Intermittent Fasting and Metabolic Health
Abstract
:1. Introduction
2. Methods
3. Metabolic Syndrome and Common Diet Approaches
4. Intermittent Fasting vs. Caloric Restriction
5. Approaches to Intermittent Fasting
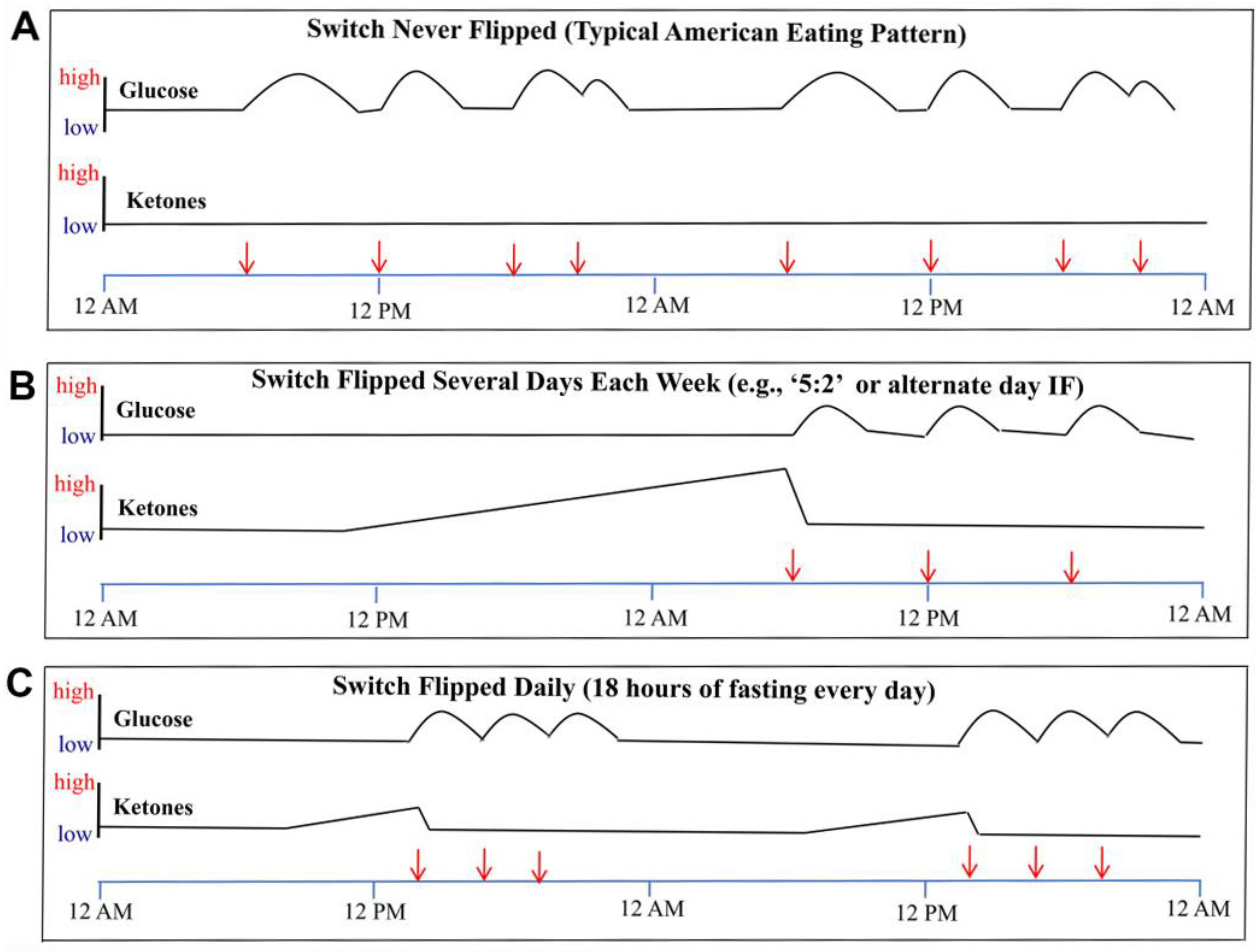
6. Physiology of Intermittent Fasting
7. Intermittent Fasting and Weight Loss
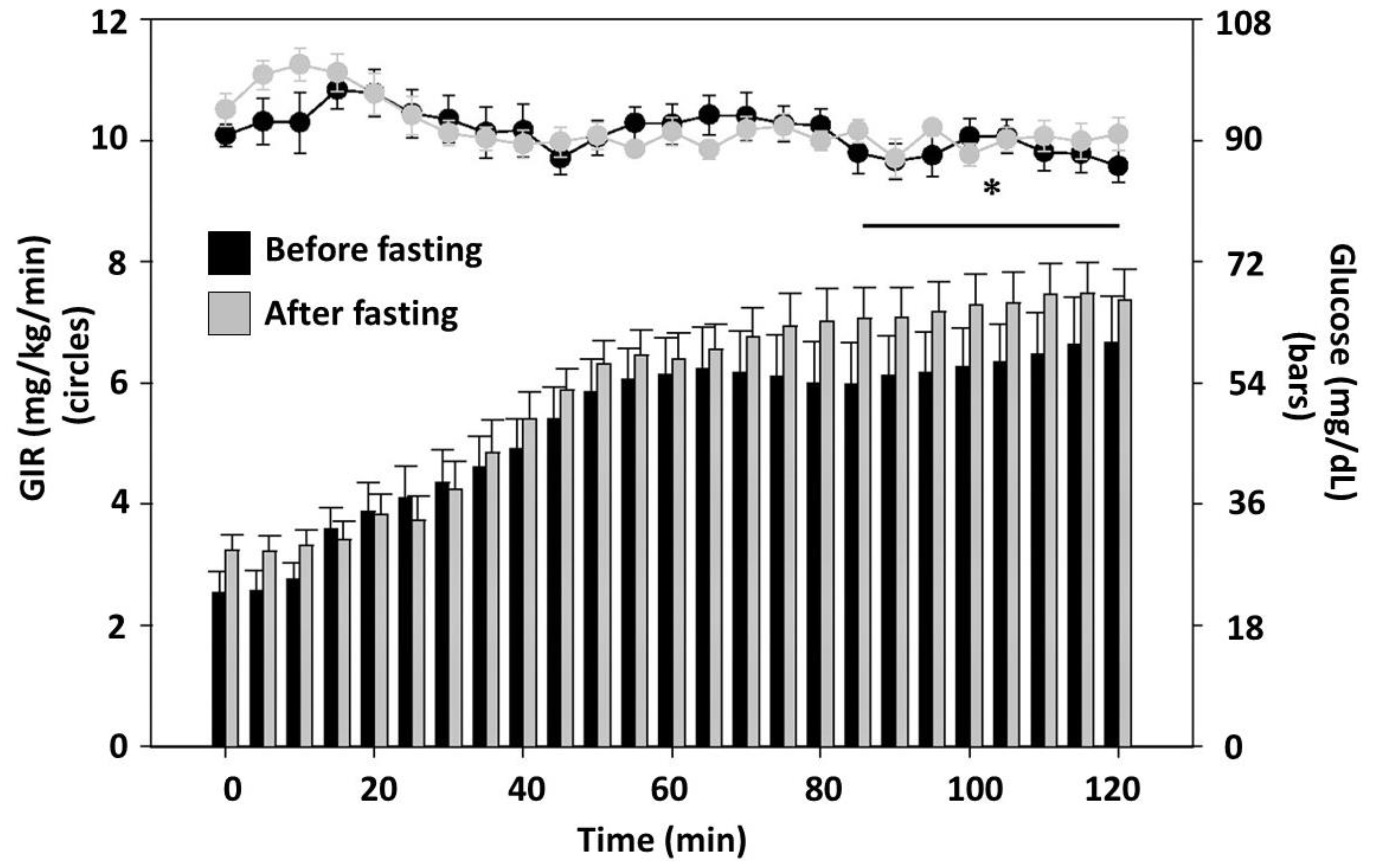
8. Intermittent Fasting and Insulin Resistance, Diabetes and Prediabetes
9. Intermittent Fasting and Cardiovascular Risk
9.1. Lipids—Mechanism of Action
9.2. HTN—Mechanism of Action
9.3. Inflammation—Mechanism of Action
10. Feasibility of Intermittent Fasting in Modern Lifestyle
11. Intermittent Fasting—Reason for Caution
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Withrow, D.; Alter, D.A. The economic burden of obesity worldwide: A systematic review of the direct costs of obesity. Obes. Rev. 2011, 12, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Cornier, M.A.; Dabelea, D.; Hernandez, T.L.; Lindstrom, R.C.; Steig, A.J.; Stob, N.R.; Van Pelt, R.E.; Wang, H.; Eckel, R.H. The metabolic syndrome. Endocr. Rev. 2008, 29, 777–822. [Google Scholar] [CrossRef] [PubMed]
- Malinowski, B.; Zalewska, K.; Węsierska, A.; Sokołowska, M.M.; Socha, M.; Liczner, G.; Pawlak-Osińska, K.; Wiciński, M. Intermittent Fasting in Cardiovascular Disorders-An Overview. Nutrients 2019, 11, 673. [Google Scholar] [CrossRef] [Green Version]
- DeBoer, M.D. Obesity, systemic inflammation, and increased risk for cardiovascular disease and diabetes among adolescents: A need for screening tools to target interventions. Nutrition 2013, 29, 379–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.H.; Lee, D.K.; Liu, M.; Portincasa, P.; Wang, D.Q. Novel Insights into the Pathogenesis and Management of the Metabolic Syndrome. Pediatr. Gastroenterol. Hepatol. Nutr. 2020, 23, 189–230. [Google Scholar] [CrossRef]
- Gurka, M.J.; Filipp, S.L.; DeBoer, M.D. Geographical variation in the prevalence of obesity, metabolic syndrome, and diabetes among US adults. Nutr. Diabetes 2018, 8, 14. [Google Scholar] [CrossRef] [Green Version]
- Tabák, A.G.; Herder, C.; Rathmann, W.; Brunner, E.J.; Kivimäki, M. Prediabetes: A high-risk state for diabetes development. Lancet 2012, 379, 2279–2290. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Beydoun, M.A.; Liang, L.; Caballero, B.; Kumanyika, S.K. Will all Americans become overweight or obese? estimating the progression and cost of the US obesity epidemic. Obesity 2008, 16, 2323–2330. [Google Scholar] [CrossRef]
