Abstract
Abrupt sunlight reduction scenarios (ASRS) following catastrophic events, such as a nuclear war, a large volcanic eruption or an asteroid strike, could prompt global agricultural collapse. There are low-cost foods that could be made available in an ASRS: resilient foods. Nutritionally adequate combinations of these resilient foods are investigated for different stages of a scenario with an effective response, based on existing technology. While macro- and micronutrient requirements were overall met, some—potentially chronic—deficiencies were identified (e.g., vitamins D, E and K). Resilient sources of micronutrients for mitigating these and other potential deficiencies are presented. The results of this analysis suggest that no life-threatening micronutrient deficiencies or excesses would necessarily be present given preparation to deploy resilient foods and an effective response. Careful preparedness and planning—such as stock management and resilient food production ramp-up—is indispensable for an effective response that not only allows for fulfilling people’s energy requirements, but also prevents severe malnutrition.
1. Introduction
Supply chain disruptions caused by the COVID-19 pandemic have emphasized the fragility of food systems as of late, serving as a scaled-down example of the potential severity of a global food system shock [1,2,3,4]. There currently is a clear need for more work on preparing for the most extreme and abrupt food system shocks. The global food production system, largely based on agriculture, is dependent on consistent environmental conditions, such as sunlight, temperature and precipitation, which all can be severely affected by both natural and anthropogenic factors. Potentially very severe risks to global food production that have been described in the food security literature are numerous, including crop pathogens, herbicide-resistant weeds, extreme crop pests, super bacteria displacing beneficial bacteria, abrupt climate change, slow but extreme climate change, mass pollinator decline or a combination of these [5,6]. Anthropogenic climate change is already intensifying the risk of concurrent severe weather events [7], negatively impacting food systems [8] and making it increasingly likely that concurrent shocks would result in a multiple bread-basket failure (MBBF) [9,10]. The ensuing significant crop yield declines would cause food prices to spike, thus exacerbating food insecurity [11].
This would be a dramatic scenario, but unfortunately there exist even more severe food-related global catastrophic risks (GCRs) than the aforementioned. A common definition of a GCR is “an event that could damage human well-being on a global scale, even endangering or destroying modern civilization” [12]. The current agriculture-based global food system is known to function in “self-undermining, self-debilitating dynamics, disrupting yields and supply chains”, thus sustaining a significant vulnerability to global food catastrophe [13]. We classified a subset of these most extreme food catastrophes under the label of abrupt sunlight reduction scenarios (ASRS), in which a sudden catastrophic event causes an immense amount of aerosol material of sulfates or black carbon (soot) to be projected and entrapped in the stratosphere for several years. The ensuing abrupt reduction in sunlight irradiation, global temperatures, and precipitation levels could swiftly trigger near-total global agricultural collapse, which would most likely push billions of people into starvation [14], given a lack of proper planning and preparedness. At least three potential mechanisms for such a catastrophe have been identified in the literature: a volcanic winter caused by a large volcanic eruption, a direct impact of an exceptionally large asteroid or comet, and a nuclear winter triggered by a nuclear war in which numerous cities have been targeted [5,12].
In the absence of significant preparation prior to one of these extreme global catastrophes taking place, there would be little opportunity for adaptation. Given there currently are enough food stocks to feed the global population for only a few months [15], and these catastrophes would likely last several years at a minimum, humanity would have to react quickly to prevent mass starvation globally. Some researchers estimate that in a full-scale nuclear winter scenario, around 75% of the global population would starve to death [14] in the absence of a rapid and effective response; and millions would likely starve from even moderate nuclear autumn from a smaller nuclear exchange [16]. Increasing planning and preparedness capabilities for scaling up food production methods that are resilient to these catastrophes has been proposed as a cost-effective solution to these GCRs [17]. This study aims at assessing the nutrition landscape in such a scenario, in order to better understand where planning and preparedness efforts should be focused to avoid massive famines, forms of malnutrition and global public health issues.
A portfolio of food solutions that are resilient to abrupt catastrophic disruptions in the food system would be a valuable resource for increasing the resilience of food systems and preparedness globally. We define resilient foods or resilient food solutions as those foods, food production methods or interventions that would allow for significant food availability in the face of a global catastrophic food shock. These solutions should be well-suited for contributing to an adequate food supply for the greatest number of people even in the worst scenarios. Thus, this study focuses on technically-viable solutions that are both scalable and open source whenever possible to facilitate rapid production ramping. A preliminary version of such a resilient food portfolio is proposed in Section 2.3, with a focus on abrupt sunlight reduction scenarios.
Affordability should also be a key factor if the majority of the population is to have adequate access to food during a global catastrophe, just as it is today [18]. Low-income populations in developing countries would expectedly be most at risk of starvation [19]. Even today, about 25,000 people die daily from hunger and related diseases [20]. Estimating food prices with a reasonable degree of precision during an extreme food catastrophe is, however, a formidable task, and strongly dependent on the details of the scenario. Nonetheless, we hypothesize that foods that are currently low cost are more likely to be available for the most underprivileged in the considered scenarios.
Another key consideration is for these food solutions to be able to contribute to a diet as nutritionally balanced as possible during the catastrophe period to prevent malnutrition, the topic of the current work. A previous study on the topic [21] proved how a balanced diet can be achieved using only resilient food sources that could be produced during an ASRS, while warning that access to such a diet may not be available to the poorest populations. In contrast, this analysis aims to improve upon these results by demonstrating how the proposed portfolio of low-cost resilient foods can also be nutritionally adequate for an ASRS, using an existing nuclear winter model [22] as an example. To do this, the nutritional needs were estimated for the global human population and then compared to the micronutrient profiles and ramp rates of potentially available traditional and alternative resilient foods. The availability was divided into three periods: (I) food stocks (vegetable and animal), (II) potatoes and traditional resilient foods ramp-up, and (III) greenhouses, crop relocation, and alternative resilient foods. Both optimistic and pessimistic diets combining resilient foods available simultaneously—according to the proposed chronology—were created and then compared to needs of proteins, fats, carbohydrates, minerals, and vitamins. The results are presented and discussed in the context of improving humanity’s resilience to major food shocks.
2. Methods
2.1. Human Nutritional Needs
For simplicity, the energy needs to be covered are based on the minimum recommended requirements of an average adult as proposed by the World Health Organization (WHO) for food management during emergencies [23]: 2100 kcal of energy intake, for a body weight of 62 kg [24], as carried out in previous food-related GCR assessments [25,26,27,28].
The intake of each nutrient was classified into three different categories based on the nutrition literature: adequate intake, moderate- and severe-risk-associated intakes (Figure 1). Complete referencing is available in the Supplementary Information (Table S2 ‘Human Nutritional Needs’).
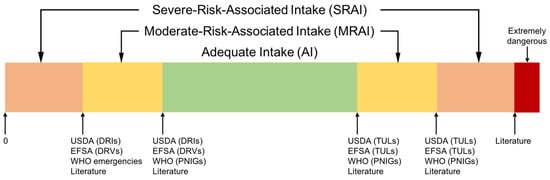
Figure 1.
The three proposed intake categories: adequate intake (AI), moderate- and severe-risk-associated intakes (MRAI and SRAI). Threshold values were found in USDA DRIs and TULs [29,30], EFSA DRVs [31,32] and TULs [33], WHO PNIGs [34,35] and emergency management handbooks [23,36], and in other literature (see Table S2 ‘Human Nutritional Needs’ in Supplementary Information).
2.1.1. Adequate Intakes (AI)
Lower adequate intake (AI) values were usually defined as the most conservative value between the dietary reference intake (DRIs) as defined by the United States Department of Agriculture (USDA) [29,30], the dietary reference values (DRVs) as defined by the European Food Safety Authority (EFSA) [31,32] and the population nutrient intake goals (PNIGs) from the WHO recommendations [34,35]. Upper AI values were derived in a similar manner from tolerable upper limits (TULs) defined by the USDA [30], the EFSA [33] and the WHO [34,35].
2.1.2. Moderate- and Severe-Risk-Associated Intakes (MRAI and SRAI)
Depending on the risk associated with out-of-range nutrient intakes, they were further classified into moderate- and severe-risk-associated intakes (MRAI and SRAI). A similar rationale as for AIs was used to define the limit between MRAI and SRAI (for both upper and lower limits), using the second most conservative value if available. When more relevant or up-to-date data were found in the literature, such as in WHO emergency management handbooks [23,36], those values were used instead (see Table S2 ‘Human Nutritional Needs’ in the Supplementary Information). Alternatively, those values were used as the upper limit for SRAIs, if they were associated with extremely severe health risks—e.g., poisoning, long-term severe damage, or death.
2.2. Catastrophic Scenario Characterization
Post-disaster scenario analysis was based on the Coupe et al. [22,37] in-depth nuclear winter climate model. In their study, 150 Tg of soot are projected above the clouds, where rain cannot wash it out [22,38]. The subsequent climatic consequences, such as large drops in temperature and precipitation levels, were used as a basis to understand how the food system might be altered in this scenario, rather than as direct mathematical inputs. For example, it was used to approximate which foods could be more important, such as crops capable of sustaining reasonably high yields with low temperatures and high water stress, or food sources independent of these conditions. The catastrophe strikes in May, which is likely a worst-case scenario. Models suggest that the climactic effect of particle emissions is larger during this period; additionally, food stocks are at their near lowest in the global North, as the Northern hemisphere harvest season has not yet started, and could potentially fail [38]. The results found using this model are also, to some extent, applicable to the volcanic winter scenario [22,37,39], even though the soot aerosols from nuclear fires result in more extreme climate effects.
2.3. Resilient Foods
Resilient foods were selected upon their expected availability in the event of an ASRS. They include traditional foods such as cool-tolerant staple crops that could be grown outside, or other major staple crops that could be grown in greenhouses, but also innovative resilient foods that are scalable and could provide a significant portion of the world’s dietary needs. Affordability and caloric availability were also taken into consideration to exclude some potential food sources, such as mushrooms, insects, canola meal, fresh fruits and vegetables, nuts, synthetic fat, leaf protein concentrate and others; these are discussed in Appendix A.
2.3.1. Traditional Resilient Foods
Potato
Potatoes are cool tolerant [40,41,42] and have been proposed as a food resilient to global cooling [43], thus it was hypothesized that their production will still be possible in the proposed sunlight reduction scenario, especially after the first year has passed, if crop relocation has been undertaken. The nutritional analysis used boiled potato flesh, cooked in skin without salt. The intake was limited to 500 kcal (575 g) per day per person to reflect limited availability, but larger intakes are safe.
Wheat, Barley, Canola
Some staple crops, namely wheat, barley and canola (a variety of rapeseed; for canola oil), could be eligible for outdoor growing, if relevant adjustments are made to relocate them to appropriate regions. In the first months after the catastrophic event, significant amounts of these would be available as food stocks. Representative examples of foods produced from these crops were used in the nutritional analysis. For wheat, white wheat flour was used in period I, while hard red spring wheat was used in periods II and III. This cultivar appears to be a good proxy for what could be grown in significant quantities in tropical latitudes in the considered scenario. Moreover, cooked pearled barley and oil from canola seeds were also used.
Rice, Maize, Soybeans
Rice, maize, and soybeans are three staple crops that would be highly demanded, as they constitute a significant part of the global population’s diets—representing respectively 13, 12 and 3% of global crop production in 2019, by weight [44]—, and the latter being rich in proteins and fats. It is hypothesized that they could potentially be grown in greenhouses, as they are warm-environment crops. They could be available in stocks first, and then via greenhouse harvesting. Scaling up of low-cost open source greenhouse agriculture during a food GCR scenario has been described elsewhere [25]. Previous research suggests that it would take between 6 months and 1 year to build a significant number of greenhouses and start growing crops. The foods used here were cooked rice (white, long-grain, regular, unenriched, in period I; and brown, long-grain in periods II and III); cooked sweet yellow corn and corn flour; and soy flour (full-fat) and mature, cooked soybeans.
