Dietetic Management of Adults with Phenylketonuria (PKU) in the UK: A Care Consensus Document
Abstract
:1. Introduction
2. Materials and Methods
3. Results
- Aims of dietetic care.
- Dietetic assessment for patient on and off treatment.
- Interventions, including the following:
- Protein substitutes.
- Avoidance of foods high in phenylalanine.
- Prescribed special low-protein foods, e.g., low-protein bread or pasta.
- Importance of including naturally low-protein foods, such as fruits and vegetables.
- Specific considerations for females.
- Adults not on treatment.
- Those returning to diet.
- Late treated PKU starting back on diet.
- Weight management/obesity.
- Eating disorders.
- Blood phenylalanine monitoring.
- Nutritional blood biochemistry/nutritional status.
- Patient follow-up.
- Dietetic contact with patients between outpatient appointments.
- Signposting to other services.
- Discharge or transfer from service.
- Outcome measures.
- Resources.
4. Discussion
- Calculation of protein requirements
| Parameter | Evidence Supporting | Protein Requirement Recommendations | |
|---|---|---|---|
| 1 | Safe protein intake per kilogram of body weight per day | [23] | 0.83 g/kg/day |
| 2 | Reference nutrient intake for protein | [26] | 0.75 g/kg/day |
| 3 | Use of Indicator Amino Acid Oxidation Method for protein requirement calculation | [27] | 0.93–1.2 g/kg/day |
| 4 | Appropriate protein requirements for older adults (> 65 years) | [26,28] | 1.2–1.5 g/kg/day |
| 5 | Protein requirements for injury and disease (adults) | [28] | 1–1.5 g/kg/day |
| 6 | Appropriate protein requirement adjustments for obesity | [3,28] | BMI > 30–75% of calculated requirements for actual body weight BMI > 50–65% of calculated requirements for actual body weight |
- Weight management and Obesity
- Disordered eating and eating disorders
- Reporting Blood Phenylalanine Concentrations
- Non-Dietary Treatments for PKU
- Maintaining Patient Engagement and Avoidance of Patients Being “Lost to Follow Up”
- Shared Care
- Monitoring and assurance of the SOP
- Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
- Glossary;
- Scope;
- Introduction to the clinical SOP;
- Aims and objectives of this SOP;
- Duties of the AIMD Dietitian;
- SOP delivery and implementation;
- 6.1
- Key stakeholders;
- 6.2
- Dietetic assessment and interventions for adults with PKU;
- 6.2.1
- Dietetic Assessment
- 6.2.2
- Interventions
- 6.2.3
- Follow up
- 6.2.4
- Potential outcome measures
- 6.2.5
- Resources
- Monitoring and assurance;
- Patient: patient or patient advocate.
- Adult Inherited Metabolic Disorders Dietitian (AIMD dietitian): a dietitian who works with adults who have an inherited metabolic disorder.
- Patient-centered approach: treating the patient as an individual and an equal partner in the healthcare management.
- Psychosocial: how social conditions affect mental health or how someone copes with PKU.
- Neurocognitive: the ability to think and reason. This includes the ability to concentrate, remember things, process information, and understand.
- Capacity: to use and understand information to make and communicate a decision.
- Protein substitutes: a medical food containing all amino acids, except/very small amount of phenylalanine.
- Prescribed low-protein foods: foods manufactured to be very low in protein only found on prescription in the UK.
- Phenylalanine exchange: one exchange is the amount of food that contains 1 g of protein or 50 mg phenylalanine.
- NSPKU: The National Society for Phenylketonuria—patient society in the UK.
- BIMDG: The British Inherited Metabolic Diseases Group—health-professionals interest group in the UK.
- ACBS: Advisory Committee on Borderline Substances—The ACBS is responsible for advising on the prescribing of borderline substances for use in the NHS primary care. Borderline substances are nutritional or dermatological products that have been specially formulated to manage medical conditions.
- This SOP outlines the dietitian care pathway for adult patients (16+ years) with Phenylketonuria (PKU) under the care of Adult Inherited Metabolic Disease (AIMD) teams in the UK.
- This SOP does not cover management of maternal and preconception patients with PKU.
- This SOP does not cover the management of acute inpatient admissions
- The role of the AIMD dietitian in PKU care is highlighted.
- This document is to be used with the clinical judgment of the dietitian to tailor it to the adult with PKU.
- The roles of other healthcare professionals are noted, although the entirety of their role in the pathway has not been included.
- PKU is the most common inborn error of protein metabolism with an incidence of approximately 1 in 10,000 births, with varying incidence across the UK. Management by restricted dietary intake of phenylalanine (natural protein), along with supplemented phenylalanine-free amino acids (L-AA) or glycomacropeptide (GMP) [3,21], remains the mainstay of management in the UK.