- Ward, Z.J.; Bleich, S.N.; Cradock, A.L.; Barrett, J.L.; Giles, C.M.; Flax, C.; Long, M.W.; Gortmaker, S.L. Projected U.S. State-Level Prevalence of Adult Obesity and Severe Obesity. N. Engl. J. Med. 2019, 381, 2440–2450. [Google Scholar] [CrossRef]
- DeBoer, M.D. Assessing and Managing the Metabolic Syndrome in Children and Adolescents. Nutrients 2019, 11, 1788. [Google Scholar] [CrossRef] [Green Version]
- Nikolopoulou, A.; Kadoglou, N.P. Obesity and metabolic syndrome as related to cardiovascular disease. Expert Rev. Cardiovasc. Ther. 2012, 10, 933–939. [Google Scholar] [CrossRef]
- Pérez-Martínez, P.; Mikhailidis, D.P.; Athyros, V.G.; Bullo, M.; Couture, P.; Covas, M.I.; de Koning, L.; Delgado-Lista, J.; Díaz-López, A.; Drevon, C.A.; et al. Lifestyle recommendations for the prevention and management of metabolic syndrome: An international panel recommendation. Nutr. Rev. 2017, 75, 307–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uusitupa, M.; Hermansen, K.; Savolainen, M.J.; Schwab, U.; Kolehmainen, M.; Brader, L.; Mortensen, L.S.; Cloetens, L.; Johansson-Persson, A.; Onning, G.; et al. Effects of an isocaloric healthy Nordic diet on insulin sensitivity, lipid profile and inflammation markers in metabolic syndrome—A randomized study (SYSDIET). J. Intern. Med. 2013, 274, 52–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerndt, P.R.; Naughton, J.L.; Driscoll, C.E.; Loxterkamp, D.A. Fasting: The history, pathophysiology and complications. West. J. Med. 1982, 137, 379–399. [Google Scholar] [PubMed]
- Nowosad, K.; Sujka, M. Effect of Various Types of Intermittent Fasting (IF) on Weight Loss and Improvement of Diabetic Parameters in Human. Curr. Nutr. Rep. 2021, 10, 146–154. [Google Scholar] [CrossRef]
- Anton, S.D.; Moehl, K.; Donahoo, W.T.; Marosi, K.; Lee, S.A.; Mainous, A.G.; Leeuwenburgh, C.; Mattson, M.P. Flipping the Metabolic Switch: Understanding and Applying the Health Benefits of Fasting. Obesity 2018, 26, 254–268. [Google Scholar] [CrossRef]
- Świątkiewicz, I.; Woźniak, A.; Taub, P.R. Time-Restricted Eating and Metabolic Syndrome: Current Status and Future Perspectives. Nutrients 2021, 13, 221. [Google Scholar] [CrossRef]
- Asher, G.; Sassone-Corsi, P. Time for food: The intimate interplay between nutrition, metabolism, and the circadian clock. Cell 2015, 161, 84–92. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Tang, Q.; Chen, G.; Xie, M.; Yu, S.; Zhao, J.; Chen, L. New Insights Into the Circadian Rhythm and Its Related Diseases. Front. Physiol. 2019, 10, 682. [Google Scholar] [CrossRef] [Green Version]
- Panda, S. Circadian physiology of metabolism. Science 2016, 354, 1008–1015. [Google Scholar] [CrossRef] [Green Version]
