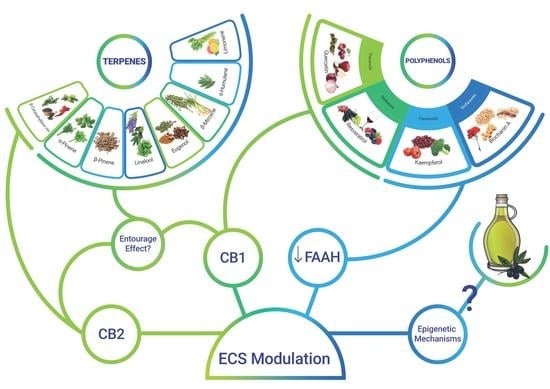
The Mediterranean Diet as a Source of Bioactive Molecules with Cannabinomimetic Activity in Prevention and Therapy Strategy
Abstract
:1. Introduction
2. Dietary Active principles with ECS-Mediated Effects
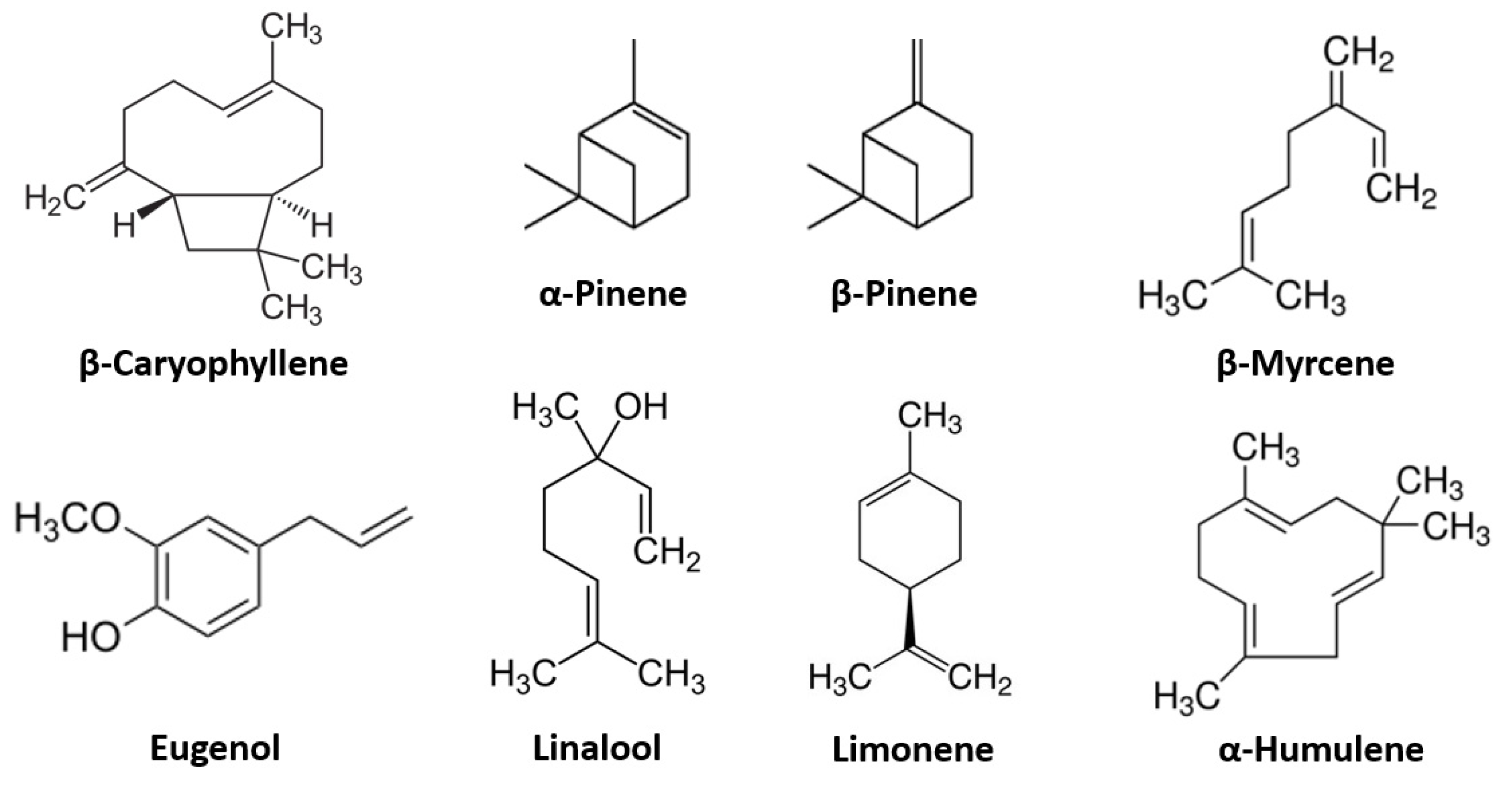
2.1. Terpenes
2.1.1. β-Caryophyllene
2.1.2. The Terpene “Entourage Effect”
2.2. Polyphenols and Flavonoids
2.2.1. Quercetin
2.2.2. Resveratrol
2.2.3. Kaempferol and Biochanin A
2.2.4. The Synergistic and Matrix Effects of Polyphenols
2.2.5. Olive Oil, Hydroxytyrosol and the ECS
3. Microbiota and ECS
3.1. Modulation of the ECS Alters the Microbiota Composition
3.2. Alteration of the Microbiota Can Modulate the ECS
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Di Marzo, V.; Silvestri, C. Lifestyle and Metabolic Syndrome: Contribution of the Endocannabinoidome. Nutrients 2019, 11, 1956. [Google Scholar] [CrossRef] [Green Version]
- Gertsch, J. Cannabimimetic phytochemicals in the diet—An evolutionary link to food selection and metabolic stress adaptation? Br. J. Pharmacol. 2017, 174, 1464–1483. [Google Scholar] [CrossRef] [Green Version]
- Tam, J.; Hinden, L.; Drori, A.; Udi, S.; Azar, S.; Baraghithy, S. The therapeutic potential of targeting the peripheral endocannabinoid/CB 1 receptor system. Eur. J. Intern. Med. 2018, 49, 23–29. [Google Scholar] [CrossRef]
- Pacher, P.; Mechoulam, R. Is lipid signaling through cannabinoid 2 receptors part of a protective system? Prog. Lipid Res. 2011, 50, 193–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atwood, B.; Mackie, K. CB2: A cannabinoid receptor with an identity crisis. J. Cereb. Blood Flow Metab. 2010, 160, 467–479. [Google Scholar] [CrossRef] [Green Version]
- Tsuboi, K.; Uyama, T.; Okamoto, Y.; Ueda, N. Endocannabinoids and related N-acylethanolamines: Biological activities and metabolism. Inflamm. Regen. 2018, 38, 28. [Google Scholar] [CrossRef] [PubMed]
- Murataeva, N.; Straiker, A.; Mackie, K. Parsing the players: 2-arachidonoylglycerol synthesis and degradation in the CNS. J. Cereb. Blood Flow Metab. 2014, 171, 1379–1391. [Google Scholar] [CrossRef] [Green Version]
- Maccarrone, M. Metabolism of the Endocannabinoid Anandamide: Open Questions after 25 Years. Front. Mol. Neurosci. 2017, 10, 166. [Google Scholar] [CrossRef] [Green Version]
- Samat, A.; Tomlinson, B.; Taheri, S.; Thomas, G.N. Rimonabant for the Treatment of Obesity. Recent Pat. Cardiovasc. Drug Discov. 2008, 3, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Soyka, M. Rimonabant and Depression. Pharmacopsychiatry 2008, 41, 204–205. [Google Scholar] [CrossRef]
- Ferber, S.G.; Namdar, D.; Hen-Shoval, D.; Eger, G.; Koltai, H.; Shoval, G.; Shbiro, L.; Weller, A. The “Entourage Effect”: Terpenes Coupled with Cannabinoids for the Treatment of Mood Disorders and Anxiety Disorders. Curr. Neuropharmacol. 2020, 18, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Gershenzon, J.; Dudareva, N. The function of terpene natural products in the natural world. Nat. Chem. Biol. 2007, 3, 408–414. [Google Scholar] [CrossRef]