- The current European Phenylketonuria Guidelines [3] recommend that all patients with PKU remain on treatment/restricted diet for life if phenylalanine is >600 µmol/L without treatment.
- Across the UK, there are recognized adult metabolic centers.
- This procedure is necessary to achieve the following:
- ○
- Standardize the dietetic care of all adult patients with PKU across the UK.
- ○
- Provide a framework to support the dietitian’s decision-making around treatment of patients with PKU.
- ○
- Assist development and supervision of AIMD dietitians in the UK and ensure that all AIMD dietitians are providing equal standards of care to patients with PKU.
- To outline the role of the AIMD dietetic team in provision of care to adults with PKU.
- To ensure equity of patient care throughout the UK.
- To agree on standards for patient care to ensure patient safety and optimal care provision by referring to European PKU guidelines 2017 [3].
- To outline the service provision required to provide optimal care.
- It is the responsibility of the AIMD dietetic service to implement the procedures and provide best practice care, as outlined in this document.
- All members of the AIMD dietetic team have a role in advocating for adults with PKU to receive the care that is aligned with this document, unless this is otherwise indicated in the course of the review.
- All members of the AIMD dietetic team are required to escalate any patient management not within the dietetic scope of practice to an appropriate IMD team member.
- The AIMD Dietitians in AIMD centers across the UK.
- The metabolic team, including physician, clinical nurse specialist, and dietetic assistant to support implementation of the SOP.
- Patients, their families, carers and advocates.
- The British Inherited Metabolic Diseases Group (BIMDG).
- National Society for Phenylketonuria (NSPKU).
- To optimize normal neurocognitive and psychosocial functioning for the patient.
- To support adults with PKU identify their personal aims and goals through the lifecycle.
- To ensure the patient is fully informed on best practice management of PKU in accordance with European PKU Guidelines 2017 [3] and to support the patient to make informed treatment decisions.
- To educate on how to maintain phenylalanine levels between 120 and 600 µmol/L [1].
- To encourage lifelong PKU management.
- To ensure the diet is nutritionally adequate.
- To help promote a healthy weight.
- Check identification of the patient and seek consent for assessment.
- Medical/surgical history.
- Psychosocial considerations, e.g., change of living circumstances.
- Anthropometry: weight, height, and body mass index.
- Current clinical issues.
- Relevant medications, including protein substitutes and prescribed low-protein foods.
- Biochemistry: nutritional status bloods (refer to European PKU guidelines [3]) and history of blood phenylalanine monitoring.
- Diet history, including the following:
- -
- Total protein intake (including food sources) and distribution over the day; prescribed and actual intake.
- -
- Quantity and timing of protein substitute, prescribed and actual intake.
- -
- How much low-protein food is being used and confidence with incorporating low-protein foods in the diet.
- -
- Menu planning and cooking skills.
- -
- Home delivery/local dispensing of protein substitutes and low-protein foods.
- -
- Discussion regarding patient’s regulation of protein intake, e.g., if he or she is using phenylalanine exchange system/counting grams of protein.
- -
- Meal timings.
- -
- Additional vitamin and mineral, omega 3 supplementation, and history of nutritional deficiencies.
- -
- Overall dietary adequacy, including assessment of total energy intake.
- Patient- and non-patient-related factors affecting treatment management and any specific concerns the patient has relating to his or her PKU.
- Discussion regarding prescription charges (if appropriate).
- To explore relationships with food if concerns are raised.
- Calculated protein requirements; refer to the European PKU guidelines [3].
- Check identification of the patient and seek consent for assessment.
- Medical/surgical history.
- Psychosocial issues.
- Anthropometry: weight, height, and body mass index.
- Current clinical issues.
- Medication (including non-prescribed medications, e.g., herbal remedies and probiotics).
- Biochemistry: nutritional bloods (and history of blood phenylalanine monitoring).
- Diet history, including the following:
- -
- Total protein intake and distribution.
- -
- Meal timings
- -
- Any extra nutritional supplementation of vitamins and minerals, trace elements, and omega 3.
- -
- Overall dietary adequacy.
- -
- Protein aversion.
- Patient- and non-patient-related factors affecting treatment management and any specific concerns the patient has relating to his or her PKU.
- Exploring barriers to being on treatment.
- Patient education/update on the management of PKU.
- Advise on adequate dose.
- Consider nutritional composition of prescribed protein substitute intake; is it nutritionally complete or is additional micronutrient supplementation required?
- Advise on when it should be taken.
- Advise on any new alternative protein substitutes—amino acid/GMP substitutes.
- Offer to arrange patient samples.
- Discuss tolerability and/or barriers to management adherence.
- Discuss new substitute regimen with patient (if required).
- Send prescription request letter to GP.
- Discuss collection options with patient, e.g., pharmacy or home delivery.
- Seek verbal or written permission to contact home-delivery company to register patient and update the company on the patient’s current prescription if appropriate.
