Impact of Infectious Disease after Lactococcus lactis Strain Plasma Intake in Vietnamese Schoolchildren: A Randomized, Placebo-Controlled, Double-Blind Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Statement of Ethics
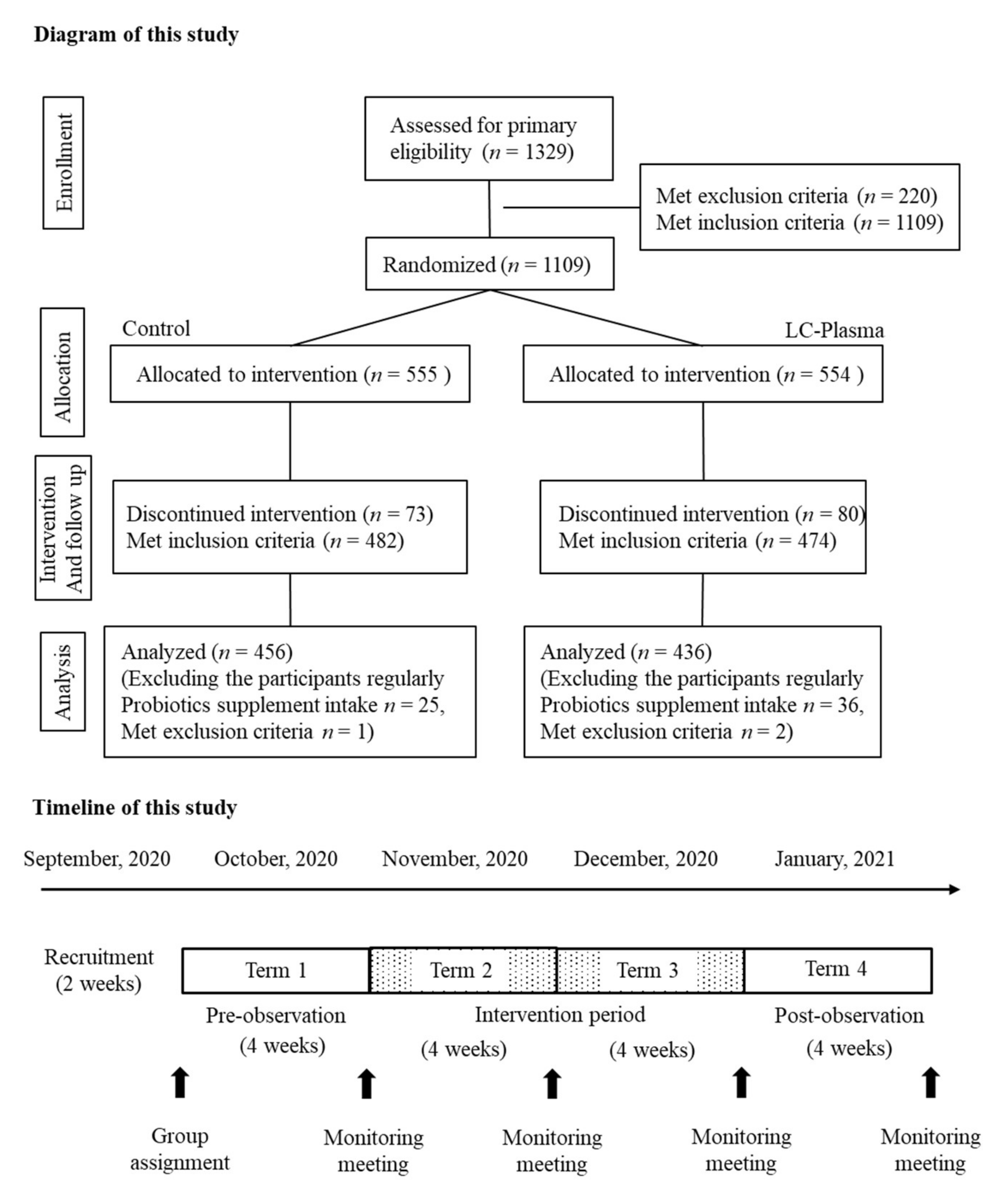
2.2. Study Design
2.3. Enrollment and Selection of Participants
2.4. Inclusion and Exclusion Criteria
- (1)
- Signed and dated informed consent form provided, after willingness of participants (guardians) confirmed;
- (2)
- Willingness to comply with all study procedures for the period of the study (both participants and guardians);
- (3)
- Aged 6 to 9 years old (elementary school grades 1 to 3);
- (4)
- Residing in Yen Mo District, Ninh Binh Province, with Vietnamese citizenship (both participants and guardians);
- (5)
- No underlying chronic sickness from respiratory disease and/or gastrointestinal disease at enrollment (participants).
