Differential Glycemic Effects of Low- versus High-Glycemic Index Mediterranean-Style Eating Patterns in Adults at Risk for Type 2 Diabetes: The MEDGI-Carb Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Eligibility Criteria
2.3. Dietary Control
2.4. Postprandial Assessments
2.5. Continuous Glucose Monitoring
2.6. Blood Collection and Analysis
2.7. Statistical Analyses
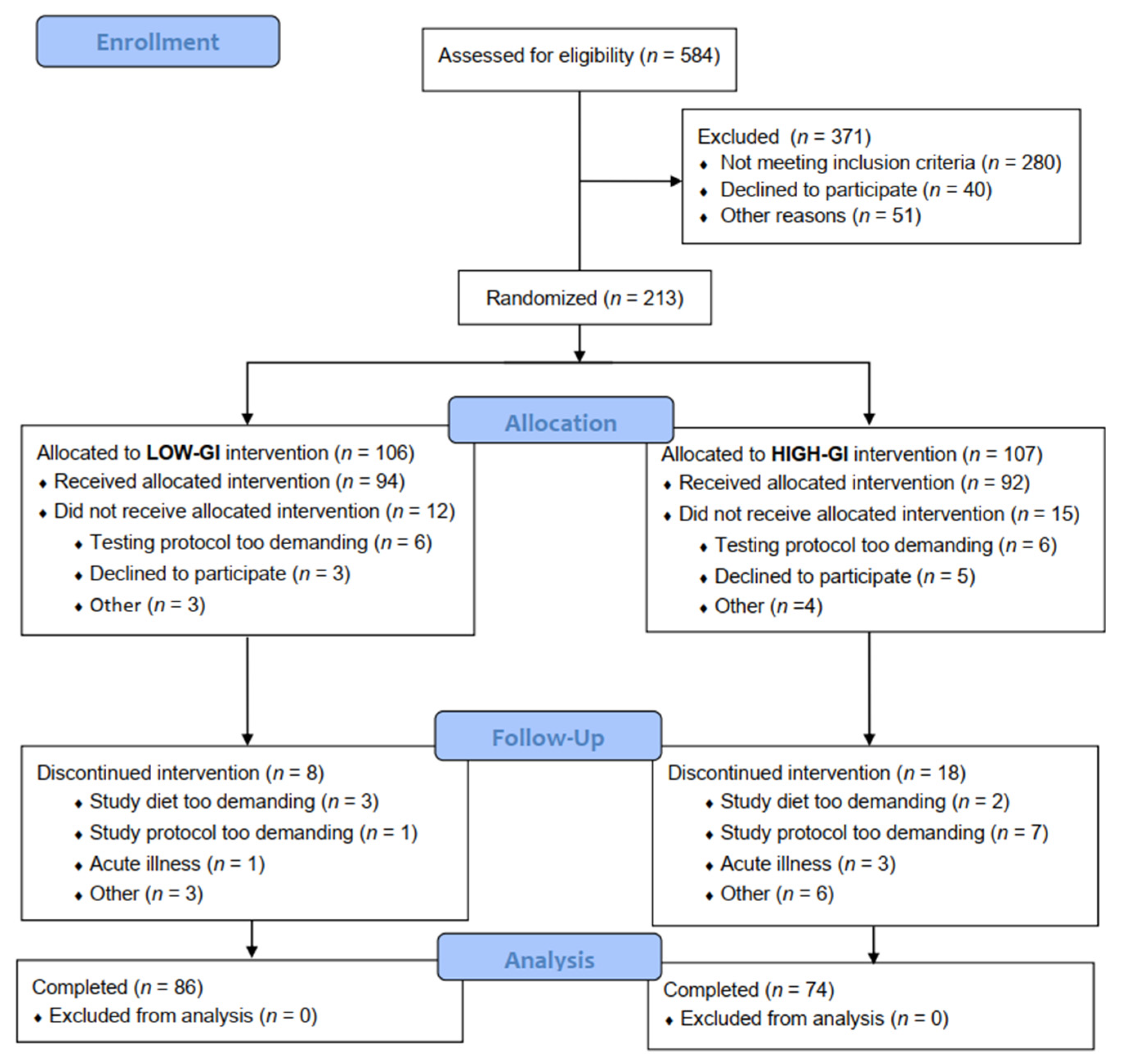
3. Results
3.1. Baseline Characteristics
3.2. Dietary Composition
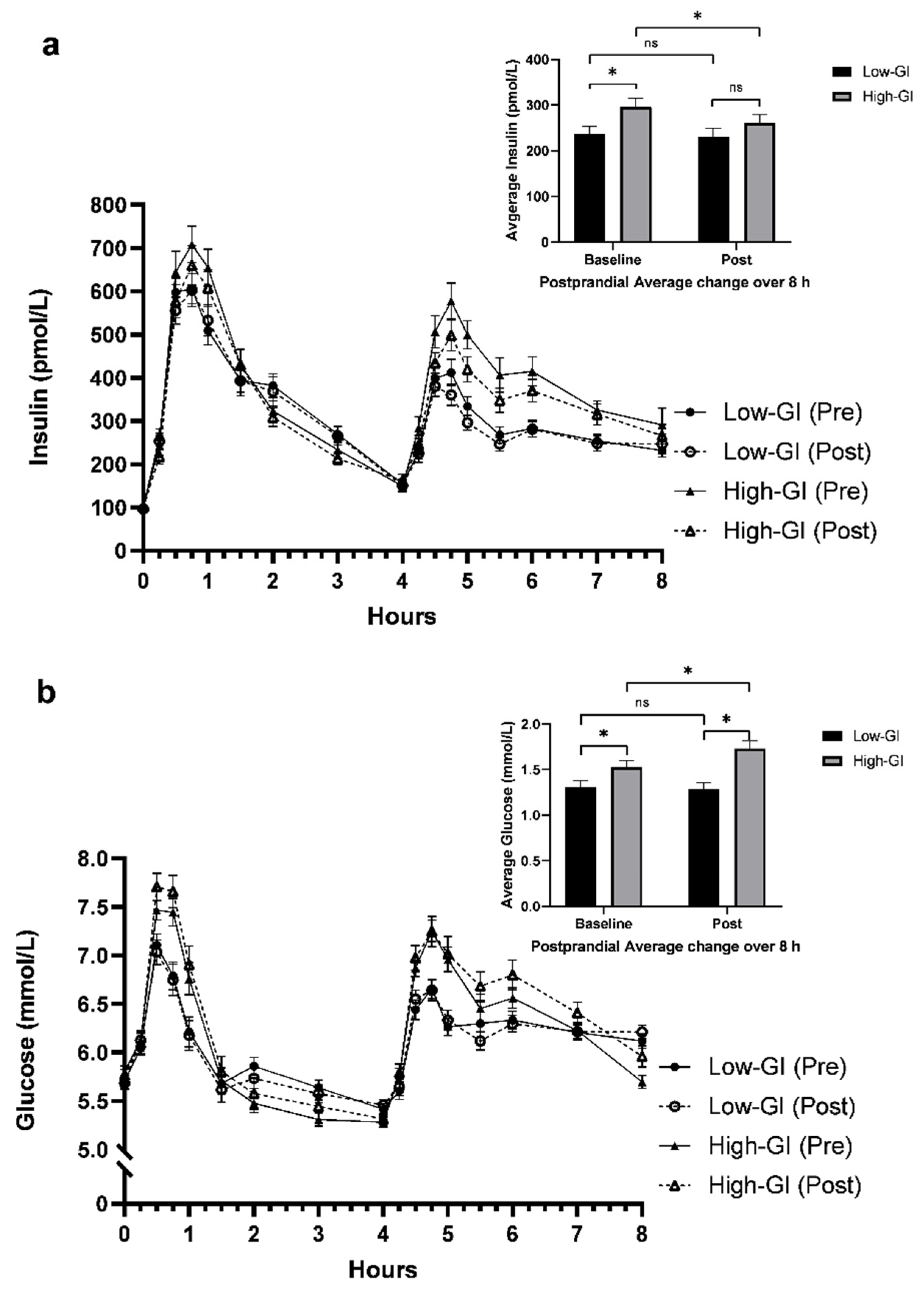
3.3. Postprandial MGTT Glucose and Insulin Responses
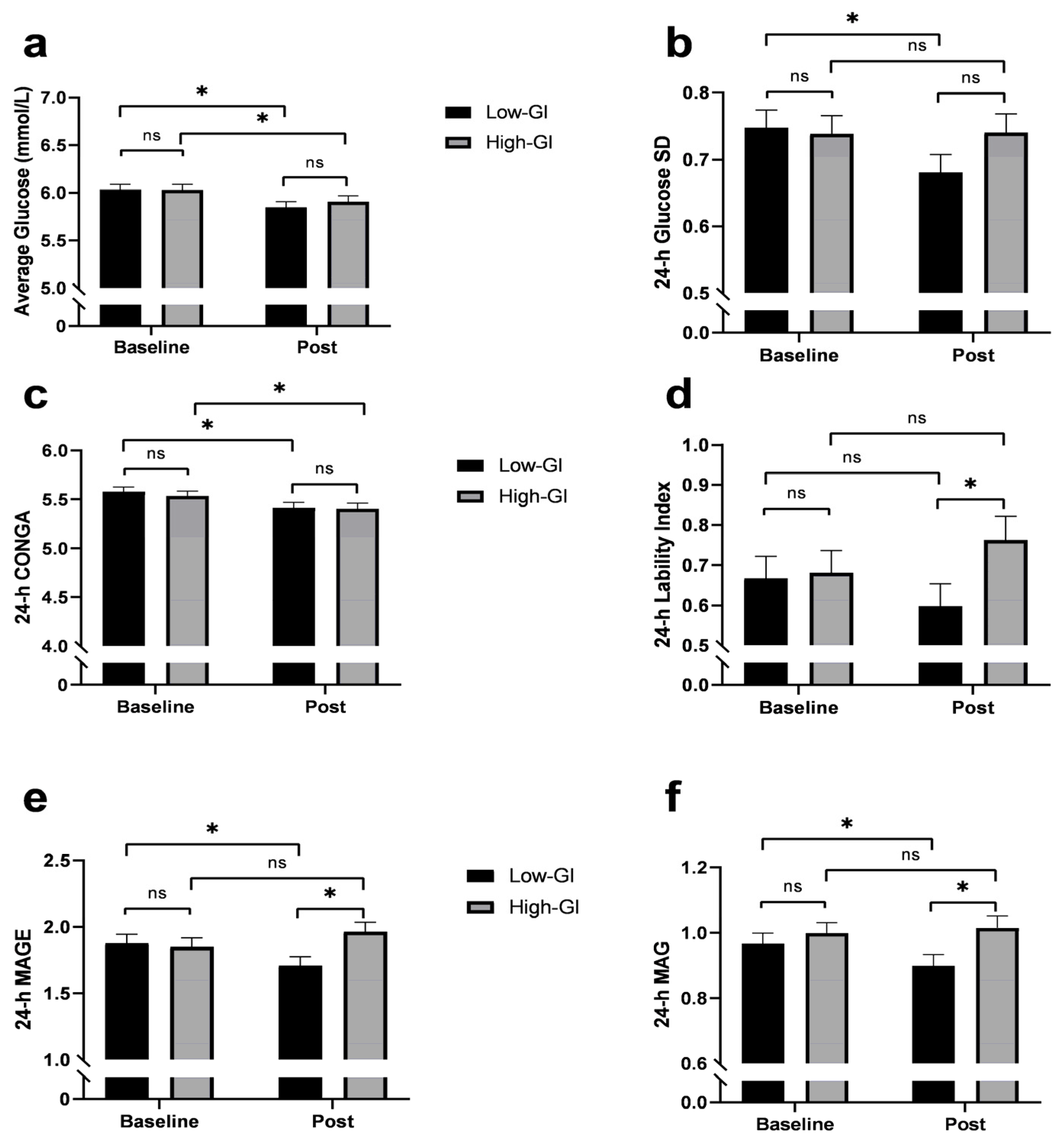
3.4. 24-h Glycemic Variability
3.5. Fasting and OGTT Glucose and Insulin Responses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaiser, A.B.; Zhang, N.; Der Pluijm, W.V. Global prevalence of type 2 diabetes over the next ten years (2018–2028). Diabetes 2018, 67 (Suppl. 1), 202-LB. [Google Scholar] [CrossRef]
- Dokken, B.B. The pathophysiology of cardiovascular disease and diabetes: Beyond blood pressure and lipids. Diabetes Spec. 2008, 21, 160–165. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Murphy, S.L.; Kockanek, K.D.; Arias, E. Mortality in the United States, 2018. NCHS Data Brief. 2020, 355, 1–8. [Google Scholar] [PubMed]
- Yamaoka, K.; Tango, T. Efficacy of lifestyle education to prevent type 2 diabetes: A meta-analysis of randomized controlled trials. Diabetes Care. 2005, 28, 2780–2786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monnier, L.; Lapinski, H.; Colette, C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: Variations with increasing levels of HbA1c. Diabetes Care 2003, 26, 881–885. [Google Scholar] [CrossRef] [Green Version]
- Blaak, E.E.; Antoine, J.M.; Benton, D.; Bjorck, I.; Bozzetto, L.; Brouns, F.; Diamant, M.; Dye, L.; Hulshof, T.; Holst, J.J.; et al. Impact of postprandial glycaemia on health and prevention of disease. Obes. Rev. 2012, 13, 923–984. [Google Scholar] [CrossRef]