- Nuutinen, T. Medicinal properties of terpenes found in Cannabis sativa and Humulus lupulus. Eur. J. Med. Chem. 2018, 157, 198–228. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.B.; Marcu, J. Cannabis Pharmacology: The Usual Suspects and a Few Promising Leads. In Advances in Pharmacology; Kendall, D., Alexander, S.P.H., Eds.; Academic Press: Cambridge, MA, USA, 2017; Volume 80, pp. 67–134. [Google Scholar] [CrossRef]
- Wagner, H. Synergy research: Approaching a new generation of phytopharmaceuticals. Fitoterapia 2011, 82, 34–37. [Google Scholar] [CrossRef]
- Booth, J.K.; Yuen, M.M.S.; Jancsik, S.; Madilao, L.L.; Page, J.E.; Bohlmann, J. Terpene Synthases and Terpene Variation in Cannabis sativa. Plant Physiol. 2020, 184, 130–147. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Sharma, R.A. Plant terpenes: Defense responses, phylogenetic analysis, regulation and clinical applications. 3 Biotech 2014, 5, 129–151. [Google Scholar] [CrossRef] [Green Version]
- Russo, E.B. The Case for the Entourage Effect and Conventional Breeding of Clinical Cannabis: No “Strain,” No Gain. Front. Plant Sci. 2019, 9, 1969. [Google Scholar] [CrossRef]
- Harb, A.A.; Bustanji, Y.; Abdalla, S.S. Hypocholesterolemic effect of β-caryophyllene in rats fed cholesterol and fat enriched diet. J. Clin. Biochem. Nutr. 2018, 62, 230–237. [Google Scholar] [CrossRef] [Green Version]
- Malingre, T.; Hendriks, H.; Batterman, S.; Bos, R.; Visser, J. The essential oil ofcannabis sativa. Planta Med. 1975, 28, 56–61. [Google Scholar] [CrossRef]
- Gertsch, J.; Leonti, M.; Raduner, S.; Racz, I.; Chen, J.-Z.; Xie, X.-Q.; Altmann, K.-H.; Karsak, M.; Zimmer, A. Beta-caryophyllene is a dietary cannabinoid. Proc. Natl. Acad. Sci. USA 2008, 105, 9099–9104. [Google Scholar] [CrossRef] [Green Version]
- Klauke, A.-L.; Racz, I.; Pradier, B.; Markert, A.; Zimmer, A.; Gertsch, J. The cannabinoid CB2 receptor-selective phytocannabinoid beta-caryophyllene exerts analgesic effects in mouse models of inflammatory and neuropathic pain. Eur. Neuropsychopharmacol. 2014, 24, 608–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aly, E.; Khajah, M.A.; Masocha, W. β-Caryophyllene, a CB2-Receptor-Selective Phytocannabinoid, Suppresses Mechanical Allodynia in a Mouse Model of Antiretroviral-Induced Neuropathic Pain. Molecules 2020, 25, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beltramo, M. Cannabinoid Type 2 Receptor as a Target for Chronic-Pain. Mini-Rev. Med. Chem. 2009, 9, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Guindon, J.; Hohmann, A.G. Cannabinoid CB2receptors: A therapeutic target for the treatment of inflammatory and neuropathic pain. Br. J. Pharmacol. 2008, 153, 319–334. [Google Scholar] [CrossRef] [Green Version]
