Abstract
:1. Introduction
2. Materials and Methods
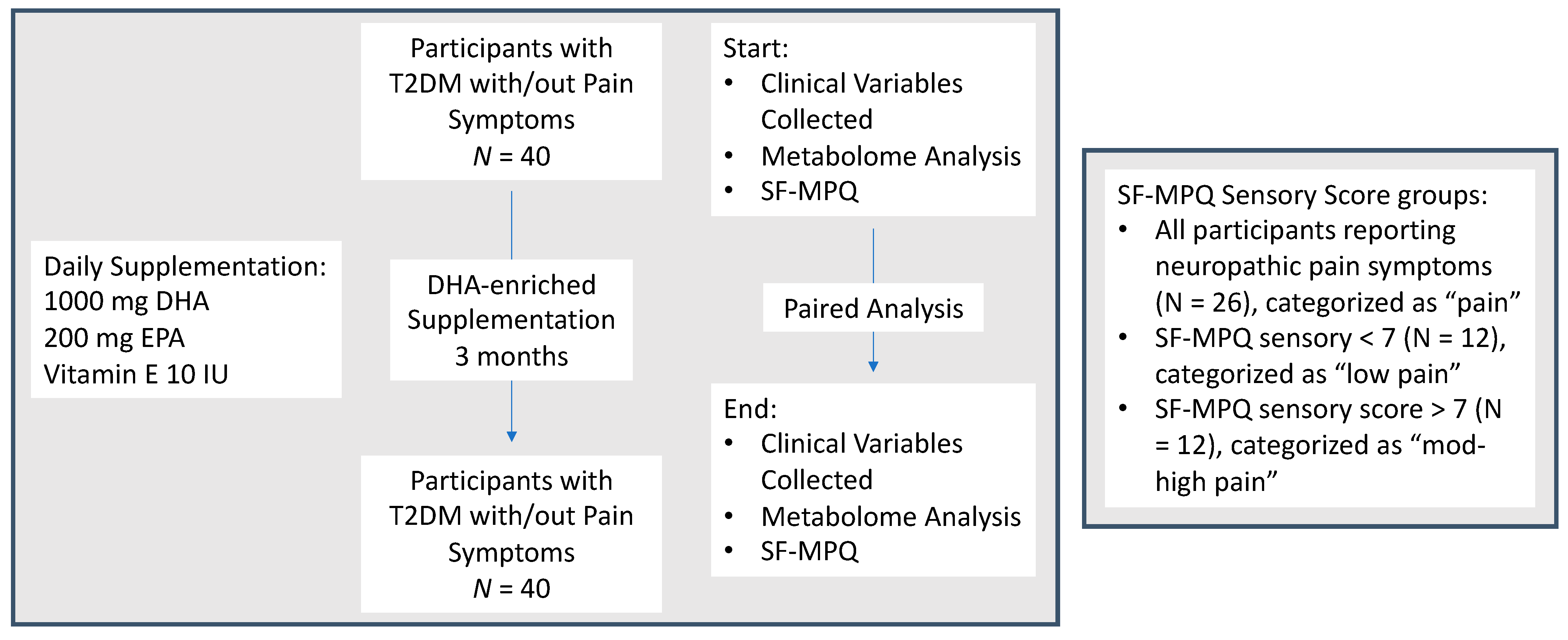
2.1. Study Design and Population
2.2. Data and Study Variables
2.3. Statistical Analysis
3. Results
3.1. Clinical Data of “En Balance-Plus” Participants Pre/Post-Omega-3 PUFAs Supplementation
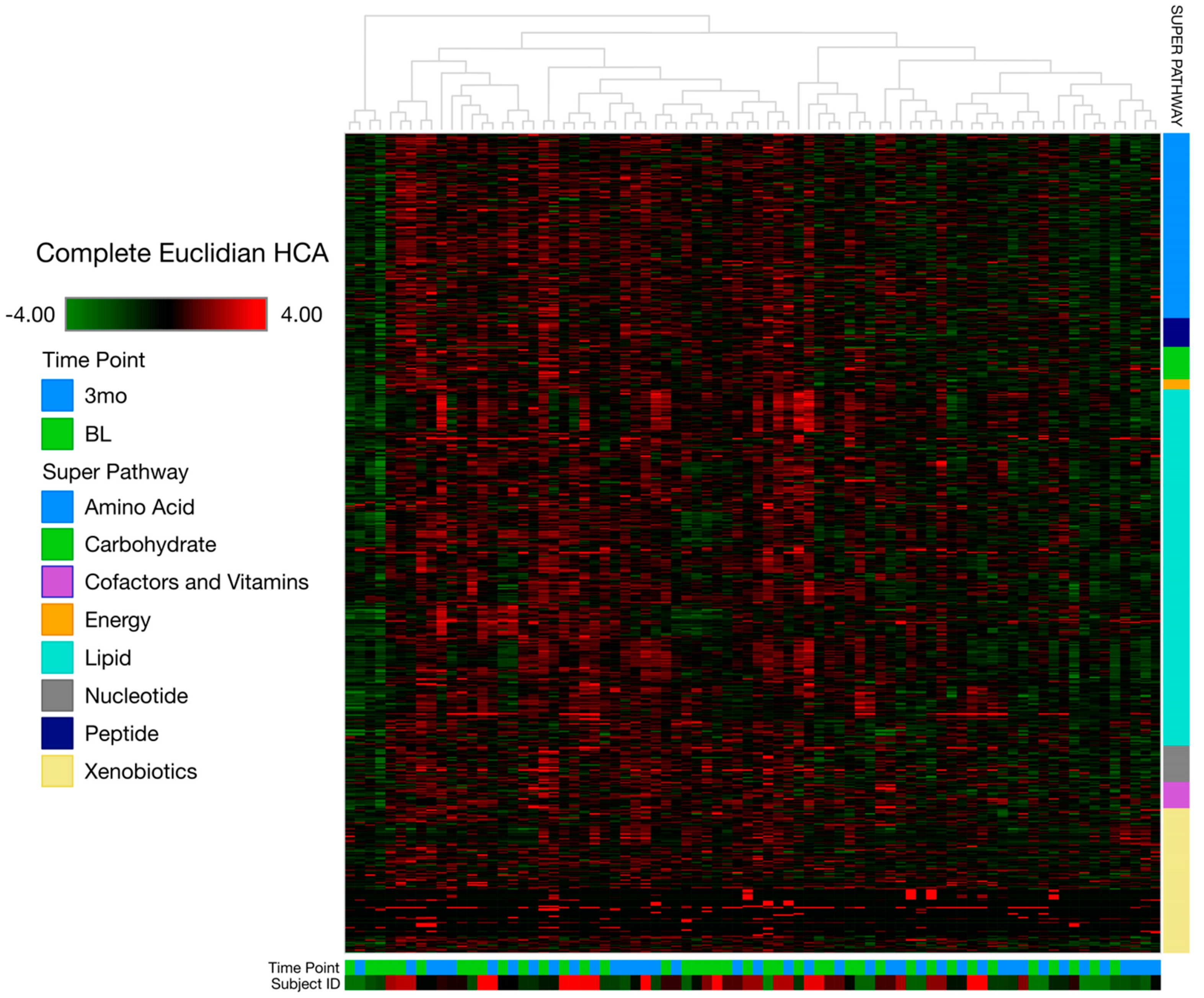
3.2. Metabolomics Data Analysis
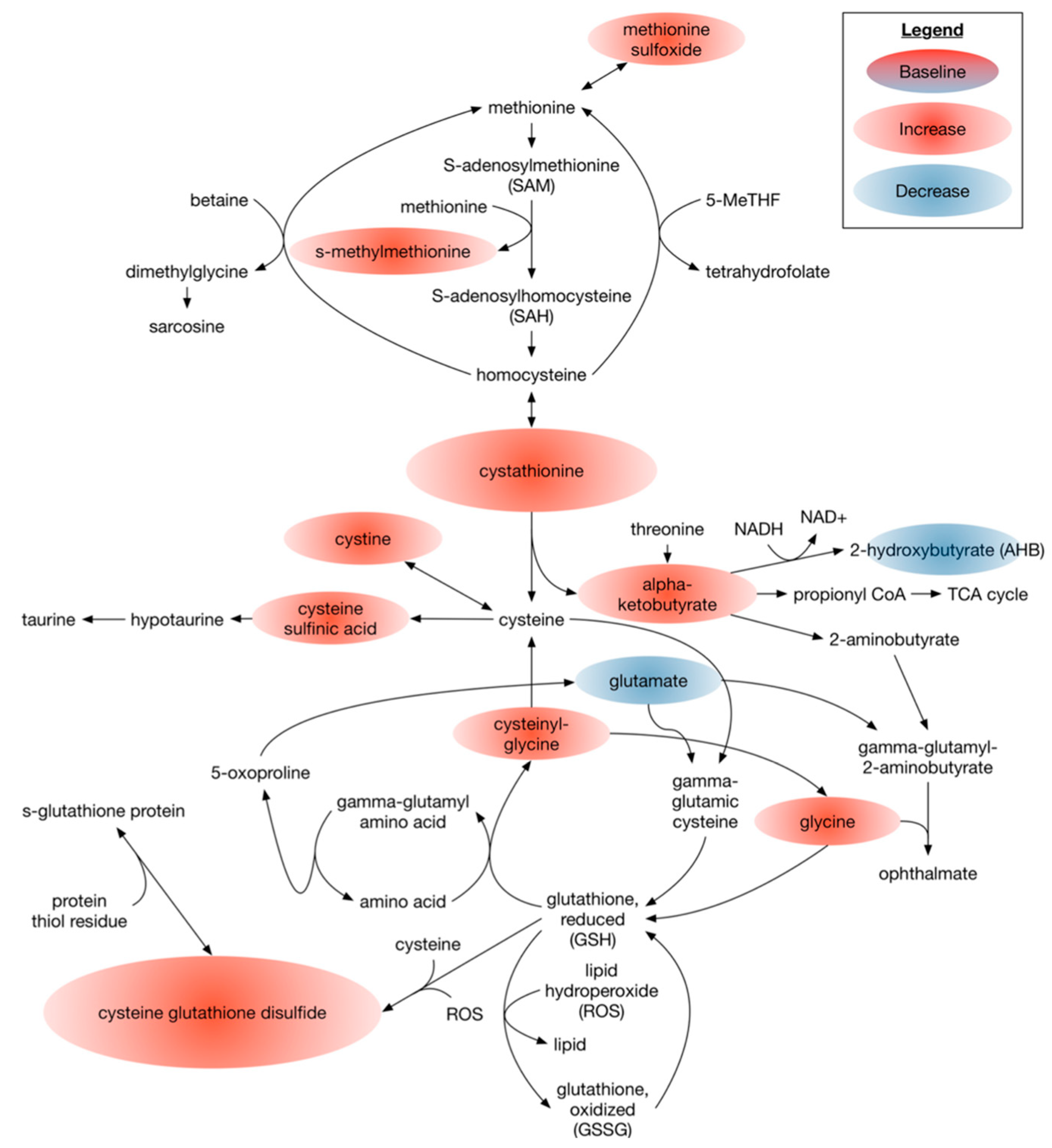
3.2.1. Omega 3 PUFAs Effect on Metabolites Associated with the Overall Cellular Oxidative State
3.2.2. Omega-3 PUFAs Effects on Biomarkers for Neurotoxicity
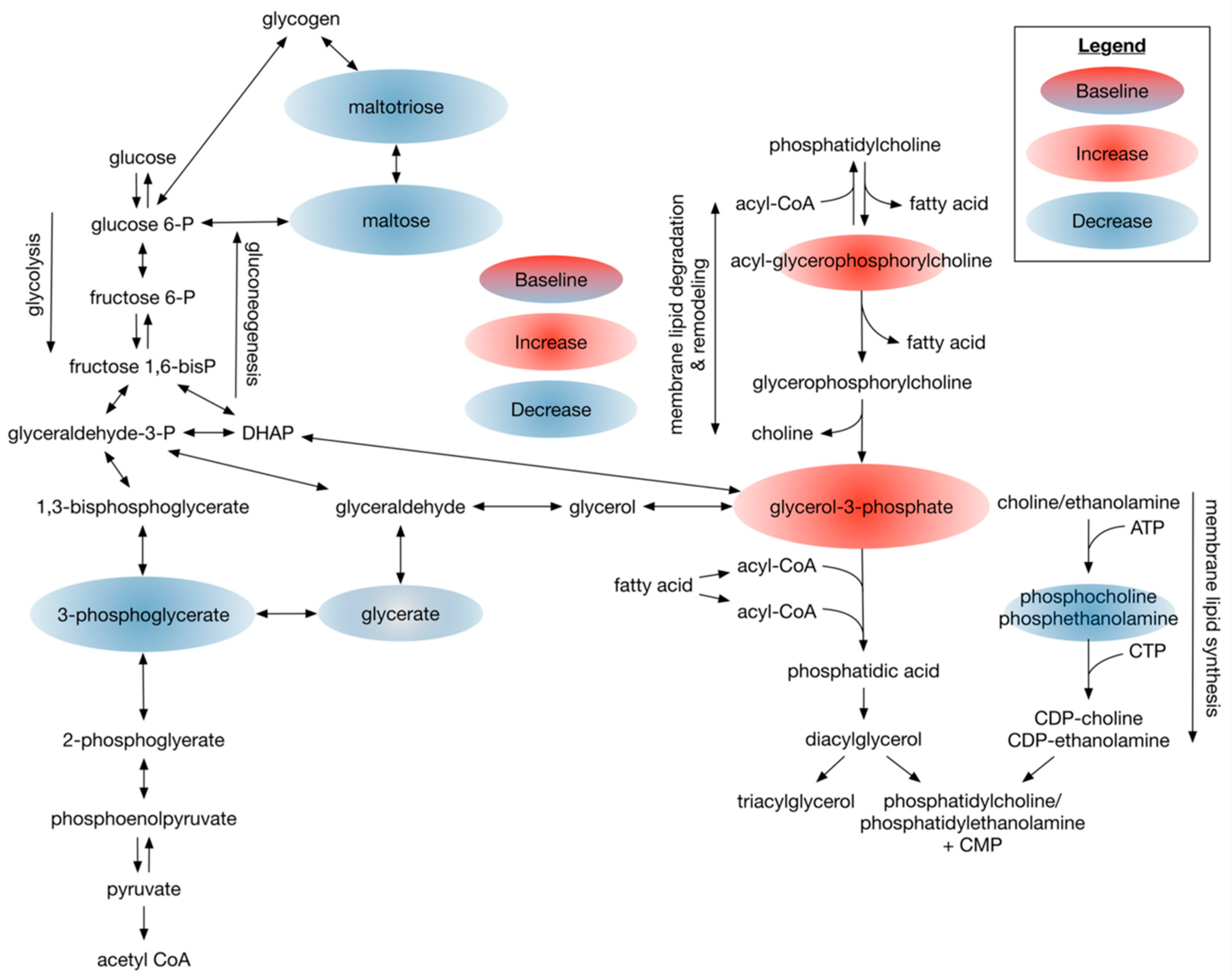
3.2.3. Dietary Omega-3 Supplementation Regulates Phospholipid Profiles in Plasma of Patients with Type 2 Diabetes
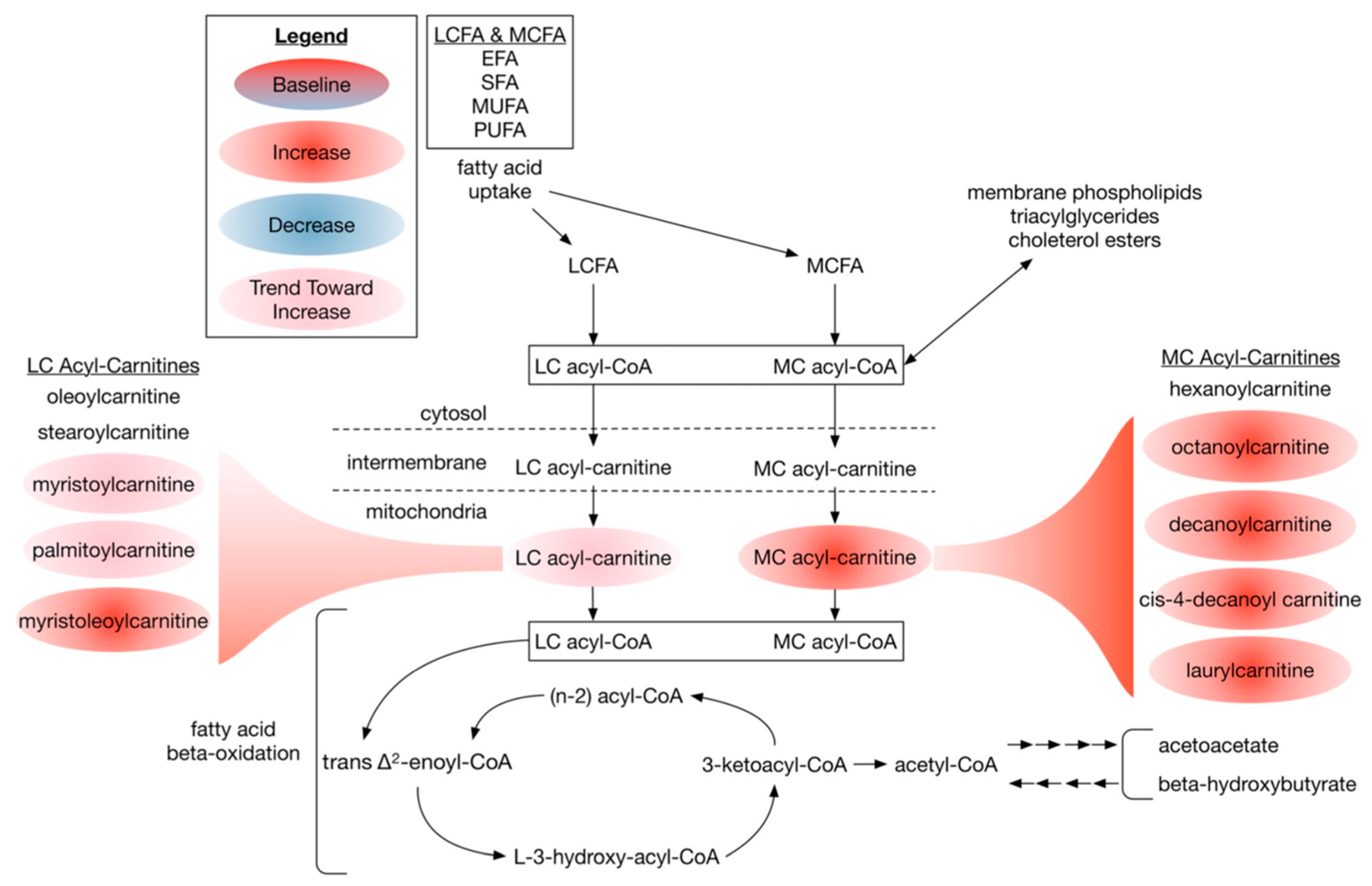
3.2.4. Dietary DHA-Enriched Supplementation Increases Acylcarnitine Species in Participants Plasma
3.3. Ingenuity Pathway Analysis (IPA)
3.4. The Associations between Top Factors Contributing to Group Separation Per RF and SF-MPQ Sensory Score
4. Discussion
4.1. Overall Metabolome Indicates Targeted Metabolomic Changes
4.2. Dietary DHA-Enriched Supplementation Leads to Improved Antioxidant Metabolic Plasma Profiles of Participants with Type 2 Diabetes
4.3. Dietary DHA-Enriched Supplementation Modulates Circulating Excitotoxic Amino Acids
4.4. Dietary DHA-Enriched Supplementation Changes Phospholipid Composition and Increases Acyl-Carnitine Levels
4.5. Top Feature Importance Metabolites Correlate with SF-MPQ Sensory Score
4.6. Potential Uses of Omega-3 Intervention beyond Pain
4.7. Study Limitations and Strengths
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jensen, T.S.; Backonja, M.M.; Jiménez, S.H.; Tesfaye, S.; Valensi, P.; Ziegler, D. New perspectives on the management of diabetic peripheral neuropathic pain. Diabetes Vasc. Dis. Res. 2006, 3, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Sloan, G.; Shillo, P.; Selvarajah, D.; Wu, J.; Wilkinson, I.D.; Tracey, I.; Anand, P.; Tesfaye, S. A new look at painful diabetic neuropathy. Diabetes Res. Clin. Pract. 2018, 144, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.Y.; Jiang, A.J.; Ma, J.L.; Wang, F.J.; Shen, G.M. Understanding the signaling pathways related to the mechanism and treatment of diabetic peripheral neuropathy. Endocrinology 2019, 160, 2119–2127. [Google Scholar] [CrossRef]
- Rosenberger, D.C.; Blechschmidt, V.; Timmerman, H.; Wolff, A.; Treede, R.D. Challenges of neuropathic pain: Focus on diabetic neuropathy. J. Neural. Transm. 2020, 127, 589–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertea, M.; Rütti, M.F.; Othman, A.; Marti-Jaun, J.; Hersberger, M.; von Eckardstein, A.; Hornemann, T. Deoxysphingoid bases as plasma markers in Diabetes mellitus. Lipids Health Dis. 2010, 9, 84. [Google Scholar] [CrossRef] [Green Version]
- Bictash, M.; Ebbels, T.M.; Chan, Q.; Loo, R.L.; Yap, I.K.; Brown, I.J.; De Iorio, M.; Daviglus, M.L.; Holmes, E.; Stamler, J.; et al. Opening up the "Black Box": Metabolic phenotyping and metabolome-wide association studies in epidemiology. J. Clin. Epidemiol. 2010, 63, 970–979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Figueroa, J.D.; Cordero, K.; Serrano-Illan, M.; Almeyda, A.; Baldeosingh, K.; Almaguel, F.G.; De Leon, M. Metabolomics uncovers dietary omega-3 fatty acid-derived metabolites implicated in anti-nociceptive responses after experimental spinal cord injury. Neuroscience 2013, 255, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Beger, R.D.; Dunn, W.; Schmidt, M.A.; Gross, S.S.; Kirwan, J.A.; Cascante, M.; Brennan, L.; Wishart, D.S.; Oresic, M.; Hankemeier, T.; et al. Metabolomics enables precision medicine: “A White Paper, Community Perspective”. Metabolomics 2016, 12, 149. [Google Scholar] [CrossRef] [Green Version]
- Dunn, W.B.; Lin, W.; Broadhurst, D.; Begley, P.; Brown, M.; Zelena, E.; Vaughan, A.A.; Halsall, A.; Harding, N.; Knowles, J.D.; et al. Molecular phenotyping of a UK population: Defining the human serum metabolome. Metabolomics 2015, 11, 9–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durán, A.M.; Salto, L.M.; Câmara, J.; Basu, A.; Paquien, I.; Beeson, W.L.; Firek, A.; Cordero-MacIntyre, Z.; De León, M. Effects of omega-3 polyunsaturated fatty-acid supplementation on neuropathic pain symptoms and sphingosine levels in Mexican-Americans with type 2 diabetes. Diabetes Metab. Syndr. Obes. 2019, 12, 109–120. [Google Scholar] [CrossRef] [Green Version]
