Sargassum plagiophyllum Extract Enhances Colonic Functions and Modulates Gut Microbiota in Constipated Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sargassum plagiophyllum Extract (SPE) Preparation
2.2. Animals and Experimental Design
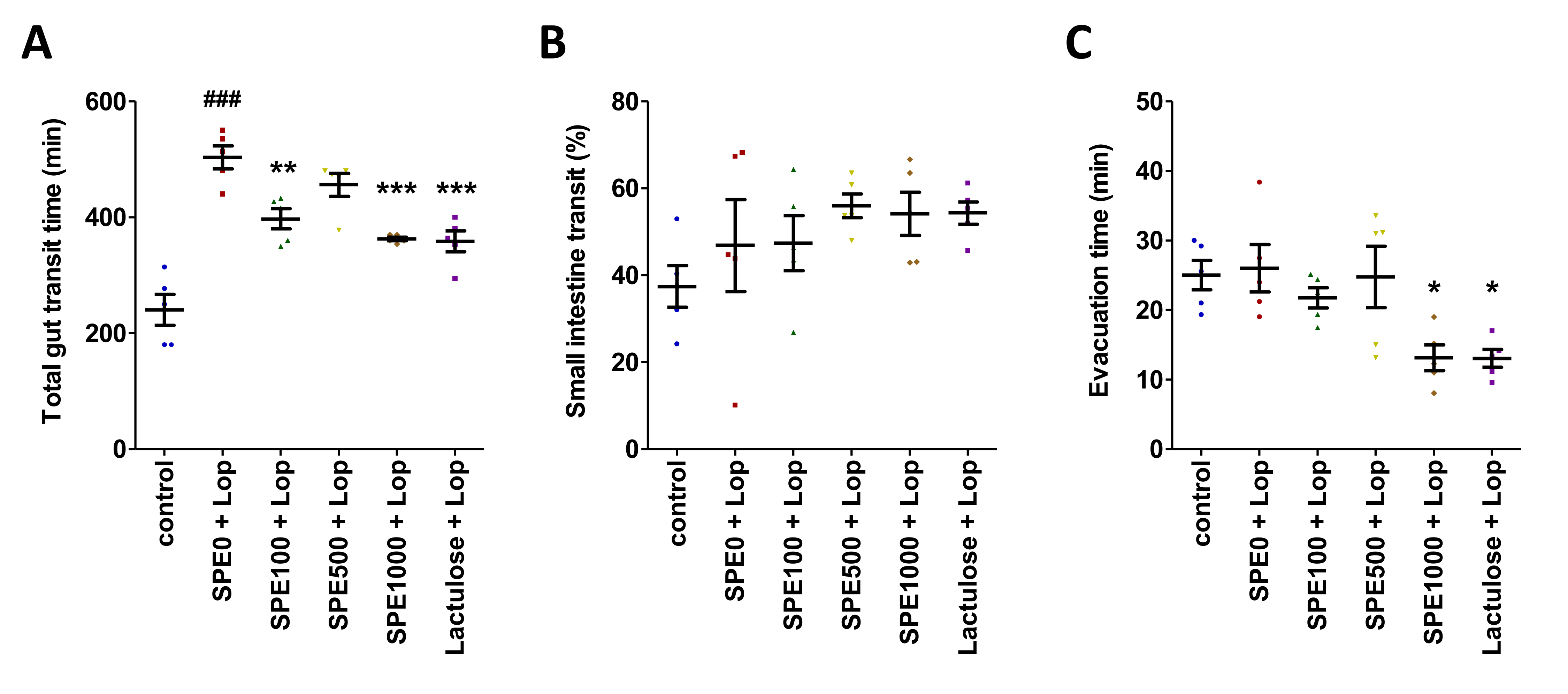
2.3. Measurement of Gastrointestinal Transit
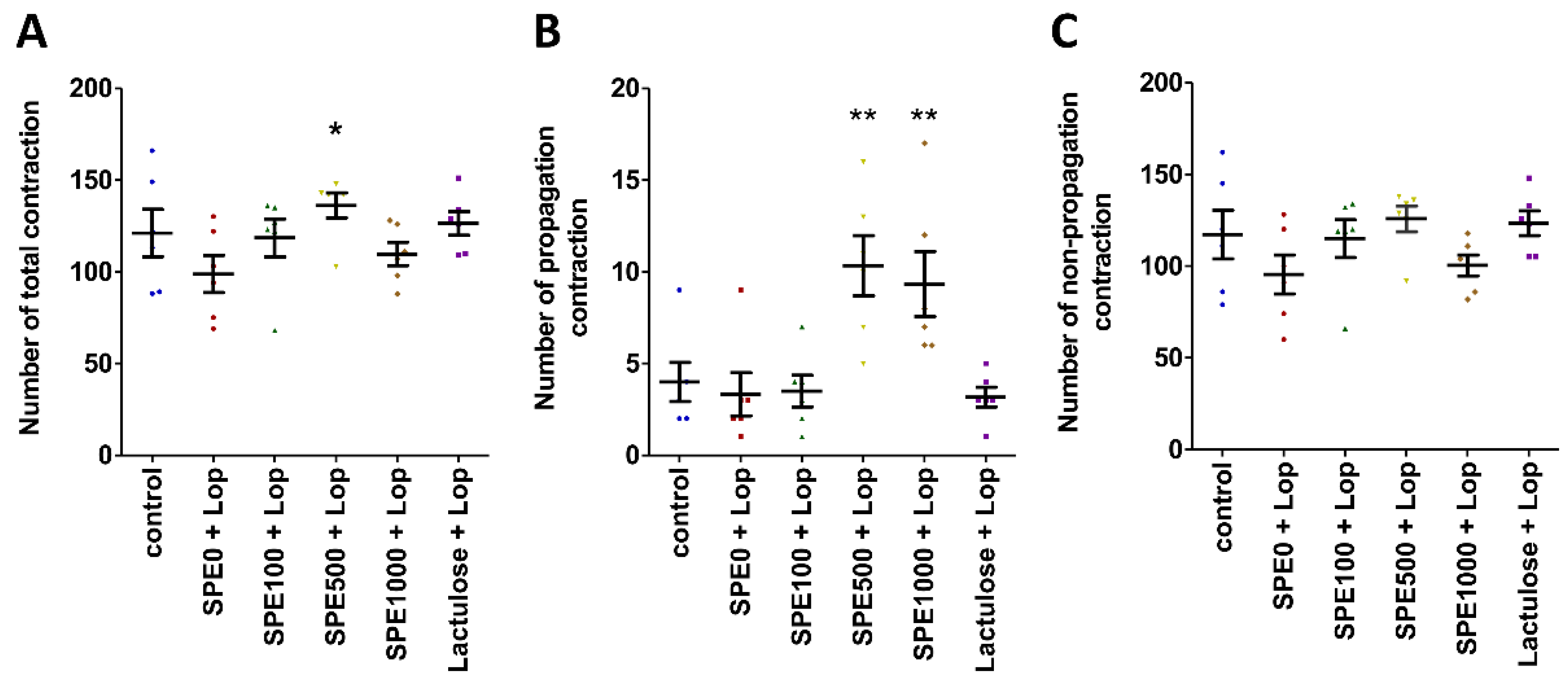
2.4. Colonic Motility Pattern
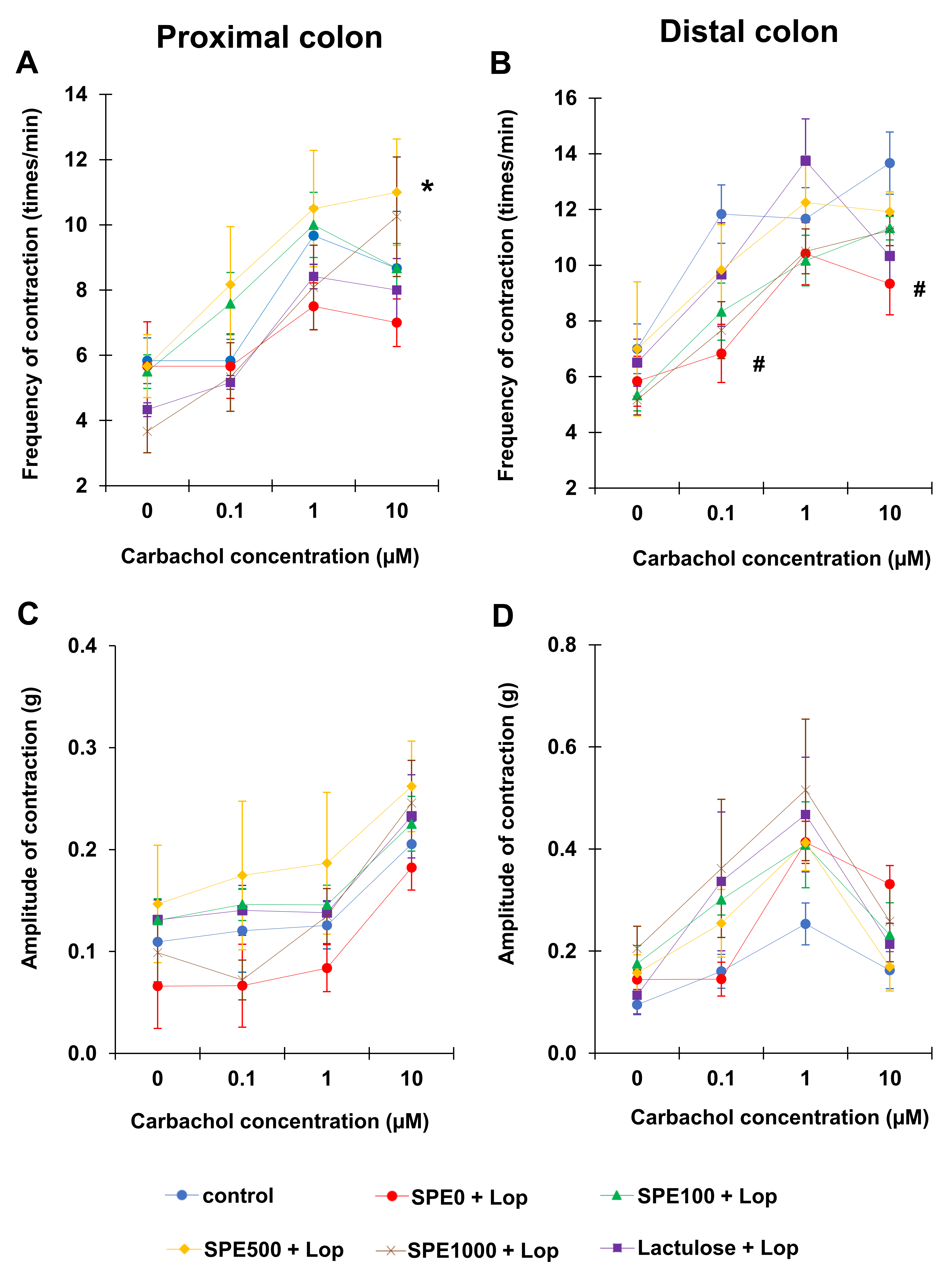
2.5. Colonic Smooth Muscle Contractility
2.6. Transepithelial Transport of Electrolytes across Cell Membranes in Distal Colon
2.7. Composition of Colonic Microbiota Analyses
2.8. Statistical Analysis
3. Results
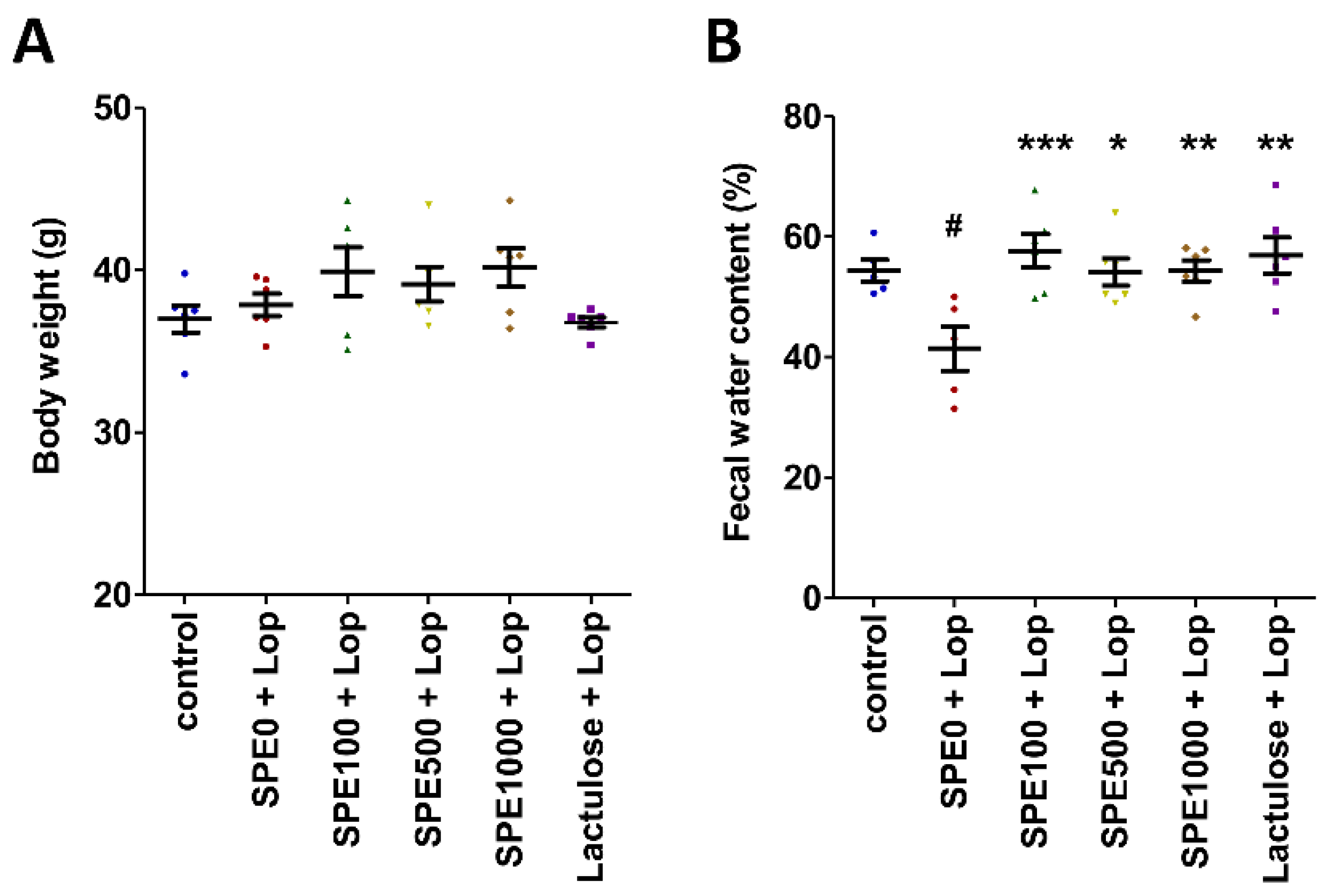
3.1. Effect of SPE Pretreatment on Body Weight, Fecal Water Content, and Gut Transit in Constipated Mice
3.2. Effect of SPE Pretreatment on Colonic Motility Pattern in Constipated Mice
3.3. Effect of SPE Pretreatment on Colonic Smooth Muscle Contractility in Constipated Mice
3.4. Effect of SPE Pretreatment on Transport of Electrolytes across Cell Membranes in Distal Colon of Constipated Mice
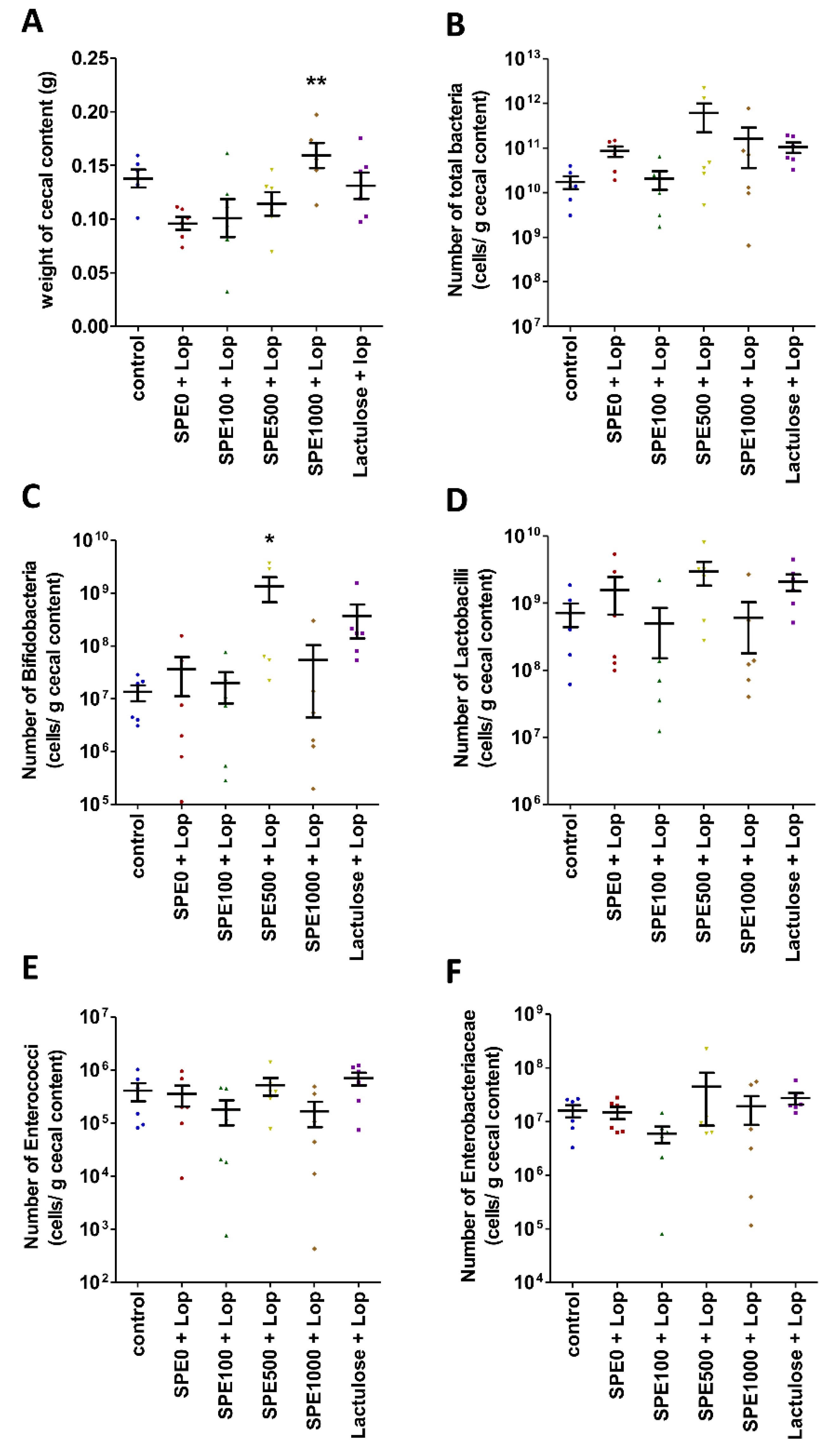
3.5. Effect of SPE Pretreatment on Composition of Gut Microbiota in Constipate Mice
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tamura, A.; Tomita, T.; Oshima, T.; Toyoshima, F.; Yamasaki, T.; Okugawa, T.; Kondo, T.; Kono, T.; Tozawa, K.; Ikehara, H.; et al. Prevalence and self-recognition of chronic constipation: Results of an internet survey. J. Neurogastroenterol. Motil. 2016, 22, 677–685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Zhong, D.; Sun, R.; Zhang, Y.; Pegg, R.B.; Zhong, G. Prevention of loperamide induced constipation in mice by KGM and the mechanisms of different gastrointestinal tract microbiota regulation. Carbohydr. Polym. 2021, 256, 117418. [Google Scholar] [CrossRef] [PubMed]
- Boland, J.W.; Boland, E.G. Constipation and malignant bowel obstruction in palliative care. Medicine 2020, 48, 18–22. [Google Scholar] [CrossRef] [Green Version]
- Milosavljevic, T.; Popovic, D.D.; Mijac, D.D.; Milovanovic, T.; Krstic, S.; Krstic, M.N. Chronic constipation, gastroenterohepatologist’s approach. Dig. Dis. 2021. [Google Scholar] [CrossRef] [PubMed]