- Monnier, L.; Colette, C.; Dunseath, G.J.; Owens, D.R. The loss of postprandial glycemic control precedes stepwise deterioration of fasting with worsening diabetes. Diabetes Care 2007, 30, 263–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenkins, D.J.; Wolever, T.M.; Taylor, R.H.; Barker, H.; Fielden, H.; Baldwin, J.M.; Bowling, A.C.; Newman, H.C.; Jenkins, A.L.; Goff, D.V. Glycemic index of foods: A physiological basis for carbohydrate exchange. Am. J. Clin. Nutr. 1981, 34, 362–366. [Google Scholar] [CrossRef] [Green Version]
- Vega-López, S.; Venn, B.J.; Slavin, J.L. Relevance of the glycemic index and glycemic load for body weight, diabetes, and cardiovascular disease. Nutrients 2018, 10, 1361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sacks, F.M.; Carey, V.J.; Anderson, C.A.; Miller, E.R., 3rd; Copeland, T.; Charleston, J.; Harshfield, B.J.; Laranjo, N.; McCarron, P.; Swain, J.; et al. Effects of high vs low glycemic index of dietary carbohydrate on cardiovascular disease risk factors and insulin sensitivity: The OmniCarb randomized clinical trial. JAMA 2014, 312, 2531–2541. [Google Scholar] [CrossRef] [PubMed]
- Nazare, J.A.; De Rougemont, A.; Normand, S.; Sauvinet, V.; Sothier, M.; Vinoy, S.; Désage, M.; Laville, M. Effect of postprandial modulation of glucose availability: Short-and long-term analysis. Br. J. Nutr. 2010, 103, 1461–1470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koloverou, E.; Esposito, K.; Giugliano, D.; Panagiotakos, D. The effect of Mediterranean diet on the development of type 2 diabetes mellitus: A meta-analysis of 10 prospective studies and 136,846 participants. Metabolism 2014, 63, 903–911. [Google Scholar] [CrossRef]