- Yorek, M.A. The potential role of fatty acids in treating diabetic neuropathy. Curr. Diab. Rep. 2018, 18, 86. [Google Scholar] [CrossRef] [PubMed]
- Chitre, N.M.; Moniri, N.H.; Murnane, K.S. Omega-3 fatty acids as druggable therapeutics for neurodegenerative disorders. CNS Neurol. Disord.-Drug Targets 2019, 18, 735–749. [Google Scholar] [CrossRef]
- Almaguel, F.G.; Liu, J.-W.; Pacheco, F.J.; Casiano, C.A.; De Leon, M. Activation and reversal of lipotoxicity in PC12 and rat cortical cells following exposure to palmitic acid. J. Neurosci. Res. 2008, 87, 1207–1218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Figueroa, J.D.; de Leon, M. Neurorestorative targets of dietary long-chain omega-3 fatty acids in neurological injury. Mol. Neurobiol. 2014, 50, 197–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Figueroa, J.; Cordero, K.; Baldeosingh, K.; Torrado, A.I.; Walker, R.L.; Miranda, J.D.; De Leon, M. Docosahexaenoic acid pretreatment confers protection and functional improvements after acute spinal cord injury in adult rats. J. Neurotrauma 2012, 29, 551–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Descorbeth, M.; Figueroa, K.; Serrano-Illán, M.; De León, M. Protective effect of docosahexaenoic acid on lipotoxicity-mediated cell death in Schwann cells: Implication of PI3K/AKT and mTORC2 pathways. Brain Behav. 2018, 8, e01123. [Google Scholar] [CrossRef] [Green Version]
- Silva, R.V.; Oliveira, J.T.; Santos, B.L.R.; Dias, F.C.; Martinez, A.M.B.; Lima, C.K.F.; Miranda, A.L.P. Long-chain omega-3 fatty acids supplementation accelerates nerve regeneration and prevents neuropathic pain behavior in mice. Front. Pharmacol. 2017, 8, 723. [Google Scholar] [CrossRef] [Green Version]
- Lewis, E.J.; Lovblom, L.E.; Cisbani, G.; Chen, D.K.; Bazinet, R.P.; Wolever, T.M.; Perkins, B.A.; Bril, V. Baseline omega-3 level is associated with nerve regeneration following 12-months of omega-3 nutrition therapy in patients with type 1 diabetes. J. Diabetes Complicat. 2021, 35, 107798. [Google Scholar] [CrossRef]
- Lewis, E.J.; Perkins, B.A.; Lovblom, L.E.; Bazinet, R.P.; Wolever, T.M.; Bril, V. Effect of omega-3 supplementation on neuropathy in type 1 diabetes: A 12-month pilot trial. Neurology 2017, 88, 2294–2301. [Google Scholar] [CrossRef]
- Britten-Jones, A.C.; Kamel, J.T.; Roberts, L.J.; Braat, S.; Craig, J.P.; MacIsaac, R.J.; Downie, L.E. Investigating the neuroprotective effect of oral omega-3 fatty acid supplementation in type 1 diabetes (nPROOFS1): A randomized placebo-controlled trial. Diabetes 2021, 70, 1794–1806. [Google Scholar] [CrossRef]
- Tesfaye, S.; Boulton, A.J.; Dickenson, A.H. Mechanisms and management of diabetic painful distal symmetrical polyneuropathy. Diabetes Care 2013, 36, 2456–2465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lohman, T.; Roache, A.; Martorell, R. Anthropometric standardization reference manual. Med. Sci. Sports Exerc. 1992, 24, 952. [Google Scholar] [CrossRef] [Green Version]
- Figueroa, J.D.; Cordero, K.; Llán, M.S.; De Leon, M. Dietary omega-3 polyunsaturated fatty acids improve the neurolipidome and restore the DHA status while promoting functional recovery after experimental spinal cord injury. J. Neurotrauma 2013, 30, 853–868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeHaven, C.D.; Evans, A.M.; Dai, H.; Lawton, K.A. Organization of GC/MS and LC/MS metabolomics data into chemical libraries. J. Cheminform. 2010, 2, 9. [Google Scholar] [CrossRef] [Green Version]
- Wishart, D.S.; Feunang, Y.D.; Marcu, A.; Guo, A.C.; Liang, K.; Vázquez-Fresno, R.; Sajed, T.; Johnson, D.; Li, C.; Karu, N.; et al. HMDB 4.0: The human metabolome database for 2018. Nucleic Acids Res. 2018, 46, D608–D617. [Google Scholar] [CrossRef]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef] [Green Version]
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.; Bourque, G.; Wishart, D.S.; Xia, J. MetaboAnalyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018, 46, W486–W494. [Google Scholar] [CrossRef] [Green Version]
- Yowtak, J.; Lee, K.Y.; Kim, H.Y.; Wang, J.; Chung, K.; Chung, J.M. Reactive oxygen species contribute to neuropathic pain by reducing spinal GABA release. Pain 2011, 152, 844–852. [Google Scholar] [CrossRef] [Green Version]