- Advise GP that a home-delivery company will manage prescription requests on behalf of the patient.
- Educate patients about the practicalities of a low-phenylalanine diet, considering individual phenylalanine tolerance and patient preferences.
- Education should include the following:
- -
- Avoiding high-phenylalanine foods.
- -
- Suitable natural low-phenylalanine foods.
- -
- Measuring and counting phenylalanine exchanges.
- -
- Avoidance of aspartame and discuss suitable phenylalanine-free sweeteners.
- -
- Appropriate alcohol consumption.
- -
- Provide sufficient resources to prepare low-phenylalanine meals.
- -
- Ensure patient understands how to read food labels.
- Ensure patients receive enough supplies via ACBS prescription to meet calorie requirements and to allow variety in the diet.
- Advise patients on the availability of new special low-protein foods.
- Provide a list of special low-protein foods available on ACBS prescription.
- Advise on the NSPKU guidance—up to 50 units per month (excluding low-protein milk alternatives and protein substitutes).
- Arrange special low-protein food samples if requested.
- Outline the system on how to obtain regular supply of special low-protein foods on prescription/home delivery, as above.
- If appropriate, discuss the importance of the strict low-phenylalanine diet for pregnancy and planning a pregnancy.
- Signpost to obtaining contraception if appropriate.
- Ensure patient knows what to do if she finds out that she is pregnant.
- Ensure adequate intakes of macro- and micronutrients.
- Discuss benefits of lifelong PKU management.
- Advise on support available.
- Discuss importance of attending annual appointments and keeping in touch.
- An appropriate step-by-step patient-centered approach should be used if a patient wishes to return to dietary treatment.
- Discuss with the patient the responsibilities of the dietitian and the patient.
- If the patient has capacity.
- Baseline behaviors and functions, communication limitations, support needs, and support/care package.
- Possible previous experience of the diet, number of phenylalanine exchanges, and protein substitute used.
- Tolerance of any monitoring, i.e., finger-prick blood taking (including blood spot and capillary).
- Number of phenylalanine exchanges, items needed on prescription.
- Key personnel/carers/cooks to teach concepts of low-phenylalanine diet.
- Devise practical menu plans for care homes or equivalent.
- Anthropometric monitoring—weekly weight charts by carers.
- Review dates for carers’ feedback on any behavior changes (improvements).
- 3-month trial to identify if the diet is helping or not.
- Identify any history of previous strategies used to manage weight or restrict diet. Discuss with the patient the efficacy of these previous strategies from the perspective of weight loss. Explore impact on mental and physical health, and quality of life.
- Assess information on previous attempts at weight loss which were unsuccessful or not sustainable. Use this information to inform the current weight-management strategy.
- Identify health issues or current medical treatment which may impact on the weight-management strategy, e.g., mental-health issues, medical conditions and treatments, socioeconomic factors, age, gender, culture, ethnicity, and personal support mechanisms.
- Assess risk of comorbidities by monitoring lipid profile, blood pressure, and HbA1c (44).
- Assess understanding of the wide range of dietary and nutritional information available. This can be overwhelming and may be a barrier to a weight-management plan.
- Tailor the education and supporting information provided to aid understanding and reduce any barriers which may have formed.
- Individually tailor patient education to help the patient identify realistic goals. This should include no more than two or three diet and lifestyle changes at a time.
- Focus education to promote understanding on food choices to support a low-protein-food diet. For example, at meals, fill half the plate with vegetables, which are naturally low in protein and calories.
- Advise on the lower calorie protein substitute options and also ensure an adequate intake of the protein substitute and micronutrients. This will help ensure nutritional balance which is essential to a healthy weight loss to ensure nutritional to and supporting weight loss.
- Regular physical activity of a moderate intensity is recommended to help support and maintain health and weight loss. NICE (2014) [44] recommends 45 to 60 min exercise, for example brisk walking of cycling, per day as part of a weight loss program.
- If there are significant barriers in place towards weight loss then discuss the possibility of delaying the weight management until a more appropriate time.
- Consider referral or signposting to other services or organizations for support if this is indicated. This could include referring to a weight-management service or program that the AIMD dietitian can then adapt to suit a low-protein diet for patients with PKU.
- Identify any history of eating disorder from referral into adult service/liaise with pediatric service for more information and if any treatment received or ongoing.
- Identify common behaviors of eating disorders, i.e., missing meals; food avoidance; bingeing behaviors; and compensatory behaviors, including laxative or diet pill misuse, vomiting, or excessive exercise. Identify any issues with body image, irregular meal pattern.
- Identify any physical signs of eating disorders, e.g., excessive tiredness, feeling cold, dizzy, digestive problems, or dental problems unrelated to PKU.
- Identify if not having periods (females) unless due to contraception method or other medical conditions.
- Identity an unusually low or high body mass index (BMI).
- Any rapid weight loss.