- Chung, H.; Chou, W.; Sears, D.D.; Patterson, R.E.; Webster, N.J.; Ellies, L.G. Time-restricted feeding improves insulin resistance and hepatic steatosis in a mouse model of postmenopausal obesity. Metabolism 2016, 65, 1743–1754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gill, S.; Le, H.D.; Melkani, G.C.; Panda, S. Time-restricted feeding attenuates age-related cardiac decline in Drosophila. Science 2015, 347, 1265–1269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaix, A.; Zarrinpar, A.; Miu, P.; Panda, S. Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab. 2014, 2, 991–1005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McAllister, M.J.; Pigg, B.L.; Renteria, L.I.; Waldman, H.S. Time-restricted feeding improves markers of cardiometabolic health in physically active college-age men: A 4-week randomized pre-post pilot study. Nutr. Res. 2020, 75, 32–43. [Google Scholar] [CrossRef]
- Moro, T.; Tinsley, G.; Bianco, A.; Marcolin, G.; Pacelli, Q.F.; Battaglia, G.; Palma, A.; Gentil, P.; Neri, M.; Paoli, A. Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. J. Transl. Med. 2016, 14, 290. [Google Scholar] [CrossRef] [PubMed]
- Tinsley, G.M.; Forsse, J.S.; Butler, N.K.; Paoli, A.; Bane, A.A.; La Bounty, P.M.; Morgan, G.B.; Grandjean, P.W. Time-restricted feeding in young men performing resistance training: A randomized controlled trial. Eur. J. Sport Sci. 2017, 17, 200–207. [Google Scholar] [CrossRef]
- Tinsley, G.M.; Moore, M.L.; Graybeal, A.J.; Paoli, A.; Kim, Y.; Gonzales, J.U.; Harry, J.R.; VanDusseldorp, T.A.; Kennedy, D.N.; Cruz, M.R. Time-restricted feeding plus resistance training in active females: A randomized trial. Am. J. Clin. Nutr. 2019, 110, 628–640. [Google Scholar] [CrossRef] [Green Version]
- Martens, C.R.; Rossman, M.J.; Mazzo, M.R.; Jankowski, L.R.; Nagy, E.E.; Denman, B.A.; Richey, J.J.; Johnson, S.A.; Ziemba, B.P.; Wang, Y.; et al. Short-term time-restricted feeding is safe and feasible in non-obese healthy midlife and older adults. Geroscience 2020, 42, 667–686. [Google Scholar] [CrossRef]
- Randle, P.J.; Garland, P.B.; Hales, C.N.; Newsholem, E.A. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1963, 1, 785–789. [Google Scholar] [CrossRef]
- Stockman, M.C.; Thomas, D.; Burke, J.; Apovian, C.M. Intermittent Fasting: Is the Wait Worth the Weight? Curr. Obes. Rep. 2018, 7, 172–185. [Google Scholar] [CrossRef]
- Meier, U.; Gressner, A.M. Endocrine regulation of energy metabolism: Review of pathobiochemical and clinical chemical aspects of leptin, ghrelin, adiponectin, and resistin. Clin. Chem. 2004, 50, 1511–1525. [Google Scholar] [CrossRef]