- Ziegler, D.; Sohr, C.G.; Nourooz-Zadeh, J. Oxidative stress and antioxidant defense in relation to the severity of diabetic polyneuropathy and cardiovascular autonomic neuropathy. Diabetes Care 2004, 27, 2178–2183. [Google Scholar] [CrossRef] [Green Version]
- Low, P.A.; Nickander, K.K.; Tritschler, H.J. The roles of oxidative stress and antioxidant treatment in experimental diabetic neuropathy. Diabetes 1997, 46 (Suppl. S2), S38–S42. [Google Scholar] [CrossRef]
- Murakami, K.; Takahito, K.; Ohtsuka, Y.; Fujiwara, Y.; Shimada, M.; Kawakami, Y. Impairment of glutathione metabolism in erythrocytes from patients with diabetes mellitus. Metabolism 1989, 38, 753–758. [Google Scholar] [CrossRef]
- Memisoğullari, R.; Taysi, S.; Bakan, E.; Capoglu, I. Antioxidant status and lipid peroxidation in type II diabetes mellitus. Cell Biochem. Funct. 2003, 21, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Martin-Lorenzo, M.; Gonzalez-Calero, L.; Ramos-Barron, A.; Sanchez-Niño, M.D.; Gomez-Alamillo, C.; García-Segura, J.M.; Ortiz, A.; Arias, M.; Vivanco, F.; Alvarez-Llamas, G. Urine metabolomics insight into acute kidney injury point to oxidative stress disruptions in energy generation and H2S availability. J. Mol. Med. 2017, 95, 1399–1409. [Google Scholar] [CrossRef]
- Reyes, R.C.; Brennan, A.M.; Shen, Y.; Baldwin, Y.; Swanson, R.A. Activation of neuronal NMDA receptors induces superoxide-mediated oxidative stress in neighboring neurons and astrocytes. J. Neurosci. 2012, 32, 12973–12978. [Google Scholar] [CrossRef] [Green Version]
- Choi, D.W.; Koh, J.Y.; Peters, S. Pharmacology of glutamate neurotoxicity in cortical cell culture: Attenuation by NMDA antagonists. J. Neurosci. 1988, 8, 185–196. [Google Scholar] [CrossRef]
- Mark, L.P.; Prost, R.W.; Ulmer, J.L.; Smith, M.M.; Daniels, D.L.; Strottmann, J.M.; Brown, W.D.; Hacein-Bey, L. Pictorial review of glutamate excitotoxicity: Fundamental concepts for neuroimaging. AJNR Am. J. Neuroradiol. 2001, 22, 1813–1824. [Google Scholar]
- Jang, J.; Kim, D.-W.; Nam, T.S.; Paik, K.S.; Leem, J. Peripheral glutamate receptors contribute to mechanical hyperalgesia in a neuropathic pain model of the rat. Neuroscience 2004, 128, 169–176. [Google Scholar] [CrossRef]
- Weijers, R.N. Membrane flexibility, free fatty acids, and the onset of vascular and neurological lesions in type 2 diabetes. J. Diabetes Metab. Disord. 2015, 15, 13. [Google Scholar] [CrossRef] [Green Version]
- Sima, A.A.; Calvani, M.; Mehra, M.; Amato, A.; Acetyl-L-Carnitine Study Group. Acetyl-L-carnitine improves pain, nerve regeneration, and vibratory perception in patients with chronic diabetic neuropathy: An analysis of two randomized placebo-controlled trials. Diabetes Care 2005, 28, 89–94. [Google Scholar] [CrossRef] [Green Version]
- McDougle, D.R.; Watson, J.E.; Abdeen, A.; Adili, R.; Caputo, M.P.; Krapf, J.E.; Johnson, R.W.; Kilian, K.A.; Holinstat, M.; Das, A. Anti-inflammatory ω-3 endocannabinoid epoxides. Proc. Natl. Acad. Sci. USA 2017, 114, E6034–E6043. [Google Scholar] [CrossRef] [Green Version]
- Krämer, A.; Green, J.; Pollard, J., Jr.; Tugendreich, S. Causal analysis approaches in ingenuity pathway analysis. Bioinformatics 2014, 30, 523–530. [Google Scholar] [CrossRef]
- Inoue, M.; Rashid, M.H.; Fujita, R.; Contos, J.J.; Chun, J.; Ueda, H. Initiation of neuropathic pain requires lysophosphatidic acid receptor signaling. Nat. Med. 2004, 10, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, B.C.; Gallagher, G.; Fridman, V.; Feldman, E.L. Diabetic neuropathy: What does the future hold? Diabetologia 2020, 63, 891–897. [Google Scholar] [CrossRef]
- Figueroa, J.D.; Serrano-Illan, M.; Licero, J.; Cordero, K.; Miranda, J.D.; De Leon, M. Fatty acid binding protein 5 modulates docosahexaenoic acid-induced recovery in rats undergoing spinal cord injury. J. Neurotrauma 2016, 33, 1436–1449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, E.J.; Thayne, K.A.; Harris, M.; Shaikh, S.R.; Darden, T.M.; Lark, D.S.; Williams, J.M.; Chitwood, W.R.; Kypson, A.P.; Rodriguez, E. Do fish oil omega-3 fatty acids enhance antioxidant capacity and mitochondrial fatty acid oxidation in human atrial myocardium via PPARγ activation? Antioxid. Redox Signal. 2014, 21, 1156–1163. [Google Scholar] [CrossRef] [Green Version]
- Nagpal, A.S.; Leet, J.; Egan, K.; Garza, R. Diabetic neuropathy: A critical, narrative review of published data from 2019. Curr. Pain Headache Rep. 2021, 25, 15. [Google Scholar] [CrossRef]