- Whether they take part in activities associated with a high risk of eating disorders (for example, professional sport, fashion, dance, or modeling).
- Other mental-health problems.
- If appropriate, educate on effects of low-calorie intake on Phe control/adverse effect on phenylalanine levels.
- Signpost to eating disorders other local resources and charities, e.g., https://www.beateatingdisorders.org.uk/ (accessed on 24 August 2021).
- Refer to appropriate local services, e.g., GP, community mental-health team. Support patient with PKU diet if going through treatment for eating disorder alongside a specialist eating disorders Dietitian.
- Review blood phenylalanine control with patient.
- Monthly blood phenylalanine sampling is recommended to support dietetic management and understanding of blood phenylalanine control [1]. Tailored plans can be discussed with patient, e.g., increased frequency, whilst dietary changes are being made.
- Ensure patient has sufficient blood-sampling equipment.
- Support independence with taking blood samples or ensuring an appropriate plan for those unable to take their own blood samples.
- Encourage the patient to take blood samples at the same time of day so that results are comparable, i.e., fasting.
- Clinical nurse specialist can help trouble shoot issues with taking blood samples.
- Agree patient preference to receiving blood phenylalanine results as per local data governance, e.g., telephone call, text, and email.
- Aim to report the blood phenylalanine result back within 3 working days of receipt of the blood result from the lab.
- Discuss with medical consultant if these are required and refer to the European PKU guidelines [3].
- To inform and discuss any new research, treatments, or guidelines as appropriate.
- Agree to follow up with patient.
- 6–12 month appointment if on treatment.
- 12 month appointment if not on treatment.
- Less frequent follow-up arrangements may be agreed upon if appropriate (e.g., male patients with hyperphenylalaninaemia).
- The option of video or telephone appointment to be considered if appropriate.
- Contact details provided for queries or help between appointments.
- Encourage patient-led approach to seek support and information as required.
- Referral to other HCPs might be needed, e.g., psychologist.
- Signposting to services outside of the NHS, e.g., IAPT (Improving Access to Psychological Therapies (England)/mental health/GP (Northern Ireland).
- Supporting letters may be needed, e.g., for travel, applying for benefits (for example PIP), employers, etc.
- Discharge arrangements will vary between services.
- PKU is a long-term condition which should have lifelong treatment and metabolic-specialist follow-up.
- Some patients may have neurological and cognitive impairments, e.g., poor working memory, meaning that they may need extra support/reminders to attend appointments.
- Dietitians will facilitate patients transfer to another center if they relocate, e.g., university students.
- Patient experience feedback.
- Knowledge and skills of managing diet.
- Attending appointments.
- Frequency of blood phenylalanine monitoring.
- Blood phenylalanine concentrations.
- Adherence to protein substitute.
- Variety in diet.
- Healthy body weight.
- No nutritional deficiencies.