- de Cabo, R.; Mattson, M.P. Effects of Intermittent Fasting on Health, Aging, and Disease. N. Engl. J. Med. 2019, 381, 2541–2551. [Google Scholar] [CrossRef] [PubMed]
- Trepanowski, J.F.; Kroeger, C.M.; Barnosky, A.; Klempel, M.C.; Bhutani, S.; Hoddy, K.K.; Gabel, K.; Freels, S.; Rigdon, J.; Rood, J.; et al. Effect of Alternate-Day Fasting on Weight Loss, Weight Maintenance, and Cardioprotection Among Metabolically Healthy Obese Adults: A Randomized Clinical Trial. JAMA Intern. Med. 2017, 177, 930–938. [Google Scholar] [CrossRef] [PubMed]
- Albosta, M.; Bakke, J. Intermittent fasting: Is there a role in the treatment of diabetes? A review of the literature and guide for primary care physicians. Clin. Diabetes Endocrinol. 2021, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Halberg, N.; Henriksen, M.; Söderhamn, N.; Stallknecht, B.; Ploug, T.; Schjerling, P.; Dela, F. Effect of intermittent fasting and refeeding on insulin action in healthy men. J. Appl. Physiol. 2005, 99, 2128–2136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catenacci, V.A.; Pan, Z.; Ostendorf, D.; Brannon, S.; Gozansky, W.S.; Mattson, M.P.; Martin, B.; MacLean, P.S.; Melanson, E.L.; Troy Donahoo, W. A randomized pilot study comparing zero-calorie alternate-day fasting to daily caloric restriction in adults with obesity. Obesity 2016, 24, 1874–1883. [Google Scholar] [CrossRef]
- Heilbronn, L.K.; Smith, S.R.; Martin, C.K.; Anton, S.D.; Ravussin, E. Alternate-day fasting in nonobese subjects: Effects on body weight, body composition, and energy metabolism. Am. J. Clin. Nutr. 2005, 81, 69–73. [Google Scholar] [CrossRef]
- Heilbronn, L.K.; Civitarese, A.E.; Bogacka, I.; Smith, S.R.; Hulver, M.; Ravussin, E. Glucose tolerance and skeletal muscle gene expression in response to alternate day fasting. Obes. Res. 2005, 13, 574–581. [Google Scholar] [CrossRef]
- Bhutani, S.; Klempel, M.C.; Kroeger, C.M.; Trepanowski, J.F.; Varady, K.A. Alternate day fasting and endurance exercise combine to reduce body weight and favorably alter plasma lipids in obese humans. Obesity 2013, 21, 1370–1379. [Google Scholar] [CrossRef]
- Varady, K.A.; Bhutani, S.; Klempel, M.C.; Kroeger, C.M.; Trepanowski, J.F.; Haus, J.M.; Hoddy, K.K.; Calvo, Y. Alternate day fasting for weight loss in normal weight and overweight subjects: A randomized controlled trial. Nutr. J. 2013, 12, 146. [Google Scholar] [CrossRef] [Green Version]