- Forootan, M.; Bagheri, N.; Darvishi, M. Chronic constipation. Medicine 2018, 97, e10631. [Google Scholar] [CrossRef]
- Hu, T.G.; Wen, P.; Fu, H.Z.; Lin, G.Y.; Liao, S.T.; Zou, Y.X. Protective effect of mulberry (Morus atropurpurea) fruit against diphenoxylate-induced constipation in mice through the modulation of gut microbiota. Food Funct. 2019, 10, 1513–1528. [Google Scholar] [CrossRef]
- Tabbers, M.M.; de Milliano, I.; Roseboom, M.G.; Benninga, M.A. Is Bifidobacterium breve effective in the treatment of childhood constipation? Results from a pilot study. Nutr. J. 2011, 10, 19. [Google Scholar] [CrossRef] [PubMed]
- Tsukahara, T.; Bukawa, W.; Kan, T.; Ushida, K. Effect of a cell preparation of Enterococcus faecalis strain EC-12 on digesta flow and recovery from constipation in a pig model and human subjects. Microb. Ecol. Health Dis. 2005, 17, 107–113. [Google Scholar]
- Zhao, Y.; Yu, Y.-B. Intestinal microbiota and chronic constipation. SpringerPlus 2016, 5, 1130. [Google Scholar] [CrossRef] [Green Version]
- Chassard, C.; Dapoigny, M.; Scott, K.P.; Crouzet, L.; Del’homme, C.; Marquet, P.; Martin, J.C.; Pickering, G.; Ardid, D.; Eschalier, A.; et al. Functional dysbiosis within the gut microbiota of patients with constipated-irritable bowel syndrome. Aliment. Pharmacol. Ther. 2012, 35, 828–838. [Google Scholar] [CrossRef]
- Parkes, G.C.; Rayment, N.B.; Hudspith, B.N.; Petrovska, L.; Lomer, M.C.; Brostoff, J.; Whelan, K.; Sanderson, J.D. Distinct microbial populations exist in the mucosa-associated microbiota of sub-groups of irritable bowel syndrome. Neurogastroenterol. Motil. 2012, 24, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Durban, A.; Abellan, J.J.; Jimenez-Hernandez, N.; Salgado, P.; Ponce, M.; Ponce, J.; Garrigues, V.; Latorre, A.; Moya, A. Structural alterations of faecal and mucosa-associated bacterial communities in irritable bowel syndrome. Environ. Microbiol. Rep. 2012, 4, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Portalatin, M.; Winstead, N. Medical Management of Constipation. Clin. Colon Rectal Surg. 2012, 25, 12–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krogh, K.; Chiarioni, G.; Whitehead, W. Management of chronic constipation in adults. United Eur. Gastroenterol. J. 2017, 5, 465–472. [Google Scholar] [CrossRef] [Green Version]
