Adherence to Dietary Recommendations of 7-Year-Old Children from a Birth Cohort in Friuli Venezia Giulia, Italy
Abstract
1. Introduction
- Standard evaluation of adequacy to the DRVs [38];
- Index-based evaluation of adequacy to DRVs at the following levels:
- Nutrient-level adequacy, through the NAR index;
- Overall-diet-level adequacy, through the MAR index;
- Percentage contribution of different food sources to macro- and micronutrient intakes.
2. Materials and Methods
2.1. Study Population
2.2. Parental and Children’s Characteristics
2.3. Dietary Assessment
2.4. Nutritional Adequacy
- For all micronutrients with one DRV indicating the minimum desirable intake (i.e., AI or AR), we truncated all NARs greater than 1 to 1 so that these nutrients could not compensate those with a NAR lower than 1 in the MAR calculation;
- For the remaining macro- and micronutrients indicating a maximum desirable intake (i.e., RI: protein, available carbohydrates, total fats, monounsaturated fatty acids (MUFAs), total PUFAs, PUFAs ω-3 and ω-6; SDT: soluble carbohydrates, saturated fatty acids (SFAs), sodium, and chloride), we followed suggestions by Hilbig [51] and redefined NARs greater than 1 (inadequate intake by excess) to be equal to: 1 minus the exceeding amount. For example, when the original NAR was equal to 1.15, our modified NAR value is equal to 0.85.