- Eid, S.; Sas, K.M.; Abcouwer, S.F.; Feldman, E.; Gardner, T.; Pennathur, S.; Fort, P.E. New insights into the mechanisms of diabetic complications: Role of lipids and lipid metabolism. Diabetologia 2019, 62, 1539–1549. [Google Scholar] [CrossRef] [Green Version]
- Chávez-Castillo, M.; Ortega, Á.; Cudris-Torres, L.; Duran, P.; Rojas, M.; Manzano, A.; Garrido, B.; Salazar, J.; Silva, A.; Rojas-Gomez, D.M.; et al. Specialized pro-resolving lipid mediators: The future of chronic pain therapy? Int. J. Mol. Sci. 2021, 22, 10370. [Google Scholar] [CrossRef] [PubMed]
- Tesfaye, S.; Kempler, P. Painful diabetic neuropathy. Diabetologia 2005, 48, 805–807. [Google Scholar] [CrossRef] [Green Version]
- Shillo, P.; Sloan, G.; Greig, M.; Hunt, L.; Selvarajah, D.; Elliott, J.; Gandhi, R.; Wilkinson, I.D.; Tesfaye, S. Painful and painless diabetic neuropathies: What is the difference? Curr. Diabetes Rep. 2019, 19, 32. [Google Scholar] [CrossRef] [Green Version]
- Felig, P.; Marliss, E.; Cahill, G.F., Jr. Plasma amino acid levels and insulin secretion in obesity. N. Engl. J. Med. 1969, 281, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Newgard, C.B.; An, J.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Lien, L.F.; Haqq, A.M.; Shah, S.H.; Arlotto, M.; Slentz, C.A.; et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009, 9, 311–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, M.S. The emerging role of branched-chain amino acids in insulin resistance and metabolism. Nutrients 2016, 8, 405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lynch, C.J.; Adams, S.H. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat. Rev. Endocrinol. 2014, 10, 723–736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrannini, E.; Natali, A.; Camastra, S.; Nannipieri, M.; Mari, A.; Adam, K.-P.; Milburn, M.V.; Kastenmüller, G.; Adamski, J.; Tuomi, T.; et al. Early metabolic markers of the development of dysglycemia and type 2 diabetes and their physiological significance. Diabetes 2013, 62, 1730–1737. [Google Scholar] [CrossRef] [Green Version]
- Menni, C.; Fauman, E.; Erte, I.; Perry, J.R.; Kastenmüller, G.; Shin, S.-Y.; Petersen, A.-K.; Hyde, C.; Psatha, M.; Ward, K.J.; et al. Biomarkers for type 2 diabetes and impaired fasting glucose using a nontargeted metabolomics approach. Diabetes 2013, 62, 4270–4276. [Google Scholar] [CrossRef] [Green Version]
- Shulman, G.I. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N. Engl. J. Med. 2014, 371, 1131–1141. [Google Scholar] [CrossRef]
- Mahendran, Y.; Cederberg, H.; Vangipurapu, J.; Kangas, A.J.; Soininen, P.; Kuusisto, J.; Uusitupa, M.; Ala-Korpela, M.; Laakso, M. Glycerol and fatty acids in serum predict the development of hyperglycemia and type 2 diabetes in Finnish men. Diabetes Care 2013, 36, 3732–3738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gromski, P.S.; Muhamadali, H.; Ellis, D.; Xu, Y.; Correa, E.; Turner, M.; Goodacre, R. A tutorial review: Metabolomics and partial least squares-discriminant analysis--a marriage of convenience or a shotgun wedding. Anal. Chim. Acta 2015, 879, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, A.; Oltvai, Z.N. Macromolecular crowding explains overflow metabolism in cells. Sci. Rep. 2016, 6, 31007. [Google Scholar] [CrossRef] [Green Version]
- Granucci, N.; Pinu, F.R.; Han, T.L.; Villas-Boas, S.G. Can we predict the intracellular metabolic state of a cell based on extracellular metabolite data? Mol. Biosyst. 2015, 11, 3297–3304. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Milburn, M.V.; Ryals, J.A.; Lonergan, S.C.; Mitchell, M.W.; Wulff, J.E.; Alexander, D.C.; Evans, A.M.; Bridgewater, B.; Miller, L.; et al. Plasma metabolomic profiles enhance precision medicine for volunteers of normal health. Proc. Natl. Acad. Sci. USA 2015, 112, E4901–E4910. [Google Scholar] [CrossRef] [Green Version]
- Beer, S.M.; Taylor, E.R.; Brown, S.E.; Dahm, C.C.; Costa, N.J.; Runswick, M.J.; Murphy, M.P. Glutaredoxin 2 catalyzes the reversible oxidation and glutathionylation of mitochondrial membrane thiol proteins: Implications for mitochondrial redox regulation and antioxidant DEFENSE. J. Biol. Chem. 2004, 279, 47939–47951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallogly, M.M.; Mieyal, J.J. Mechanisms of reversible protein glutathionylation in redox signaling and oxidative stress. Curr. Opin. Pharmacol. 2007, 7, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Reynaert, N.; Ckless, K.; Guala, A.; Wouters, E.F.; van der Vliet, A.; Janssen-Heininger, Y.M. In situ detection of S-glutathionylated proteins following glutaredoxin-1 catalyzed cysteine derivatization. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2006, 1760, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Yoshiba-Suzuki, S.; Sagara, J.-I.; Bannai, S.; Makino, N. The dynamics of cysteine, glutathione and their disulphides in astrocyte culture medium. J. Biochem. 2011, 150, 95–102. [Google Scholar] [CrossRef]