- (1)
- Unable to provide signed and dated informed consent form (guardian);
- (2)
- Unable to comply with all the study procedures (participants and guardians);
- (3)
- Allergic to milk (contained within the study products) (participants);
- (4)
- Regularly using steroid drugs (immune suppressive drugs) (participants);
- (5)
- Those with serious respiratory and/or gastrointestinal disease (participants);
- (6)
- Any persons deemed inappropriate as participants by the principal investigators of this study;
- (7)
- Those currently participating in other clinical trials (participants) or those who are planning to participate in other clinical trials during the study period (participants).
2.5. Pre-Intervention Period (Term 1; T1)
2.6. Intervention Period (Term 2; First 4 Weeks as T2, Term 3; Latter 4 Weeks as T3) and Post-Intervention Period (Term 4; T4)
2.7. Data Collection
2.8. Study Products
2.9. Definition of Score
2.10. Statistical Analysis
3. Results
3.1. Participant Characteristics and Background Data Analysis
3.2. Analysis of Cumulative Days Absent from School
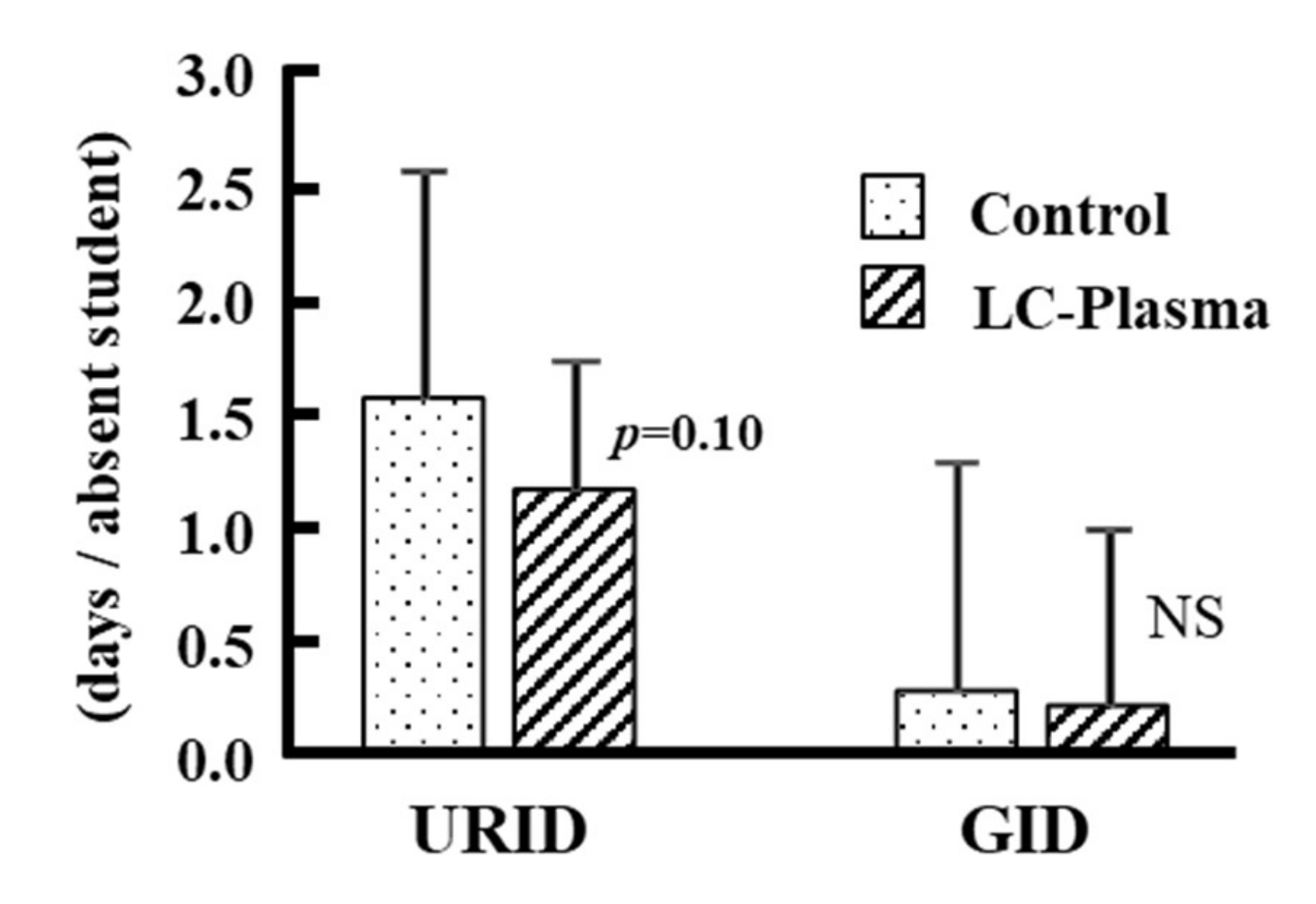
3.3. Disease Score Analysis and Cumulative Days with or without Symptoms
3.4. General Wellbeing
3.5. Safety of LC-Plasma
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Barik, S. New treatments for influenza. BMC Med. 2012, 10, 104–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vo, T.Q.; Chaikledkaew, U.; Van Hoang, M.; Riewpaiboon, A. Social and economic burden of patients with influenza-like illness and clinically diagnosed flu treated at various health facilities in Vietnam. Clinicoecon. Outcomes Res. 2017, 19, 423–432. [Google Scholar]
- Nghia, N.D.; Tu, N.H.; Phuong, H.V.M.; Thanh, T.N.; Tuan, N.H.; Duong, T.N. Influenza like illness sentinel surveillance in northern Vietnam, 2016–2018. Viet. J. Prev. Med. 2019, 12, 86–97. (In Vietnamese) [Google Scholar]
- Yen, N.T.T.; Mai, L.Q.; Duong, T.N.; Tuan, N.H.; Thanh, N.P.; Thuy, N.B.; Hang, N.L.K.; Thiem, V.D.; Ha, V.H.; Huu, T.N.; et al. Epidemiological characteristics of seasonal influenza in Vietnam, period 2006–2013. Viet. J. Prev. Med. 2015, 3, 56–64. (In Vietnamese) [Google Scholar]
- Tamura, T.; Nishikawa, M.; Anh, D.D.; Suzuki, H. Molecular epidemiological study of rotavirus and norovirus infections among children with acute gastroenteritis in Nha Trang, Vietnam, December 2005–June 2006. Jpn. J. Infect. Dis. 2010, 63, 405–411. [Google Scholar]