- Eshghinia, S.; Mohammadzadeh, F. The effects of modified alternate-day fasting diet on weight loss and CAD risk factors in overweight and obese women. J. Diabetes Metab. Disord. 2013, 12, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoddy, K.K.; Kroeger, C.M.; Trepanowski, J.F.; Barnosky, A.; Bhutani, S.; Varady, K.A. Meal timing during alternate day fasting: Impact on body weight and cardiovascular disease risk in obese adults. Obesity 2014, 22, 2524–2531. [Google Scholar] [CrossRef] [PubMed]
- Hoddy, K.K.; Gibbons, C.; Kroeger, C.M.; Trepanowski, J.F.; Barnosky, A.; Bhutani, S.; Gabel, K.; Finlayson, G.; Varady, K.A. Changes in hunger and fullness in relation to gut peptides before and after 8 weeks of alternate day fasting. Clin. Nutr. 2016, 35, 1380–1385. [Google Scholar] [CrossRef] [PubMed]
- Kroeger, C.M.; Trepanowski, J.F.; Klempel, M.C.; Barnosky, A.; Bhutani, S.; Gabel, K.; Varady, K.A. Eating behavior traits of successful weight losers during 12 months of alternate-day fasting: An exploratory analysis of a randomized controlled trial. Nutr. Health 2018, 24, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Trepanowski, J.F.; Kroeger, C.M.; Barnosky, A.; Klempel, M.; Bhutani, S.; Hoddy, K.K.; Rood, J.; Ravussin, E.; Varady, K.A. Effects of alternate-day fasting or daily calorie restriction on body composition, fat distribution, and circulating adipokines: Secondary analysis of a randomized controlled trial. Clin. Nutr. 2018, 37, 1871–1878. [Google Scholar] [CrossRef]
- Varady, K.A.; Bhutani, S.; Church, E.C.; Klempel, M.C. Short-term modified alternate-day fasting: A novel dietary strategy for weight loss and cardioprotection in obese adults. Am. J. Clin. Nutr. 2009, 90, 1138–1143. [Google Scholar] [CrossRef] [Green Version]
- Carter, S.; Clifton, P.M.; Keogh, J.B. The effects of intermittent compared to continuous energy restriction on glycaemic control in type 2 diabetes; a pragmatic pilot trial. Diabetes Res. Clin. Pract. 2016, 122, 106–112. [Google Scholar] [CrossRef]
- Harvie, M.N.; Pegington, M.; Mattson, M.P.; Frystyk, J.; Dillon, B.; Evans, G.; Cuzick, J.; Jebb, S.A.; Martin, B.; Cutler, R.G.; et al. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: A randomized trial in young overweight women. Int. J. Obes. 2011, 35, 714–727. [Google Scholar] [CrossRef] [Green Version]
- Harvie, M.; Wright, C.; Pegington, M.; McMullan, D.; Mitchell, E.; Martin, B.; Cutler, R.G.; Evans, G.; Whiteside, S.; Maudsley, S.; et al. The effect of intermittent energy and carbohydrate restriction v. daily energy restriction on weight loss and metabolic disease risk markers in overweight women. Br. J. Nutr. 2013, 110, 1534–1547. [Google Scholar] [CrossRef] [Green Version]
- Harvie, M.N.; Howell, T. Could Intermittent Energy Restriction and Intermittent Fasting Reduce Rates of Cancer in Obese, Overweight, and Normal-Weight Subjects? A Summary of Evidence. Adv. Nutr. 2016, 7, 690–705. [Google Scholar] [CrossRef]