2.5. Statistical Analysis
3. Results
3.1. Lifestyle and Anthropometric Characteristics of the Study Population
3.2. Description of Daily Dietary Nutrient Intake and Standard Comparison with the Italian Dietary Reference Values
3.2.1. Energy and Macronutrients
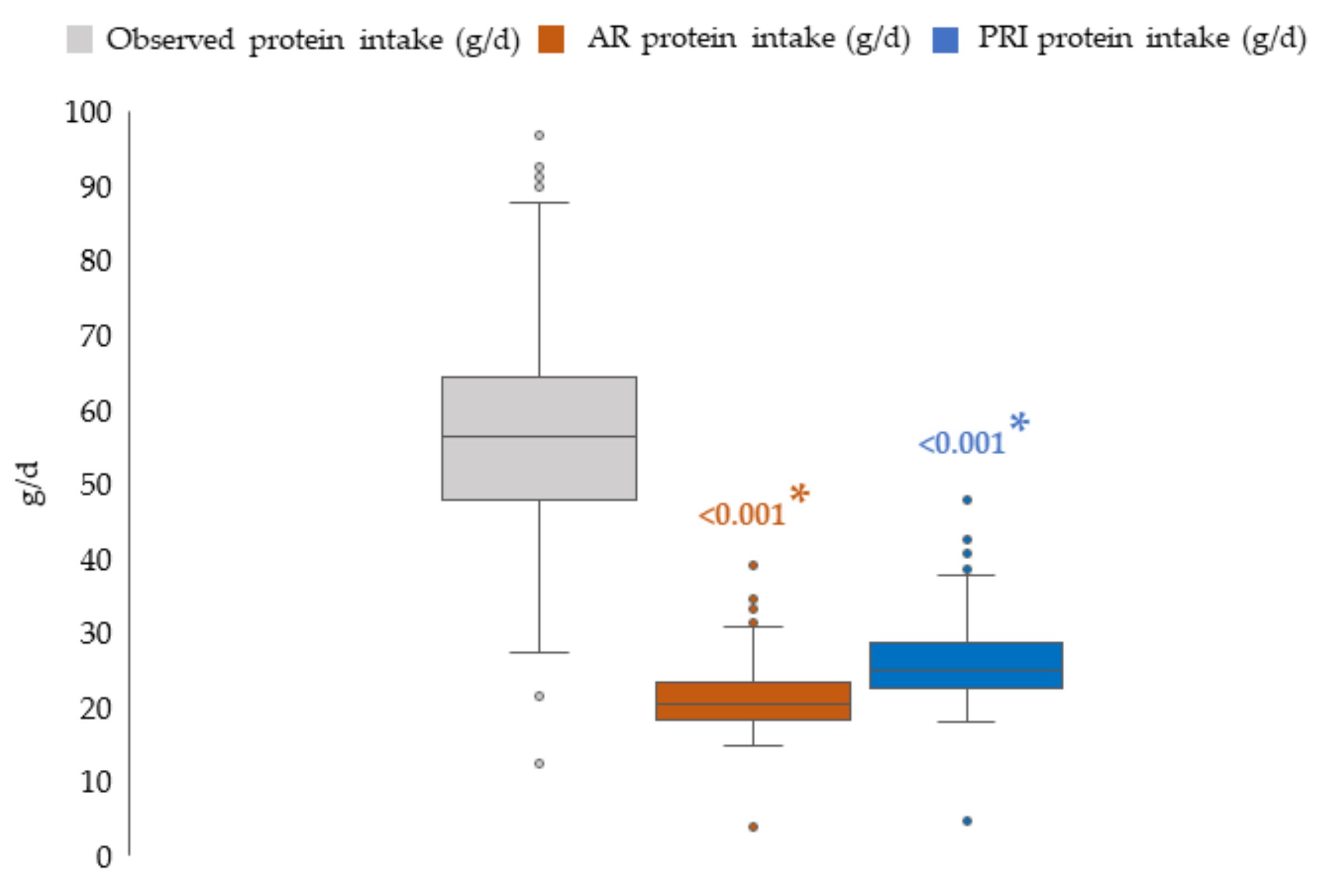
3.2.2. Protein Intake: Comparison with Average Requirement and Population Reference Intake
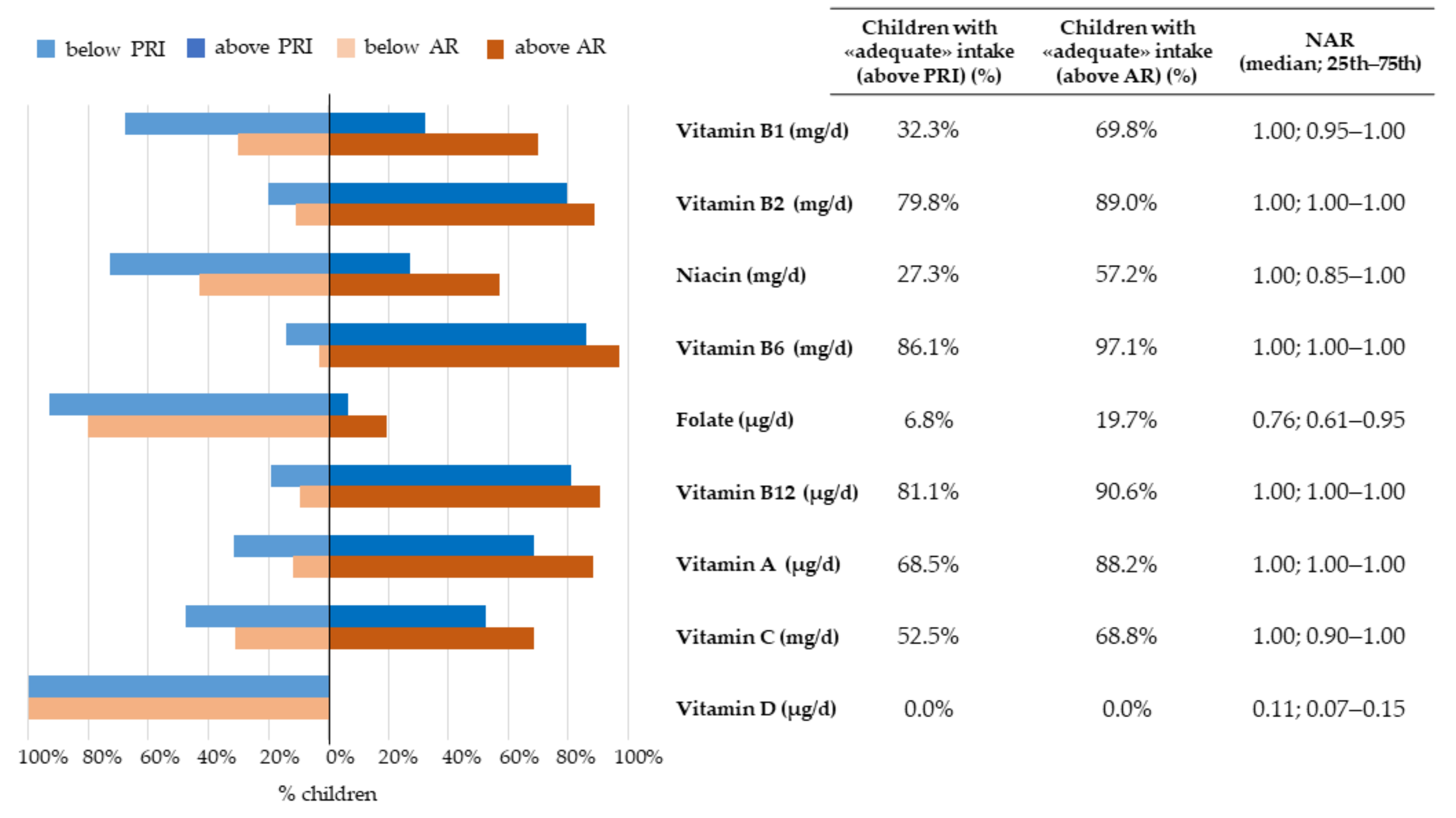
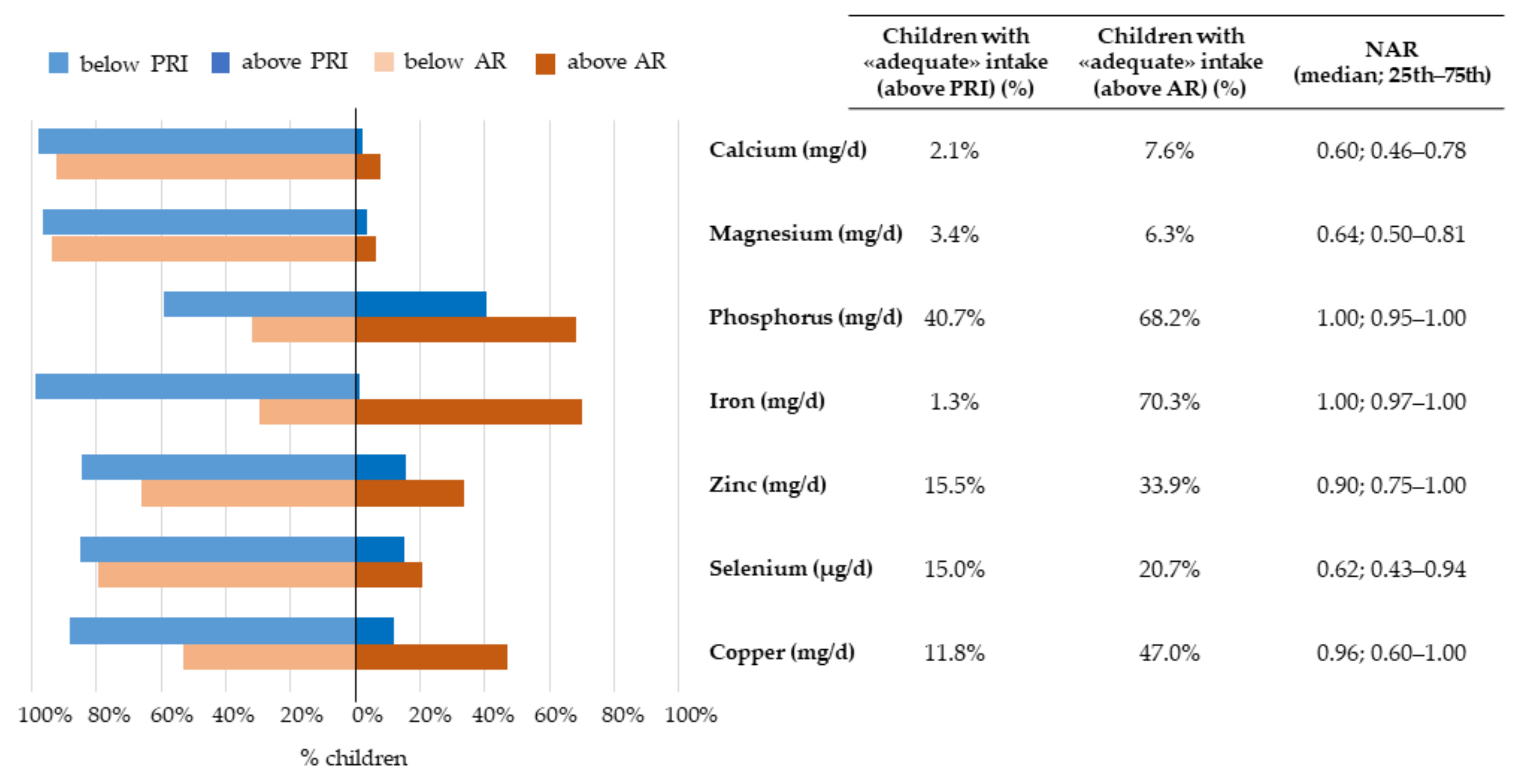
3.2.3. Micronutrients
3.3. Index-Based Evaluation of Diet Adequacy to Dietary Reference Values
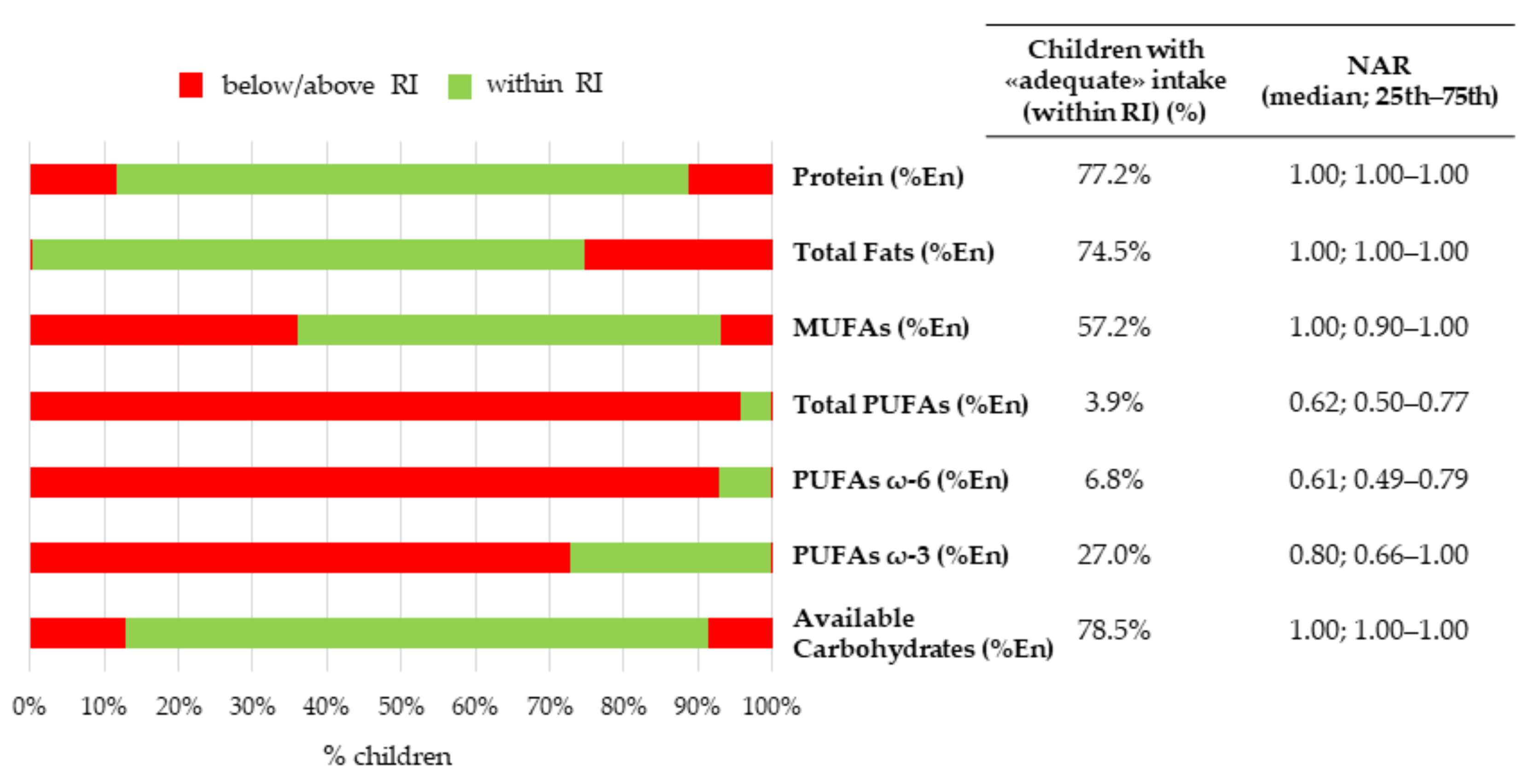
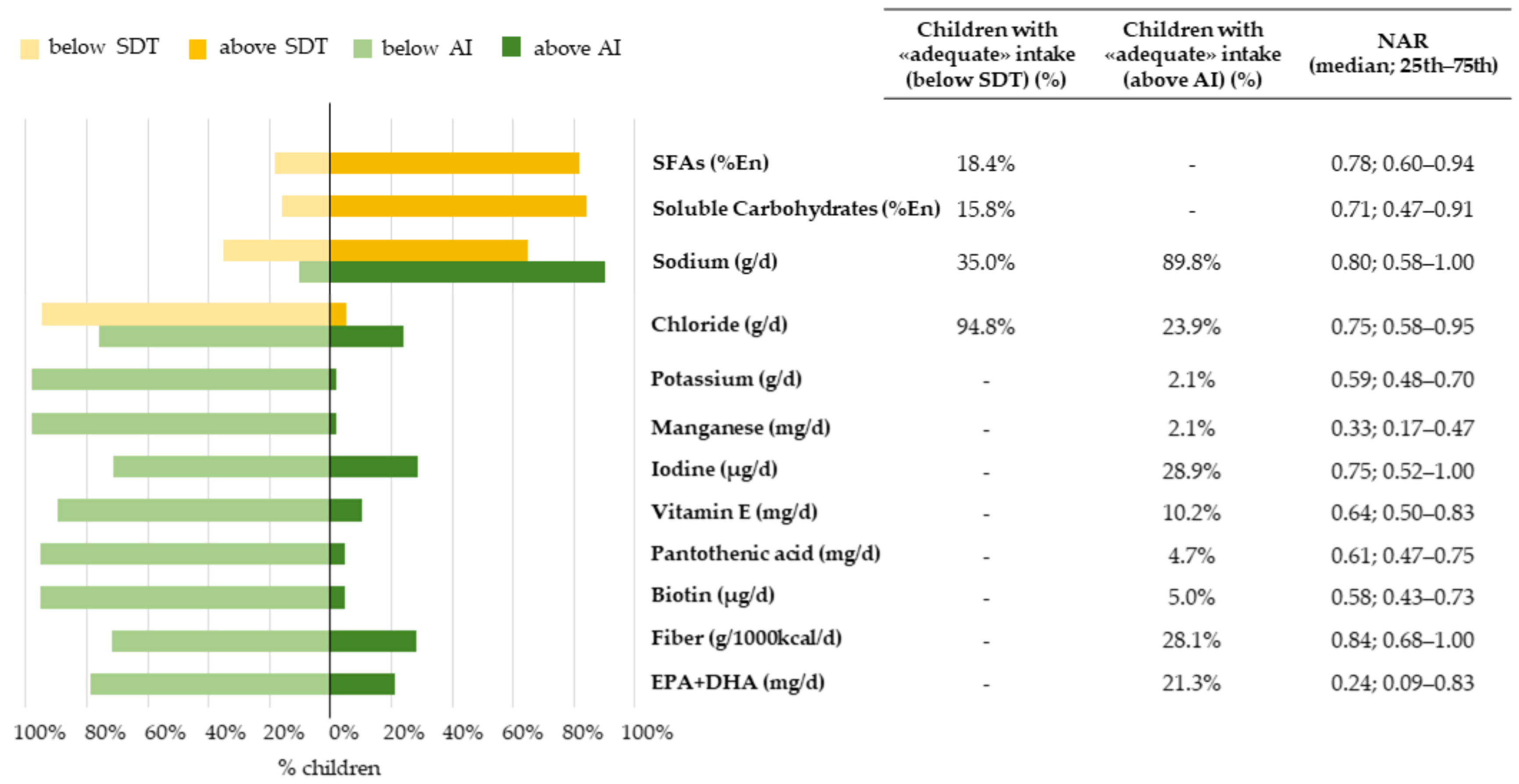
3.3.1. Nutrient-Level Adequacy
3.3.2. Overall-Diet-Level Adequacy
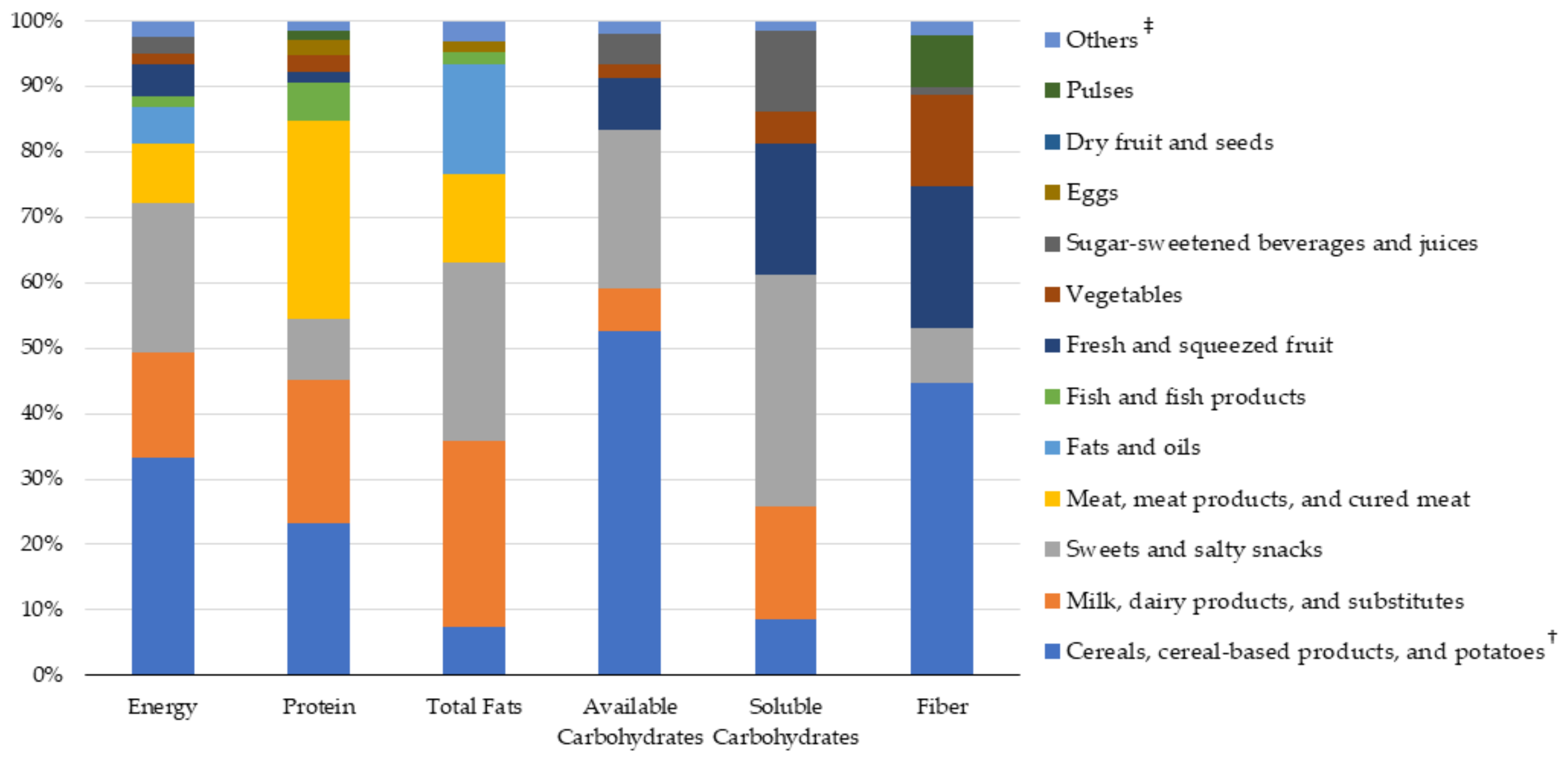
3.4. Sources of Nutrient Intakes: Food Groups
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Drozdz, D.; Alvarez-Pitti, J.; Wójcik, M.; Borghi, C.; Gabbianelli, R.; Mazur, A.; Herceg-čavrak, V.; Lopez-Valcarcel, B.G.; Brzeziński, M.; Lurbe, E.; et al. Obesity and cardiometabolic risk factors: From childhood to adulthood. Nutrients 2021, 13, 4176. [Google Scholar] [CrossRef] [PubMed]