- Gall, W.E.; Beebe, K.; Lawton, K.A.; Adam, K.-P.; Mitchell, M.; Nakhle, P.J.; Ryals, J.A.; Milburn, M.V.; Nannipieri, M.; Camastra, S.; et al. alpha-hydroxybutyrate is an early biomarker of insulin resistance and glucose intolerance in a nondiabetic population. PLoS ONE 2010, 5, e10883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cobb, J.; Eckhart, A.; Motsinger-Reif, A.; Carr, B.; Groop, L.; Ferrannini, E. α-hydroxybutyric acid is a selective metabolite biomarker of impaired glucose tolerance. Diabetes Care 2016, 39, 988–995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herder, C.; Karakas, M.; Koenig, W. Biomarkers for the prediction of type 2 diabetes and cardiovascular disease. Clin. Pharmacol. Ther. 2011, 90, 52–66. [Google Scholar] [CrossRef]
- Song, Z.; Fu, D.T.W.; Chan, Y.-S.; Leung, S.Y.; Chung, S.S.M.; Chung, S.K. Transgenic mice overexpressing aldose reductase in Schwann cells show more severe nerve conduction velocity deficit and oxidative stress under hyperglycemic stress. Mol. Cell. Neurosci. 2003, 23, 638–647. [Google Scholar] [CrossRef]
- Feldman, E.L.; Nave, K.-A.; Jensen, T.S.; Bennett, D.L.H. New horizons in diabetic neuropathy: Mechanisms, bioenergetics, and pain. Neuron 2017, 93, 1296–1313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stevens, M.J.; Obrosova, I.; Cao, X.; Van Huysen, C.; Greene, D.A. Effects of DL-alpha-lipoic acid on peripheral nerve conduction, blood flow, energy metabolism, and oxidative stress in experimental diabetic neuropathy. Diabetes 2000, 49, 1006–1015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inquimbert, P.; Moll, M.; Latremoliere, A.; Tong, C.-K.; Whang, J.; Sheehan, G.F.; Smith, B.M.; Korb, E.; Athie, M.C.P.; Babaniyi, O.; et al. NMDA receptor activation underlies the loss of spinal dorsal horn neurons and the transition to persistent pain after peripheral nerve injury. Cell Rep. 2018, 23, 2678–2689. [Google Scholar] [CrossRef] [Green Version]
- Choi, D.W. Glutamate neurotoxicity and diseases of the nervous system. Neuron 1988, 1, 623–634. [Google Scholar] [CrossRef]
- Tomiyama, M.; Furusawa, K.-I.; Kamijo, M.; Kimura, T.; Matsunaga, M.; Baba, M. Upregulation of mRNAs coding for AMPA and NMDA receptor subunits and metabotropic glutamate receptors in the dorsal horn of the spinal cord in a rat model of diabetes mellitus. Mol. Brain Res. 2005, 136, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Daulhac, L.; Mallet, C.; Courteix, C.; Etienne, M.; Duroux, E.; Privat, A.-M.; Eschalier, A.; Fialip, J. Diabetes-induced mechanical hyperalgesia involves spinal mitogen-activated protein kinase activation in neurons and microglia via N-methyl-D-aspartate-dependent mechanisms. Mol. Pharmacol. 2006, 70, 1246–1254. [Google Scholar] [CrossRef] [Green Version]
- Zhang, E.; Kim, J.-J.; Shin, N.; Yin, Y.; Nan, Y.; Xu, Y.; Hong, J.; Hsu, T.M.; Chung, W.; Ko, Y.; et al. High Omega-3 Polyunsaturated fatty acids in fat-1 mice reduce inflammatory pain. J. Med. Food. 2017, 20, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Alexander, G.M.; Reichenberger, E.; Peterlin, B.L.; Perreault, M.J.; Grothusen, J.R.; Schwartzman, R.J. Plasma amino acids changes in complex regional pain syndrome. Pain Res. Treat. 2013, 2013, 742407. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.T.; Li, C.; Peng, X.P.; Guo, J.; Yue, S.J.; Liu, W.; Zhao, F.Y.; Han, J.Z.; Huang, Y.H.; Cheng, Q.M.; et al. An excessive increase in glutamate contributes to glucose-toxicity in beta-cells via activation of pancreatic NMDA receptors in rodent diabetes. Sci. Rep. 2017, 7, 44120. [Google Scholar] [CrossRef]
- Yaksh, T.L. Behavioral and autonomic correlates of the tactile evoked allodynia produced by spinal glycine inhibition: Effects of modulatory receptor systems and excitatory amino acid antagonists. Pain 1989, 37, 111–123. [Google Scholar] [CrossRef]