Fish
Fishing would presumably still be possible in the first year after the catastrophe [45]—although yields would be reduced compared to current values [37,45]. Anchovies were integrated into the model, as it is the top fished species (Engraulis ringens) with 7.0 million tons caught in 2018—10% of the global marine capture [46]. It is foreseen that there would be some availability during the first year that will decrease over time during the rest of the catastrophe, due to a reduction in their food sources from lower photosynthetic capacity in low sunlight scenarios. Raw European anchovy (Engraulis encrasicholus) nutritional values were used as a proxy for any fish that might be consumed.
Meat and Animal Organs
As food will be scarce, a much higher number of animals than usual could be slaughtered at the beginning of the catastrophe due to lack of resources to feed them, as animal farming is a low-efficiency food conversion method (the caloric conversion efficiency typically ranges between 3–17%) [47]. A significant number of ruminants, however, could be primarily fed non-human-edible material, such as cellulose-rich agricultural residues [15,48], though investing these resources on milk rather than meat production would be ~6 times more efficient on a caloric basis [47]. Non-ruminating animals were not included here as they feed primarily on human-edible resources and would thus act as competitors rather than primary food sources. The animals that could not be fed this way would likely be slaughtered for food and used for both their meat and their organs, making these foods highly available during the first few months. After that, meat and organs would likely become scarce and the costs would climb dramatically due to limited means for feeding the animals. The foods used in the analysis here were lean meat, fat, and organ composite from cattle, calculated using the relative proportions of each organ in cattle as well as their specific nutrient content.
Milk
Maintenance of some amount of dairy livestock could be crucial as milk could be one of the few sources of fats in the diet, especially during the first year, as oil-producing plants are not guaranteed to be available in very significant quantities. Again, a significant number of dairy livestock could be maintained on a diet primarily based on agricultural residues [48]. Milk production might be available for an extended period if sufficient grazing areas and sustained generation of cellulosic residues are present, but probably in limited quantities. Unfortified whole milk with 3.25% milkfat was used.
Sugar Beet
Sugar beets have been proposed as a food resilient to global cooling [43], and might be feasible to grow outdoors in some areas even during an ASRS, as they tolerate lower temperatures and sunlight levels than cereal crops. They could potentially make a significant contribution to people’s energy uptake, thus helping to bridge a possible caloric gap.
2.3.2. Alternative Resilient Foods
Seaweed (Macroalgae)
Seaweed can grow with low sunlight [49] and would be protected from ultraviolet light possibly generated by a nuclear winter [50]. Developing seaweed farming has been proposed as a potentially very significant contribution to the dietary intakes of the global population in this situation [38]. Seaweed consumption has been advocated by the FAO for decades [51,52,53]. It is fast-growing and highly scalable [54,55], and seaweed aquaculture does not compete with terrestrial agriculture for fresh water, land, or pesticides [56]. In 2012, seaweed harvests accounted for 3 million dry tonnes [54], so there is already substantial demand and cultural acceptance as a food in many regions of the globe. With significant up-scaling effort, substantial seaweed production could be possible as soon as 6 months after the disaster [38]. The seaweed used were emi-tsunomata, laver, and wakame.
Single-Cell Protein from Bacteria
Certain single-cell organisms—some species of bacteria, microalgae, and fungi—can be leveraged for production of protein-rich foods. Single-cell protein from agriculture-independent sources such as methane- or hydrogen-oxidizing bacteria could complement or replace animal proteins [57]. Ramping up this technology has the potential to make a significant contribution to fulfilling the protein requirements of the global population [26,28]. Here it is estimated that it would be available around one year after the catastrophe. Nutritional data from the methane-oxidizing bacteria of Unibio A/S was used as a proxy [58]. Spirulina (Arthrospira platensis) was used as well as a representative example of the group of cyanobacteria sometimes considered to be microalgae, including spirulina, chlorella, and others.
Lignocellulosic Sugar
Quick deployment of technologies to produce sugar from lignocellulosic biomass could make an important contribution to fulfilling the caloric requirements of the global population during the catastrophe period. A previous analysis suggested this food source could be ramped up to a significant amount 6 months after the onset of the catastrophe [59]. A typical Atwater factor of 4 kcal/g was used [60].
2.4. Investigation of Suitable Diets
2.4.1. Chronology of the Resilient Foods’ Availability
A chronology of the availability of these resilient foods in the context of the proposed ASRS is presented in Table 1. The time following the catastrophe was divided into three periods, each expected to last between 3 and 9 months. There remains significant uncertainty in the timing of the potential availability of the proposed food sources, but overlapping availability could be sufficient to consider combining the associated food sources in post-catastrophe diets. Food in Period I (food stocks-vegetable and animal), following the catastrophe, would mainly be from previously stored stocks of major staple crops (those typically used to provide a dominant portion of many human diets globally, such as wheat, barley, rice, canola, maize and soybeans). Animal stocks would also be available and might be slaughtered in great numbers due to plummeting feed availability. Meat, organs, fish and milk are presumed available. Potatoes might still be grown in the scenario considered, and sugar should be available with sugar beet crops. After stocks are nearly or completely depleted—between 3 and 6 months after the shock [15,61]—and a significant share of existing livestock has been slaughtered globally, Period II is projected to start, where diets might be made of mainly potatoes, with marginal contributions of the major staple crops’ stocks and harvests (from outdoor and greenhouse growing). White rice and wheat flour were replaced by brown rice—that needs less processing—and spring wheat—that should be easier to grow under the new climatic conditions, respectively. Other available foods are animal products (meat, organ, milk, fish), and seaweed [38]. Lignocellulosic sugar and seaweed could technically undergo significant production ramp-up during this period, potentially making a substantial contribution to the global food requirement [38,59]. A few months after that, if crop relocation and/or greenhouse ramp-up [25] are successful, a significant amount of staple crops could become available again, and possibly significant quantities of single cell protein as well, if industrial production ramp-up succeeds [26,28]. This would be Period III, which might extend longer than the two first periods. Other potential resilient foods, which might also be available, are discussed in Appendix A.

Table 1.
Proposed chronology of resilient foods’ availability—in red: not available in significant quantities; in yellow: possibly available in significant quantities; in green: available. Each period is expected to last between 3 and 9 months.
2.4.2. Nutrient Analysis
Table 2 provides the nutrients selected for analysis. Nutritional values for all of the resilient foods listed in Table 1 were obtained mainly from Food Data Central [62] and complemented by various academic literature sources (see Table S1 ‘Food Nutritional Content’ in Supplementary Information).

Table 2.
Nutrients selected for analysis of foods and for which AIs, MRAIs and SRAIs were determined (see Supplementary Information).
Diets combining foods available simultaneously—according to the proposed chronology—were created. Relative proportions of foods were tuned to meet the 2100 kcal energy intake and as many AIs as possible, or at least MRAIs.
3. Results and Discussion
3.1. Proposed Diet Combinations Based on Resilient Foods
In this Section, some feasible diets are presented for the different periods defined in Section 2.4.1. The foods expected to be available simultaneously were combined and the resulting nutrient profile was analyzed, with a focus on potential deficiencies. For each period, two diets were proposed: one in an optimistic scenario, where all the expected resilient foods are effectively available and can be combined together; and a pessimistic one where one key resilient food source is missing. The proposed food combinations are presented in Table 3, while the nutrient profile of each combination is shown in Figure 2. Amino acid profiles were not presented here, as none of the diets presented any amino acid deficiencies. Further discussion is provided in Appendix B (Table A1 and Table A2).

Table 3.
Distribution of caloric and weight intake of resilient foods in optimistic and pessimistic scenarios in each period.
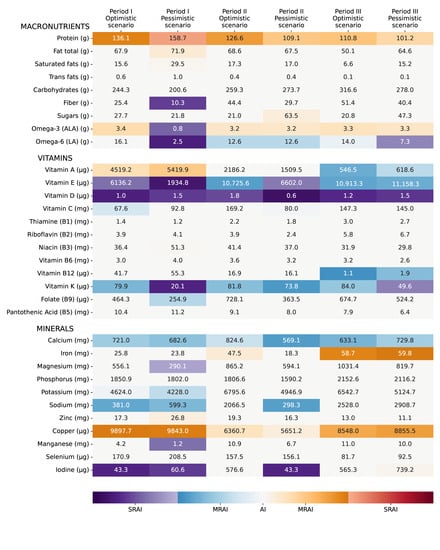
Figure 2.
Nutrient distribution in optimistic and pessimistic scenarios for each period.
The words ‘moderate(ly)’ and ‘severe(ly)’ are used to refer to MRAIs and SRAIs.
3.1.1. Period I
Optimistic Scenario
In an optimistic scenario for Period I, the included foods come from cattle (meat, organs, milk), fish, as well as staple crops (as stocks: rice, barley, canola, soy, wheat) and potatoes (Table 3). Many of the nutrient AIs were met (Figure 2), with deficiencies in vitamins D, E, C, and K, particularly D and E (SRAIs). Calcium intake was moderately low, but within WHO recommendations during emergencies.
Pessimistic Scenario—Without Staple Crops’ Stocks
In a pessimistic scenario for Period I, it was proposed that stocks of staple crops (wheat, barley, canola, rice, corn, and soy) would be unavailable and that most people would feed on potatoes, fish, cattle, and sugar (Table 3). The diet proposed is, in this case, deficient in several micronutrients (Figure 2). Essential fatty acid intake was severely low (alpha-linolenic acid (ALA) and linoleic acid (LA)), which could be palliated by bacteria and microalgae-based foods. Fiber intake was severely low as well, but this could be countered by consuming whole grains, skins, and husks. Protein intakes appear dangerously high. Vitamins D, E, and K are again problematic, as well as manganese, magnesium, and, to a lesser extent, potassium.
3.1.2. Period II
Optimistic Scenario
It is envisioned that during an optimistic Period II, people could still feed on potatoes, major staple crops (wheat, barley, canola, rice, corn, and soybeans), fish, and animals, but at lower levels than Period I (Table 3). It means that stocks are not completely depleted and/or harvesting was rendered possible by relocating crops and/or building greenhouses. Seaweed is expected to start making a significant contribution to the diet. The nutrient intake seemed adequate, with only one SRAI—vitamin D (Figure 2).
Pessimistic Scenario—Without Seaweed
The pessimistic diet that was investigated for Period II was if seaweed had not been scaled up sufficiently to be proposed (Table 3). As a consequence, overall food availability could be reduced and feeding everyone might prove even more difficult. They have been replaced in their entirety by an intake of lignocellulosic sugar, to make up for the missing calories. The nutrient profile is similar to the optimistic scenario, except for mineral intakes (Figure 2). Again, vitamins D, E, C, and K are missing, with the first two reaching SRAI levels. Calcium intake is also low.
3.1.3. Period III
Optimistic Scenario
During Period III, it is projected that staple crops would be available in higher quantities, but fish and animal meat and organs would be lacking. Moreover, seaweed would be available, as well as single-cell protein (here from methane-oxidizing bacteria), and optimistically milk from grazing cattle too (Table 3). In the proposed diet, two nutrients reached SRAIs: vitamin D and manganese (Figure 2). Vitamins E and K and calcium are again moderately deficient, and vitamin A becomes deficient in this period compared to the others due to lower availability of animal-sourced foods (e.g., cattle organs and milk).
Pessimistic Scenario—Without Greenhouses
For Period III, the pessimistic scenario assumes a failure of ramping up greenhouse production sufficiently (Table 3). Hence, rice, maize, and soybeans are not available. The nutrient profile is quite similar to the optimistic scenario, except that some LA and vitamin K are severely lacking (Figure 2).
3.2. Risks Associated with Inadequate Intakes
3.2.1. Macronutrients
Proteins
In all proposed diet combinations, proteins are generally in excess, mostly due to the high availability of meat in the first two periods of the ASRS from the presumed slaughter of most farmed animals due to lack of feed. The MRAI upper limit was set to 140 g per WHO recommendations [34]; however, Bilsborough and Mann reported that obtaining over 35% of the energy intake as proteins (184 g for 2100 kcal) is ‘dangerously excessive’ while 25% (131 g) is safe [63], hence, while this does not appear to be risk-free, it is likely tolerable for a certain amount of time, especially during a catastrophe.