- Heindel, J.J.; Balbus, J.; Birnbaum, L.; Brune-Drisse, M.N.; Grandjean, P.; Gray, K.; Landrigan, P.J.; Sly, P.D.; Suk, W.; Slechta, D.C.; et al. Developmental origins of health and disease: Integrating environmental influences. Endocrinology 2015, 156, 3416–3421. [Google Scholar] [CrossRef] [PubMed]
- Peña-Romero, A.C.; Navas-Carrillo, D.; Marín, F.; Orenes-Piñero, E. The future of nutrition: Nutrigenomics and nutrigenetics in obesity and cardiovascular diseases. Crit. Rev. Food Sci. Nutr. 2018, 58, 3030–3041. [Google Scholar] [CrossRef] [PubMed]
- Gingras, V.; Hivert, M.F.; Oken, E. Early-Life Exposures and Risk of Diabetes Mellitus and Obesity. Curr. Diab. Rep. 2018, 18, 89. [Google Scholar] [CrossRef] [PubMed]
- Kelishadi, R.; Poursafa, P. A review on the genetic, environmental, and lifestyle aspects of the early-life origins of cardiovascular disease. Curr. Probl. Pediatr. Adolesc. Health Care 2014, 44, 54–72. [Google Scholar] [CrossRef]
- Maynard, M.; Gunnell, D.; Emmett, P.; Frankel, S.; Davey Smith, G. Fruit, vegetables, and antioxidants in childhood and risk of adult cancer: The Boyd Orr cohort. J. Epidemiol. Community Health 2003, 57, 218–225. [Google Scholar] [CrossRef]