- Riewpaiboon, A.; Shin, S.; Le, T.P.; Vu, D.T.; Nguyen, T.H.; Alexander, N.; Dang, D.A. Rotavirus Economic Study Group. Cost of rotavirus diarrhea for programmatic evaluation of vaccination in Vietnam. BMC Public Health 2016, 16, 777–784. [Google Scholar] [CrossRef] [Green Version]
- Phu, P.T.; Vinh, D.N.; Son, V.T.; Hanh, N.T.; Lan, N.H.; Van Vinh, T.; Trang, N.T.M.; Ha, D.T.M.; Thwaites, G.E.; Thuong, N.T.T. Risk factors for poor treatment outcomes of 2266 multidrug-resistant tuberculosis cases in Ho Chi Minh City: A retrospective study. BMC Infect. Dis. 2020, 20, 164–174. [Google Scholar]
- Nakamori, M.; Ninh, N.X.; Khan, N.C.; Huong, C.T.; Tuan, N.A.; Le, B.M.; Hien, V.T.T.; Nhung, B.T.; Nakano, T.; Yoshiike, N.; et al. Nutritional status, feeding practice and incidence of infectious diseases among children aged 6 to 18 months in northern mountainous. Vietnam. J. Med. Investig. 2010, 57, 45–53. [Google Scholar] [CrossRef] [Green Version]
- Heyman, M. Effect of lactic acid bacteria on diarrheal diseases. J. Am. Coll. Nutr. 2000, 9 (Suppl. 2), 137S–146S. [Google Scholar] [CrossRef]
- Savaiano, D.A. Lactose digestion from yogurt: Mechanism and relevance. Am. J. Clin. Nutr. 2014, 99 (Suppl. 5), 1251S–1255S. [Google Scholar] [CrossRef] [Green Version]
- Kanauchi, O.; Andoh, A.; AbuBakar, S.; Yamamoto, N. Probiotics and Paraprobiotics in Viral Infection: Clinical Application and Effects on the Innate and Acquired Immune Systems. Curr. Pharm. Des. 2018, 24, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Santamarina, A.; Alexandre, L.; Mondragón, A.D.C.; Cardelle-Cobas, A.; Regal, P.; Rodriguez-Avila, J.A.; Miranda, J.M.; Franco, C.M.; Cepeda, A. Probiotic Effects against Virus Infections: New Weapons for an Old War. Foods 2021, 10, 130. [Google Scholar] [CrossRef] [PubMed]
- Jounai, K.; Ikado, K.; Sugimura, T.; Ano, Y.; Braun, J.; Fujiwara, D. Spherical lactic acid bacteria activate plasmacytoid dendritic cells immunomodulatory function via TLR9-dependent crosstalk with myeloid dendritic cells. PLoS ONE 2012, 7, e32588. [Google Scholar] [CrossRef] [PubMed]
- Jounai, K.; Sugimura, T.; Ohshio, K.; Fujiwara, D. Oral administration of Lactococcus lactis subsp lactis JCM5805 enhances lung immune response resulting in protection from murine parainfluenza virus infection. PLoS ONE 2015, 6, e0119055. [Google Scholar]