- Good quality of life, e.g., PKU Quality of Life Survey.
- NSPKU diet booklet.
- Relevant center resources.
- Picture booklets on the NSPKU website.
- Company literature/websites and recipe books.
- Apps for smart phones or tablets.
Appendix B
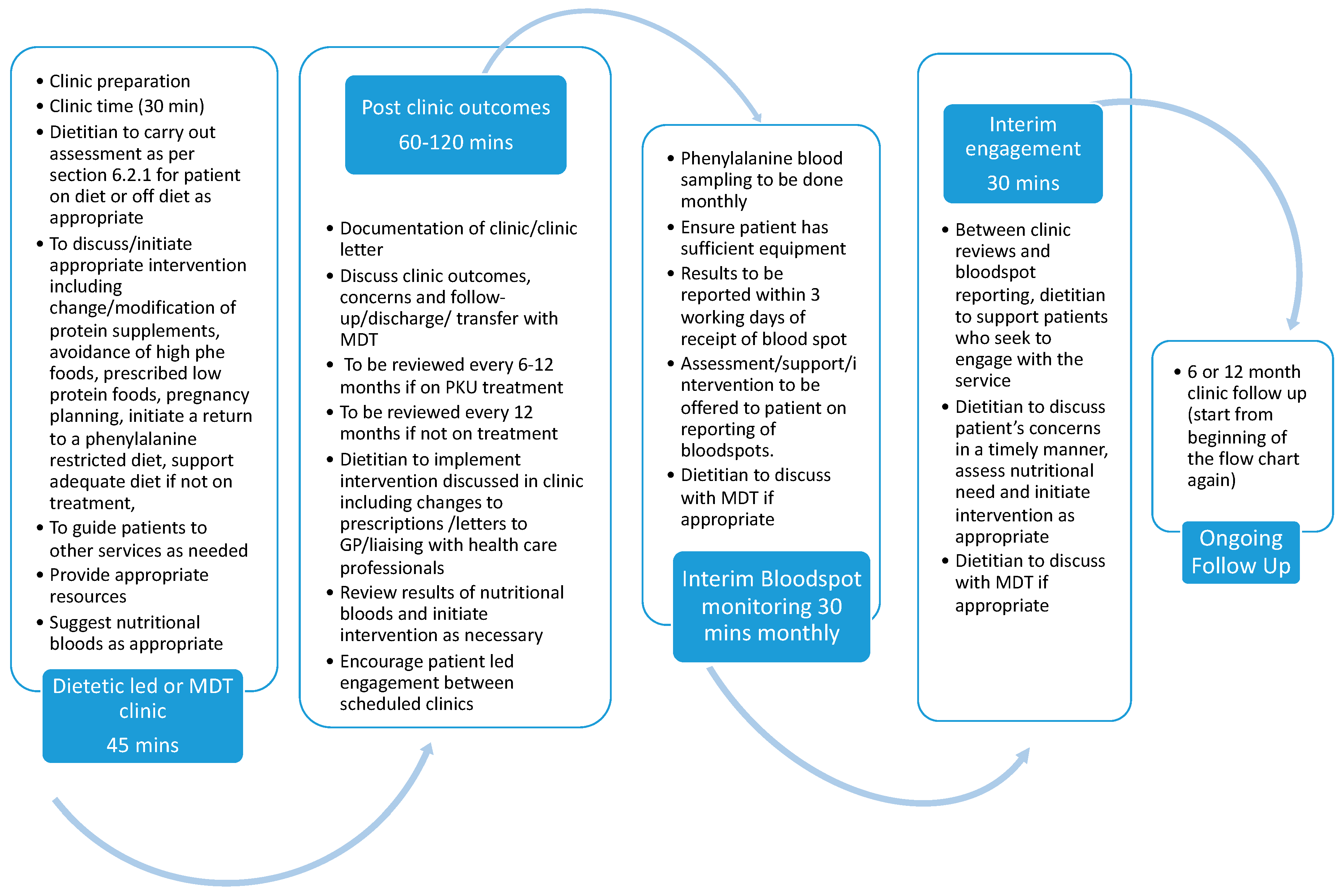
Appendix C
| Auditing adherence to the SOP for the Dietetic Management of Adults with Phenylketonuria (PKU) in the UK | ||||||
| Date audit completed: | ||||||
| Time period covered from: | To: | |||||
| Frequency of dietetic clinic consultation (MDT or Dietetic led) | ||||||
| Patient identifier | Date of most recent clinic appointment offered (D1) | Date of previous clinicappointment offered (D2) | Is the most recent appointment (D1) a rescheduled appointment due to the patient requesting a change of appointment? Y/N | If yes, what was the date of the appointment offered prior to D2 (D3) | Time (weeks) betweenappointments offered to patient (D2–D1 or D3–D1) | Recommended timeframe achieved? Y/N |
| Frequency of dietetic clinic consultation (MDT- or Dietetic-led)—simplified table | ||||||
| Patient identifier | D1 | D2 | Is D1 pt requested reschedule? Y/N | If Y, D3 | Time b/w D2–D1 or D3–D1 | Recommendation achieved? Y/N? |
| Time taken to report phenylalanine blood sampling | ||||||
| Patient identifier | Date result reported by lab (DA) | Date result reported to patient (DB) | Time (days) between date result reported by lab (DA) and date result reported to patient (DB) | Recommendation achieved? Y/N? | ||
References
- Pandor, A.; Eastham, J.; Beverley, C.; Chilcott, J.; Paisley, S. Clinical Effectiveness and Cost-Effectiveness of Neonatal Screening for Inborn Errors of Metabolism Using Tandem Mass Spectrometry: A Systematic Review. In NIHR Health Technology Assessment Programme: Executive Summaries; NIHR Journals Library: Southampton, UK, 2004; Volume 8. [Google Scholar]
- van Spronsen, F.J.; van Wegberg, A.M.J.; Ahring, K.; Bélanger-Quintana, A.; Blau, N.; Bosch, A.M.; Burlina, A.; Campistol, J.; Feillet, F.; Giżewska, M.; et al. Key European Guidelines for the Diagnosis and Management of Patients with Phenylketonuria. Lancet Diabetes Endocrinol. 2017, 5, 743–756. [Google Scholar] [CrossRef] [Green Version]
- van Wegberg, A.M.J.; MacDonald, A.; Ahring, K.; Bélanger-Quintana, A.; Blau, N.; Bosch, A.M.; Burlina, A.; Campistol, J.; Feillet, F.; Giżewska, M.; et al. The Complete European Guidelines on Phenylketonuria: Diagnosis and Treatment. Orphanet J. Rare Dis. 2017, 12, 162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enns, G.M.; Koch, R.; Brumm, V.; Blakely, E.; Suter, R.; Jurecki, E. Suboptimal Outcomes in Patients with PKU Treated Early with Diet Alone: Revisiting the Evidence. Mol. Genet. Metab. 2010, 101, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.C.J.; Guest, J.F. Economic Impact of Feeding a Phenylalanine-Restricted Diet to Adults with Previously Untreated Phenylketonuria. J. Intellect. Disabil. Res. 1999, 43, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Eijgelshoven, I.; Demirdas, S.; Smith, T.A.; van Loon, J.M.T.; Latour, S.; Bosch, A.M. The Time Consuming Nature of Phenylketonuria: A Cross-Sectional Study Investigating Time Burden and Costs of Phenylketonuria in the Netherlands. Mol. Genet. Metab. 2013, 109, 237–242. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, A.; Smith, T.A.; de Silva, S.; Alam, V.; van Loon, J.M.T. The Personal Burden for Caregivers of Children with Phenylketonuria: A Cross-Sectional Study Investigating Time Burden and Costs in the UK. Mol. Genet. Metab. Rep. 2016, 9, 1–5. [Google Scholar] [CrossRef]