- Juonala, M.; Magnussen, C.G.; Berenson, G.S.; Venn, A.; Burns, T.L.; Sabin, M.A.; Srinivasan, S.R.; Daniels, S.R.; Davis, P.H.; Chen, W.; et al. Childhood Adiposity, Adult Adiposity, and Cardiovascular Risk Factors. N. Engl. J. Med. 2011, 365, 1876–1885. [Google Scholar] [CrossRef]
- WHO. Childhood Obesity Surveillance Initiative (COSI); WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Nittari, G.; Scuri, S.; Petrelli, F.; Pirillo, I.; Di Luca, N.M.; Grappasonni, I. Fighting obesity in children from European world health organization member states. Epidemiological data, medical-social aspects, and prevention programs. Clin. Ter. 2019, 170, E223–E230. [Google Scholar] [CrossRef]
- Spinelli, A.; Buoncristiano, M.; Nardone, P.; Starc, G.; Hejgaard, T.; Júlíusson, P.B.; Fismen, A.S.; Weghuber, D.; Musić Milanović, S.; García-Solano, M.; et al. Thinness, overweight, and obesity in 6- to 9-year-old children from 36 countries: The World Health Organization European Childhood Obesity Surveillance Initiative—COSI 2015–2017. Obes. Rev. 2021, 22, 1–15. [Google Scholar] [CrossRef]
- Nardone, P.; Spinelli, A. OKkio Alla Salute (Indagine Nazionale 2019). Available online: https://www.epicentro.iss.it/okkioallasalute/indagine-2019-dati#writers (accessed on 22 December 2021).