- Esposito, K.; Maiorino, M.I.; Bellastella, G.; Chiodini, P.; Panagiotakos, D.; Giugliano, D. A journey into a Mediterranean diet and type 2 diabetes: A systematic review with meta-analyses. BMJ Open 2015, 5, e008222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuttolomondo, A.; Simonetta, I.; Daidone, M.; Mogavero, A.; Ortello, A.; Pinto, A. Metabolic and Vascular Effect of the Mediterranean Diet. Int. J. Mol. Sci. 2019, 20, 4716. [Google Scholar] [CrossRef] [Green Version]
- Bergia, R.E.; Biskup, I.; Giacco, R.; Costabile, G.; Gray, S.; Wright, A.; Vitale, M.; Campbell, W.W.; Landberg, R.; Riccardi, G. The MEDGICarb-Study: Design of a multi-center randomized controlled trial to determine the differential health-promoting effects of low- and high-glycemic index Mediterranean-style eating patterns. Contemp. Clin. Trials Commun. 2020, 19, 100640. [Google Scholar] [CrossRef]
- Grundy, S.M.; Brewer, H.B.; Cleeman, J.I.; Smith, S.C.; Lenfant, C. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation 2004, 109, 433–438. [Google Scholar] [CrossRef] [Green Version]
- Brand-Miller, J.; Wolever, T.M.; Colagiuri, S.; Foster-Powell, K. The New Glucose Revolution: The Glycemic Index Solution for Optimum Health; Hodder, 2002; Available online: https://books.google.com/books?id=noWOSQAACAAJ (accessed on 6 June 2021).
- Giacco, R.; Costabile, G.; Della Pepa, G.; Anniballi, G.; Griffo, E.; Mangione, A.; Cipriano, P.; Viscovo, D.; Clemente, G.; Landberg, R.; et al. A whole-grain cereal-based diet lowers postprandial plasma insulin and triglyceride levels in individuals with metabolic syndrome. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 837–844. [Google Scholar] [CrossRef]
- Matthan, N.R.; Ausman, L.M.; Meng, H.; Tighiouart, H.; Lichtenstein, A.H. Estimating the reliability of glycemic index values and potential sources of methodological and biological variability. Am. J. Clin. Nutr. 2016, 104, 1004–1013. [Google Scholar] [CrossRef]
- Little, R.J.A.; Rubin, D.B. Bayes and Multiple Imputation. In Statistical Analysis with Missing Data, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2002; pp. 200–220. [Google Scholar]