- Jounai, K.; Sugimura, T.; Morita, Y.; Ohshio, K.; Fujiwara, D. Administration of Lactococcus lactis strain Plasma induces maturation of plasmacytoid dendritic cells and protection from rotavirus infection in suckling mice. Int. Immunopharmacol. 2018, 56, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Sugimura, T.; Takahashi, H.; Jounai, K.; Ohshio, K.; Kanayama, M.; Tazumi, K.; Tanihata, Y.; Miura, Y.; Fujiwara, D.; Yamamoto, N. Effects of oral intake of plasmacytoid dendritic cells-stimulative lactic acid bacterial strain on pathogenesis of influenza-like illness and immunological response to influenza virus. Br. J. Nutr. 2015, 114, 727–733. [Google Scholar] [CrossRef] [Green Version]
- Sakata, K.; Sasaki, Y.; Jounai, K.; Fujii, T.; Fujiwara, D. Preventive Effect of Lactococcus lactis subsp lactis JCM 5805 Yogurt Intake on Influenza Infection among Schoolchildren. Health 2017, 9, 756–762. [Google Scholar]
- Tanaka, K.; Suzuki, H.; Kanayama, M.; Fujii, T.; Fujiwara, D.; Nozawa, H.; Sugimura, H. The Safety Evaluation of Long-term or Excessive Intake of the Beverage Containing Lactococcus lactis subsp. lactis JCM 5805 and Resistant Maltodextrin—A Randomized, Double-blind, Placebo-controlled, Parallel-group Trial. Jpn. Pharmacol. Ther. 2015, 43, 1711–1727. (In Japanese) [Google Scholar]
- Kato, Y.; Kanayama, M.; Yanai, S.; Nozawa, H.; Kanauchi, O.; Suzuki, S. Safety Evaluation of Excessive Intake of Lactococcus lactis Subsp lactis JCM 5805: A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Trial. Food Nutr. Sci. 2018, 9, 403–419. [Google Scholar]
- Shibata, T.; Kanayama, M.; Haida, M.; Fujimoto, S.; Oroguchi, T.; Sata, K.; Mita, N.; Kutsuzawa, T.; Ikeuchi, M.; Kondo, M.; et al. Lactococcus lactis JCM5805 activates anti-viral immunity and reduces symptoms of common cold and influenza in healthy adults in a randomized controlled trial. J. Func. Foods 2016, 24, 492–500. [Google Scholar] [CrossRef]
- Loeb, M.; Dang, A.D.; Thiem, V.D.; Thanabalan, V.; Wang, B.; Nguyen, N.B.; Tran, H.T.M.; Luong, T.M.; Singh, P.; Smieja, M.; et al. Effect of Vitamin D supplementation to reduce respiratory infections in children and adolescents in Vietnam: A randomized controlled trial. Influenza Other Respir Viruses 2019, 13, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Barrett, B.; Brown, R.L.; Mundt, M.P.; Thomas, G.R.; Barlow, S.K.; Highstrom, A.D.; Bahrainian, M. Validation of a short form Wisconsin Upper Respiratory Symptom Survey (WURSS-21). Health Qual. Life