- Tropini, C.; Moss, E.L.; Merrill, B.D.; Ng, K.M.; Higginbottom, S.K.; Casavant, E.P.; Gonzalez, C.G.; Fremin, B.; Bouley, D.M.; Elias, J.E.; et al. Transient osmotic perturbation causes long-term alteration to the gut microbiota. Cell 2018, 173, 1742–1754. [Google Scholar] [CrossRef] [Green Version]
- Vriesman, M.H.; Koppen, I.J.N.; Camilleri, M.; Di Lorenzo, C.; Benninga, M.A. Management of functional constipation in children and adults. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 21–39. [Google Scholar] [CrossRef]
- Ngatu, R.N.; Ikeda, M.; Watanabe, H.; Tanaka, M.; Inoue, M. Laxative effects of dietary supplementation with sujiaonori algal biomaterial in Japanese adult women with functional constipation: A case study. J. Funct. Biomater. 2017, 8, 15. [Google Scholar] [CrossRef] [Green Version]
- Peñalver, R.; Lorenzo, J.M.; Ros, G.; Amarowicz, R.; Pateiro, M.; Nieto, G. Seaweeds as a functional ingredient for a healthy diet. Mar. Drugs 2020, 18, 301. [Google Scholar] [CrossRef]
- Dewinta, A.F.; Susetya, I.E.; Suriani, M. Nutritional profile of Sargassum sp. from Pane Island, Tapanuli Tengah as a component of functional food. J. Phys. Conf. Ser. 2020, 1542, 012040. [Google Scholar] [CrossRef]
- K-da, S.; Peerakietkhajorn, S.; Siringoringo, B.; Muangnil, P.; Wichienchot, S.; Khuituan, P. Oligosaccharides from Gracilaria fisheri ameliorate gastrointestinal dysmotility and gut dysbiosis in colitis mice. J. Funct. Foods 2020, 71, 104021. [Google Scholar] [CrossRef]
- Siringoringo, B.; Huipao, N.; Tipbunjong, C.; Nopparat, J.; Wichienchot, S.; Hutapea, A.M.; Khuituan, P. Gracilaria fisheri oligosaccharides ameliorate inflammation and colonic epithelial barrier dysfunction in mice with acetic acid-induced colitis. Asian Pac. J. Trop. Biomed. 2021, 11, 440–449. [Google Scholar]
- Chamidah, A. Prebiotic index evaluation of crude laminaran of Sargassum sp. using feces of wistar rats. IOP Conf. Ser. Earth Environ. Sci. 2018, 139, 012043. [Google Scholar] [CrossRef]
- Ramnani, P.; Chitarrari, R.; Tuohy, K.; Grant, J.; Hotchkiss, S.; Philp, K.; Campbell, R.; Gill, C.; Rowland, I. In vitro fermentation and prebiotic potential of novel low molecular weight polysaccharides derived from agar and alginate seaweeds. Anaerobe 2012, 18, 1–6. [Google Scholar] [CrossRef]