- Sherman, S.E.; Loomis, C.W. Morphine insensitive allodynia is produced by intrathecal strychnine in the lightly anesthetized rat. Pain 1994, 56, 17–29. [Google Scholar] [CrossRef]
- Loomis, C.W.; Khandwala, H.; Osmond, G.; Hefferan, M.P. Coadministration of intrathecal strychnine and bicuculline effects synergistic allodynia in the rat: An isobolographic analysis. J. Pharmacol. Exp. Ther. 2001, 296, 756–761. [Google Scholar]
- Rondón, L.J.; Farges, M.C.; Davin, N.; Sion, B.; Privat, A.M.; Vasson, M.P.; Eschalier, A.; Courteix, C. l-Arginine supplementation prevents allodynia and hyperalgesia in painful diabetic neuropathic rats by normalizing plasma nitric oxide concentration and increasing plasma agmatine concentration. Eur. J. Nutr. 2018, 57, 2353–2363. [Google Scholar] [CrossRef] [PubMed]
- Zeilhofer, H.U.; Ralvenius, W.T.; Acuña, M.A. Restoring the spinal pain gate: GABA(A) receptors as targets for novel analgesics. Adv. Pharmacol. 2015, 73, 71–96. [Google Scholar] [PubMed]
- Zeilhofer, H.U.; Benke, D.; Yevenes, G.E. Chronic pain states: Pharmacological strategies to restore diminished inhibitory spinal pain control. Annu. Rev. Pharmacol. Toxicol. 2012, 52, 111–133. [Google Scholar] [CrossRef] [PubMed]
- Coste, T.C.; Gerbi, A.; Vague, P.; Maixent, J.M.; Pieroni, G.; Raccah, D. Peripheral diabetic neuropathy and polyunsaturated fatty acid supplementations: Natural sources or biotechnological needs? Cell. Mol. Biol. 2004, 50, 845–853. [Google Scholar] [PubMed]
- Salto, L.M.; Cordero-MacIntyre, Z.; Beeson, L.; Schulz, E.; Firek, A.; De Leon, M. En balance participants decrease dietary fat and cholesterol intake as part of a culturally sensitive Hispanic diabetes education program. Diabetes Educ. 2011, 37, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Viader, A.; Sasaki, Y.; Kim, S.; Strickland, A.; Workman, C.S.; Yang, K.; Gross, R.W.; Milbrandt, J. Aberrant Schwann cell lipid metabolism linked to mitochondrial deficits leads to axon degeneration and neuropathy. Neuron 2013, 77, 886–898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ueda, H. Lysophosphatidic acid signaling is the definitive mechanism underlying neuropathic pain. Pain 2017, 158 (Suppl. S1), S55–S65. [Google Scholar] [CrossRef]
- Hammad, S.M.; DCCT/EDIC Group of Investigators; Baker, N.L.; El Abiad, J.M.; Spassieva, S.D.; Pierce, J.S.; Rembiesa, B.; Bielawski, J.; Lopes-Virella, M.F.; Klein, R.L. Increased plasma levels of select deoxy-ceramide and ceramide species are associated with increased odds of diabetic neuropathy in type 1 diabetes: A pilot study. Neuromol. Med. 2017, 19, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Diabetes Prevention Program Research Group. 10-year follow-up of diabetes incidence and weight loss in the diabetes prevention program outcomes study. Lancet 2009, 374, 1677–1686. [Google Scholar] [CrossRef] [Green Version]
- Varma, R.; Chung, J.; Foong, A.W.; Torres, M.; Choudhury, F.; Azen, S.P. Four-year incidence and progression of visual impairment in Latinos: The Los Angeles Latino Eye Study. Am. J. Ophthalmol. 2010, 149, 713–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schoen, K.; Horvat, N.; Guerreiro, N.F.C.; de Castro, I.; De Giassi, K.S. Spectrum of clinical and radiographic findings in patients with diagnosis of H1N1 and correlation with clinical severity. BMC Infect. Dis. 2019, 19, 964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.-K.; Feng, Y.; Yuan, M.Y.; Fu, H.J.; Wu, B.Y.; Sun, G.Z.; Yang, G.R.; Zhang, X.L.; Wang, L.; Xu, X.P.; et al. Plasma glucose levels and diabetes are independent predictors for mortality and morbidity in patients with SARS. Diabet. Med. 2006, 23, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; The Northwell COVID-19 Research Consortium. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef]
- Yin, Y.; Rohli, K.E.; Shen, P.; Lu, H.; Liu, Y.; Dou, Q.; Zhang, L.; Kong, X.; Yang, S.; Jia, P. The epidemiology, pathophysiological mechanisms, and management toward COVID-19 patients with Type 2 diabetes: A systematic review. Prim. Care Diabetes 2021, 15, 899–909. [Google Scholar] [CrossRef]
- Vahid, F.; Rahmani, D. Can an anti-inflammatory diet be effective in preventing or treating viral respiratory diseases? A systematic narrative review. Clin. Nutr. ESPEN 2021, 43, 9–15. [Google Scholar] [CrossRef]