Outcomes 2009, 7, 76–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komano, Y.; Shimada, K.; Naito, H.; Fukao, K.; Ishihara, Y.; Fujii, T.; Kokubo, T.; Daida, H. Efficacy of heat-killed Lactococcus lactis JCM 5805 on immunity and fatigue during consecutive high intensity exercise in male athletes: A randomized, placebo-controlled, double-blinded trial. J. Int. Soc. Sports Nutr. 2018, 15, 39–48. [Google Scholar] [CrossRef] [Green Version]
- Stojanovic, J.; Boucher, V.G.; Boyle, J.; Enticott, J.; Lavoie, K.L.; Bacon, S.L. COVID-19 is not the flu: Four graphs from four countries. Front. Public Health 2021, 9, 628479. [Google Scholar] [CrossRef]
- Alves, G.M.G.; Rocha, C.S.M.A.; Alves, D.C.A.J. Antibiotics for preventing suppurative complications from undifferentiated acute respiratory infections in children under five years of age. Cochrane Database Syst. Rev. 2016, 29, CD007880. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Ge, T.; Xiao, Y.; Liao, Y.; Cui, Y.; Zhang, Y.; Ho, W.; Yu, G.; Zhang, T. Probiotics for prevention and treatment of respiratory tract infections in children: A systematic review and meta-analysis of randomized controlled trials. Medicine 2016, 95, e4509. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, R.; Yamamoto, N.; Yamada, S.; Fujii, T.; Yamamoto, N.; Kanauchi, O. Induction of anti-viral genes mediated by humoral factors upon stimulation with Lactococcus lactis strain plasma results in repression of dengue virus replication in vitro. Antivir. Res. 2018, 160, 101–108. [Google Scholar] [CrossRef]
- Teijaro, J.R.; Walsh, K.B.; Rice, S.; Rosen, H.; Oldstone, M.B. Mapping the innate signaling cascade essential for cytokine storm during influenza virus infection. Proc. Natl. Acad. Sci. USA 2014, 111, 3799–3804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moriyama, M.; Hugentobler, W.J.; Iwasaki, A. Seasonality of Respiratory Viral Infections. Annu. Rev. Virol. 2020, 7, 83–101. [Google Scholar] [CrossRef]
- Galanti, M.; Birger, R.; Ud-Dean, M.; Filip, I.; Morita, H.; Comito, D.; Anthony, S.; Freyer, G.A.; Ibrahim, S.; Lane, B.; et al. Rates of asymptomatic respiratory virus infection across age groups. Epidemiol. Infect. 2019, 147, e176. [Google Scholar] [CrossRef] [Green Version]
- Lien, D.T.K.; Nhung, B.T.; Khan, N.C.; Hop, L.T.; Nga, N.T.Q.; Hung, N.T.; Kiers, J.; Shigeru, Y.; te Biesebeke, R. Impact of milk consumption on performance and health of primary school children in rural Vietnam. Asia Pac. J. Clin. Nutr. 2009, 18, 326–334. [Google Scholar]