- Shang, Q.; Shan, X.; Cai, C.; Hao, J.; Li, G. Dietary fucoidan modulates the gut microbiota in mice by increasing the abundance of Lactobacillus and Ruminococcaceae. Food Funct. 2016, 7, 3224–3232. [Google Scholar] [CrossRef] [PubMed]
- Yangthong, M.; Hutadilok-Towatana, N.; Thawonsuwan, J.; Wutiporn, P. An aqueous extract from Sargassum sp. enhances the immune response and resistance against Streptococcus iniae in the Asian sea bass (Lates calcarifer Bloch). J. Appl. Phycol. 2016, 28, 3587–3598. [Google Scholar] [CrossRef]
- Zheng, L.X.; Chen, X.Q.; Cheong, K.L. Current trends in marine algae polysaccharides: The digestive tract, microbial catabolism, and prebiotic potential. Int. J. Biol. Macromol. 2020, 151, 344–354. [Google Scholar] [CrossRef]
- Liu, L.; Heinrich, M.; Myers, S.; Dworjanyn, S.A. Towards a better understanding of medicinal uses of the brown seaweed Sargassum in traditional Chinese medicine: A phytochemical and pharmacological review. J. Ethnopharmacol. 2012, 142, 591–619. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, J.; Yan, L.; Cheng, Y.; Li, Q.; Wu, S.; Chen, L.; Thring, R.W.; Yang, Y.; Gao, Y.; et al. Sargassum fusiforme fucoidan alleviates high-fat diet-induced obesity and insulin resistance associated with the improvement of hepatic oxidative stress and gut microbiota profile. J. Agric. Food Chem. 2020, 68, 10626–10638. [Google Scholar] [CrossRef]
- Edison, E.; Diharmi, A.; Ariani, N.M.; Ilza, M. Komponen bioactive dan aktivitas antioksidan ekstrak kasar Sargassum plagyophyllum. J. Pengolah. Has. Perikan. Indones. 2020, 20, 58–66. [Google Scholar]
- Saeton, U.; Nontasak, P.; Palasin, K.; Wonglapsuwan, M.; Mayakun, J.; Pongparadon, S.; Chotigeat, W. Potential health benefits of fucoidan from the brown seaweeds Sargassum plagiophyllum and Sargassum polycystum. J. Appl. Phycol. 2021, 33, 3357–3364. [Google Scholar] [CrossRef]
- Suresh, V.; Senthilkumar, N.; Thangam, R.; Rajkumar, M.; Anbazhagan, C.; Rengasamy, R.; Gunasekaran, P.; Kannan, S.; Palani, P. Separation, purification and preliminary characterization of sulfated polysaccharides from Sargassum plagiophyllum and its in vitro anticancer and antioxidant activity. Process Biochem. 2013, 48, 364–373. [Google Scholar] [CrossRef]