- Palermo, L.; MacDonald, A.; Limback, E.; Robertson, L.; Howe, S.; Geberhiwot, T.; Romani, C. Emotional Health in Early-Treated Adults with Phenylketonuria (PKU): Relationship with Cognitive Abilities and Blood Phenylalanine. J. Clin. Exp. Neuropsychol. 2020, 42, 142–159. [Google Scholar] [CrossRef]
- Rose, A.M.; Grosse, S.D.; Garcia, S.P.; Bach, J.; Kleyn, M.; Simon, N.-J.E.; Prosser, L.A. The Financial and Time Burden Associated with Phenylketonuria Treatment in the United States. Mol. Genet. Metab. Rep. 2019, 21, 100523. [Google Scholar] [CrossRef]
- Aitkenhead, L.; Krishna, G.; Ellerton, C.; Moinuddin, M.; Matcham, J.; Shiel, L.; Hossain, S.; Kiffin, M.; Foley, J.; Skeath, R.; et al. Long-Term Cognitive and Psychosocial Outcomes in Adults with Phenylketonuria. J. Inherit. Metab. Dis. 2021, 44, 1353–1368. [Google Scholar] [CrossRef]
- Burlina, A.P.; Lachmann, R.H.; Manara, R.; Cazzorla, C.; Celato, A.; van Spronsen, F.J.; Burlina, A. The Neurological and Psychological Phenotype of Adult Patients with Early-Treated Phenylketonuria: A Systematic Review. J. Inherit. Metab. Dis. 2019, 42, 209–219. [Google Scholar] [CrossRef]
- Hofman, D.L.; Champ, C.L.; Lawton, C.L.; Henderson, M.; Dye, L. A Systematic Review of Cognitive Functioning in Early Treated Adults with Phenylketonuria. Orphanet J. Rare Dis. 2018, 13, 150. [Google Scholar] [CrossRef] [PubMed]
- Mütze, U.; Thiele, A.G.; Baerwald, C.; Ceglarek, U.; Kiess, W.; Beblo, S. Ten Years of Specialized Adult Care for Phenylketonuria—A Single-Centre Experience. Orphanet J. Rare Dis. 2016, 11, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peres, M.; Almeida, M.F.; Pinto, É.J.; Carmona, C.; Rocha, S.; Guimas, A.; Ribeiro, R.; Martins, E.; Bandeira, A.; MacDonald, A.; et al. Implementing a Transition Program from Paediatric to Adult Services in Phenylketonuria: Results after Two Years of Follow-up with an Adult Team. Nutrients 2021, 13, 799. [Google Scholar] [CrossRef] [PubMed]
- Wood, G.; Pinto, A.; Evans, S.; Daly, A.; Adams, S.; Costelloe, S.; Gribben, J.; Ellerton, C.; Emm, A.; Firman, S.; et al. Special Low Protein Foods Prescribed in England for PKU Patients: An Analysis of Prescribing Patterns and Cost. Nutrients 2021, 13, 3977. [Google Scholar] [CrossRef]
- Ford, S.; O’Driscoll, M.; MacDonald, A. Living with Phenylketonuria: Lessons from the PKU Community. Mol. Genet. Metab. Rep. 2018, 17, 57–63. [Google Scholar] [CrossRef] [PubMed]
- NHS Creative. Guidance for Standard Operating Procedure (SOP) for Management of COVID-19 Risk Assessments. Available online: https://www.england.nhs.uk/south-east/wp-content/uploads/sites/45/2021/05/Standard-Operating-Process-for-Management-and-COVID.pdf (accessed on 4 December 2021).
- British Dietetic Association. Model and Process for Nutrition and Dietetic Practice. The Model and Process. Available online: https://www.bda.uk.com/practice-and-education/nutrition-and-dietetic-practice/professional-guidance/model-and-process-for-dietetic-practice.html (accessed on 4 December 2021).