- Van Sickle, M.D.; Duncan, M.; Kingsley, P.J.; Mouihate, A.; Urbani, P.; Mackie, K.; Stella, N.; Makriyannis, A.; Piomelli, D.; Davison, J.S.; et al. Identification and Functional Characterization of Brainstem Cannabinoid CB 2 Receptors. Science 2005, 310, 329–332. [Google Scholar] [CrossRef] [Green Version]
- Marco, E.M.; García-Gutiérrez, M.S.; Bermúdez-Silva, F.-J.; Moreira, F.; Guimarães, F.; Manzanares, J.; Viveros, M.-P. Endocannabinoid system and psychiatry: In search of a neurobiological basis for detrimental and potential therapeutic effects. Front. Behav. Neurosci. 2011, 5, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galdino, P.M.; Nascimento, M.V.M.; Florentino, I.F.; Lino, R.C.; Fajemiroye, J.O.; Chaibub, B.A.; de Paula, J.R.; de Lima, T.C.M.; Costa, E.A. The anxiolytic-like effect of an essential oil derived from Spiranthera odoratissima A. St. Hil. leaves and its major component, β-caryophyllene, in male mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2012, 38, 276–284. [Google Scholar] [CrossRef] [Green Version]
- Bahi, A.; Al Mansouri, S.; Al Memari, E.; Al Ameri, M.; Nurulain, S.M.; Ojha, S. β-Caryophyllene, a CB2 receptor agonist produces multiple behavioral changes relevant to anxiety and depression in mice. Physiol. Behav. 2014, 135, 119–124. [Google Scholar] [CrossRef]
- Youssef, D.A.; El-Fayoumi, H.M.; Mahmoud, M.F. Beta-caryophyllene alleviates diet-induced neurobehavioral changes in rats: The role of CB2 and PPAR-γ receptors. Biomed. Pharmacother. 2018, 110, 145–154. [Google Scholar] [CrossRef]
- Eyileten, C.; Kaplon-Cieslicka, A.; Mirowska-Guzel, D.; Małek, L.; Postula, M. Antidiabetic Effect of Brain-Derived Neurotrophic Factor and Its Association with Inflammation in Type 2 Diabetes Mellitus. J. Diabetes Res. 2017, 2017, 2823671. [Google Scholar] [CrossRef]
- Jung, J.I.; Kim, E.J.; Kwon, G.T.; Jung, Y.J.; Park, T.; Kim, Y.; Yu, R.; Choi, M.-S.; Chun, H.S.; Kwon, S.-H.; et al. β-Caryophyllene potently inhibits solid tumor growth and lymph node metastasis of B16F10 melanoma cells in high-fat diet–induced obese C57BL/6N mice. Carcinogenesis 2015, 36, 1028–1039. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Czerwinski, P.; Xia, N.; Förstermann, U.; Li, H. Downregulation of BDNF Expression by PKC and by TNF-? in Human Endothelial Cells. Pharmacology 2015, 96, 1–10. [Google Scholar] [CrossRef]
- Zheng, X.; Sun, T.; Wang, X. Activation of type 2 cannabinoid receptors (CB2R) promotes fatty acid oxidation through the SIRT1/PGC-1α pathway. Biochem. Biophys. Res. Commun. 2013, 436, 377–381. [Google Scholar] [CrossRef]