- Kalasariya, H.S.; Yadav, V.K.; Yadav, K.K.; Tirth, V.; Algahtani, A.; Islam, S.; Gupta, N.; Jeon, B.-H. Seaweed-based molecules and their potential biological activities: An eco-sustainable cosmetics. Molecules 2021, 26, 5313. [Google Scholar] [CrossRef] [PubMed]
- Kumar, Y.; Tarafdar, A.; Badgujar, P.C. Seaweed as a source of natural antioxidants: Therapeutic activity and food applications. Hindawi 2021, 5753391. [Google Scholar] [CrossRef]
- Sengkhim, R.; Peerakietkhajorn, S.; Jeanmard, N.; Pongparadorn, S.; Khuituan, P.; Thitiphatphuvanon, T.; Surinlert, P.; Tipbunjong, C. Effects of Sargassum plagiophyllum extract pretreatment on tissue histology of constipated mice. Trop. J. Pharm. Res. 2021, 20, 2339–2346. [Google Scholar] [CrossRef]
- Zahra, R.; Mehrnaz, M.; Farzaneh, V.; Kohzad, S. Antioxidant activity of extract from a brown alga, Sargassum boveanum. Afr. J. Biotechnol. 2007, 6, 2740–2745. [Google Scholar]
- Hayeeawaema, F.; Wichienchot, S.; Khuituan, P. Amelioration of gut dysbiosis and gastrointestinal motility by konjac oligo-glucomannan on loperamide-induced constipation in mice. Nutrition 2020, 73, 110715. [Google Scholar] [CrossRef]
- Khuituan, P.; K-da, S.; Bannob, K.; Hayeeawaema, F.; Peerakietkhajorn, S.; Tipbunjong, C.; Charoenphandhu, N. Prebiotic oligosaccharides from dragon fruits alter gut motility in mice. Biomed. Pharmacother. 2019, 114, 108821. [Google Scholar] [CrossRef]
- Li, H.; Sheppard, D.N.; Hug, M.J. Transepithelial electrical measurements with the Ussing chamber. J. Cyst. Fibros 2004, 3, 123–126. [Google Scholar] [CrossRef] [Green Version]
- Clarke, L.L. A guide to Ussing chamber studies of mouse intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G1151–G1166. [Google Scholar] [CrossRef] [Green Version]
- Peerakietkhajorn, S.; Jeanmard, N.; Chuenpanitkit, P.; K-da, S.; Bannob, K.; Khuituan, P. Effects of plant oligosaccharides derived from dragon fruit on gut microbiota in proximal and distal colon of mice. Sains Malays. 2020, 49, 603–611. [Google Scholar] [CrossRef]