- Kolb, H.; Kempf, K.; Röhling, M.; Martin, S. Insulin: Too much of a good thing is bad. BMC Med. 2020, 18, 224. [Google Scholar] [CrossRef]
- Rautio, N.; Jokelainen, J.; Oksa, H.; Saaristo, T.; Peltonen, M.; Puolijoki, H.; Tuomilehto, J.; Vanhala, M.; Moilanen, L.; Uusitupa, M.; et al. Changes in glucose metabolism in people with different glucose metabolism disorders at baseline: Follow-up results of a Finnish national diabetes prevention programme. Diabet Med. 2015, 32, 1611–1616. [Google Scholar] [CrossRef] [PubMed]
- Selvin, E.; Steffes, M.W.; Zhu, H.; Matsushita, K.; Wagenknecht, L.; Pankow, J.; Coresh, J.; Brancati, F.L. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N. Engl. J. Med. 2010, 362, 800–811. [Google Scholar] [CrossRef] [PubMed]
- Guess, N.D. Dietary interventions for the prevention of type 2 diabetes in high-risk groups: Current state of evidence and future research needs. Nutrients 2018, 10, 1245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solomon, T.P.J.; Haus, J.M.; Kelly, K.R.; Cook, M.D.; Filion, J.; Rocco, M.; Kashyap, S.R.; Watanabe, R.M.; Barkoukis, H.; Kirwan, J.P. A low-glycemic index diet combined with exercise reduces insulin resistance, postprandial hyperinsulinemia, and glucose-dependent insulinotropic polypeptide responses in obese, prediabetic humans. Am. J. Clin. Nutr. 2010, 92, 1359–1368. [Google Scholar] [CrossRef] [PubMed]
- Brand-Miller, J.; Hayne, S.; Petocz, P.; Colagiuri, S. Low-glycemic index diets in the management of diabetes: A meta-analysis of randomized controlled trials. Diabetes Care 2003, 26, 2261–2267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Augustin, L.S.A.; Kendall, C.W.C.; Jenkins, D.J.A.; Willett, W.C.; Astrup, A.; Barclay, A.W.; Björck, I.; Brand-Miller, J.C.; Brighenti, F.; Buyken, A.E.; et al. Glycemic index, glycemic load and glycemic response: An International Scientific Consensus Summit from the International Carbohydrate Quality Consortium (ICQC). Nutr. Metab. Cardiovasc. Dis. 2015, 25, 795–815. [Google Scholar] [CrossRef] [Green Version]