- Sladdin, I.; Ball, L.; Bull, C.; Chaboyer, W. Patient-Centred Care to Improve Dietetic Practice: An Integrative Review. J. Hum. Nutr. Diet. 2017, 30, 453–470. [Google Scholar] [CrossRef]
- World Health Organization. People-Centred and Integrated Health Services: An Overview of the Evidence. Services Deliver and Safety; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- MacDonald, A.; van Wegberg, A.M.J.; Ahring, K.; Beblo, S.; Bélanger-Quintana, A.; Burlina, A.; Campistol, J.; Coşkun, T.; Feillet, F.; Giżewska, M.; et al. PKU Dietary Handbook to Accompany PKU Guidelines. Orphanet J. Rare Dis. 2020, 15, 171. [Google Scholar] [CrossRef]
- Daly, A.; Evans, S.; Chahal, S.; Santra, S.; Pinto, A.; Gingell, C.; Rocha, J.C.; van Spronsen, F.; Jackson, R.; MacDonald, A. The Effect of Glycomacropeptide versus Amino Acids on Phenylalanine and Tyrosine Variability over 24 Hours in Children with PKU: A Randomized Controlled Trial. Nutrients 2019, 11, 520. [Google Scholar] [CrossRef] [Green Version]
- Joint WHO/FAO/UNU Expert Consultation. Protein and Amino Acid Requirements in Human Nutrition; WHO Technical Report Series, No. 935; World Health Organization: Geneva, Switzerland, 2007; pp. 1–265. [Google Scholar]
- Firman, S.; Witard, O.C.; O’Keeffe, M.; Ramachandran, R. Dietary Protein and Protein Substitute Requirements in Adults with Phenylketonuria: A Review of the Clinical Guidelines. Clin. Nutr. 2021, 40, 702–709. [Google Scholar] [CrossRef]
- Gokmen Ozel, H.; Ahring, K.; Bélanger-Quintana, A.; Dokoupil, K.; Lammardo, A.M.; Robert, M.; Rocha, J.C.; Almeida, M.F.; van Rijn, M.; MacDonald, A. Overweight and Obesity in PKU: The Results from 8 Centres in Europe and Turkey. Mol. Genet. Metab. Rep. 2014, 1, 483–486. [Google Scholar] [CrossRef]
- Dietary Reference Values for Food Energy and Nutrients for the United Kingdom. Report of the Panel on Dietary Reference Values of the Committee on Medical Aspects of Food Policy. Rep. Health Soc. Subj. 1991, 41, 1–210. [Google Scholar]
- Elango, R.; Humayun, M.A.; Ball, R.O.; Pencharz, P.B. Evidence That Protein Requirements Have Been Significantly Underestimated. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 52–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parenteral and Enteral Nutrition Group. A Specialist Group of the British Dietetic Association. A Pocket Guide to Clinical Nutrition, 5th ed.; Parenteral and Enteral Nutrition Group: South Yorkshire, UK, 2018. [Google Scholar]
- White, J.E.; Kronmal, R.A.; Acosta, P.B. Excess Weight among Children with Phenylketonuria. J. Am. Coll. Nutr. 1982, 1, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Burrage, L.C.; McConnell, J.; Haesler, R.; O’Riordan, M.A.; Sutton, V.R.; Kerr, D.S.; McCandless, S.E. High Prevalence of Overweight and Obesity in Females with Phenylketonuria. Mol. Genet. Metab. 2012, 107, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Camatta, G.C.; de Cássia Kanufre, V.; Alves, M.R.A.; Soares, R.D.L.; de Carvalho Norton, R.; de Aguiar, M.J.B.; Starling, A.L.P. Body Fat Percentage in Adolescents with Phenylketonuria and Associated Factors. Mol. Genet. Metab. Rep. 2020, 23, 100595. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.; Pinto, A.; Faria, A.; Teixeira, D.; van Wegberg, A.M.J.; Ahring, K.; Feillet, F.; Calhau, C.; MacDonald, A.; Moreira-Rosário, A.; et al. Is the Phenylalanine-Restricted Diet a Risk Factor for Overweight or Obesity in Patients with Phenylketonuria (PKU)? A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 3443. [Google Scholar] [CrossRef]
- Williams, R.A.; Hooper, A.J.; Bell, D.A.; Mamotte, C.D.S.; Burnett, J.R. Plasma Cholesterol in Adults with Phenylketonuria. Pathology 2015, 47, 134–137. [Google Scholar] [CrossRef]
- Mazzola, P.N.; Teixeira, B.C.; Schirmbeck, G.H.; Reischak-Oliveira, A.; Derks, T.G.J.; van Spronsen, F.J.; Dutra-Filho, C.S.; Schwartz, I.V.D. Acute Exercise in Treated Phenylketonuria Patients: Physical Activity and Biochemical Response. Mol. Genet. Metab. Rep. 2015, 5, 55–59. [Google Scholar] [CrossRef]