- Kaufmann, P.; Pfefferkorn, A.; Teuber, M.; Meile, L. Identification and quantification of Bifidobacterium species isolated from food with genus-specific 16S rRNA-targeted probes by colony hybridization and PCR. Appl. Environ. Microbiol. 1997, 63, 1268–1273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuki, T.; Watanabe, K.; Fujimoto, J.; Kado, Y.; Takada, T.; Matsumoto, K.; Tanaka, R. Quantitative PCR with 16S rRNA-gene-targeted species-specific primers for analysis of human intestinal bifidobacteria. Appl. Environ. Microbiol. 2004, 70, 167–173. [Google Scholar] [CrossRef] [Green Version]
- Fu, C.J.; Carter, J.N.; Li, Y.; Porter, J.H.; Kerley, M.S. Comparison of agar plate and real-time PCR on enumeration of Lactobacillus, Clostridium perfringens and total anaerobic bacteria in dog faeces. Lett. Appl. Microbiol. 2006, 42, 490–494. [Google Scholar] [CrossRef]
- Leser, T.D.; Amenuvor, J.Z.; Jensen, T.K.; Lindecrona, R.H.; Boye, M.; Møller, K. Culture-independent analysis of gut bacteria: The pig gastrointestinal tract microbiota revisited. Appl. Environ. Microbiol. 2002, 68, 673–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sghir, A.; Gramet, G.; Suau, A.; Rochet, V.; Pochart, P.; Dore, J. Quantification of bacterial groups within human fecal flora by oligonucleotide probe hybridization. Appl. Environ. Microbiol. 2000, 66, 2263–2266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, X.; Liu, L.; Gamallat, Y.; Zhang, B.; Xin, Y. Enteromorpha and polysaccharides from enteromorpha ameliorate loperamide-induced constipation in mice. Biomed. Pharmacother. 2017, 96, 1075–1081. [Google Scholar] [CrossRef] [PubMed]
- Cherry, P.; Yadav, S.; Strain, C.R.; Allsopp, P.J.; Mcsorley, E.M.; Ross, R.P.; Stanton, C. Prebiotics from seaweeds: An ocean of opportunity? Mar. Drugs 2019, 17, 327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Binn, N. Role of the GI tract microbiota in health and disease. In Probiotics, Prebiotics and the Gut Microbiota; Gibson, G.R., Ed.; International Life Science Institute Europe: Brussels, Belgium, 2013; pp. 4–10. [Google Scholar]
- Rivière, A.; Selak, M.; Lantin, D.; Leroy, F.; De Vuyst, L. Bifidobacteria and butyrate-producing colon bacteria: Importance and strategies for their stimulation in the human gut. Front. Microbiol. 2016, 7, 979. [Google Scholar] [CrossRef] [Green Version]