- Sutton, E.F.; Beyl, R.; Early, K.S.; Cefalu, W.T.; Ravussin, E.; Peterson, C.M. Early Time-Restricted Feeding Improves Insulin Sensitivity, Blood Pressure, and Oxidative Stress Even without Weight Loss in Men with Prediabetes. Cell Metab. 2018, 27, 1212–1221.e1213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janssen, I.; Powell, L.H.; Crawford, S.; Lasley, B.; Sutton-Tyrrell, K. Menopause and the metabolic syndrome: The Study of Women’s Health Across the Nation. Arch. Intern. Med. 2008, 168, 1568–1575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carter, S.; Clifton, P.M.; Keogh, J.B. Effect of Intermittent Compared With Continuous Energy Restricted Diet on Glycemic Control in Patients With Type 2 Diabetes: A Randomized Noninferiority Trial. JAMA Netw. Open 2018, 1, e180756. [Google Scholar] [CrossRef] [PubMed]
- Shulman, G.I. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N. Engl. J. Med. 2014, 371, 2237–2238. [Google Scholar] [CrossRef] [PubMed]
- Ingelsson, E.; Sundström, J.; Arnlöv, J.; Zethelius, B.; Lind, L. Insulin resistance and risk of congestive heart failure. JAMA 2005, 294, 334–341. [Google Scholar] [CrossRef] [Green Version]
- Santos, H.O.; Macedo, R.C.O. Impact of intermittent fasting on the lipid profile: Assessment associated with diet and weight loss. Clin. Nutr. ESPEN 2018, 24, 14–21. [Google Scholar] [CrossRef]
- Wan, R.; Weigand, L.A.; Bateman, R.; Griffioen, K.; Mendelowitz, D.; Mattson, M.P. Evidence that BDNF regulates heart rate by a mechanism involving increased brainstem parasympathetic neuron excitability. J. Neurochem. 2014, 129, 573–580. [Google Scholar] [CrossRef] [Green Version]
- Teng, N.I.; Shahar, S.; Rajab, N.F.; Manaf, Z.A.; Johari, M.H.; Ngah, W.Z. Improvement of metabolic parameters in healthy older adult men following a fasting calorie restriction intervention. Aging Male 2013, 16, 177–183. [Google Scholar] [CrossRef]
- Erdem, Y.; Özkan, G.; Ulusoy, Ş.; Arıcı, M.; Derici, Ü.; Şengül, Ş.; Sindel, Ş.; Ertürk, Ş.; Diseases, T.S.o.H.a.R. The effect of intermittent fasting on blood pressure variability in patients with newly diagnosed hypertension or prehypertension. J. Am. Soc. Hypertens 2018, 12, 42–49. [Google Scholar] [CrossRef]
- Kesztyüs, D.; Vorwieger, E.; Schönsteiner, D.; Gulich, M.; Kesztyüs, T. Applicability of time-restricted eating for the prevention of lifestyle-dependent diseases in a working population: Results of a pilot study in a pre-post design. Ger. Med. Sci. 2021, 19, 4. [Google Scholar] [CrossRef]