- Pontiroli, A.E. Type 2 diabetes mellitus is becoming the most common type of diabetes in school children. Acta Diabetol. 2004, 41, 85–90. [Google Scholar] [CrossRef]
- Chen, L.; Magliano, D.J.; Zimmet, P.Z. The worldwide epidemiology of type 2 diabetes mellitus—Present and future perspectives. Nat. Rev. Endocrinol. 2012, 8, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Bryan, J.; Osendarp, S.; Hughes, D.; Calvaresi, E.; Baghurst, K.; Van Klinken, J.W. Nutrients for cognitive development in school-aged children. Nutr. Rev. 2004, 62, 295–306. [Google Scholar] [CrossRef] [PubMed]
- John, C.C.; Black, M.M.; Nelson, C.A. Neurodevelopment: The impact of nutrition and inflammation during early to middle childhood in low-resource settings. Pediatrics 2017, 139, S59–S71. [Google Scholar] [CrossRef] [PubMed]
- Rask-Nissilä, L.; Jokinen, E.; Terho, P.; Tammi, A.; Hakanen, M.; Rönnemaa, T.; Viikari, J.; Seppänen, R.; Välimäki, I.; Helenius, H.; et al. Effects of diet on the neurologic development of children at 5 years of age: The STRIP project. J. Pediatr. 2002, 140, 328–333. [Google Scholar] [CrossRef]
- Lam, L.F.; Lawlis, T.R. Feeding the brain—The effects of micronutrient interventions on cognitive performance among school-aged children: A systematic review of randomized controlled trials. Clin. Nutr. 2017, 36, 1007–1014. [Google Scholar] [CrossRef]
- Mattei, D.; Pietrobelli, A. Micronutrients and Brain Development. Curr. Nutr. Rep. 2019, 8, 99–107. [Google Scholar] [CrossRef]
- Leidy, H.J.; Gwin, J.A. Growing up strong: The importance of physical, mental, and emotional strength during childhood and adolescence with focus on dietary factors1. Appl. Physiol. Nutr. Metab. 2020, 45, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Van Stralen, M.M.; Yildirim, M.; Wulp, A.; te Velde, S.J.; Verloigne, M.; Doessegger, A.; Androutsos, O.; Kovács, É.; Brug, J.; Chinapaw, M.J.M. Measured sedentary time and physical activity during the school day of European 10- to 12-year-old children: The ENERGY project. J. Sci. Med. Sport 2014, 17, 201–206. [Google Scholar] [CrossRef]
- Beets, M.W.; Shah, R.; Weaver, R.G.; Huberty, J.; Beighle, A.; Moore, J.B. Physical activity in after-school programs: Comparison with physical activity policies. J. Phys. Act. Health 2015, 12, 1–7. [Google Scholar] [CrossRef]
- Maher, C.; Virgara, R.; Okely, T.; Stanley, R.; Watson, M.; Lewis, L. Physical activity and screen time in out of school hours care: An observational study. BMC Pediatr. 2019, 19, 283. [Google Scholar] [CrossRef]
- Rangelov, N.; Marques-Vidal, P.; Suggs, L.S. Reporting children’s food consumption: A comparison of reliability between a 2-day food record and a 7-day food diary. Nutrire 2018, 43, 1–5. [Google Scholar] [CrossRef]
- Sette, S.; Le Donne, C.; Piccinelli, R.; Arcella, D.; Turrini, A.; Leclercq, C. The third Italian National Food Consumption Survey, INRAN-SCAI 2005–06—Part 1: Nutrient intakes in Italy. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 922–932. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, M.B.E.; Robson, P.J.; Wallace, J.M.W. Issues in dietary intake assessment of children and adolescents. Br. J. Nutr. 2004, 92, S213–S222. [Google Scholar] [CrossRef]
- Willett, W. Nutritional Epidemiology; OXFORD University Press: New York, NY, USA, 2013; ISBN 9780199979448. [Google Scholar]
- Börnhorst, C.; Huybrechts, I.; Hebestreit, A.; Krogh, V.; De Decker, A.; Barba, G.; Moreno, L.A.; Lissner, L.; Tornaritis, M.; Loit, H.M.; et al. Usual energy and macronutrient intakes in 2–9-year-old European children. Int. J. Obes. 2014, 38, S115–S123. [Google Scholar] [CrossRef]
- Madrigal, C.; Soto-Méndez, M.J.; Hernández-Ruiz, Á.; Valero, T.; Ávila, J.M.; Ruiz, E.; Villoslada, F.L.; Leis, R.; de Victoria, E.M.; Moreno, J.M.; et al. Energy intake, macronutrient profile and food sources of spanish children aged one to <10 years—Results from the esnupi study. Nutrients 2020, 12, 893. [Google Scholar] [CrossRef]
- Alexy, U.; Sichert-Hellert, W.; Kersting, M. Fifteen-year time trends in energy and macronutrient intake in German children and adolescents: Results of the DONALD study. Br. J. Nutr. 2002, 87, 595–604. [Google Scholar] [CrossRef]
- Glynn, L.; Emmett, P.; Rogers, I. Food and nutrient intakes of a population sample of 7-year-old children in the south-west of England in 1999/2000—What difference does gender make? J. Hum. Nutr. Diet. 2005, 18, 7–19. [Google Scholar] [CrossRef]
- Zaragoza-Jordana, M.; Closa-Monasterolo, R.; Luque, V.; Ferré, N.; Grote, V.; Koletzko, B.; Pawellek, I.; Verduci, E.; ReDionigi, A.; Socha, J.; et al. Micronutrient intake adequacy in children from birth to 8 years. Data from the Childhood Obesity Project. Clin. Nutr. 2018, 37, 630–637. [Google Scholar] [CrossRef]
- Verduci, E.; Radaelli, G.; Stival, G.; Salvioni, M.; Giovannini, M.; Scaglioni, S. Dietary macronutrient intake during the first 10 years of life in a cohort of Italian children. J. Pediatr. Gastroenterol. Nutr. 2007, 45, 90–95. [Google Scholar] [CrossRef]
- Bertoli, S.; Petroni, M.L.; Pagliato, E.; Mora, S.; Weber, G.; Chiumello, G.; Testolin, G. Validation of food frequency questionnaire for assessing dietary macronutrients and calcium intake in Italian children and adolescents. J. Pediatr. Gastroenterol. Nutr. 2005, 40, 555–560. [Google Scholar] [CrossRef]
- Verduci, E.; Banderali, G.; Montanari, C.; Canani, R.B.; Caserta, L.C.; Corsello, G.; Mosca, F.; Piazzolla, R.; Rescigno, M.; Terracciano, L.; et al. Childhood dietary intake in Italy: The epidemiological “MY FOOD DIARY” survey. Nutrients 2019, 11, 1129. [Google Scholar] [CrossRef]
- Rosi, A.; Mena, P.; Castello, F.; Del Rio, D.; Scazzina, F. Comprehensive dietary evaluation of Italian primary school children: Food consumption and intake of energy, nutrients and phenolic compounds. Int. J. Food Sci. Nutr. 2021, 72, 70–81. [Google Scholar] [CrossRef]
- Collo, A.; Ferro, A.; Belci, P.; Cerutti, F.; Rabbone, I.; Ignaccolo, M.G.; Carletto, G.; Vallini, C.; Cadario, F.; Savastio, S.; et al. Nutritional behavior in Italian and immigrant children. Minerva Pediatr. 2019, 71, 481–487. [Google Scholar] [CrossRef]
- Sette, S.; Le Donne, C.; Piccinelli, R.; Mistura, L.; Ferrari, M.; Leclercq, C.; Arcella, D.; Bevilacqua, N.; Buonocore, P.; Capriotti, M.; et al. The third National Food Consumption Survey, INRAN-SCAI 2005-06: Major dietary sources of nutrients in Italy. Int. J. Food Sci. Nutr. 2013, 64, 1014–1021. [Google Scholar] [CrossRef]
- SINU. Livelli di Assunzione di Riferimento ed Energia per la Popolazione Italiana (LARN); SICS: Milano, Italy, 2014; ISBN 9788890685224. [Google Scholar]
- Madden, P.; Yoder, M. Program Evaluation: Food Stamps and Commodity Distribution in Rural Areas of Central Pennsylvania; ERIC: Philadelphia, PA, USA, 1971. [Google Scholar]
- Hatløy, A.; Torheim, L.E.; Oshaug, A. Food variety—A good indicator of nutritional adequacy of the diet? A case study from an urban area in Mali, West Africa. Eur. J. Clin. Nutr. 1998, 52, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Eldridge, A.L.; Catellier, D.J.; Hampton, J.C.; Dwyer, J.T.; Bailey, R.L. Trends in mean nutrient intakes of US infants, toddlers, and young children from 3 Feeding Infants and Toddlers Studies (FITS). J. Nutr. 2019, 149, 1230–1237. [Google Scholar] [CrossRef]
- Valent, F.; Mariuz, M.; Bin, M.; Little, D.; Mazej, D.; Tognin, V.; Tratnik, J.; McAfee, A.J.; Mulhern, M.S.; Parpinel, M.; et al. Associations of prenatal mercury exposure from maternal fish consumption and polyunsaturated fatty acids with child neurodevelopment: A prospective cohort study in Italy. J. Epidemiol. 2013, 23, 360–370. [Google Scholar] [CrossRef]
- Valent, F.; Horvat, M.; Sofianou-Katsoulis, A.; Spiric, Z.; Mazej, D.; Little, D.; Prasouli, A.; Mariuz, M.; Tamburlini, G.; Nakou, S.; et al. neurodevelopmental effects of low-level prenatal mercury exposure from maternal fish consumption in a mediterranean cohort: Study rationale and design. J. Epidemiol. 2013, 23, 146–152. [Google Scholar] [CrossRef]
- Brumatti, L.V.; Rosolen, V.; Mariuz, M.; Piscianz, E.; Valencic, E.; Bin, M.; Athanasakis, E.; D’Adamo, P.; Fragkiadoulaki, E.; Calamandrei, G.; et al. Impact of Methylmercury and Other Heavy Metals Exposure on Neurocognitive Function in Children Aged 7 Years: Study Protocol of the Follow-up. J. Epidemiol. 2021, 31, 157–163. [Google Scholar] [CrossRef]
- Concina, F.; Pani, P.; Carletti, C.; Bravo, G.; Knowles, A.; Parpinel, M.; Ronfani, L.; Barbone, F. Dietary Intake of the Italian PHIME Infant Cohort: How We Are Getting Diet Wrong from as Early as Infancy. Nutrients 2021, 13, 4430. [Google Scholar] [CrossRef]
- Lobstein, T.; Jewell, J. What is a “high” prevalence of obesity? Two rapid reviews and a proposed set of thresholds for classifying prevalence levels. Obes. Rev. 2021, 23, e13363. [Google Scholar] [CrossRef] [PubMed]
- Cole, T.J.; Lobstein, T. Extended international (IOTF) body mass index cut-offs for thinness, overweight and obesity. Pediatr. Obes. 2012, 7, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Gnagnarella, P.; Salvini, S.; Parpinel, M. Food Composition Database for Epidemiological Studies in Italy. Available online: http://www.bda-ieo.it/ (accessed on 1 November 2021).