- Hall, A.; Hanh, T.T.; Farley, K.; Quynh, T.P.; Valdivia, F. An evaluation of the impact of a school nutrition programme in Vietnam. Public Health Nutr. 2007, 10, 819–826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Age (Year) | Gender (Male/Female) | Body Weight (kg) | Body Height (cm) | |
|---|---|---|---|---|
| Control | 6.86 ± 0.91 | 237/219 | 21.7 ± 4.4 | 119.0 ± 6.8 |
| LC-Plasma | 6.78 ± 0.95 | 218/218 | 21.5 ± 4.7 | 118.8 ± 6.6 |
| p-value | 0.16 | 0.56 | 0.22 | 0.51 |
| Z-Score | ||||
| Weight/Age | Height/Age | Weight/Height/Age | ||
| Control | −0.66 ± 1.16 | −0.68 ± 0.87 | −0.39 ± 1.23 | |
| LC-Plasma | −0.68 ± 1.27 | −0.67 ± 0.90 | −0.44 ± 1.31 | |
| p-value | 0.32 | 0.75 | 0.21 | |
| Absent by | Terms | Group | Cumulative Days | p-Value | Odds Ratio (95% CI) | |
|---|---|---|---|---|---|---|
| Not Absent | Absent | |||||
| URID/GID | Term 2 | Control | 12,698 | 70 ** | 0.004 | 0.57 (0.38~0.84) |
| LC-Plasma | 12,170 | 38 | ||||
| Term 3 | Control | 12,739 | 29 | 0.49 | 1.19 (0.73~1.95) | |
| LC-Plasma | 12,175 | 33 | ||||
| URID | Term 2 | Control | 12,706 | 62 ** | 0.005 | 0.56 (0.37~0.85) |
| LC-Plasma | 12,175 | 33 | ||||
| Term 3 | Control | 12,746 | 22 | 0.38 | 1.28 (0.74~2.24) | |
| LC-Plasma | 12,181 | 27 | ||||
| GID | Term 2 | Control | 12,760 | 8 | 0.45 | 0.65 (0.23~1.89) |
| LC-Plasma | 12,203 | 5 | ||||
| Term 3 | Control | 12,761 | 7 | 0.84 | 0.90 (0.32~2.54) | |
| LC-Plasma | 12,203 | 6 | ||||
| Symptom | Terms | Group | Cumulative Days | p-Value | Odds Ratio (95% CI) | |
|---|---|---|---|---|---|---|
| Negative | Positive | |||||
| Fever | Term 2 | Control | 12,660 | 108 ** | 0.001 | 0.58 (0.42~0.79) |
| LC-Plasma | 12,148 | 60 | ||||
| Term 3 | Control | 12,728 | 40 | 0.93 | 1.02 (0.66~1.58) | |
| LC-Plasma | 12,169 | 39 | ||||
| Cough | Term 2 | Control | 10,340 | 2428 | 0.85 | 1.01 (0.95~1.07) |
| LC-Plasma | 9875 | 2333 | ||||
| Term 3 | Control | 10,196 | 2572 | 0.22 | 1.04 (0.98~1.11) | |
| LC-Plasma | 9673 | 2535 | ||||
| Runny nose | Term 2 | Control | 10,811 | 1957 # | 0.07 | 0.94 (0.87~1.01) |
| LC-Plasma | 10,437 | 1771 | ||||
| Term 3 | Control | 10,688 | 2080 | 0.83 | 1.01 (0.94~1.08) | |
| LC-Plasma | 10,207 | 2001 | ||||
| Constipation | Term 2 | Control | 9705 | 3063 $ | 0.03 | 1.06 (1.01~1.13) |
| LC-Plasma | 9138 | 3070 | ||||