Fats and Fatty Acids
Omega 6 fatty acids (LA) are insufficient in all diets, except for the optimistic one in Period I. It reaches SRAI in Period III if greenhouses are not available. Severe deficiency has been linked to dermatitis in animal models [64], and imbalances in the ratio of omega 3 and omega 6 fatty acids have been linked to atopic, allergic, visual, attentional, emotional, and sleep problems [65]. Vegetable oils (e.g., soybean, corn, canola) are major omega 6 sources that could palliate this issue.
Fiber
Low fiber intake, observed in Period I pessimistic scenario (13 g), is associated with constipation and digestive tract disorders. Interestingly, the average intake of fiber is 15 g/day for American adults [66], while 25 g is recommended [31]. It is concluded that this issue would be tolerable for at least a short amount of time, e.g., one period of 3 to 9 months, and it could be alleviated by consuming whole grains, skins, and husks.
3.2.2. Micronutrients
Potential micronutrient deficiencies based on the proposed diets are presented in Table 4, as well as strategies to mitigate them. Further discussion is provided in Appendix C.

Table 4.
Micronutrient deficiencies that could be present in diets resilient to ASRS and selected potential sources and mitigation strategies.
The current analysis highlights the difficulty of providing an adequate supply of some micronutrients, markedly vitamins D and E. Deficiencies of these vitamins, however, are largely common across vast portions of the global population today [72,73,74], indicating that population survivability may not be hampered during the catastrophe even if these deficiencies were present. More research on efficient, adequate interventions such as fortification and supplementation schemes is useful for both addressing this problem today and during a catastrophe. Strategic micronutrient supplement stockpiles could be a useful resource for addressing this problem during global food catastrophes.
Some micronutrients were in excess. Niacin is often in excess but is associated with low toxicity—mainly flushing of the face, which is a mild effect [75]. Vitamin B12 was in excess in Period I, but it has not been linked to significant side effects [75]. Moreover, as the availability of animal foods will decline with time, body storage of vitamin B12 for future physiological needs might be a way of preventing later deficiencies. Vitamin A was in excess in Period I, which is associated with various disorders depending on the actual source of the vitamin [76]. Copper was usually in excess; this might affect antioxidant status and immune function [77]. Manganese was slightly in excess in Period III, but studies show no evidence of toxicity from levels higher than these [78]. Iodine was also in excess in Period III, mostly due to seaweed intake, but boiling the seaweed can reduce iodine content significantly [79].
3.3. Suitability of Each Diet and Comparisons among Periods
It appears that adequate diets can be provided during Period I in an optimistic scenario. In this scenario, only vitamins D and E were severely lacking and means of mitigation were discussed in Section 3.2.2. In the pessimistic scenario, however, many deficiencies are present, and missing calories were replaced by potentially harmful amounts of proteins. Significant efforts should be made to carefully manage staple crops’ stocks in order to avoid global famine and malnutrition and more effort is needed in identifying resilient sources of balanced nutrition.
For Period II, the optimistic scenario seemed to be nutritionally adequate. However, some chronic deficiencies, e.g., in vitamin D, might appear. The pessimistic scenario seemed relatively complete too, but it should be noted that, even though the nutritional balance appears adequate, food availability would be lower in this scenario, hence increasing famine and malnutrition incidence. Chronic deficiencies might kick in during this period if some nutrients cannot be provided over several periods.
Fairly balanced diets were found for Period III, provided that people manage to make up for potential chronic deficiencies—e.g., in vitamins D, E, K, omega 6, maybe calcium—and that potential new deficiencies—vitamins A and B12—are dealt with in time. In the optimistic scenario, manganese excess should be handled too. Here again, it should be pointed out that the pessimistic scenario diet, as nutritionally adequate as it might seem, has the flaw of not being able to feed as many people as the optimistic scenario diet should.
It is worth restating that these prospective diet combinations are not considered optimal nor exclusive. They simply aim to represent combinations of reasonable amounts of food sources thought to be available simultaneously during an abrupt sunlight reduction catastrophe based on the current state of the research while attempting to fulfill as many essential nutrient requirements as possible, especially the nutrients whose deficiencies are linked to severe health disorders. Further investigation and optimization of these diets would be a significant contribution to ASRS preparedness. For example, methods such as gradient descent over multi-dimensional weighted distance-to-optimal-intake function or genetic algorithms could be useful. These could be incorporated into future scenario analyses for ASRS response integrating different resilient foods to study nutritional considerations globally or regionally.
3.4. Uncertainties and Mitigation
3.4.1. Catastrophe Scenario Considered
A scenario of 150 Tg of soot injected into the upper troposphere and stratosphere during a nuclear war was used as a basis, which corresponds to only one possible outcome of a range of potential sunlight reduction scenarios. Given the absence of empirical data on the severity of a nuclear winter scenario, works like this must rely on climate models of post-catastrophic climate [22,37] to infer the global dietary resources and needs, with the inherent uncertainty this brings. Other sources of uncertainty, related to the severity of the scenario, include the number of target cities, the amount of smoke produced from this, how much of the soot ends up in the atmosphere, the degree to which these would alter temperature, light, and precipitation levels and the duration of the effects [80]. Similar concerns apply to the other ASRS catastrophes.
Other significant uncertainties in this study include the degree to which global industry and energy, trade and international relationships, etc. are affected. During the COVID-19 pandemic, substantial disruptions have been identified [81,82,83,84]. There can also be counterproductive policy [85]. Uncertainties may vary with, e.g., the nature of the abrupt sunlight reduction shock (volcanic winter, nuclear winter, asteroid/comet impact, or an unknown scenario not covered in literature), or for the nuclear war scenario: involved countries, duration and extent of the conflict, weapons used, but also remaining diplomatic relationships and trade, etc. In the analysis shown here, international cooperation and trade were assumed. Global industrial and energy producing infrastructure were considered to remain largely unaffected. In a more extreme scenario in which these are disabled in combination with the ASRS, a different food solutions portfolio would have to be proposed [86].
Regarding the severity of ionizing radiation, the internal dose (which is mostly from food) would be negligible compared with the external dose following a major nuclear war [87]. There is still live discussion in the scientific literature about cancer deaths from the long-term effects of radiation in Hiroshima and Nagasaki. Sutou states that people who lived in Hiroshima and Nagasaki areas after the atomic bombing did not have a shorter life expectancy than the general Japanese population [88]. Other articles estimated cancer deaths from 430 [89] to 1900 [90]. For comparison, deaths from the immediate effects of the atomic bombs in Hiroshima and Nagasaki were around 200,000.
Nonetheless, the analysis is applicable to any scenario involving a catastrophic abrupt sunlight reduction, whether it happens following a nuclear war, a large volcanic eruption, a very large asteroid or comet impact.
3.4.2. Nutritional Values
There is no guarantee that the nutritional needs of the population will remain the same in the event of an abrupt sunlight reduction catastrophe as described here, nor that, if they were to remain unchanged, the selected values are applicable across continents and cultures. Additionally, increasing prevalence of malnutrition and disease in such a starvation-prone period might affect digestibility and bioavailability of several nutrients [91], potentially influencing the suitability of the diet combinations. Moreover, there is uncertainty regarding whether the nutritional values of the resilient foods would remain the same; reduced sunlight and temperatures could significantly affect the nutrient content of crops and is an area in need of further research. For instance, immature grains could be more nutrient-dense, but less calorie-dense than mature grains, as is the case for rice [92]. This would affect the overall nutrient profile of the diets. Lower sunlight and temperatures may not be sufficient for the crops to reach maturity. Moreover, lower ultraviolet irradiation could reduce the vitamin D content of foods. Whether this takes place would depend on the nature and severity of the scenario.
Moreover, for the sake of simplicity, this analysis tackles only a subset of adequate nutrition, namely 2100 kcal for the global average 62 kg [24] of body weight. However, there is variability in nutritional needs across ages, genders and conditions, e.g. it would be insufficient for most adult men. Many subgroups (e.g., infants, children, pregnant or lactating women) should be considered in future research, as they have different nutritional needs [29,31,34]. This choice and its appropriateness are further discussed in Appendix D.
It is worth pointing out that nutrition science and recommendations are greatly debated in the scientific community as they are often based on surveys rather than more accurate, but more difficult to obtain physiological data [93,94,95]. Adding this to the uncertainties around nutrition in a post-catastrophic situation, caution is recommended in handling the results of this work, as they are prone to revision due to changes and improvements in the scientific understanding of nutrition, and the understanding of the potential of resilient foods, which ought to be addressed in future work. Moreover, billions of people worldwide do not have healthy—or even nutritionally adequate—diets according to the recommendations used here [29,30,31,32,33,34,35], as reported by Our World in Data [96]. Hence, it is difficult to assess how severe some deficiencies could be. Additionally, while regional or cultural acceptance of foods such as seaweed or meat might be limiting, it should be noted that the primary determinants of food choice in extreme situations are perceived nutritional value and feeling of fullness provided by the foods [97].
Uncertainties concerning protein digestibility are discussed in Appendix D, as well as the role of antinutrients—substances that can interfere with nutrient absorption through various mechanisms—and contaminants.
3.4.3. Availability of Resilient Foods
It is worth restating that the availability of different foods over time during an ASRS as proposed in this work represents a reasonable and technically feasible scenario in which quick response and cooperation allow humanity to avoid mass global starvation. This cooperation needs to be centered not only in trade between areas that can produce resilient foods and those that cannot but also in terms of making information and technologies openly available so that all those that have the potential to produce resilient foods have the adequate knowledge to do it. There is no guarantee, however, that such cooperation will effectively be available if such an event occurs, as there are many uncertainties concerning how such a scenario would develop. Many variables can impact these conclusions, such as the extent of the sunlight decrease (which could force relocation to lower latitudes and therefore changed photoperiods, rendering some crops impossible to grow, though a small amount of supplemental artificial light could ameliorate this [25]), the global and regional temperature decreases (that would impact transport distances and regional food availabilities) and the degree of cooperation for industry up-scaling (that could impact single cell protein, cellulosic sugar, and/or greenhouse production). Another uncertainty is the degree of information and willingness of the population to use distributed food production techniques to meet their needs as in the case of the COVID-19 pandemic [98,99]. It should also be pointed out that the selected timing of the catastrophe is only one possible scenario; a different timing would likely imply a somewhat higher food availability at the onset, due to different food stock levels, degree of aerosol lofting, and harvest timing.
Chronological analysis was performed on periods of several months—rather than month-by-month—making results less precise but more robust to potential changes in the knowledge of an ASRS, such as the suitability and potential of the foods proposed. Some uncertainty still remains on whether the availability of different resilient foods would overlap in this way, and on whether the foods would be available in sufficient quantities. These will be addressed in future work. Moreover, this analysis included a somewhat large variety of foods, since dietary diversity is a key element of an adequate nutrition intake [100]; if a lower amount of food sources would prove to be available, the outcome of this study would be worse, as individual nutrient requirements could be more difficult to fulfill.
3.4.4. Other Resilient Foods
There are several other classes of resilient foods that could bring useful nutritional contributions in these contexts that were not included in the diet combinations analyzed. They are discussed in Appendix A.
3.5. Future Work
The dietary combinations proposed in this study are not prescriptions, rather they serve to determine how feasible a nutritionally balanced diet is in the proposed scenario, based on low-cost foods that would be available provided there is sufficient preparedness and a fairly quick and coordinated response. Finding out in advance how the foods likely to be available to the most vulnerable in these most extreme scenarios can be adequately combined could help prevent mass starvation. This would be an important step not only towards fulfilling the ‘Zero Hunger’ sustainable development goal [101], but also towards achieving the more ambitious ideal of feeding everyone no matter what.