- Hurst, N.R.; Kendig, D.M.; Murthy, K.S.; Grider, J.R. The Short chain fatty acids, butyrate and propionate, have differential effects on the motility of the guinea pig colon. Neurogastroenterol. Motil. 2014, 26, 1586–1596. [Google Scholar] [CrossRef]
- Shimotoyodome, A.; Meguro, S.; Hase, T.; Tokimitsu, I.; Sakata, T. Decreased colonic mucus in rats with loperamide-induced constipation. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2000, 126, 203–212. [Google Scholar] [CrossRef]
- Barcelo, A.; Claustre, J.; Moro, F.; Chayvialle, J.A.; Cuber, J.C.; Plaisancié, P. Mucin secretion is modulated by luminal factors in the isolated vascularly perfused rat colon. Gut 2000, 46, 218–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Kandasamy, S.; Zhang, J.; Kirby, C.W.; Karakach, T.; Hafting, J.; Critchley, A.T.; Evans, F.; Prithiviraj, B. Prebiotic effects of diet supplemented with the cultivated red seaweed Chondrus crispus or with fructo-oligo-saccharide on host immunity, colonic microbiota and gut microbial metabolites. BMC Complement. Altern. Med. 2015, 15, 279. [Google Scholar] [CrossRef] [PubMed]
- Jabeen, A.; Baig, M.T.; Shaikh, S.; Sarosh, N.A.; Kashif, S.S.; Shahnaz, S.; Vengus, P.; Soomro, H.; Shahid, U. In vivo study on laxative effect of Prunus amygdalus oil. Int. J. Med. Res. Health Sci. 2019, 8, 121–125. [Google Scholar]
- Yu, B.; Jiang, Y.; Jin, L.; Ma, T.; Yang, H. Role of quercetin in modulating chloride transport in the intestine. Front. Physiol. 2016, 7, 549. [Google Scholar] [CrossRef] [Green Version]
- Billet, A.; Hanrahan, J.W. The secret life of CFTR as a calcium-activated chloride channel. J. Physiol. 2013, 591, 5273–5278. [Google Scholar] [CrossRef] [PubMed]
- Ranjani, D.M.; Longanathan, P.; Arputharaj, P.; Kalaiarasi, J. Pharmacognostical and phytochemical analysis of Sargassum cinereum (Turner) C. Agardh. J. Pharmacogn. Phytochem. 2018, 7, 2233–2238. [Google Scholar]
- Yue, G.G.L.; Yip, T.W.N.; Huang, Y.; Ko, W.H. Cellular mechanism for potentiation of Ca2+-mediated Cl− secretion by the flavonoid baicalein in intestinal epithelia. J. Biol. Chem. 2004, 279, 39310–39316. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Wen, G.; Tuo, B.; Zhang, F.; Wan, H.; He, J.; Yang, S.; Dong, H. Molecular mechanisms of calcium signaling in the modulation of small intestinal ion transports and bicarbonate secretion. Oncotarget 2018, 9, 3727–3740. [Google Scholar] [CrossRef] [Green Version]