| Term 3 | Control | 9798 | 2970 # | 0.050 | 0.94 (0.89~1.00) | |
| LC-Plasma | 9495 | 2713 | ||||
| Diarrhea | Term 2 | Control | 12,477 | 263 | 0.11 | 0.86 (0.72~1.03) |
| LC-Plasma | 11,990 | 218 | ||||
| Term 3 | Control | 12,471 | 269 ** | 0.01 | 0.78 (0.65~0.94) | |
| LC-Plasma | 12,005 | 203 | ||||
| Abdominal pain | Term 2 | Control | 12,399 | 369 | 0.56 | 1.04 (0.90~1.21) |
| LC-Plasma | 11,840 | 368 | ||||
| Term 3 | Control | 12,470 | 298 # | 0.08 | 0.86 (0.73~1.02) | |
| LC-Plasma | 11,962 | 246 | ||||
| Group | Terms | Cumulative Days of General Wellbeing | p-Value | Odds Ratio (95% CI) | |
|---|---|---|---|---|---|
| Positive 1 | Negative 2 | ||||
| Control | Term 2 | 10,506 | 2262 * | 0.03 | 0.93 (0.87~0.99) |
| LC-Plasma | 10,175 | 2033 | |||
| Control | Term 3 | 10,776 | 1992 * | 0.04 | 0.93 (0.87~0.99) |
| LC-Plasma | 10,416 | 1792 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thu, N.N.; Mai, T.T.; Trang, T.T.T.; Tuan, N.A.; Quyen, T.C.; Hanh, N.L.; Hoan, N.H.; Lan, B.T.H.; Hau, P.T.; Tue, H.H.; et al. Impact of Infectious Disease after Lactococcus lactis Strain Plasma Intake in Vietnamese Schoolchildren: A Randomized, Placebo-Controlled, Double-Blind Study. Nutrients 2022, 14, 552. https://doi.org/10.3390/nu14030552
Thu NN, Mai TT, Trang TTT, Tuan NA, Quyen TC, Hanh NL, Hoan NH, Lan BTH, Hau PT, Tue HH, et al. Impact of Infectious Disease after Lactococcus lactis Strain Plasma Intake in Vietnamese Schoolchildren: A Randomized, Placebo-Controlled, Double-Blind Study. Nutrients. 2022; 14(3):552. https://doi.org/10.3390/nu14030552
Chicago/Turabian StyleThu, Nghiem Nguyet, Truong Tuyet Mai, Tran Thị Thu Trang, Nguyen Anh Tuan, Tran Chau Quyen, Nguyen Lien Hanh, Nguyen Huu Hoan, Bui Thi Huong Lan, Phung Thi Hau, Ha Huy Tue, and et al. 2022. "Impact of Infectious Disease after Lactococcus lactis Strain Plasma Intake in Vietnamese Schoolchildren: A Randomized, Placebo-Controlled, Double-Blind Study" Nutrients 14, no. 3: 552. https://doi.org/10.3390/nu14030552
APA StyleThu, N. N., Mai, T. T., Trang, T. T. T., Tuan, N. A., Quyen, T. C., Hanh, N. L., Hoan, N. H., Lan, B. T. H., Hau, P. T., Tue, H. H., Dung, T. V., Tsuji, R., Watanabe, Y., Yamamoto, N., & Kanauchi, O. (2022). Impact of Infectious Disease after Lactococcus lactis Strain Plasma Intake in Vietnamese Schoolchildren: A Randomized, Placebo-Controlled, Double-Blind Study. Nutrients, 14(3), 552. https://doi.org/10.3390/nu14030552