- Wegman, M.; Guo, M.H.; Bennion, D.M.; Shankar, M.N.; Chrzanowski, S.; Goldberg, L.A.; Xu, J.; Williams, T.A.; Lu, X.; Hsu, S.I.; et al. Practicality of Intermittent Fasting in Humans and its Effect on Oxidative Stress and Genes Related to Aging and Metabolism. Rejuvenation Res. 2015, 18, 162–172. [Google Scholar] [CrossRef] [Green Version]
- Beshyah, S.A.; Hassanein, M.; Ahmedani, M.Y.; Shaikh, S.; Ba-Essa, E.M.; Megallaa, M.H.; Afandi, B.; Ibrahim, F.; Al-Muzaffar, T. Diabetic hypoglycaemia during Ramadan fasting: A trans-national observational real-world study. Diabetes Res. Clin. Pract. 2019, 150, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Dardano, A.; Penno, G.; Del Prato, S.; Miccoli, R. Optimal therapy of type 2 diabetes: A controversial challenge. Aging 2014, 6, 187–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, M.E.; Williamson, J.D.; Gerstein, H.C.; Byington, R.P.; Cushman, W.C.; Ginsberg, H.N.; Ambrosius, W.T.; Lovato, L.; Applegate, W.B. Effects of randomization to intensive glucose control on adverse events, cardiovascular disease, and mortality in older versus younger adults in the ACCORD Trial. Diabetes Care 2014, 37, 634–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Protocol | Frequency | Duration | Additional Considerations |
|---|---|---|---|
| Alternate day | Every other day | 24 h | |
| 5:2 | Two days weekly | 24 h each day | 2 other days involve a very low calorie diet |
| Time-restricted feeding | Every day | 14–18 h | Food is consumed over a 6-h period |
| B2 regimen | Everyday | 14 h | 2 large meals per day: breakfast between 06:00 a.m. and 10:00 a.m. and lunch between 12:00 p.m. and 04:00 p.m. and no dinner |
| Weekly 1 day Fasting | Once a week | 24 h | Water-only diet 1 day per week and regular eating on the other 6 days of the week |
| Intermittent VLCD therapy | Variable | 24 h | 1-d VLCD: VLCD for 1 day a week and 5-d VLCD: VLCD for 5 consecutive days a week, repeated every 5 weeks. * |
| Case Series | Effects of IF vs. Baseline |
|---|---|
| Furmli et al. (7 months) | Baseline Values: HbA1C: 11%, 96.7 mmol/mol Body weight: 83.8 kg After 7 months of intermittent fasting: - HbA1C decreased by 4% - Body weight decreased by 10 kg |
| Furmli et al. (11 months) | Average Baseline Values: HbA1C: 6.8%, 50.8 mmol/mol Body weight: 97.1 kg After 11 months of intermittent fasting: - HbA1C decreased by 1.2% - Body weight decreased by 10.6 kg |
| Lichtash et al. (14 months) | Average Baseline Values HbA1C: 9.3% Body Weight: 55.3 kg After 14 months of intermittent fasting: - HbA1C decreased by 3.5% - Body weight decreased by 1.8 kg |