- Danne, T.; Nimri, R.; Battelino, T.; Bergenstal, R.M.; Close, K.L.; DeVries, J.H.; Garg, S.; Heinemann, L.; Hirsch, I.; Amiel, S.A.; et al. International consensus on use of continuous glucose monitoring. Diabetes Care 2017, 40, 1631–1640. [Google Scholar] [CrossRef] [Green Version]
- Gorst, C.; Kwok, C.S.; Aslam, S.; Buchan, I.; Kontopantelis, E.; Myint, P.K.; Heatlie, G.; Loke, Y.; Rutter, M.K.; Mamas, M.A. Long-term glycemic variability and risk of adverse outcomes: A systematic review and meta-analysis. Diabetes Care 2015, 38, 2354–2369. [Google Scholar] [CrossRef] [Green Version]
- Lachin, J.M.; Bebu, I.; Bergenstal, R.M.; Pop-Busui, R.; Service, F.J.; Zinman, B.; Nathan, D.M. Association of glycemic variability in type 1 diabetes with progression of microvascular outcomes in the diabetes control and complications trial. Diabetes Care 2017, 40, 777–783. [Google Scholar] [CrossRef] [Green Version]
- Chakarova, N.; Dimova, R.; Grozeva, G.; Tankova, T. Assessment of glucose variability in subjects with prediabetes. Diabetes Res. Clin. Pract. 2019, 151, 56–64. [Google Scholar] [CrossRef] [Green Version]
- Salas-Salvadó, J.; Bulló, M.; Babio, N.; Martínez-González, M.Á.; Ibarrola-Jurado, N.; Basora, J.; Estruch, R.; Covas, M.I.; Corella, D.; Arós, F.; et al. Reduction in the incidence of type 2 diabetes with the mediterranean diet: Results of the PREDIMED-Reus nutrition intervention randomized trial. Diabetes Care 2011, 34, 14–19. [Google Scholar] [CrossRef] [Green Version]
- Schwingshackl, L.; Chaimani, A.; Hoffmann, G.; Schwedhelm, C.; Boeing, H. A network meta-analysis on the comparative efficacy of different dietary approaches on glycaemic control in patients with type 2 diabetes mellitus. Eur. J. Epidemiol. 2018, 33, 157–170. [Google Scholar] [CrossRef] [Green Version]