- Alli, A.A.; Bao, H.F.; Liu, B.C.; Yu, L.; Aldrugh, S.; Montgomery, D.S.; Ma, H.P.; Eaton, D.C. Calmodulin and CaMKII modulate ENaC activity by regulating the association of MARCKS and the cytoskeleton with the apical membrane. Am. J. Physiol.-Ren. Physiol. 2015, 309, 456–463. [Google Scholar] [CrossRef] [Green Version]
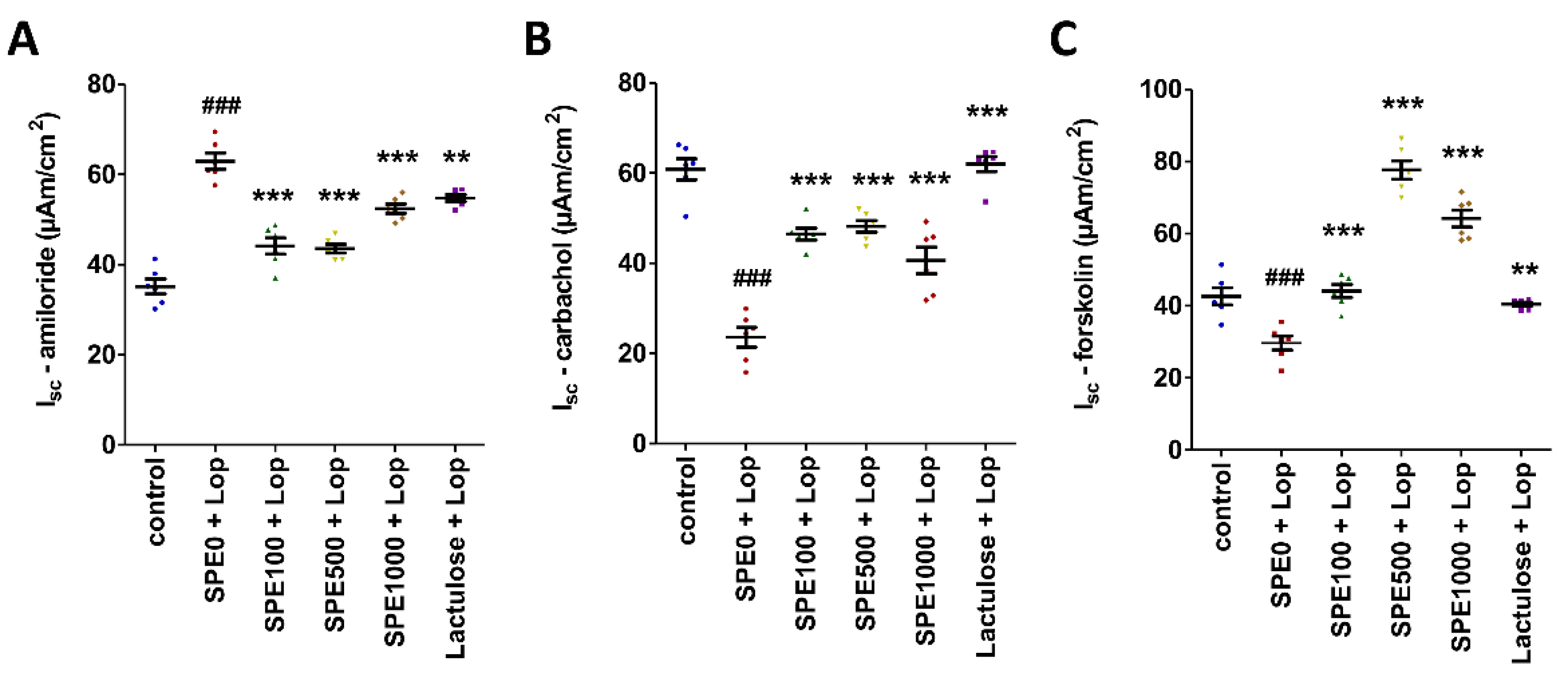
| Treatment | Vte (V) | Rte (Ω.cm2) | Isc (µA/cm2) |
|---|---|---|---|
| Control | 8.33 ± 1.89 | 71.57 ± 7.28 | 119.74 ± 23.50 |
| 0 mg/kg SPE + Loperamide | 8.22 ± 1.63 | 57.23 ± 7.38 | 147.51 ± 25.31 |
| 100 mg/kg SPE + Loperamide | 8.53 ± 1.93 | 59.47 ± 6.55 | 136.40 ± 22.98 |
| 500 mg/kg SPE + Loperamide | 6.51 ± 1.18 | 58.68 ± 5.45 | 108.59 ± 14.58 |
| 1000 mg/kg SPE + Loperamide | 8.58 ± 1.35 | 72.18 ± 10.24 | 121.79 ± 12.04 |
| 500mg/kg Lactulose + Loperamide | 5.37 ± 0.88 | 57.72 ± 8.19 | 100.08 ± 21.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khuituan, P.; Huipao, N.; Jeanmard, N.; Thantongsakul, S.; Promjun, W.; Chuthong, S.; Tipbunjong, C.; Peerakietkhajorn, S. Sargassum plagiophyllum Extract Enhances Colonic Functions and Modulates Gut Microbiota in Constipated Mice. Nutrients 2022, 14, 496. https://doi.org/10.3390/nu14030496
Khuituan P, Huipao N, Jeanmard N, Thantongsakul S, Promjun W, Chuthong S, Tipbunjong C, Peerakietkhajorn S. Sargassum plagiophyllum Extract Enhances Colonic Functions and Modulates Gut Microbiota in Constipated Mice. Nutrients. 2022; 14(3):496. https://doi.org/10.3390/nu14030496
Chicago/Turabian StyleKhuituan, Pissared, Nawiya Huipao, Nilobon Jeanmard, Sitthiwach Thantongsakul, Warittha Promjun, Suwarat Chuthong, Chittipong Tipbunjong, and Saranya Peerakietkhajorn. 2022. "Sargassum plagiophyllum Extract Enhances Colonic Functions and Modulates Gut Microbiota in Constipated Mice" Nutrients 14, no. 3: 496. https://doi.org/10.3390/nu14030496
APA StyleKhuituan, P., Huipao, N., Jeanmard, N., Thantongsakul, S., Promjun, W., Chuthong, S., Tipbunjong, C., & Peerakietkhajorn, S. (2022). Sargassum plagiophyllum Extract Enhances Colonic Functions and Modulates Gut Microbiota in Constipated Mice. Nutrients, 14(3), 496. https://doi.org/10.3390/nu14030496