The overarching goal of this line of research is to strengthen resilience and response for food-related GCRs. Resilience and response strengthening have been proposed as important facets of a robust defense against existential risk as well [102] as they could help reduce existential risk factors, of which social unrest due to global famine could be an example [103]. Preparing for worst-case scenario climatic catastrophes could also potentially inform food researchers and policymakers on how to reduce the death toll of food shocks, such as MBBF, that are quite severe, but that do not risk endangering the survival or future of humanity.
The current model is based on partially validated conclusions of ongoing research on the topic of catastrophe-resilient foods. More work is needed in several areas: on the one hand, nutritional values need to be assessed with more confidence and precision, especially for application in a catastrophe scenario; on the other hand, reducing uncertainty in the potential availability of foods is paramount, perhaps via complete integration of climate models and regional analyses based on Geographic Information System (GIS) methods to assess the situation on a regional rather than global basis. GIS-based analysis can also be helpful for identifying targets for research in resilient foods [104]. Better understanding of micronutrient deficiency mitigation pathways, such as industrial production ramp-up, supplementation and fortification schemes, can also greatly improve preparedness in food-related GCRs.
As previously mentioned, this analysis is focused mainly on the technical feasibility of providing adequate nutrition during the most extreme global food catastrophes. Including more economic considerations and modeling would improve the understanding of the food landscape after a food catastrophe originating from an abrupt sunlight reduction. Finally, understanding some other, more uncertain factors, such as the degree of post-catastrophe coordination between regions, the degree of government intervention in food systems, such as via price ceilings, rationing or food export bans, could help further strengthen catastrophe preparedness. This work aims to serve as a basis for the nutritional study of future scenario analyses incorporating the considerations discussed in this section.
By combining the methodology presented in this study with analyses of specific catastrophic food scenarios based on concrete sets of assumptions, the specific forms of malnutrition that can be expected will be highlighted, as well as specific foods and interventions that could be implemented. Upcoming research will integrate this type of nutritional analysis with a parallel work analyzing food production, availability, and affordability in specific ASRS scenarios.
4. Conclusions
The aim of this work is to pave the way towards a better understanding of the nutrition landscape in an abrupt sunlight reduction scenario (ASRS), such as a nuclear or volcanic winter. A nuclear winter scenario as proposed in the literature is used as a model ASRS to study how even an effective response to this extreme food crisis would likely result in nutritional insufficiencies, what these are likely to be, and how to mitigate them. While many uncertainties remain regarding the scenario of a sudden decrease in sunlight reaching the surface of the Earth, the role of planning and preparedness in the outcome of such a catastrophe is clearly vital. Indeed, the subsequent collapse of agriculture would drive humanity into starvation if proper preparation were absent.
This study analyzes potential combinations of resilient foods into feasible, balanced diets that would help mitigate the impact of this catastrophic scenario given an effective response to it, including rapid food production ramp-up. A list of low-cost resilient foods based on current technologies is proposed, from which nutritionally adequate diets appear technically achievable, even for the most at-risk populations in the most extreme food catastrophes. No life-threatening nutrient deficiencies or excesses were found, meaning that given sufficient availability of resilient foods, the worst consequences of malnutrition appear avoidable. However, some micronutrients were found to be potentially deficient over long periods of time, e.g., vitamin D and E. Careful management and cooperation over our food sources—storage, trade, production ramp-up, etc.—would be essential to ensure food availability and prevent the worst outcomes. Adequate preparedness is indispensable in achieving the goal of feeding everyone no matter what. This study aims to serve as a basis for the nutritional study of scenario analyses during future research.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu14030492/s1, Table S1: Food Nutritional Content; Table S2: Human Nutritional Needs; Table S3: Cattle (organs) calculations.
Author Contributions
A.P.: conceptualization, methodology, formal analysis, writing—original draft, writing—review and editing, visualization, funding acquisition; J.B.G.M.: conceptualization, methodology, formal analysis, writing—original draft, writing—review and editing, project administration; V.B.: conceptualization, methodology, formal analysis, writing—original draft, writing—review and editing; R.S.: formal analysis, writing—review and editing; J.M.P.: formal analysis, writing—review and editing; D.C.D.: conceptualization, methodology, formal analysis, writing—review and editing, supervision, funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded in part by the Alliance to Feed the Earth in Disasters (ALLFED). Alix Pham was funded as a research intern by the Stanford Existential Risks Initiative (SERI) and Joshua Pearce was supported by the Thompson Endowment.
Acknowledgments
The authors would like to thank Mike Hinge, Aron Mill, Morgan Rivers, and Leslie Shallcross for their useful inputs and comments.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| ASRS | Abrupt sunlight reduction scenario |
| AI | Adequate intake |
| ALA | Alpha-linolenic acid |
| DRIs | Dietary reference intake |
| DRVs | Dietary reference values |
| EFSA | European Food Safety Authority |
| FAO | Food and Agriculture Organization |
| GIS | Geographic Information System |
| GCR | Global catastrophic risk |
| LA | Linoleic acid |
| MRAI | Moderate-risk-associated intake |
| MBBF | Multiple bread-basket failure |
| PNIGs | Population nutrient intake goals |
| SRAI | Severe-risk-associated intake |
| TUL | Tolerable upper limit |
| USDA | United States Department of Agriculture |
| UV | Ultraviolet |
| WHO | World Health Organization |
Appendix A. Other Resilient Foods
There are several other classes of resilient foods that could bring useful nutritional contributions in these contexts that were not included in our diet combinations. A brief outline is provided for each of these potential resilient foods along with the reasoning of why they were excluded and/or left for future work.
Appendix A.1. Mushrooms
Mushrooms are highly climate-resilient and have been repeatedly advocated as potential foods in an abrupt sunlight reduction scenario [5,15,105,106], and have been recognized as generally risk-resilient [4]. However, their low energy content limits their potential for making up a significant share of the overall total energy intake of the population globally. Indeed, currently, production costs per unit of dry product are very high, which could mean little availability for the majority of the population [17]. Since the current analysis is aiming at providing healthy diet combinations for everyone, in particular for the poorest populations who would likely be at a much larger risk of starvation, they were excluded. Nonetheless, they could be considered as occasional foods as they could help fill possible nutrient gaps such as fiber, vitamin D (if UV treated), phosphorus, potassium, and zinc (shiitake and oyster mushrooms were taken as proxies).
Appendix A.2. Insects
Similarly, insects are currently costly to produce [17], and analogously to meat production most would be a net calorie sink. Those capable of digesting cellulose—whose feed does not compete with food for human consumption—appear to have limited potential to scale to significant global production in the proposed scenario for various reasons [15], as well as high cost [17]. However, they are also risk-resilient due to their potential to be scaled up over time and automated [107], and low sensitivity to extreme weather events if bred in stackable multi-compartment units [4]. More research would be needed to clarify affordability at scale and ramp-up potential.
Appendix A.3. Canola Meal
Canola (rapeseed) meal was considered as a potential food as it is a by-product of canola oil. It is high in protein and fiber and is currently used as animal feed. It has been proposed as a human food source [108], which would be of great interest if canola crops could be scaled up significantly during the catastrophe. However, canola meal contains a significant amount of glucosinolates [108], which could prevent the absorption of iodine [109]. While it could be of interest in a seaweed-rich diet as iodine toxicity is of concern (see Section 3.2 of main text), it was excluded. More research is needed on the exact interaction between glucosinolates, iodine, and other nutrients before canola meal can be safely included as a high-potential resilient food. However, there are processes that could help reduce the amount of antinutrients to improve the nutritional content of canola meal [110,111], and there is ongoing research on increasing its palatability [108,112]. Future work ought to determine if canola meal is leverageable in an ASRS.
Appendix A.4. Fresh Vegetables, Fruits, and Nuts
Soon after the catastrophe, there may be some yield of vegetables, fruits and nuts from already planted crops. A greater variety could be grown in greenhouses after about five months, including long day plants with a very small amount of supplemental light [25]. Also, cool tolerant crops such as carrots, cabbages and peas could be cultivated outdoors in the tropics. The most challenging would likely be food grown on trees. Either the trees could be relocated to the tropics or a greenhouse could be erected over them with significant supplemental light and likely heating. This would be challenging and thus these foods would become very expensive. In general, fruits and vegetables are already more expensive per calorie, and would likely increase significantly in a catastrophe. These foods were not considered in the analysis due to potentially low availability, particularly for the poorest populations most at risk of starvation due to high post-catastrophe prices and demand. However, a small amount of vegetables/fruits/nuts could be consumed, and they could help reduce deficiencies in fibers, vitamin A, E, C, and K [62], so nutrition would generally be better than considered here.
Appendix A.5. Synthetic Fat
Previous research has suggested the conversion of hydrocarbon-derived waxes to produce digestible fats—similar to margarine or cooking oils—independently of sunlight and conventional agriculture [113]. Given the low number of fat-rich foods that are potentially resilient to abrupt sunlight reduction, it might prove difficult to provide enough fat to the global population in these scenarios. Synthetic fat technologies could be an interesting avenue to bridge this nutritional gap. However, more research is needed prior to using fat derived from petroleum wax as a safe food for GCRs: feasibility needs to be demonstrated with a proof of concept, and nutritional studies should also be conducted to assess the safety and potential as a food. A very similar food was produced and consumed in Germany during the 19th century [113].
Appendix A.6. Leaf Protein Concentrate
Leaf protein concentrate (LPC) from forest trees has been proposed as a resilient food [6,21] as it could be another source of protein, vitamins and minerals. It could be particularly useful in the transition between Period I and II, when stored foods have run out but resilient foods may have not fully ramped up. For example, pine trees are geographically widespread in the northern hemisphere, and species such as Pinus koraiensis and Pinus morrisonicola are nontoxic and widely consumed today. Pine needle concentrate is high in vitamin C [114]. Alfalfa concentrate [115] is high in vitamins K and E [116]. In addition, it is also possible to extract LPC from agricultural residues. Moreover, populations could produce LPC at the household level, making it a decentralized solution [117]. This has the added benefit of providing a potential source of food even without industry or wide-spread cooperation, while these were optimistically assumed in the rest of this analysis. As was observed during shortages of some foods, families pivoted to decentralized food production as there were runs on supplies for chicken and rabbit production in the U.S. [99]. Decentralized production of protein from chickens and rabbits can supply a substantial amount of calories and proteins. Ongoing research is focusing on its nutritional value and possible toxicity, as well as the potential of LPC as a resilient food.
Appendix A.7. Other Foods
Other food solutions, which have not been included in the present analysis, might be of interest in the suggested scenario. For instance, repurposing of by-products from slaughterhouses currently used for animal feed production—e.g., meat meal and bone meal—could potentially be redirected to feed human populations during the catastrophe. Other animal-based food production methods could include rats fed on cellulose and food waste, or shipworms.
The full potential of fermentation technologies to leverage a variety of microorganisms such as bacteria, fungi, or microalgae for the production of nutrient-rich foods was not included in this analysis. However, bacterial single-cell protein from methane-oxidizing bacteria and from spirulina have been included as a representative example [28]. Similar microbial protein can be produced from CO2 and hydrogen [26]. Other emerging microbial foods often referred to as microalgae other than spirulina such as chlorella and nannochloropsis grown in photobioreactors could also bring useful nutritional contributions, such as fats and essential fatty acids. However, the energy efficiency values (electricity to calories) of cultivating these species in closed environments are expected to be much lower than for the abovementioned hydrogen-based single cell protein (4% vs. 18%) [118], which also contain fats and essential fatty acids [119]. Microbial electrosynthesis could be used for the production of acetic acid, and potentially longer-chain, more nutritionally-rich fatty acids as the technology develops [27]. Similarly, food ingredients such as sugars and glycerol (glycerin) could be produced with non-biological processes from agriculture-independent sources, such as CO2 [120] or hydrocarbons [113].
Appendix B. Amino Acid Requirements
Amino acids were never deficient. Some were provided at very high levels, but it should be mentioned that knowledge of adverse effects of excessive amino acid intake is scarce (see Table A1 and Table A2). While it is advisable to consume no more than twice the lower AI, many individuals—especially in high-income countries—consume 3–4 times this quantity, presumably without severe symptoms, even though it cannot be assumed to be risk-free [35]. Amino acid values were compared to a literature review on human hazards of excessive amino acid intake [121], and no significant hazard was uncovered, as shown in the tables below.