- Bozzetto, L.; Alderisio, A.; Giorgini, M.; Barone, F.; Giacco, A.; Riccardi, G.; Rivellese, A.A.; Annuzzi, G. Extra-virgin olive oil reduces glycemic response to a high-glycemic index meal in patients with type 1 diabetes: A randomized controlled trial. Diabetes Care 2016, 39, 518–524. [Google Scholar] [CrossRef] [Green Version]
- Dickinson, S.; Colagiuri, S.; Faramus, E.; Petocz, P.; Brand-Miller, J.C. Postprandial hyperglycemia and insulin sensitivity differ among lean young adults of different ethnicities. J. Nutr. 2002, 132, 2574–2579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Appel, L.J.; Moore, T.J.; Obarzanek, E.; Vollmer, W.M.; Svetkey, L.P.; Sacks, F.M.; Bray, G.A.; Vogt, T.M.; Cutler, J.A.; Windhauser, M.M.; et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N. Engl. J. Med. 1997, 336, 1117–1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estruch, R.; Ros, E.; Salas-Salvado, J.; Covas, M.I.; Corella, D.; Aros, F.; Gomez-Gracia, E.; Ruiz-Gutierrez, V.; Fiol, M.; Lapetra, J.; et al. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N. Engl. J. Med. 2018, 21, 378.e34. [Google Scholar] [CrossRef]
- Rodríguez-Rejón, A.I.; Castro-Quezada, I.; Ruano-Rodríguez, C.; Ruiz-López, M.D.; Sánchez-Villegas, A.; Toledo, E.; Artacho, R.; Estruch, R.; Salas-Salvadó, J.; Covas, M.I.; et al. Effect of a Mediterranean diet intervention on dietary glycemic load and dietary glycemic index: The PREDIMED study. J. Nutr. Metab. 2014, 2014, 985373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Demographic Characteristics | Low-GI (n = 102) | High-GI (n = 111) |
|---|---|---|
| Age at randomization (years) | 55 ± 10 | 55 ± 11 |
| Female n (%) | 55 (53.9%) | 64 (57.7%) |
| Weight (kg) | 92 ± 14 | 88 ± 14 |
| BMI (kg/m2) | 31.1 ± 3.1 | 30.3 ± 3.0 |
| Waist Circumference (cm) | 106 ± 8 | 105 ± 9 |
| Metabolic characteristics | ||
| Glucose (mmol/L) | 5.4 ± 0.6 | 5.4 ± 0.7 |