- Concina, F.; Carletti, C.; Pani, P.; Knowles, A.; Barbone, F.; Parpinel, M. Development of a food composition database to study complementary feeding: An Italian experience. J. Food Compos. Anal. 2016, 46, 96–102. [Google Scholar] [CrossRef]
- Talamini, R.; Polesel, J.; Montella, M.; Dal Maso, L.; Crovatto, M.; Crispo, A.; Spina, M.; Canzonieri, V.; La Vecchia, C.; Franceschi, S. Food groups and risk of non-Hodgkin lymphoma: A multicenter, case-control study in Italy. Int. J. Cancer 2006, 118, 2871–2876. [Google Scholar] [CrossRef]
- Hilbig, A.; Drossard, C.; Kersting, M.; Alexy, U. Nutrient Adequacy and Associated Factors in a Nationwide Sample of German Toddlers. J. Pediatr. Gastroenterol. Nutr. 2015, 61, 130–137. [Google Scholar] [CrossRef]
- Madrigal, C.; Soto-méndez, M.J.; Hernández-ruiz, Á.; Valero, T.; Villoslada, F.L.; Leis, R.; de Victoria, E.M.; Moreno, J.M.; Ortega, R.M.; Ruiz-lópez, M.D.; et al. Dietary intake, nutritional adequacy, and food sources of protein and relationships with personal and family factors in spanish children aged one to <10 years: Findings of the esnupi study. Nutrients 2021, 13, 1062. [Google Scholar] [CrossRef]
- Lin, Y.; Bolca, S.; Vandevijvere, S.; Van Oyen, H.; Van Camp, J.; De Backer, G.; Foo, L.H.; De Henauw, S.; Huybrechts, I. Dietary sources of animal and plant protein intake among Flemish preschool children and the association with socio-economic and lifestyle-related factors. Nutr. J. 2011, 10, 97. [Google Scholar] [CrossRef][Green Version]
- Fabiani, R.; Naldini, G.; Chiavarini, M. Dietary patterns and metabolic syndrome in adult subjects: A systematic review and meta-analysis. Nutrients 2019, 11, 2056. [Google Scholar] [CrossRef]
- Rippin, H.L.; Hutchinson, J.; Jewell, J.; Breda, J.J.; Cade, J.E. Child and adolescent nutrient intakes from current national dietary surveys of European populations. Nutr. Res. Rev. 2019, 32, 38–69. [Google Scholar] [CrossRef] [PubMed]
- Fidler Mis, N.; Braegger, C.; Bronsky, J.; Campoy, C.; Domellöf, M.; Embleton, N.D.; Hojsak, I.; Hulst, J.; Indrio, F.; Lapillonne, A.; et al. Sugar in Infants, Children and Adolescents: A Position Paper of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2017, 65, 681–696. [Google Scholar] [CrossRef]
- Angelino, D.; Rosi, A.; Ruggiero, E.; Nucci, D.; Paolella, G.; Pignone, V.; Pellegrini, N.; Martini, D. Analysis of food labels to evaluate the nutritional quality of bread products and substitutes sold in italy: Results from the food labelling of italian products (flip) study. Foods 2020, 9, 1905. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshini, V. Purchasing practice of the consumers towards ready to eat food products. Asian J. Home Sci. 2015, 10, 290–295. [Google Scholar] [CrossRef]
- Basurra, R.S.; Tunung, R.; Kavita, C.; Ribka, A.; Chandrika, M.; Ubong, A. Consumption practices and perception of ready-to-eat food among university students and employees in Kuala Lumpur, Malaysia. Food Res. 2021, 5, 246–251. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Committee on Strategies to Reduce Sodium Intake. Strategies to Reduce Sodium Intake in the United States. In Taste and Flavor Roles of Sodium in Foods: A Unique Challenge to Reducing Sodium Intake; Henney, J.E., Taylor, C.L., Boon, C.S., Eds.; National Academies Press: Washington, DC, USA, 2010; Volume 3. Available online: https://www.ncbi.nlm.nih.gov/books/NBK50958/ (accessed on 30 November 2021).