- Kishi, T.; Hirooka, Y.; Nagayama, T.; Isegawa, K.; Katsuki, M.; Takesue, K.; Sunagawa, K. Calorie Restriction Improves Cognitive Decline via Up-Regulation of Brain-Derived Neurotrophic Factor. Int. Heart J. 2015, 56, 110–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, F.; Bellini, G.; Luongo, L.; Manzo, I.; Tolone, S.; Tortora, C.; Bernardo, M.E.; Grandone, A.; Conforti, A.; Docimo, L.; et al. Cannabinoid Receptor 2 as Antiobesity Target: Inflammation, Fat Storage, and Browning Modulation. J. Clin. Endocrinol. Metab. 2016, 101, 3469–3478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Besseiche, A.; Riveline, J.-P.; Gautier, J.-F.; Bréant, B.; Blondeau, B. Metabolic roles of PGC-1α and its implications for type 2 diabetes. Diabetes Metab. 2015, 41, 347–357. [Google Scholar] [CrossRef]
- Sharma, C.M.; Al Kaabi, J.M.; Nurulain, S.N.; Goyal, S.; Amjad Kamal, M.; Ojha, S. Polypharmacological Properties and Therapeutic Potential of β-Caryophyllene: A Dietary Phytocannabinoid of Pharmaceutical Promise. Curr. Pharm. Des. 2016, 22, 3237–3264. [Google Scholar] [CrossRef]
- Mueller, M.; Jungbauer, A. Culinary plants, herbs and spices—A rich source of PPARγ ligands. Food Chem. 2009, 117, 660–667. [Google Scholar] [CrossRef]
- Galaj, E.; Bi, G.-H.; Moore, A.; Chen, K.; He, Y.; Gardner, E.; Xi, Z.-X. Beta-caryophyllene inhibits cocaine addiction-related behavior by activation of PPARα and PPARγ: Repurposing a FDA-approved food additive for cocaine use disorder. Neuropsychopharmacology 2020, 46, 860–870. [Google Scholar] [CrossRef] [PubMed]
- Irrera, N.; D’Ascola, A.; Pallio, G.; Bitto, A.; Mazzon, E.; Mannino, F.; Squadrito, V.; Arcoraci, V.; Minutoli, L.; Campo, G.M.; et al. β-Caryophyllene Mitigates Collagen Antibody Induced Arthritis (CAIA) in Mice Through a Cross-Talk between CB2 and PPAR-γ Receptors. Biomolecules 2019, 9, 326. [Google Scholar] [CrossRef] [Green Version]
- Irrera, N.; D’Ascola, A.; Pallio, G.; Bitto, A.; Mannino, F.; Arcoraci, V.; Rottura, M.; Ieni, A.; Minutoli, L.; Metro, D.; et al. β-Caryophyllene Inhibits Cell Proliferation through a Direct Modulation of CB2 Receptors in Glioblastoma Cells. Cancers 2020, 12, 1038. [Google Scholar] [CrossRef] [Green Version]
- Baron, E.P. Medicinal Properties of Cannabinoids, Terpenes, and Flavonoids in Cannabis, and Benefits in Migraine, Headache, and Pain: An Update on Current Evidence and Cannabis Science. Headache J. Head Face Pain 2018, 58, 1139–1186. [Google Scholar] [CrossRef]
- Khalil, A.A.; Rahman, U.U.; Khan, M.R.; Sahar, A.; Mehmood, T.; Khan, M. Essential oil eugenol: Sources, extraction techniques and nutraceutical perspectives. RSC Adv. 2017, 7, 32669–32681. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, E.C.D.; Baldasso, G.M.; Bicca, M.A.; Paes, R.S.; Capasso, R.; Dutra, R.C. Terpenoids, Cannabimimetic Ligands, beyond the Cannabis Plant. Molecules 2020, 25, 1567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nallathambi, R.; Mazuz, M.; Namdar, D.; Shik, M.; Namintzer, D.; Vinayaka, A.C.; Ion, A.; Faigenboim, A.; Nasser, A.; Laish, I.; et al. Identification of Synergistic Interaction Between Cannabis-Derived Compounds for Cytotoxic Activity in Colorectal Cancer Cell Lines and Colon Polyps That Induces Apoptosis-Related Cell Death and Distinct Gene Expression. Cannabis Cannabinoid Res. 2018, 3, 120–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santiago, M.; Sachdev, S.; Arnold, J.C.; Mcgregor, I.S.; Connor, M. Absence of Entourage: Terpenoids Commonly Found inCannabis sativaDo Not Modulate the Functional Activity of Δ9-THC at Human CB1and CB2Receptors. Cannabis Cannabinoid Res. 2019, 4, 165–176. [Google Scholar] [CrossRef] [Green Version]
- Finlay, D.B.; Sircombe, K.J.; Nimick, M.; Jones, C.; Glass, M. Terpenoids From Cannabis Do Not Mediate an Entourage Effect by Acting at Cannabinoid Receptors. Front. Pharmacol. 2020, 11, 359. [Google Scholar] [CrossRef]
- Heblinski, M.; Santiago, M.; Fletcher, C.; Stuart, J.; Connor, M.; McGregor, I.S.; Arnold, J.C. Terpenoids Commonly Found in Cannabis sativa Do Not Modulate the Actions of Phytocannabinoids or Endocannabinoids on TRPA1 and TRPV1 Channels. Cannabis Cannabinoid Res. 2020, 5, 305–317. Available online: https://home.liebertpub.com/can (accessed on 2 January 2021). [CrossRef] [PubMed] [Green Version]
- De Petrocellis, L.; Ligresti, A.; Moriello, A.S.; Allarà, M.; Bisogno, T.; Petrosino, S.; Stott, C.G.; Di Marzo, V. Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br. J. Pharmacol. 2011, 163, 1479–1494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smart, D.; Gunthorpe, M.J.; Jerman, J.C.; Nasir, S.; Gray, J.; Muir, A.I.; Chambers, J.K.; Randall, A.; Davis, J.B. The endogenous lipid anandamide is a full agonist at the human vanilloid receptor (hVR1). J. Cereb. Blood Flow Metab. 2000, 129, 227–230. [Google Scholar] [CrossRef] [Green Version]
- De Petrocellis, L.; Di Marzo, V. Non-CB1, Non-CB2 Receptors for Endocannabinoids, Plant Cannabinoids, and Synthetic Cannabimimetics: Focus on G-protein-coupled Receptors and Transient Receptor Potential Channels. J. Neuroimmune Pharmacol. 2009, 5, 103–121. [Google Scholar] [CrossRef] [PubMed]
- Starkus, J.; Jansen, C.; Shimoda, L.M.N.; Stokes, A.J.; Small-Howard, A.L.; Turner, H. Diverse TRPV1 responses to cannabinoids. Channels 2019, 13, 172–191. [Google Scholar] [CrossRef] [Green Version]
- Jansen, C.; Shimoda, L.M.N.; Kawakami, J.K.; Ang, L.; Bacani, A.J.; Baker, J.D.; Badowski, C.; Speck, M.; Strokes, A.J.; Small-Howard, A.L.; et al. Myrcene and terpene regulation of TRPV1. Channels 2019, 13, 344–366. [Google Scholar] [CrossRef] [Green Version]
- Riera, C.E.; Menozzi-Smarrito, C.; Affolter, M.; Michlig, S.; Munari, C.; Robert, F.; Vogel, H.; Simon, S.A.; Le Coutre, J. Compounds from Sichuan and Melegueta peppers activate, covalently and non-covalently, TRPA1 and TRPV1 channels. Br. J. Pharmacol. 2009, 157, 1398–1409. [Google Scholar] [CrossRef] [Green Version]