| Total cholesterol (mmol/L) | 4.9 ± 1.0 | 5.0 ± 0.9 |
| Triglycerides (mmol/L) | 1.3 ± 0.6 | 1.3 ± 0.6 |
| HDL (mmol/L) | 1.3 ± 0.4 | 1.3 ± 0.4 |
| LDL (mmol/L) | 3.2 ± 0.8 | 3.3 ± 0.7 |
| Systolic blood pressure (mm Hg) | 126 ± 14 | 126 ± 14 |
| Diastolic blood pressure (mm Hg) | 82 ± 9 | 83 ± 8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bergia, R.E.; Giacco, R.; Hjorth, T.; Biskup, I.; Zhu, W.; Costabile, G.; Vitale, M.; Campbell, W.W.; Landberg, R.; Riccardi, G. Differential Glycemic Effects of Low- versus High-Glycemic Index Mediterranean-Style Eating Patterns in Adults at Risk for Type 2 Diabetes: The MEDGI-Carb Randomized Controlled Trial. Nutrients 2022, 14, 706. https://doi.org/10.3390/nu14030706
Bergia RE, Giacco R, Hjorth T, Biskup I, Zhu W, Costabile G, Vitale M, Campbell WW, Landberg R, Riccardi G. Differential Glycemic Effects of Low- versus High-Glycemic Index Mediterranean-Style Eating Patterns in Adults at Risk for Type 2 Diabetes: The MEDGI-Carb Randomized Controlled Trial. Nutrients. 2022; 14(3):706. https://doi.org/10.3390/nu14030706
Chicago/Turabian StyleBergia, Robert E., Rosalba Giacco, Therese Hjorth, Izabela Biskup, Wenbin Zhu, Giuseppina Costabile, Marilena Vitale, Wayne W. Campbell, Rikard Landberg, and Gabriele Riccardi. 2022. "Differential Glycemic Effects of Low- versus High-Glycemic Index Mediterranean-Style Eating Patterns in Adults at Risk for Type 2 Diabetes: The MEDGI-Carb Randomized Controlled Trial" Nutrients 14, no. 3: 706. https://doi.org/10.3390/nu14030706
APA StyleBergia, R. E., Giacco, R., Hjorth, T., Biskup, I., Zhu, W., Costabile, G., Vitale, M., Campbell, W. W., Landberg, R., & Riccardi, G. (2022). Differential Glycemic Effects of Low- versus High-Glycemic Index Mediterranean-Style Eating Patterns in Adults at Risk for Type 2 Diabetes: The MEDGI-Carb Randomized Controlled Trial. Nutrients, 14(3), 706. https://doi.org/10.3390/nu14030706