- Wagner, E.F.; Swenson, C.C.; Henggeler, S.W. Practical and methodological challenges in validating community-based interventions. Child. Serv. 2000, 3, 211–231. [Google Scholar] [CrossRef]
- Stevens, J.R.; Herrick, J.S.; Wolff, R.K.; Slattery, M.L. Power in pairs: Assessing the statistical value of paired samples in tests for differential expression. BMC Genom. 2018, 19, 953. [Google Scholar] [CrossRef] [Green Version]
- Hartweg, J.; Perera, R.; Montori, V.M.; Dinneen, S.F.; Neil, A.H.; Farmer, A.J. Omega-3 polyunsaturated fatty acids (PUFA) for type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2008, 1, CD003205. [Google Scholar] [CrossRef]

| Clinical Parameter | Baseline (BL) | 3 Months (3Mo) | Difference 3Mo—BL | Significance, p |
|---|---|---|---|---|
| Age (year) | 55.5 ± 11.8 | - | - | - |
| Male%, Female% | 43%, 57% | - | - | - |
| % Hispanic | 100 | - | - | - |
| BMI, kg/m2 | 29.7 ± 5.5 | 29.8 ± 5.7 | 0.1 | 0.706 1 |
| Cholesterol, mg/dL | 176.3 ± 35.7 | 179 ± 34.3 | 2.7 | 0.294 |
| LDL, mg/dL | 117.1 ± 32.5 | 111.6 ± 27.9 | −5.5 | 0.083 |
| HDL, mg/dL | 47.2 ± 13 | 48.2 ± 14 | 1 | 0.600 |
| Cholesterol:HDL | 3.9 ± 1.0 | 3.9 ± 1.1 | 0 | 0.561 |
| Triglycerides, mg/dL | 169.3 ± 83.9 | 163.7 ± 123 | −5.6 | 0.347 |
| Fasting Glucose, mg/dL | 154.1 ± 71.9 | 142.7 ± 65.5 | −11.4 | 0.095 |
| HbA1c% | 7.6 ± 2.3 | 7.4 ± 2.1 | −0.2 | 0.014 |
| Baseline (BL) | 3 Months (3Mo) | Difference 3Mo—BL | Significance, p | |
|---|---|---|---|---|
| GC-MS | ||||
| DHA | 0.71 ± 0.4 | 1.85 ± 1.1 | 1.114 | <0.001 |
| EPA | 1.03 ± 0.68 | 1.62 ± 1.1 | 0.59 | 0.001 |
| Dietary Intake | ||||
| DHA, mg | 57.6 ± 98.9 | 1035.2 ± 30.1 | 977.6 | 0.0001 |
| EPA, mg | 26.8 ± 65.5 | 211.7 ± 11.3 | 184.9 | 0.0001 |
| Total Biochemicals Identified | 695 |
| Total Biochemicals p ≤ 0.05, matched paired t-Test | 106 |
| Biochemicals (↑↓) | 69|37 |
| Super Pathway | Sub Pathway | Biochemical Name | Fold Change 3Mo BL | % Change |
|---|---|---|---|---|
| Amino Acid | Glycine, Serine, and Threonine | glycine | 1.11 | +11% |
| Glutamate | glutamate | 0.89 | −11% | |
| glutamine | 1.07 | - | ||
| Leucine, Isoleucine, and Valine | isoleucine | 1.09 | - | |
| Methionine, Cysteine, SAM, and Taurine | methionine | 1.1 | - | |
| s-methylmethionine | 1.07 | +7% | ||
| methionine sulfoxide | 1.19 | +19% | ||
| cystathionine | 1.9 | +90% | ||
| alpha-ketobutyrate | 1.36 | +36% | ||
| 2-aminobutyrate | 0.94 | - | ||
| cysteine | 1.01 | - | ||
| cystine | 1.2 | +20% | ||
| cysteine s-sulfate | 0.86 | −14% | ||
| hypotaurine | 0.94 | - | ||
| taurine | 0.93 | - | ||
| 2-hydroxybutyrate/2-hydroxyisobutyrate | 0.82 | −18% | ||
| Arginine and Proline | Arginine | 1.1 | +10% | |
| Glutathione | cysteine-glutathione disulfide | 2.56 | +156% | |
| cysteinylglycine | 1.19 | +19% | ||
| Carbohydrate | Fructose, Mannose, and Galactose | fructose | 0.8 | - |
| Lipid | Glycerlipid | glycerol 3-phosphate | 1.78 | +78% |
| Super Pathway | Sub Pathway | Biochemical Name | Fold Change 3Mo BL | % Change |
|---|---|---|---|---|
| Carbohydrate | Glycolysis, Gluconeogenesis, and Pyruvate Metabolism | glucose | 0.92 | - |
| 3-phosphoglycerate | 0.43 | −57% | ||
| pyruvate | 1.09 | - | ||
| lactate | 0.95 | - | ||
| glycerate | 0.85 | −15% | ||
| Glycogen Metabolism | maltotriose | 0.44 | −56% | |
| maltose | 0.52 | −48% | ||
| Lipid | Polyunsaturated Fatty Acid (n3 and n6) | eicosapentaenoate (EPA; 20:5n3) | 1.57 | +57% |
| docosapentaenate (n3 DPA; 22:5n3) | 0.97 | - | ||
| docosahexaenoate (DHA; 22:6n3) | 2.62 | +162% | ||
| docosatrienoate (22:3n3) | 0.67 | −33% | ||
| arachidonate (20:4n6) | 0.83 | −17% | ||
| adrenate (22:4n6) | 0.82 | - | ||
| Phospholipid Metabolism | phosphoethanolamine | 0.81 | −19% | |
| glycerophosphoinositol | 0.5 | −50% | ||
| 1,2-dipalmitoyl-GPC (16:0/16:0) | 1.15 | +15% | ||
| 1-palmitoyl-2-oleoyl-GPC (16:0/18:1) | 1.14 | +14% | ||
| 1-stearoyl-2-oleoyl-GPC (18:0/18:1) | 1.26 | +26% | ||
| 1-stearoyl-2-linoleoyl-GPC (18:0/18:2) | 1.13 | +13% | ||
| 1-linoleoyl-2-linolenoyl-GPC (18:2/18:3) | 1.32 | +32% | ||
| 1-palmitoyl-2-linoleoyl-GPI (18:2/18:3) | 1.22 | +22% | ||
| 1-oleoyl-2-linoleoyl-GPI (18:1/18:2) | 1.42 | +42% | ||
| 1-stearoyl-2-linoleoyl-GPI (18:0/18:2) | 1.18 | +18% | ||
| 1-stearoyl-2-arachidonoyl-GPE (18:0/20:4) | 0.86 | −14% | ||
| 1-palmitoyl-2-arachidonoyl-GPE (16:0/20:4) | 0.86 | −14% | ||
| 1-palmitoyl-2-stearoyl-GPC (16:0/18:0) | 1.15 | +15% | ||
| 1-palmitoyl-2-oleoyl-GPI (16:0/18:1) | 1.23 | +23% | ||