| Authors (Year) | Number Enrolled | Study Design/Fasting Protocol Used | Description of Participants | Study Duration | Effect on Lipids | Effect on BP | Effect on Inflammatory Markers |
|---|---|---|---|---|---|---|---|
| Harvie et al. (2011) [48] | 107 | RCT Intermittent energy restriction | Overweight or obese women (premenopausal) | 6 months | ↓TC (p < 0.01); NS: LDL, TGs, HDL | ↓Systolic (p = 0.99); ↓Diastolic (p = 0.84) | |
| Varady et al. (2013) [40] | 15 | RCT Alternate day fasting | Individuals with BMI 20–29.9 kg/m2 | 12 weeks | ↓LDL (p < 0.01); ↓TGs (p < 0.01); NS: HDL | ↓ (p = 0.51) | ↓CRP (p = 0.01) ↓Leptin (p = 0.03) ↑Adiponectin (p < 0.01) |
| Bhutani et al. (2013) [39] | 83 | RCT Alternate day fasting plus endurance exercise (exercise control) | Individuals with obesity BMI 30–39.9 kg/m2 | 12 weeks | ↓LDL (p < 0.05); ↑HDL (p < 0.05); NS: TC, TGs | ↓Systolic (p = 0.254); ↓Diastolic (p = 0.570) | NS CRP |
| Eshghinia et al. (2013) [41] | 15 | Observation over 8 weeks with alternating day fasting | Overweight or obese women BMI ≥ 25 kg/m2 | 8 weeks | NS: LDL, TGs, HDL | ↓Systolic (p < 0.001) | |
| Teng et al. (2013) [58] | 28 | RCT Fasting caloric restriction (300–500 cal/day) vs. control | Men in Malaysia BMI 23–29.9 kg/m2 | 12 weeks | ↓TC (p < 0.001) ↓LDL (p < 0.05) NS: HDL, TGs | ↓Systolic (p < 0.05); ↓Diastolic (p < 0.05) | |
| Harvie et al. (2013) [49] | 77 | RCT Intermittent energy and carbohydrate restriction vs. control | Overweight or obese women | 3 months | NS: LDL, TGs, HDL | NS: IL6, TNFα, leptin, adiponectin | |
| Erdem et al. (2018) [59] | 60 | Prospective cohort (observational study) | Individuals from the Cappadocia cohort with prehypertension and hypertension (SBP 120–139 and ≥140; DBP 80–89 and ≥90 mmHg | At least 1 week | ↓Systolic (p < 0.001); ↓Diastolic (p < 0.039) | ||
| Hoddy et al. (2016) [43] | 59 | 8 week alternating day fasting Protocol | Obese individuals BMI 30–39.9 kg/m2 | 8 weeks | ↓Leptin (p < 0.05) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasim, I.; Majeed, C.N.; DeBoer, M.D. Intermittent Fasting and Metabolic Health. Nutrients 2022, 14, 631. https://doi.org/10.3390/nu14030631
Vasim I, Majeed CN, DeBoer MD. Intermittent Fasting and Metabolic Health. Nutrients. 2022; 14(3):631. https://doi.org/10.3390/nu14030631
Chicago/Turabian StyleVasim, Izzah, Chaudry N. Majeed, and Mark D. DeBoer. 2022. "Intermittent Fasting and Metabolic Health" Nutrients 14, no. 3: 631. https://doi.org/10.3390/nu14030631
APA StyleVasim, I., Majeed, C. N., & DeBoer, M. D. (2022). Intermittent Fasting and Metabolic Health. Nutrients, 14(3), 631. https://doi.org/10.3390/nu14030631