- Sichert-Hellert, W.; Kersting, M.; Manz, F. Changes in time-trends of nutrient intake from fortified and non-fortified food in German children and adolescents—15 Year results of the DONALD Study. Eur. J. Nutr. 2001, 40, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Gibson, R.S.; Hess, S.Y.; Hotz, C.; Brown, K.H. Indicators of zinc status at the population level: A review of the evidence. Br. J. Nutr. 2008, 99, 14–23. [Google Scholar] [CrossRef]
- Vierucci, F.; Del Pistoia, M.; Fanos, M.; Erba, P.; Saggese, G. Prevalence of hypovitaminosis D and predictors of vitamin D status in Italian healthy adolescents. Ital. J. Pediatr. 2014, 40, 1–9. [Google Scholar] [CrossRef]
- Rutigliano, I.; De Filippo, G.; De Giovanni, D.; Campanozzi, A. Is sunlight enough for sufficient vitamin D status in children and adolescents? A survey in a sunny region of southern Italy. Nutrition 2021, 84, 111101. [Google Scholar] [CrossRef]
- Pani, P.; Carletti, C. OKkio alla SALUTE Risultati Dell’ Indagine 2016—Regione Friuli Venezia Giulia; Istituto Superiore di Sanità: Roma, Italy, 2016; pp. 1–56. [Google Scholar]
- Nardone, P.; Spinelli, A.; Buoncristiano, M.; Lauria, L.; Pierannunzio, D.; Galeone, D. Il Sistema di Sorveglianza OKkio Alla SALUTE: Risultati 2016; Istituto Superiore di Sanità: Roma, Italy, 2018; p. 83. [Google Scholar]
- Royo-Bordonada, M.A.; Gorgojo, L.; Ortega, H.; Martín-Moreno, J.M.; Lasunción, M.A.; Garcés, C.; Gil, A.; Rodríguez-Artalejo, F.; De Oya, M. Greater dietary variety is associated with better biochemical nutritional status in Spanish children: The Four Provinces Study. Nutr. Metab. Cardiovasc. Dis. 2003, 13, 357–364. [Google Scholar] [CrossRef]
- Castro-Quezada, I.; Román-Viñas, B.; Serra-Majem, L. The mediterranean diet and nutritional adequacy: A review. Nutrients 2014, 6, 231–248. [Google Scholar] [CrossRef]
- Peng, W.; Berry, E.M.; Goldsmith, R. Adherence to the Mediterranean diet was positively associated with micronutrient adequacy and negatively associated with dietary energy density among adolescents. J. Hum. Nutr. Diet. 2019, 32, 41–52. [Google Scholar] [CrossRef]
| Median | 25th–75th Centile | |
|---|---|---|
| Child weight at 7 years (kg) 1 | 25.5 | 22.8–29.2 |
| Child height at 7 years (cm) 1 | 124.3 | 121.0–128.5 |
| N | % | |
| Sex | ||
| Male | 197 | 51.7 |
| Female | 184 | 48.3 |
| Weight status 1 | ||
| Underweight | 9 | 2.6 |
| Normal weight | 255 | 72.9 |
| Overweight | 67 | 19.1 |
| Obese | 19 | 5.4 |
| Extra-curricular sport or play activities | ||
| Never | 15 | 3.9 |
| 1–3 days/week | 303 | 79.5 |
| >4 days/week | 58 | 15.2 |
| Not reported | 5 | 1.3 |
| Videogames activity | ||
| Never | 106 | 27.8 |
| <1 h/day | 172 | 45.1 |
| 1–2 h/day | 64 | 17.6 |
| 3 h/day | 5 | 1.3 |
| Not reported | 34 | 8.9 |
| Television use | ||
| Never | 15 | 3.9 |
| <1 h/day | 105 | 27.6 |
| 1–2 h/day | 233 | 61.2 |
| 3–4 h/day | 22 | 5.8 |
| Not reported | 6 | 1.6 |
| Food consumption while screen-time activities | ||
| Yes | 103 | 27.0 |
| No | 274 | 71.9 |
| Not reported | 4 | 1.0 |
| Caregiver (weekdays) 2 | ||
| Mother | 326 | 86.2 |
| Father | 146 | 38.7 |
| Grandparents | 135 | 35.8 |
| School | 164 | 43.4 |
| Baby-sitter | 13 | 3.4 |
| Others | 19 | 5.1 |
| Females (N = 184) | Males (N = 197) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Median | 25th | 75th | DRVs | Median | 25th | 75th | Median | 25th | 75th | p-Value | |
| Energy (kJ/d) | 6291.8 | 5593.2 | 6982.2 | 6139.2 | 5261 | 6862.2 | 6404.4 | 5773.6 | 7113.2 | 0.002 * | |
| Energy (kcal/d) | 1503.0 | 1336.2 | 1668.0 | 1466.6 | 1256.8 | 1639.3 | 1530.0 | 1379.3 | 1699.3 | 0.002 * | |
| Protein (g/d) | 55.6 | 47.2 | 64.3 | 54.0 | 46.3 | 63.4 | 56.8 | 48.4 | 64.5 | 0.077 | |
| Protein (%En) | 14.8 | 13.2 | 16.5 | 12–18 %En (RI) 1 | 14.9 | 13.3 | 16.9 | 14.8 | 13.0 | 16.3 | 0.165 |
| Total fats (g/d) | 52.2 | 42.6 | 61.7 | 51.1 | 42.0 | 60.4 | 53.3 | 43.4 | 63.7 | 0.084 | |
| Total fats (%En) | 31.3 | 27.4 | 35.1 | 20–35 %En (RI) | 32.0 | 27.7 | 35.1 | 30.8 | 27.2 | 35.1 | 0.309 |
| Saturated fatty acids (g/d) | 20.5 | 16.5 | 24.5 | 20.2 | 14.9 | 24.0 | 20.9 | 17.0 | 25.0 | 0.055 | |
| Saturated fatty acids (%En) | 12.2 | 10.6 | 14.0 | <10 %En (SDT) | 12.2 | 10.8 | 14.0 | 12.2 | 10.4 | 14.1 | 0.865 |
| Monounsaturated fatty acids (g/d) | 18.0 | 14.5 | 22.1 | 18.0 | 14.0 | 21.7 | 18.1 | 14.8 | 22.1 | 0.307 | |
| Monounsaturated fatty acids (%En) | 10.8 | 9.3 | 12.6 | 10–15 %En (RI) 2 | 11.1 | 9.4 | 12.7 | 10.7 | 9.3 | 12.4 | 0.137 |
| Oleic acid (g/d) | 16.5 | 13.4 | 20.1 | 16.6 | 13.2 | 20.0 | 16.5 | 13.8 | 20.1 | 0.405 | |
| Polyunsaturated fatty acids (g/d) | 5.2 | 4.1 | 6.6 | 5.3 | 4.1 | 6.7 | 5.1 | 4.1 | 6.5 | 0.792 | |
| Polyunsaturated fatty acids (%En) | 3.1 | 2.5 | 3.9 | 5–10 %En (RI) | 3.3 | 2.6 | 4.0 | 3.0 | 2.4 | 3.7 | 0.021 * |
| Arachidonic acid (mg/d) | 146.3 | 95.6 | 219.7 | 143.2 | 96.4 | 206.5 | 154.2 | 94.5 | 226.9 | 0.503 | |
| Linoleic acid (g/d) | 3.9 | 3.1 | 5.2 | 3.9 | 3.1 | 5.3 | 3.8 | 3.0 | 5.2 | 0.509 | |
| PUFAs ω-6 (%En) | 2.4 | 2.0 | 3.2 | 4–8 %En (RI) | 2.6 | 2.1 | 3.3 | 2.3 | 1.9 | 3.0 | 0.007 * |
| Alpha-linolenic acid (g/d) | 0.6 | 0.4 | 0.7 | 0.5 | 0.4 | 0.7 | 0.6 | 0.5 | 0.7 | 0.101 | |