| 1-oleoyl-2-arachidonyl-GPE (18:1/20:4) | 0.7 | −30% | ||
| Plasmalogen | 1-(1-enyl-palmitoyl)-2-eicosapentaenoyl-GPE (p-16:0/20:5) | 1.88 | +88% | |
| 1-(1-enyl-palmitoyl)-2-palmitoleoyl-GPC (p-16:0/16:1) | 1.13 | +13% | ||
| Glycerolipid | glycerol 3-phosphate | 1.78 | +78% | |
| glycerophosphoglycerol | 0.56 | −44% | ||
| Diacylglycerol | linoleoyl-docosahexaenoyl-glycerol (18:2/22:6) [1] | 3.52 | +252% | |
| linoleoyl-docosahexaenoyl-glycerol (18:2/22:6) [2] | 4.61 | +361% |
| Super Pathway | Sub Pathway | Biochemical Name | Fold Change 3Mo BL | % Change |
|---|---|---|---|---|
| Lipid | Fatty Acid Metabolism (Acyl Carnitine) | octanoylcarnitine | 1.39 | 39% |
| decanoylcarnitine | 1.37 | 37% | ||
| cis-4-decenoyl carnitine | 1.2 | 20% | ||
| laurylcarnitine | 1.31 | 31% | ||
| myristoylcarnitine | 1.17 | - | ||
| palmitoylcarnitine | 1.13 | - | ||
| myristoleoylcarnitine | 1.26 | 26% | ||
| Ketone Bodies | acetoacetate | 0.64 | - | |
| 3-hydroxybutyrate (BHBA) | 0.81 | - | ||
| Fatty Acid, Monohydroxy | 3-hydroxyhexanoate | 1.1 | - | |
| 3-hydroxyoctanoate | 1.3 | - | ||
| 3-hydroxydecanoate | 1.19 | - | ||
| 3-hydroxylaurate | 1.1 | - | ||
| Nucleotide | Purine Metabolism, Adenosine Containing | adenosine 5′-monophosphate | 0.58 | 42% |
| Bio Function Categories | Diseases or Functions Annotation | p-Value | Activation Z-Score | Number of Biochemicals |
|---|---|---|---|---|
| Free Radical Scavenging | Synthesis of reactive oxygen species | 7.21 × 10−7 | −1.69 | 13 |
| Formation of reactive oxygen species | 4.19 × 10−4 | −1.96 | 4 | |
| Biosynthesis of hydrogen peroxide | 4.95 × 10−5 | −1.95 | 5 | |
| Cellular Compromise, Lipid Metabolism, Small Molecule Biochemistry | Peroxidation of Lipids | 2.25 × 10−5 | −1.94 | 6 |
| Cell-to-Cell Signaling and Interaction, Hematological System Development and Function, Inflammatory Response | Aggregation of blood platelets | 5.7 × 10−5 | −1.78 | 7 |
| Carbohydrate Metabolism, Molecular Transport, Small Molecule Biochemistry | Uptake of D-glucose | 1.19 × 10−4 | −1.72 | 6 |
| Cell Signaling, Molecular Transport, Vitamin and Mineral Metabolism | Quantity of Ca2+ | 1.52 × 10−4 | −1.83 | 9 |
| Entrance of Ca2+ | 2.15 × 10−4 | −1.97 | 4 | |
| Drug Metabolism, Molecular Transport, Small Molecule Biochemistry | Concentration of glutathione | 3.43 × 10−4 | 1.97 | 5 |
| Metabolite | Correlation | p-Value |
|---|---|---|
| cysteine-glutathione disulfide | −0.460 | <0.001 |
| linoleoyl-docosahexaenoyl-glycerol (18:2/22:6) [1] | −0.266 | 0.059 |
| Sphingosine | 0.489 | <0.001 |
| 3-phosphoglycerate | 0.572 | <0.001 |
| Glycerol 3-phosphate | −0.163 | 0.252 |
| 1-linoleoyl-GPA (18:2) | 0.406 | 0.003 |
| 2′-deoxyuridine | −0.492 | <0.001 |
| Maltose | 0.360 | 0.009 |
| Super Pathway | Sub Pathway | Biochemical Name | Fold Change 3Mo BL | % Change |
|---|---|---|---|---|
| Lipid | Sphingolipid Metabolism | sphingosine | 0.45 | −55% |
| Lysolipid | 1-linoleoyl-GPA (18:2) | 0.43 | −57% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durán, A.M.; Beeson, W.L.; Firek, A.; Cordero-MacIntyre, Z.; De León, M. Dietary Omega-3 Polyunsaturated Fatty-Acid Supplementation Upregulates Protective Cellular Pathways in Patients with Type 2 Diabetes Exhibiting Improvement in Painful Diabetic Neuropathy. Nutrients 2022, 14, 761. https://doi.org/10.3390/nu14040761
Durán AM, Beeson WL, Firek A, Cordero-MacIntyre Z, De León M. Dietary Omega-3 Polyunsaturated Fatty-Acid Supplementation Upregulates Protective Cellular Pathways in Patients with Type 2 Diabetes Exhibiting Improvement in Painful Diabetic Neuropathy. Nutrients. 2022; 14(4):761. https://doi.org/10.3390/nu14040761
Chicago/Turabian StyleDurán, Alfonso M., W. Lawrence Beeson, Anthony Firek, Zaida Cordero-MacIntyre, and Marino De León. 2022. "Dietary Omega-3 Polyunsaturated Fatty-Acid Supplementation Upregulates Protective Cellular Pathways in Patients with Type 2 Diabetes Exhibiting Improvement in Painful Diabetic Neuropathy" Nutrients 14, no. 4: 761. https://doi.org/10.3390/nu14040761
APA StyleDurán, A. M., Beeson, W. L., Firek, A., Cordero-MacIntyre, Z., & De León, M. (2022). Dietary Omega-3 Polyunsaturated Fatty-Acid Supplementation Upregulates Protective Cellular Pathways in Patients with Type 2 Diabetes Exhibiting Improvement in Painful Diabetic Neuropathy. Nutrients, 14(4), 761. https://doi.org/10.3390/nu14040761