Table A1.
Daily amino acid intakes estimated for the proposed diets based on resilient foods.
Table A1.
Daily amino acid intakes estimated for the proposed diets based on resilient foods.
| Period I Optimistic Scenario | Period I Pessimistic Scenario | Period II Optimistic Scenario | Period II Pessimistic Scenario | Period III Optimistic Scenario | Period III Pessimistic Scenario | |
|---|---|---|---|---|---|---|
| Histidine (g) | 3.27 | 4.45 | 3.26 | 2.89 | 2.53 | 2.27 |
| Isoleucine (g) | 5.44 | 6.67 | 5.37 | 4.64 | 4.81 | 4.45 |
| Leucine (g) | 9.87 | 12.00 | 9.53 | 8.18 | 8.58 | 7.72 |
| Lysine (g) | 9.33 | 13.54 | 8.44 | 7.70 | 5.72 | 5.33 |
| Methionine (g) | 2.89 | 4.23 | 2.72 | 2.33 | 2.16 | 2.30 |
| Cysteine (g) | 1.64 | 1.64 | 1.77 | 1.47 | 1.49 | 1.29 |
| Phenylalanine (g) | 5.53 | 6.01 | 5.52 | 4.66 | 5.19 | 4.68 |
| Tyrosine (g) | 4.41 | 5.32 | 4.37 | 3.73 | 3.92 | 3.58 |
| Threonine (g) | 5.53 | 6.97 | 5.09 | 4.40 | 4.52 | 4.23 |
| Tryptophan (g) | 1.65 | 1.83 | 1.49 | 1.35 | 1.78 | 1.86 |
| Valine (g) | 5.44 | 6.63 | 6.12 | 5.06 | 6.02 | 5.82 |

Table A2.
Notes on safety of amino acid levels in our diets compared to a review of hazards. Note: some of these are direct quotes from the hazards study.
Table A2.
Notes on safety of amino acid levels in our diets compared to a review of hazards. Note: some of these are direct quotes from the hazards study.
| Amino Acid | Comment (All Values Are per Day Unless Otherwise Specified) |
|---|---|
| Histidine | Histidine appears to be one of the more toxic amino acids. However, there were no overt side effects when up to 4.5 g/d of histidine was given as treatment. Values in our diets are generally well below this value. |
| Leucine | No signs of toxicity observed in studies administering leucine (5–6 g, i.v. or orally). The values in our diet are somewhat above this, but when considering the additional leucine intake of the patients of the study from their diet, the sum would likely be larger than the proposed diets. Some uncertainty remains but no significant hazard appears to be present. |
| Lysine | Lysine intakes of the proposed diets are well below the values used in chronic studies. Lysine generally shows very low toxicity. |
| Methionine | Methionine is generally considered the most toxic amino acid. However, 5 g/d of methionine for several weeks was reportedly innocuous in studies, and proposed diets are well below this value. |
| Cysteine | In studies on humans, 5–10-g doses of cysteine induced nausea, lightheadedness, and dissociation. However, the proposed diets are well below this value. |
| Phenylalanine | In humans given single oral doses of up to 10 g, no adverse effects were noted. Proposed diets show values well below this value. |
| Tyrosine | A study showed oral doses of 100 mg/kg in adults did not change blood pressure or pulse rate, and there were no other reported side effects. The proposed diets are below this value. |
| Threonine | Threonine appears to be one of the least toxic amino acids. No serious side effects were reported when up to 6 g of threonine was given daily for 2 weeks to patients with spasticity. The threonine intakes of the proposed diets are below the total value these patients were receiving. No toxicity data appear to be available for healthy adults, except headaches and backaches occurred when subjects were given up to 22.5 g of threonine i.v.). |
| Tryptophan | No evidence exists of serious adverse effects attributable directly to tryptophan in humans, and tryptophan is widely sold as a sleep aid. |
| Isoleucine and Valine | Many studies to date sought clinical or physiological benefits from leucine or BCAA mixtures. Few, if any, adverse effects are reported. For example, several investigators administered leucine (5–6 g, i.v. or orally) and observed no signs of toxicity). |
Appendix C. Comments on Micronutrient Deficiencies
Table A3 contains the current production levels of various micronutrients based on sources resilient to ASRS, and comparing them with the minimum amount required for the global population to consume in order to prevent deficiency. The minimum requirements were estimated based on the adequate intake per person and a global population of 8 billion. It shows existing vitamin production is already significant and could be used to palliate deficiencies with efficient distribution schemes. It can also be scaled up as needed based on proven technologies.

Table A3.
Comparison of current production of micronutrients compared to the minimum required to fulfill the requirements of the global population.
Table A3.
Comparison of current production of micronutrients compared to the minimum required to fulfill the requirements of the global population.
| MicroNutrient | Estimated Requirement (Tonne/y) | Current Production (Tonne/y) | Sources and Notes |
|---|---|---|---|
| Vitamin D | 44 | 97 | Based on Sheep Wool [122]. May Not Be ASRS-Resilient. |
| Vitamin E | 43,800 | Up To 42,600 | [123] |
| Vitamin A | 2000 | 7500 | [124] |
| Vitamin B12 | 12 | 15 | [125] |
| Calcium | 2,920,000 | 67,120,000 | Based on Calcium Carbonate [126] and Corrected For 40% Calcium Content. |
| Vitamin C | 240,900 | 220,000 | [127] |
| Vitamin B1 | 3400 | 4000 | [125] |
| Vitamin B6 | 4800 | 2500 | [125] |
| Iron | 39,400 | 67,900 | Based on Ferrous Sulphate [128] and Corrected for 33% Iron Content. |
| Zinc | 27,700 | 35,400 | Based on Zinc Sulphate Monohydrate [129] and Corrected for 36% Zinc Content. |
Appendix C.1. Vitamins
First, vitamin D was severely lacking for all the suggested diets. It is even more serious when considering a low sunlight scenario, as exposure to sunlight is a major source of vitamin D for most people. Indeed, solar ultraviolet-B radiation stimulates the production of vitamin D, both within humans, but also within our foods [66]. Sources of vitamin D include fatty fish (mackerel, salmon, sardines) as well as mushrooms exposed to sunlight or UV light. Supplements are widely available today, with vitamin D3 made from lanolin from sheep wool, and vitamin D2 from ergosterol in yeast. Current supplies of vitamin D, however, can cover the needs of 0.44% of the global population; it appears difficult to up-scale such an industry to be able to fulfill a worldwide need of this extent. Sources of vitamin D2 from yeast or D3 from lichen should be further investigated as well as means of distributed production. Even if most Americans are deficient in this vitamin [74], it is crucial to research if humans can be provided sufficient amounts of vitamin D to survive, and if possible, stay healthy, in such a catastrophe. Moreover, this study may have underestimated total vitamin D intake as the only considered fish source is anchovy, for which vitamin D content is not given by Food Data Central, and some other fish have large amounts of it. Future work should quantify the vitamin D in the fish that would be available.
Vitamin E was low in all periods, especially in Period I. Even though severe deficiency is rare and symptoms have not been observed in individuals consuming low vitamin E diets, this deficiency needs to be mitigated, as it is associated with neurological symptoms—such as damage to sensory nerves, muscles, and retina. Nonetheless, it is estimated that 90% of American adults get less than 9.8 mg/day of alpha-tocopherol (vitamin E) through their food, excluding enriched/fortified foods and supplements [74], which is why the MRAI lower limit was set at 10 mg. Seeds and nuts, as well as vegetable oils, are rich in vitamin E. Moreover, it seems that the current production of vitamin E in China could be sufficient to provide supplements for the global population—that would need approximately 45,000 t/year (see Table A3).
Vitamin C deficiency is linked to scurvy, which can be fatal; however, this disease can be prevented with intakes as low as 10 mg/day [130]. The amount of vitamin C in this diet has not yet been linked to serious diseases and is thus considered as tolerable, at least for a relatively short period of time. Moreover, vitamin C production could be enough to fulfill global needs (250,000 t/year at 82.5 mg/pers) (see Table A3).
Vitamin K deficiency is linked to impaired blood clotting; however, it has been reported that 50% of Americans have lower vitamin K intakes than the one presented here [74] and this value was higher than the EU recommendation [31] by 3 µg. Hence, this was assumed to be tolerable. Canola and soybean oil contain significant quantities of it [66], so careful management of these food sources could be crucial to avoid severe vitamin K deficiency—as observed for the first and third periods’ pessimistic scenarios. Additionally, fermenting food can increase the vitamin K content, and could be a means of mitigation at the household level.
Folate intake needs careful consideration as deficiency of it is associated with severe problems such as congenital and chronic diseases; one avenue could be to fortify some foods, such as flour, with it, if possible, as it has proven efficient in the past [66].
Besides, most of the vitamins in our diet exist in different forms (vitamers), e.g., vitamin D2 and D3 have different degrees of absorbability. Significant uncertainty is present regarding how this would affect the nutrient intake recommendations as well as their absorption and digestibility and is considered beyond the scope of the current work.
Appendix C.2. Minerals
A tablespoon of salt could be used in several abovementioned diets to bridge sodium and iodine mineral requirement gaps. Iodine supplementation of salt should not be a problem during a catastrophic scenario as reserves of this mineral are very large [131].
Calcium was generally low; calcium deficiency is associated with abnormal parathyroid function. Interestingly, intakes of naturally occurring calcium are lower than 0.83 g for half of the American adults [74], while lower AI is set at 1 mg per USDA recommendations [29]. Calcium levels were always above the WHO recommendations for emergency management [23]; but if it were to last for a very long period, this deficiency should be addressed to prevent skeletal and chronic diseases.
Manganese deficiency is associated with various disorders (osteoporosis, diabetes, epilepsy, atherosclerosis, impaired wound healing, and cataracts [66]) and could be managed by feeding unrefined grains, nuts, and leafy vegetables. This underlines the need to carefully control the stored grains so that the populations have constant access to them.
Appendix D. Nutritional Values
Appendix D.1. Diet Appropriateness
On top of many subgroups not being taken into account, other considerations were not included in the scope. There are three different kinds of recommendations that should be considered simultaneously: absolute intake (g/day), intake related to body weight (g/kg/day), and intake as a fraction of total energy (% energy) [63]. Selecting one energy intake and body weight couple—namely 2100 kcal and 62 kg—simplified the analysis, but this omission should be dealt with in future work.
Moreover, the WHO advises adapting the targeted caloric intake to temperatures during emergencies [23]. Indeed, cold can affect our energy requirements; however, appropriate clothing can mitigate this issue [132], so this was not considered here. Nonetheless, this could be an interesting avenue for future research on the topic of emergency nutrition in ASRS.
Appendix D.2. Protein Digestibility
Protein digestibility can come in the way of proper protein intake. Not all foods are equal in terms of protein absorption in our digestive tract [133]. Thus, protein and amino acid content of the foods explored here were corrected for protein digestibility as described in the Food and Drug Administration (FDA) Federal Register [134] or in other research papers if not available in the main source (see Table S1 ‘Food Nutritional Content’ in the Supplementary Information). Amino acid profiles of the foods are quantitatively incorporated in the model.
Uncertainties remain regarding the digestibility of proteins and amino acids. Values chosen here come from different sources that do not use the same indices (ranging from true protein digestibility measured at the end of the digestive tract, or at the end of the small intestine, to in vitro analyses using enzymes). As there is no unified analysis method for determining protein digestibility, this analysis selected a set of values as coherent as possible. More precise methods might yield better-suited values in the future.
Appendix D.3. Antinutrients
Antinutrients are various substances that can interfere with nutrient absorption through various mechanisms—e.g., by binding to minerals or inhibiting enzyme activity [135,136]. These were not taken into account.