| EPA + DHA (mg/d) | 61.0 | 23.7 | 208.3 | 250 mg/d (AI) | 53.9 | 24.0 | 207.3 | 67.3 | 23.7 | 210.0 | 0.620 |
| PUFAs ω-3 (%En) | 0.4 | 0.3 | 0.5 | 0.5–2.0 %En (RI) | 0.4 | 0.3 | 0.5 | 0.4 | 0.3 | 0.5 | 0.572 |
| Cholesterol (mg/d) | 185.3 | 143.0 | 224.8 | 186 | 141.9 | 225.5 | 183.0 | 143.3 | 224.4 | 0.852 | |
| Available carbohydrates (g/d) | 197.6 | 163.7 | 223.9 | 189.3 | 153.0 | 215.8 | 204.1 | 172.0 | 230.4 | <0.001 * | |
| Available carbohydrates (%En) | 51.8 | 48.3 | 56.6 | 45–60 %En (RI) | 51.3 | 47.7 | 55.6 | 52.5 | 48.7 | 57.3 | 0.095 |
| Soluble carbohydrates (g/d) | 72.5 | 59.0 | 87.5 | 71.2 | 58.6 | 85.8 | 74.7 | 60.1 | 91.3 | 0.057 | |
| Soluble carbohydrates (%En) | 19.4 | 16.4 | 23.0 | <15 %En (SDT) | 19.4 | 16.3 | 23.3 | 19.4 | 16.4 | 22.7 | 0.948 |
| Fiber (g/1000 kcal/d) | 7.0 | 5.7 | 8.7 | 8.4 g/1000 kcal (AI) | 7.2 | 5.8 | 8.9 | 7.0 | 5.4 | 8.5 | 0.139 |
| Females (N = 184) (N = 197) | Males (N = 197) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Median | 25th | 75th | DRVs | Median | 25th | 75th | Median | 25th | 75th | p-Value | |
| Sodium (g/d) | 1.7 | 1.3 | 2.1 | 1.1 g/d (AI); 1.5 g/d (SDT) | 1.7 | 1.3 | 2.1 | 1.8 | 1.4 | 2.1 | 0.207 |
| Potassium (g/d) | 1.8 | 1.4 | 2.1 | 3 g/d (AI) | 1.7 | 1.4 | 2.1 | 1.8 | 1.5 | 2.1 | 0.719 |
| Calcium (mg/d) | 537.8 | 409.6 | 706.3 | 900 mg/d (AR); 1100 mg/d (PRI) | 515.9 | 392.4 | 657.0 | 567.7 | 420.1 | 721.7 | 0.034 * |
| Magnesium (mg/d) | 83.5 | 65.5 | 105.8 | 130 mg/d (AR); 150 mg/d (PRI) | 81.8 | 61.7 | 104.2 | 85.3 | 67.0 | 106.0 | 0.193 |
| Phosphorus (mg/d) | 819.0 | 693.8 | 966.9 | 730 mg/d (AR); 875 mg/d (PRI) | 778.7 | 668.5 | 941.4 | 846.2 | 715.7 | 975.8 | 0.020 * |
| Iron (mg/d) | 5.9 | 4.8 | 7.2 | 5 mg/d (AR); 13 mg/d (PRI) | 5.6 | 4.6 | 7.1 | 6.1 | 5.0 | 7.5 | 0.066 |
| Zinc (mg/d) | 6.3 | 5.3 | 7.4 | 7 mg/d (AR); 8 mg/d (PRI) | 6.0 | 5.1 | 7.3 | 6.6 | 5.6 | 7.4 | 0.004 * |
| Selenium (μg/d) | 18.5 | 13.0 | 28.2 | 30 μg/d (AR); 34 μg/d (PRI) | 17.4 | 13.2 | 27.4 | 19.1 | 12.9 | 28.7 | 0.336 |
| Copper (mg/d) | 0.4 | 0.2 | 0.5 | 0.4 mg/d (AR); 0.6 mg/d (PRI) | 0.4 | 0.2 | 0.5 | 0.4 | 0.3 | 0.5 | 0.288 |
| Chloride (g/d) | 1.3 | 1.0 | 1.7 | 1.7 g/d (AI); 2.3 g/d (SDT) | 1.2 | 1.0 | 1.7 | 1.3 | 1.0 | 1.7 | 0.331 |
| Manganese (mg/d) | 0.4 | 0.2 | 0.6 | 1.2 mg/d (AI) | 0.4 | 0.2 | 0.6 | 0.4 | 0.2 | 0.6 | 0.650 |
| Iodine (μg/d) | 75.0 | 51.8 | 104.6 | 100 μg/d (AI) | 69.3 | 47.2 | 104.8 | 80.0 | 55.1 | 104.6 | 0.077 |
| Vitamin B1 (mg/d) | 0.7 | 0.6 | 0.9 | 0.6 mg/d (AR); 0.8 mg/d (PRI) | 0.7 | 0.6 | 0.8 | 0.7 | 0.6 | 0.9 | 0.127 |
| Vitamin B2 (mg/d) | 1.1 | 0.8 | 1.3 | 0.7 mg/d (AR); 0.8 mg/d (PRI) | 1.0 | 0.8 | 1.3 | 1.1 | 0.9 | 1.3 | 0.014 * |
| Niacin (mg/d) | 9.6 | 7.7 | 12.2 | 9 mg/d (AR); 12 mg/d (PRI) | 9.6 | 7.6 | 12.1 | 9.5 | 7.7 | 12.3 | 0.890 |
| Pantothenic acid (mg/d) | 2.1 | 1.6 | 2.6 | 3.5 mg/d (AI) | 2.2 | 1.7 | 2.7 | 2.1 | 1.6 | 2.6 | 0.979 |
| Vitamin B6 (mg/d) | 1.3 | 1.0 | 1.5 | 0.7 mg/d (AR); 0.9 mg/d (PRI) | 1.2 | 1.0 | 1.5 | 1.3 | 1.1 | 1.5 | 0.394 |
| Biotin (μg/d) | 11.5 | 8.6 | 14.7 | 20 μg/d (AI) | 11.5 | 8.4 | 14.8 | 11.5 | 8.7 | 14.6 | 0.929 |
| Folate (μg/d) | 160.6 | 127.5 | 199.7 | 210 μg/d (AR); 250 μg/d (PRI) | 155.1 | 125.5 | 194.1 | 165.7 | 135.0 | 204.2 | 0.072 |
| Vitamin B12 (μg/d) | 2.4 | 1.8 | 3.3 | 1.3 μg/d (AR); 1.6 μg/d (PRI) | 2.4 | 1.7 | 3.1 | 2.5 | 1.9 | 3.4 | 0.133 |
| Vitamin A (μg/d) 1 | 603.7 | 438.8 | 853.4 | 350 μg/d (AR); 500 μg/d (PRI) | 589.6 | 421.8 | 856.2 | 617.4 | 450.1 | 853.1 | 0.419 |
| Vitamin C (mg/d) | 63.2 | 40.7 | 98.4 | 45 mg/d (AR); 60 mg/d (PRI) | 61.6 | 41.0 | 97.5 | 63.7 | 40.4 | 98.4 | 0.984 |
| Vitamin D (μg/d) | 1.1 | 0.7 | 1.5 | 10 μg/d (AR); 15 μg/d (PRI) | 1.0 | 0.7 | 1.3 | 1.1 | 0.8 | 1.6 | 0.142 |
| Vitamin E (mg/d) 2 | 5.1 | 4.0 | 6.6 | 8 mg/d (AI) | 5.2 | 4.1 | 6.6 | 5.1 | 4.0 | 6.6 | 0.496 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giordani, E.; Marinoni, M.; Fiori, F.; Concina, F.; Ronfani, L.; Dalmin, P.; Barbone, F.; Edefonti, V.; Parpinel, M. Adherence to Dietary Recommendations of 7-Year-Old Children from a Birth Cohort in Friuli Venezia Giulia, Italy. Nutrients 2022, 14, 515. https://doi.org/10.3390/nu14030515
Giordani E, Marinoni M, Fiori F, Concina F, Ronfani L, Dalmin P, Barbone F, Edefonti V, Parpinel M. Adherence to Dietary Recommendations of 7-Year-Old Children from a Birth Cohort in Friuli Venezia Giulia, Italy. Nutrients. 2022; 14(3):515. https://doi.org/10.3390/nu14030515
Chicago/Turabian StyleGiordani, Elisa, Michela Marinoni, Federica Fiori, Federica Concina, Luca Ronfani, Patrizia Dalmin, Fabio Barbone, Valeria Edefonti, and Maria Parpinel. 2022. "Adherence to Dietary Recommendations of 7-Year-Old Children from a Birth Cohort in Friuli Venezia Giulia, Italy" Nutrients 14, no. 3: 515. https://doi.org/10.3390/nu14030515
APA StyleGiordani, E., Marinoni, M., Fiori, F., Concina, F., Ronfani, L., Dalmin, P., Barbone, F., Edefonti, V., & Parpinel, M. (2022). Adherence to Dietary Recommendations of 7-Year-Old Children from a Birth Cohort in Friuli Venezia Giulia, Italy. Nutrients, 14(3), 515. https://doi.org/10.3390/nu14030515