- Daly, A.; Evans, S.; Pinto, A.; Jackson, R.; Ashmore, C.; Rocha, J.C.; MacDonald, A. The Impact of the Use of Glycomacropeptide on Satiety and Dietary Intake in Phenylketonuria. Nutrients 2020, 12, 2704. [Google Scholar] [CrossRef]
- Robertson, L.V.; McStravick, N.; Ripley, S.; Weetch, E.; Donald, S.; Adam, S.; Micciche, A.; Boocock, S.; MacDonald, A. Body Mass Index in Adult Patients with Diet-Treated Phenylketonuria. J. Hum. Nutr. Diet. 2013, 26 (Suppl. S1), 1–6. [Google Scholar] [CrossRef]
- Cazzorla, C.; Bensi, G.; Biasucci, G.; Leuzzi, V.; Manti, F.; Musumeci, A.; Papadia, F.; Stoppioni, V.; Tummolo, A.; Vendemiale, M.; et al. Living with Phenylketonuria in Adulthood: The PKU ATTITUDE Study. Mol. Genet. Metab. Rep. 2018, 16, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Luu, S.; Breunig, T.; Drilias, N.; Kuhl, A.; Scott Schwoerer, J.; Cody, P. A Survey of Eating Attitudes and Behaviors in Adolescents and Adults with Phenylalanine Hydroxylase Deficiency. WMJ 2020, 119, 37–43. [Google Scholar] [PubMed]
- NICE 2020. Eating Disorders: Recognition and Treatment NICE Guideline NG69. 2020. Available online: https://www.nice.org.uk/guidance/ng69 (accessed on 4 November 2021).
- SIGN Evidence-Based Guidelines. Eating Disorders, A National Clinical Guideline, Consultation Draft May 2021. 2021. Available online: https://www.sign.ac.uk/media/1849/20210511-ed-draft-v115-peer-review.pdf (accessed on 10 December 2021).
- NHS England. Highly Specialised Services Quality Dashboards—Women and Children Metric Definitions for 2020/21. 2020. Available online: https://www.england.nhs.uk/publication/highly-specialised-services-quality-dashboards-women-and-children-metric-definitions-for-2020-21 (accessed on 30 November 2021).
- Inwood, A.C.; Balasubramaniam, S.; Wiley, V.; Kreis, C.; Harrigan, K. Australasian Consensus Guidelines for the Management of Phenylketonuria (PKU) throughout the Lifespan. Available online: https://www.mdda.org.au/wp-content/uploads/2017/10/Australasian-PKU-Lifespan-Guidelines-FINAL-Endorsed-5.10.2017.pdf (accessed on 4 November 2021).
- MacDonald, A.; Ahring, K.; Almeida, M.F.; Belanger-Quintana, A.; Blau, N.; Burlina, A.; Cleary, M.; Coskum, T.; Dokoupil, K.; Evans, S.; et al. The Challenges of Managing Coexistent Disorders with Phenylketonuria: 30 Cases. Mol. Genet. Metab. 2015, 116, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Obesity: Identification, Assessment and Management Clinical Guideline. Available online: https://www.nice.org.uk/guidance/cg189/resources/obesity-identification-assessment-and-management-pdf-35109821097925 (accessed on 4 November 2021).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robertson, L.; Adam, S.; Ellerton, C.; Ford, S.; Hill, M.; Randles, G.; Woodall, A.; Young, C.; MacDonald, A. Dietetic Management of Adults with Phenylketonuria (PKU) in the UK: A Care Consensus Document. Nutrients 2022, 14, 576. https://doi.org/10.3390/nu14030576
Robertson L, Adam S, Ellerton C, Ford S, Hill M, Randles G, Woodall A, Young C, MacDonald A. Dietetic Management of Adults with Phenylketonuria (PKU) in the UK: A Care Consensus Document. Nutrients. 2022; 14(3):576. https://doi.org/10.3390/nu14030576
Chicago/Turabian StyleRobertson, Louise, Sarah Adam, Charlotte Ellerton, Suzanne Ford, Melanie Hill, Gemma Randles, Alison Woodall, Carla Young, and Anita MacDonald. 2022. "Dietetic Management of Adults with Phenylketonuria (PKU) in the UK: A Care Consensus Document" Nutrients 14, no. 3: 576. https://doi.org/10.3390/nu14030576
APA StyleRobertson, L., Adam, S., Ellerton, C., Ford, S., Hill, M., Randles, G., Woodall, A., Young, C., & MacDonald, A. (2022). Dietetic Management of Adults with Phenylketonuria (PKU) in the UK: A Care Consensus Document. Nutrients, 14(3), 576. https://doi.org/10.3390/nu14030576