Appendix D.4. Contaminants
Some contaminants—mainly heavy metals or other toxic chemicals—were liable to appear in significant quantities in some of the selected foods as mentioned in the FAO [137]. These were not included in our analysis.
References
- Hailu, G. Economic Thoughts on COVID-19 for Canadian Food Processors. Can. J. Agric. Econ. 2020, 68, 163–169. [Google Scholar] [CrossRef]
- De Paulo Farias, D.; dos Santos Gomes, M.G. COVID-19 Outbreak: What Should Be Done to Avoid Food Shortages? Trends Food Sci. Technol. 2020, 102, 291. [Google Scholar] [CrossRef] [PubMed]
- Laborde, D.; Martin, W.; Swinnen, J.; Vos, R. COVID-19 Risks to Global Food Security. Science 2020, 369, 500–502. [Google Scholar] [CrossRef] [PubMed]
- Tzachor, A.; Richards, C.E.; Holt, L. Future Foods for Risk-Resilient Diets. Nat. Food 2021, 2, 326–329. [Google Scholar] [CrossRef]
- Denkenberger, D.C.; Pearce, J.M. Feeding Everyone: Solving the Food Crisis in Event of Global Catastrophes That Kill Crops or Obscure the Sun. Futures 2015, 72, 57–68. [Google Scholar] [CrossRef]
- Pearce, J.M.; Khaksari, M.; Denkenberger, D. Preliminary Automated Determination of Edibility of Alternative Foods: Non-Targeted Screening for Toxins in Red Maple Leaf Concentrate. Plants 2019, 8, 110. [Google Scholar] [CrossRef]
- Masson-Delmotte, V.; Zhai, P.; Pirani, A.; Connors, S.L.; Péan, C.; Berger, S.; Caud, N.; Chen, Y.; Goldfarb, L.; Gomis, M.I.; et al. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Bailey, R.; Benton, T.G.; Challinor, A.; Elliott, J.; Gustafson, D.; Hiller, B.; Jones, A.; Kent, C.; Lewis, K.; Meacham, T.; et al. Extreme Weather and Resilience of the Global Food System: Final Project Report from the UK-US Taskforce on Extreme Weather and Global Food System Resilience; UK Global Food Security Programme: Swindon, UK, 2015; Available online: https://www.stat.berkeley.edu/~aldous/157/Papers/extreme_weather_resilience.pdf (accessed on 21 December 2021).
- Gaupp, F.; Hall, J.; Mitchell, D.; Dadson, S. Increasing Risks of Multiple Breadbasket Failure under 1.5 and 2 °C Global Warming. Agric. Syst. 2019, 175, 34–45. [Google Scholar] [CrossRef]
- Gaupp, F.; Hall, J.; Hochrainer-Stigler, S.; Dadson, S. Changing Risks of Simultaneous Global Breadbasket Failure. Nat. Clim. Chang. 2020, 10, 54–57. [Google Scholar] [CrossRef]
- Janetos, A.; Justice, C.; Jahn, M.; Obersteiner, M.; Glauber, J.; Mulhern, W. The Risks of Multiple Breadbasket Failures in the 21st Century: A Science Research Agenda; The Frederick S. Pardee Center for the Study of the Longer-Range Future: Boston, MA, USA, 2017; ISBN 978-1-936727-14-8. [Google Scholar]
- Bostrom, N.; Cirkovic, M.M. Global Catastrophic Risks; Oxford University Press: Oxford, UK, 2011; ISBN 0-19-960650-1. [Google Scholar]
- Tzachor, A. A System Dynamics Perspective of Food Systems, Environmental Change and Global Catastrophic Risks. Available online: https://www.researchgate.net/profile/Asaf_Tzachor/publication/357735667_A_System_Dynamics_Perspective_of_Food_Systems_Environmental_Change_and_Global_Catastrophic_Risks/links/61dd2b2c323a2268f997c734/A-System-Dynamics-Perspective-of-Food-Systems-Environmental-Change-and-Global-Catastrophic-Risks.pdf (accessed on 21 December 2021). [CrossRef]
- Xia, L.; Robock, A.; Scherrer, K.; Harrison, C.; Jaegermeyr, J.; Bardeen, C.; Toon, O.; Heneghan, R. Global Famine after Nuclear War. Nat. Food Preprint. [CrossRef]
- Denkenberger, D.C.; Pearce, J.M. Feeding Everyone No Matter What: Managing Food Security after Global Catastrophe; Academic Press: Cambridge, MA, USA, 2014; ISBN 978-0-12-802358-7. [Google Scholar]
- Pearce, J.M.; Denkenberger, D.C. A National Pragmatic Safety Limit for Nuclear Weapon Quantities. Safety 2018, 4, 25. [Google Scholar] [CrossRef]
- Denkenberger, D.; Pearce, J.; Taylor, A.R.; Black, R. Food without Sun: Price and Life-Saving Potential. Foresight 2019, 21, 118–129. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. The State of Food Security and Nutrition in the World 2020: Transforming Food Systems for Affordable Healthy Diets; The State of Food Security and Nutrition in the World (SOFI); FAO, IFAD, UNICEF, WFP and WHO: Rome, Italy, 2020; ISBN 978-92-5-132901-6. [Google Scholar]
- Helfand, I. Nuclear Famine: Two Billion People at Risk? International Physicians for the Prevention of Nuclear War: Somerville, MA, USA, 2013; Available online: https://www.psr.org/wp-content/uploads/2018/04/two-billion-at-risk.pdf (accessed on 21 December 2021).
- Holmes, J. Losing 25,000 to Hunger Every Day. Available online: https://www.un.org/en/chronicle/article/losing-25000-hunger-every-day (accessed on 7 December 2021).
- Denkenberger, D.C.; Pearce, J.M. Micronutrient Availability in Alternative Foods during Agricultural Catastrophes. Agriculture 2018, 8, 169. [Google Scholar] [CrossRef]
- Coupe, J.; Bardeen, C.G.; Robock, A.; Toon, O.B. Nuclear Winter Responses to Nuclear War between the United States and Russia in the Whole Atmosphere Community Climate Model Version 4 and the Goddard Institute for Space Studies ModelE. J. Geophys. Res. Atmos. 2019, 124, 8522–8543. [Google Scholar] [CrossRef]
- WHO. Food and Nutrition Needs in Emergencies; World Health Organization: Copenhagen, Denmark, 2004. [Google Scholar]
- Walpole, S.C.; Prieto-Merino, D.; Edwards, P.; Cleland, J.; Stevens, G.; Roberts, I. The Weight of Nations: An Estimation of Adult Human Biomass. BMC Public Health 2012, 12, 439. [Google Scholar] [CrossRef]
- Alvarado, K.A.; Mill, A.; Pearce, J.M.; Vocaet, A.; Denkenberger, D. Scaling of Greenhouse Crop Production in Low Sunlight Scenarios. Sci. Total Environ. 2020, 707, 136012. [Google Scholar] [CrossRef]
- García Martínez, J.B.; Egbejimba, J.; Throup, J.; Matassa, S.; Pearce, J.M.; Denkenberger, D.C. Potential of Microbial Protein from Hydrogen for Preventing Mass Starvation in Catastrophic Scenarios. Sustain. Prod. Consum. 2021, 25, 234–247. [Google Scholar] [CrossRef]
- García Martínez, J.B.; Brown, M.M.; Christodoulou, X.; Alvarado, K.A.; Denkenberger, D.C. Potential of Microbial Electrosynthesis for Contributing to Food Production Using CO2 during Global Agriculture-Inhibiting Disasters. Clean. Eng. Technol. 2021, 4, 100139. [Google Scholar] [CrossRef]
- García Martínez, J.B.; Pearce, J.M.; Throup, J.; Cates, J.; Denkenberger, D.C. Methane Single Cell Protein: Securing Protein Supply during Global Food Catastrophes. OSF Preprints 2020. [Google Scholar] [CrossRef]
- USDA. 2015–2020 Dietary Guidelines for Americans; US Department of Health and Human Services and US Department of Agriculture: Washington, DC, USA, 2015; 144p.
- USDA. Dietary Recommended Intakes (DRIs); US Department of Health and Human Services and US Department of Agriculture: Washington, DC, USA, 2015.
- EFSA. Dietary Reference Values for Nutrients Summary Report. EFSA Support. Publ. 2017, 14, e15121E. [Google Scholar] [CrossRef]
- EFSA. Labelling Reference Intake Values for N-3 and n-6 Polyunsaturated Fatty Acids. EFSA J. 2009, 7, 1176. [Google Scholar] [CrossRef]
- EFSA. Tolerable Upper Intake Levels for Vitamins and Minerals; EFSA, Europäische Kommission, Scientific Panel on Dietetic Products, Nutrition and Allergies, Eds.; European Food Safety Authority: Parma, Italy, 2006; ISBN 978-92-9199-014-6.
- World Health Organization. Diet, Nutrition, and the Prevention of Chronic Diseases: Report of a WHO-FAO Expert Consultation; [Joint WHO-FAO Expert Consultation on Diet, Nutrition, and the Prevention of Chronic Diseases, 2002, Geneva, Switzerland]. In Expert Consultation on Diet, Nutrition, and the Prevention of Chronic Diseases; WHO, FAO, Eds.; WHO Technical Report Series; World Health Organization: Geneva, Switzerland, 2003; ISBN 978-92-4-120916-8. [Google Scholar]
- World Health Organization. Protein and Amino Acid Requirements in Human Nutrition: Report of a Joint WHO/FAO/UNU Expert Consultation, [Geneva, 9–16 April 2002]; WHO, FAO, United Nations, Eds.; WHO Technical Report Series; WHO: Geneva, Switzerland, 2007; ISBN 978-92-4-120935-9. [Google Scholar]
- WHO. The Management of Nutrition in Major Emergencies; World Health Organization: Geneva, Switzerland, 2000; ISBN 978-92-4-154520-4. [Google Scholar]
- Coupe, J.; Stevenson, S.; Lovenduski, N.S.; Rohr, T.; Harrison, C.S.; Robock, A.; Olivarez, H.; Bardeen, C.G.; Toon, O.B. Nuclear Niño Response Observed in Simulations of Nuclear War Scenarios. Commun. Earth Environ. 2021, 2, 18. [Google Scholar] [CrossRef]
- Mill, A.; Harrison, C.; James, S.; Shah, S.; Fist, T.; Alvarado, K.; Taylor, A.; Denkenberger, D. Preventing Global Famine in Case of Sun-Blocking Scenarios: Seaweed as an Alternative Food Source; In Proceedings of the Effective Altruism Global, London, UK, 18–19 October 2019.
- Newhall, C.; Self, S.; Robock, A. Anticipating Future Volcanic Explosivity Index (VEI) 7 Eruptions and Their Chilling Impacts. Geosphere 2018, 14, 572–603. [Google Scholar] [CrossRef]
- Yamori, W.; Noguchi, K.; Hikosaka, K.; Terashima, I. Cold-Tolerant Crop Species Have Greater Temperature Homeostasis of Leaf Respiration and Photosynthesis than Cold-Sensitive Species. Plant Cell Physiol. 2009, 50, 203–215. [Google Scholar] [CrossRef]
- Fleisher, D.H.; Condori, B.; Quiroz, R.; Alva, A.; Asseng, S.; Barreda, C.; Bindi, M.; Boote, K.J.; Ferrise, R.; Franke, A.C.; et al. A Potato Model Intercomparison across Varying Climates and Productivity Levels. Glob. Chang. Biol. 2017, 23, 1258–1281. [Google Scholar] [CrossRef]
- Haverkort, A.J. Potato Crop Response to Radiation and Daylength. In Potato Biology and Biotechnology; Elsevier: Amsterdam, The Netherlands, 2007; pp. 353–365. [Google Scholar]
- Jägermeyr, J.; Robock, A.; Elliott, J.; Müller, C.; Xia, L.; Khabarov, N.; Folberth, C.; Schmid, E.; Liu, W.; Zabel, F.; et al. A Regional Nuclear Conflict Would Compromise Global Food Security. Proc. Natl. Acad. Sci. USA 2020, 117, 7071–7081. [Google Scholar] [CrossRef]
- FAO FAOSTAT. Available online: http://www.fao.org/faostat/en/#data/FO/visualize (accessed on 29 June 2021).
- Scherrer, K.J.N.; Harrison, C.S.; Heneghan, R.F.; Galbraith, E.; Bardeen, C.G.; Coupe, J.; Jägermeyr, J.; Lovenduski, N.S.; Luna, A.; Robock, A.; et al. Marine Wild-Capture Fisheries after Nuclear War. Proc. Natl. Acad. Sci. USA 2020, 117, 29748–29758. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture; Food and Agriculture Organization of the United Nations: Rome, Italy, 2014; p. 223. [Google Scholar]
- Shepon, A.; Eshel, G.; Noor, E.; Milo, R. Energy and Protein Feed-to-Food Conversion Efficiencies in the US and Potential Food Security Gains from Dietary Changes. Environ. Res. Lett. 2016, 11, 105002. [Google Scholar] [CrossRef]
- Tieman, R.; Fist, T.; O’Leary, N.; Kucer, S.; Birkholz, C.; Pearce, J.M.; Denkenberger, D. Stalks, Stems, Leaves—Assessing the Potential to Sustain Global Ruminant Populations from Agricultural Residue Inventories. To be published.
- Bird, N.L.; Chen, L.C.-M.; McLachlan, J. Effects of Temperature, Light and Salinity on Growth in Culture of Chondrus crispus. Furcellaria lumbricalis, Gracilaria tikvahiae (Gigartinales, Rhodophyta), and Fucus serratus (Fucales, Phaeophyta). Bot. Mar. 1979, 22, 521–527. [Google Scholar] [CrossRef]
- Mills, M.J.; Toon, O.B.; Turco, R.P.; Kinnison, D.E.; Garcia, R.R. Massive Global Ozone Loss Predicted Following Regional Nuclear Conflict. Proc. Natl. Acad. Sci. USA 2008, 105, 5307–5312. [Google Scholar] [CrossRef]
- DeBoer, J.A. The Marine Plant Resources and Their Aquaculture Potential in the Bahamas: A Report to the Fisheries Training and Development Project (BHA/78/001), Nassau, Bahamas; FAO: Rome, Italy, 1981. [Google Scholar]
- McHugh, D.J. A Guide to the Seaweed Industry; Food and Agriculture Organization of the United Nations: Rome, Italy, 2003; ISBN 978-92-5-104958-7. [Google Scholar]
- Ferdouse, F.; Holdt, S.L.; Smith, R.; Murúa, P.; Yang, Z. Food and Agriculture Organization of the United Nations The Global Status of Seaweed Production, Trade and Utilization; Globlefish Research Programme: Rome, Italy, 2018; Volume 124, ISBN 978-92-5-130870-7. [Google Scholar]
- Yarish, C.; Brummett, R.; Hansen, S.; Bjerregaard, R.; Valderrama, D.; Sims, N.; Radulovich, R.; Diana, J.; Capron, M.; Forster, J.; et al. Seaweed Aquaculture for Food Security, Income Generation and Environmental Health in Tropical Developing Countries; World Bank Group: Washington, DC, USA, 2016. [Google Scholar]
- John, R.P.; Anisha, G.S.; Nampoothiri, K.M.; Pandey, A. Micro and Macroalgal Biomass: A Renewable Source for Bioethanol. Bioresour. Technol. 2011, 102, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Fedoroff, N.V.; Battisti, D.S.; Beachy, R.N.; Cooper, P.J.M.; Fischhoff, D.A.; Hodges, C.N.; Knauf, V.C.; Lobell, D.; Mazur, B.J.; Molden, D.; et al. Radically Rethinking Agriculture for the 21st Century. Science 2010, 327, 833–834. [Google Scholar] [CrossRef] [PubMed]
- Ritala, A.; Häkkinen, S.T.; Toivari, M.; Wiebe, M.G. Single Cell Protein—State-of-the-Art, Industrial Landscape and Patents 2001–2016. Front. Microbiol. 2017, 8, 2009. [Google Scholar] [CrossRef] [PubMed]
- Unibio Group Chemical Composition of Uniprotein. Available online: https://www.unibio.dk/end-product/chemical-composition-1/ (accessed on 14 May 2020).
- Throup, J.; García Martínez, J.B.; Bals, B.; Cates, J.; Pearce, J.M.; Denkenberger, D.C. Rapid Repurposing of Pulp and Paper Mills, Biorefineries, and Breweries for Lignocellulosic Sugar Production in Global Food Catastrophes. Food Bioprod. Process. 2022, 131, 22–39. [Google Scholar] [CrossRef]
- Atwater, W.; Bryant, A. The Availability and Food Values of Food Materials; 12th Annual Report of the Storrs, CT Agricultural Experimental Station; University of Connecticut: Storrs, CT, USA, 1900. [Google Scholar]
- Robock, A.; Toon, O.B. Local Nuclear War, Global Suffering. Sci. Am. 2010, 302, 74–81. [Google Scholar] [CrossRef]
- A.R.S. Food Data Central; U.S. Department of Agriculture: Washington, DC, USA, 2019.
- Bilsborough, S.; Mann, N. A Review of Issues of Dietary Protein Intake in Humans. Int. J. Sport Nutr. Exerc. Metab. 2006, 16, 129–152. [Google Scholar] [CrossRef]
- Stroud, C.K.; Nara, T.Y.; Roqueta-Rivera, M.; Radlowski, E.C.; Lawrence, P.; Zhang, Y.; Cho, B.H.; Segre, M.; Hess, R.A.; Brenna, J.T.; et al. Disruption of FADS2 Gene in Mice Impairs Male Reproduction and Causes Dermal and Intestinal Ulceration. J. Lipid Res. 2009, 50, 1870–1880. [Google Scholar] [CrossRef]
- Richardson, A.; Ross, M. Physical Signs of Fatty Acid Deficiency; Food and Behaviour Research: Oxford, UK, 2003. [Google Scholar]
- Erdman, J.W., Jr.; Macdonald, I.A.; Zeisel, S.H. Present Knowledge in Nutrition; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Tulchinsky, T.H. Micronutrient Deficiency Conditions: Global Health Issues. Public Health Rev. 2010, 32, 243–255. [Google Scholar] [CrossRef]
- Eggersdorfer, M.; Laudert, D.; Létinois, U.; McClymont, T.; Medlock, J.; Netscher, T.; Bonrath, W. One Hundred Years of Vitamins—A Success Story of the Natural Sciences. Angew. Chem. Int. Ed. 2012, 51, 12960–12990. [Google Scholar] [CrossRef]
- Tarvainen, M.; Fabritius, M.; Yang, B. Determination of Vitamin K Composition of Fermented Food. Food Chem. 2019, 275, 515–522. [Google Scholar] [CrossRef]
- El-Mansi, M. Fermentation Microbiology and Biotechnology: An Historical Perspective. In Fermentation Microbiology and Biotechnology, 4th ed.; CRC Press: Boca Raton, FL, USA, 2019; ISBN 978-0-429-50698-7. [Google Scholar]
- Silverman, J. Single Cell Protein: A Sustainable Approach to Meeting the Growing Protein Demand. AQUAFEED Adv. Process. Formul. 2020, 7, 10–14. [Google Scholar]
- Palacios, C.; Gonzalez, L. Is Vitamin D Deficiency a Major Global Public Health Problem? J. Steroid Biochem. Mol. Biol. 2014, 144 Pt A, 138–145. [Google Scholar] [CrossRef]
- Péter, S.; Friedel, A.; Roos, F.F.; Wyss, A.; Eggersdorfer, M.; Hoffmann, K.; Weber, P. A Systematic Review of Global Alpha-Tocopherol Status as Assessed by Nutritional Intake Levels and Blood Serum Concentrations. Int. J. Vitam. Nutr. Res. 2015, 85, 261–281. [Google Scholar] [CrossRef] [PubMed]
- Fulgoni, V.L.; Keast, D.R.; Bailey, R.L.; Dwyer, J. Foods, Fortificants, and Supplements: Where Do Americans Get Their Nutrients? J. Nutr. 2011, 141, 1847–1854. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine. Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and Its Panel on Folate, Other B Vitamins, and Choline Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline; The National Academies Collection: Reports Funded by National Institutes of Health; National Academies Press: Washington, DC, USA, 1998; ISBN 978-0-309-06411-8. [Google Scholar]
- Penniston, K.L.; Tanumihardjo, S.A. The Acute and Chronic Toxic Effects of Vitamin A. Am. J. Clin. Nutr. 2006, 83, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Turnlund, J.R.; Jacob, R.A.; Keen, C.L.; Strain, J.J.; Kelley, D.S.; Domek, J.M.; Keyes, W.R.; Ensunsa, J.L.; Lykkesfeldt, J.; Coulter, J. Long-Term High Copper Intake: Effects on Indexes of Copper Status, Antioxidant Status, and Immune Function in Young Men. Am. J. Clin. Nutr. 2004, 79, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Finley, J.W.; Penland, J.G.; Pettit, R.E.; Davis, C.D. Dietary Manganese Intake and Type of Lipid Do Not Affect Clinical or Neuropsychological Measures in Healthy Young Women. J. Nutr. 2003, 133, 2849–2856. [Google Scholar] [CrossRef]
- Zava, T.T.; Zava, D.T. Assessment of Japanese Iodine Intake Based on Seaweed Consumption in Japan: A Literature-Based Analysis. Thyroid Res. 2011, 4, 14. [Google Scholar] [CrossRef]
- Ord, T. The Precipice: Existential Risk and the Future of Humanity; Hachette Books: New York, NY, USA, 2020; ISBN 978-0-316-48489-3. [Google Scholar]
- Baldwin, R.; Tomiura, E. Thinking Ahead about the Trade Impact of COVID-19. In Economics in the Time of COVID-19; CEPR Press: London, UK, 2020; pp. 59–71. [Google Scholar]
- Kerr, W.A. The COVID-19 Pandemic and Agriculture: Short-and Long-Run Implications for International Trade Relations. Can. J. Agric. Econ. 2020, 68, 225–229. [Google Scholar] [CrossRef]
- Brosemer, K.; Schelly, C.; Gagnon, V.; Arola, K.L.; Pearce, J.M.; Bessette, D.; Schmitt Olabisi, L. The Energy Crises Revealed by COVID: Intersections of Indigeneity, Inequity, and Health. Energy Res. Soc. Sci. 2020, 68, 101661. [Google Scholar] [CrossRef]
- Henry, R. Innovations in Agriculture and Food Supply in Response to the COVID-19 Pandemic. Mol. Plant 2020, 13, 1095. [Google Scholar] [CrossRef] [PubMed]
- Glauber, J.; Laborde Debucquet, D.; Martin, W.; Vos, R. COVID-19: Trade Restrictions Are Worst Possible Response to Safeguard Food Security. In COVID-19 and Global Food Security; IFPRI Book Chapters; International Food Policy Research Institute: Paris, France, 2020; pp. 66–68. [Google Scholar]
- Denkenberger, D.C.; Cole, D.D.; Abdelkhaliq, M.; Griswold, M.; Hundley, A.B.; Pearce, J.M. Feeding Everyone If the Sun Is Obscured and Industry Is Disabled. Int. J. Disaster Risk Reduct. 2017, 21, 284–290. [Google Scholar] [CrossRef]
- Peterson, K.R.; Shapiro, C.S. Internal Dose Following a Major Nuclear War. Health Phys. 1992, 62, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Sutou, S. Correction to: Low-Dose Radiation from A-Bombs Elongated Lifespan and Reduced Cancer Mortality Relative to Un-Irradiated Individuals. Genes Environ. 2019, 41, 12. [Google Scholar] [CrossRef]
- US National Academy of Sciences. Department of Homeland Security Nuclear Attack Fact Sheet. Available online: https://www.dhs.gov/publication/nuclear-attack-fact-sheet (accessed on 15 November 2021).
- Radiation Effects Research Foundation (RERF). Frequently Asked Questions about the Atomic-Bomb Survivor Research Program. Available online: https://www.rerf.or.jp/en/faq/ (accessed on 15 November 2021).
- Preedy, V.R.; Patel, V.B. (Eds.) Handbook of Famine, Starvation, and Nutrient Deprivation: From Biology to Policy; Springer: Cham, Switzerland, 2019; ISBN 978-3-319-55386-3. [Google Scholar]
- Miraji, K.F.; Linnemann, A.R.; Fogliano, V.; Laswai, H.S.; Capuano, E. Nutritional Quality and in Vitro Digestion of Immature Rice-Based Processed Products. Food Funct. 2020, 11, 7611–7625. [Google Scholar] [CrossRef]
- Woolston, C. Dietary Data under Fire for Being Unreliable. Nature 2015, 522, 259. [Google Scholar] [CrossRef]
- Archer, E.; Blair, S.N. Implausible Data, False Memories, and the Status Quo in Dietary Assessment. Adv. Nutr. 2015, 6, 229–230. [Google Scholar] [CrossRef]
- Archer, E.; Hand, G.A.; Blair, S.N. Validity of U.S. Nutritional Surveillance: National Health and Nutrition Examination Survey Caloric Energy Intake Data, 1971–2010. PLoS ONE 2013, 8, e76632. [Google Scholar] [CrossRef]
- Roser, M.; Ritchie, H. Food Prices; Our World in Data: Oxford, UK, 2013. [Google Scholar]
- Douglas, M. Food Choice during Scarcity. In Food Preferences and Taste: Continuity and Change; Berghahn Books: New York, NY, USA, 1997; Volume 2, pp. 86–92. ISBN 1-57181-958-4. [Google Scholar]
- Rodriguez-Leyva, D.; Pierce, G.N. The Impact of Nutrition on the COVID-19 Pandemic and the Impact of the COVID-19 Pandemic on Nutrition. Nutrients 2021, 13, 1752. [Google Scholar] [CrossRef]
- Meyer, T.K.; Pascaris, A.; Denkenberger, D.; Pearce, J.M. US Potential of Sustainable Backyard Distributed Animal and Plant Protein Production during and after Pandemics. Sustainability 2021, 13, 5067. [Google Scholar] [CrossRef]
- Ruel, M.T. Operationalizing Dietary Diversity: A Review of Measurement Issues and Research Priorities. J. Nutr. 2003, 133, 3911S–3926S. [Google Scholar] [CrossRef]
- United Nations Goal 2: Zero Hunger. Available online: https://www.un.org/sustainabledevelopment/hunger/ (accessed on 7 December 2021).
- Cotton-Barratt, O.; Daniel, M.; Sandberg, A. Defence in Depth against Human Extinction: Prevention, Response, Resilience, and Why They All Matter. Glob. Policy 2020, 11, 271–282. [Google Scholar] [CrossRef]
- Baum, S.; Barrett, A. A Model for the Impacts of Nuclear War; Social Science Research Network: Rochester, NY, USA, 2018. [Google Scholar]
- Fist, T.; Adesanya, A.A.; Denkenberger, D.; Pearce, J.M. Global Distribution of Forest Classes and Leaf Biomass for Use as Alternative Foods to Minimize Malnutrition. World Food Policy 2021, 7, 128–146. [Google Scholar] [CrossRef]
- Denkenberger, D.C.; Pearce, J.M. Cost-Effectiveness of Interventions for Alternate Food to Address Agricultural Catastrophes Globally. Int. J. Disaster Risk Sci. 2016, 7, 205–215. [Google Scholar] [CrossRef]
- Denkenberger, D.C.; Pearce, J.M. Cost-Effectiveness of Interventions for Alternate Food in the United States to Address Agricultural Catastrophes. Int. J. Disaster Risk Reduct. 2018, 27, 278–289. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Tran, G.; Heuzé, V.; Ankers, P. State-of-the-Art on Use of Insects as Animal Feed. Anim. Feed Sci. Technol. 2014, 197, 1–33. [Google Scholar] [CrossRef]
- Hald, C.; Dawid, C.; Tressel, R.; Hofmann, T. Kaempferol 3-O-(2‴-O-Sinapoyl-β-Sophoroside) Causes the Undesired Bitter Taste of Canola/Rapeseed Protein Isolates. J. Agric. Food Chem. 2019, 67, 372–378. [Google Scholar] [CrossRef]
- Schöne, F.; Rajendram, R. Chapter 16—Iodine in Farm Animals. In Comprehensive Handbook of Iodine; Preedy, V.R., Burrow, G.N., Watson, R., Eds.; Academic Press: San Diego, CA, USA, 2009; pp. 151–170. ISBN 978-0-12-374135-6. [Google Scholar]
- Kalaydzhiev, H.; Ivanova, P.; Stoyanova, M.; Pavlov, A.; Rustad, T.; Silva, C.L.; Chalova, V.I. Valorization of Rapeseed Meal: Influence of Ethanol Antinutrients Removal on Protein Extractability, Amino Acid Composition and Fractional Profile. Waste Biomass Valoriz. 2019, 11, 2709–2719. [Google Scholar] [CrossRef]
- Kalaydzhiev, H.; Brandão, T.R.S.; Ivanova, P.; Silva, C.L.M.; Chalova, V.I. A Two-Step Factorial Design for Optimization of Protein Extraction from Industrial Rapeseed Meal after Ethanol-Assisted Reduction of Antinutrients. Int. Food Res. J. 2019, 26, 1155–1163. [Google Scholar]
- Nandasiri, R.; Eskin, N.A.M.; Komatsu, E.; Perreault, H.; Thiyam-Holländer, U. Valorization of Canola By-Products: Concomitance of Flavor-Active Bitter Phenolics Using Pressurized Heat Treatments. LWT 2021, 138, 110397. [Google Scholar] [CrossRef]
- García Martínez, J.B.; Alvarado, K.A.; Denkenberger, D.C. Synthetic Fat from Petroleum as a Resilient Food for Global Catastrophes: Preliminary Techno-Economic Assessment and Technology Roadmap. Chem. Eng. Res. Des. 2022, 177, 255–272. [Google Scholar] [CrossRef]
- Chung, H.-S.; Lee, J.H. Comparative Evaluation of Physicochemical Properties of Pine Needle Powders Prepared by Different Drying Methods. Prev. Nutr. Food Sci. 2015, 20, 143–147. [Google Scholar] [CrossRef][Green Version]
- Leaf for Life; Industrial Leaf Concentrate Process (France). Available online: https://www.leafforlife.org/gen/industrial-scale-lc.html (accessed on 15 November 2021).
- Grela, E.; Pietrzak, K. Production Technology, Chemical Composition and Use of Alfalfa Protein-Xanthophyll Concentrate as Dietary Supplement. J. Food Process. Technol. 2014, 5, 10. [Google Scholar] [CrossRef]
- Kennedy, D. Leaf Concentrate: A Field Guide for Small-Scale Programs; Leaf for Life: Interlachen, FL, USA, 1993. [Google Scholar]
- Alvarado, K.A.; García Martínez, J.B.; Matassa, S.; Egbejimba, J.; Denkenberger, D. Food in Space from Hydrogen-Oxidizing Bacteria. Acta Astronaut. 2021, 180, 260–265. [Google Scholar] [CrossRef]
- Duchene, L. Bridging the Omega-3 Gap with Methane, Microalgae; Global Seafood: Portsmouth, NH, USA, 2016; Available online: https://www.globalseafood.org/advocate/bridging-the-omega-3-gap-with-methane-microalgae/ (accessed on 21 December 2021).
- García Martínez, J.B.; Alvarado, K.A.; Christodoulou, X.; Denkenberger, D.C. Chemical Synthesis of Food from CO2 for Space Missions and Food Resilience. J. CO2 Util. 2021, 53, 101726. [Google Scholar] [CrossRef]
- Garlick, P.J. The Nature of Human Hazards Associated with Excessive Intake of Amino Acids. J. Nutr. 2004, 134, 1633S–1639S. [Google Scholar] [CrossRef]
- Hirsch, A.L. Chapter 6—Industrial Aspects of Vitamin D. In Vitamin D, 3rd ed.; Feldman, D., Pike, J.W., Adams, J.S., Eds.; Academic Press: San Diego, CA, USA, 2011; pp. 73–93. ISBN 978-0-12-381978-9. [Google Scholar]
- CEIC. China Production: Year to Date: Vitamin E. Available online: https://www.ceicdata.com/en/china/pharmaceutical-production-ytd-antiparasitics-vitamins-and-minerals/cn-production-ytd-vitamin-e (accessed on 15 November 2021).
- Searby, L. BASF Picks Germany to Boost Global Vitamin A Capacity. Available online: https://www.nutraingredients.com/Article/2016/10/06/BASF-picks-Germany-to-boost-global-vitamin-A-capacity (accessed on 15 November 2021).
- Demain, A.L.; Sánchez, S. Microbial Synthesis of Primary Metabolites: Current Trends and Future Prospects. In Fermentation Microbiology and Biotechnology, 4th ed.; CRC Press: Boca Raton, FL, USA, 2019; ISBN 978-0-429-50698-7. [Google Scholar]
- Grand View Research. Calcium Carbonate Market Size. Available online: https://www.grandviewresearch.com/industry-analysis/calcium-carbonate-market (accessed on 28 May 2020).
- PW Consulting. Analysis of Market Status and Development Trend of Vitamin C Industry in China. Available online: https://pmarketresearch.com/analysis-of-market-status-and-development-trend-of-vitamin-c-industry-in-china/ (accessed on 15 November 2021).
- ICIS. Chemical Profile Ferrous Sulfate. Available online: https://www.icis.com/explore/resources/news/2005/12/02/517817/chemical-profile-ferrous-sulfate (accessed on 15 November 2021).
- ICIS. Chemical Profile: Zinc Sulfate. Available online: https://www.icis.com/explore/resources/news/2006/03/13/2013005/chemical-profile-zinc-sulfate (accessed on 15 November 2021).
- Sauberlich, H.E. A history of scurvy and vitamin C. In Vitamin C in Health and Disease; Packer, L., Fuchs, J., Eds.; Marcel Dekker: New York, NY, USA, 1997; pp. 1–24. [Google Scholar]
- Hora, K. Iodine Production and Industrial Applications; IDD Newsletter, August 2016; IGN: Ottawa, ON, Canada, 2016. [Google Scholar]
- Joint FAO/WHO/UNU Expert Consultation on Energy and Protein Requirements. Energy and Protein Requirements: Report of a Joint FAO/WHO/UNU Expert Consultation [Held in Rome from 5 to 17 October 1981]; World Health Organization: Geneva, Switzerland, 1985; ISBN 978-92-4-120724-9. [Google Scholar]
- Dallas, D.C.; Sanctuary, M.R.; Qu, Y.; Khajavi, S.H.; Van Zandt, A.E.; Dyandra, M.; Frese, S.A.; Barile, D.; German, J.B. Personalizing Protein Nourishment. Crit. Rev. Food Sci. Nutr. 2017, 57, 3313–3331. [Google Scholar] [CrossRef]
- FDA Federal Register. 1993. Available online: https://www.govinfo.gov/app/details/FR-1993-01-06 (accessed on 21 December 2021).
- Akande, K.E.; Doma, U.D.; Agu, H.; Adamu, H.M. Major Antinutrients Found in Plant Protein Sources: Their Effect on Nutrition. Pak. J. Nutr. 2010, 9, 827–832. [Google Scholar] [CrossRef]
- Popova, A.; Mihaylova, D. Antinutrients in Plant-Based Foods: A Review. Open Biotechnol. J. 2019, 13, 68–76. [Google Scholar] [CrossRef]
- Commission Regulation (EC) No. 1881/2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs. Available online: https://www.ecolex.org/details/legislation/commission-regulation-ec-no-18812006-setting-maximum-levels-for-certain-contaminants-in-foodstuffs-lex-faoc068134/ (accessed on 27 July 2021).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).