- Galeotti, N.; Mannelli, L.D.C.; Mazzanti, G.; Bartolini, A.; Ghelardini, C. Menthol: A natural analgesic compound. Neurosci. Lett. 2001, 322, 145–148. [Google Scholar] [CrossRef]
- Liu, B.; Fan, L.; Balakrishna, S.; Sui, A.; Morris, J.B.; Jordt, S.-E. TRPM8 is the principal mediator of menthol-induced analgesia of acute and inflammatory pain. Pain 2013, 154, 2169–2177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lowe, H.; Steele, B.; Bryant, J.; Toyang, N.; Ngwa, W. Non-Cannabinoid Metabolites of Cannabis sativa L. with Therapeutic Potential. Plants 2021, 10, 400. [Google Scholar] [CrossRef]
- Cardinali, A.; Lattanzio, V.; Lattanzio, V.M.T. Role of Phenolics in the Resistance Mechanisms of Plants against Fungal Pathogens and Insects. Res. Signpost 2006, 37. Available online: https://www.researchgate.net/publication/303270594 (accessed on 3 September 2021).
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hart, J.H. Inhibition of Wood-Rotting Fungi by Stilbenes and Other Polyphenols in Eucaly ptus sideroxylon. Phytopathology 1974, 64, 939–984. [Google Scholar] [CrossRef]
- El Gharras, H. Polyphenols: Food sources, properties and applications—A review. Int. J. Food Sci. Technol. 2009, 44, 2512–2518. [Google Scholar] [CrossRef]
- Carullo, G.; Cappello, A.R.; Frattaruolo, L.; Badolato, M.; Armentano, B.; Aiello, F. Quercetin and derivatives: Useful tools in inflammation and pain management. Futur. Med. Chem. 2017, 9, 79–93. [Google Scholar] [CrossRef]
- Tapas, A.; Sakarkar, D.; Kakde, R. Flavonoids as Nutraceuticals: A Review. Trop. J. Pharm. Res. 2008, 7, 1089–1099. [Google Scholar] [CrossRef]
- Leyva-López, N.; Gutierrez-Grijalva, E.P.; Ambriz-Perez, D.L.; Heredia, J.B. Flavonoids as Cytokine Modulators: A Possible Therapy for Inflammation-Related Diseases. Int. J. Mol. Sci. 2016, 17, 921. [Google Scholar] [CrossRef]
- Fürst, R.; Zündorf, I. Plant-Derived Anti-Inflammatory Compounds: Hopes and Disappointments regarding the Translation of Preclinical Knowledge into Clinical Progress. Mediat. Inflamm. 2014, 2014, 146832. [Google Scholar] [CrossRef] [Green Version]
- Hirpara, K.V.; Aggarwal, P.; Mukherjee, A.J.; Joshi, N.; Burman, A.C. Quercetin and Its Derivatives: Synthesis, Pharmacological Uses with Special Emphasis on Anti-Tumor Properties and Prodrug with Enhanced Bio-Availability. Anti-Cancer Agents Med. Chem. 2009, 9, 138–161. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, G. Quercetin: A flavonol with multifaceted therapeutic applications? Fitoterapia 2015, 106, 256–271. [Google Scholar] [CrossRef] [PubMed]
- Shrinivasan, M.; Skariyachan, S.; Aparna, V.; Kolte, V.R. Homology modelling of CB1 receptor and selection of potential inhibitor against Obesity. Bioinformation 2012, 8, 523–528. [Google Scholar] [CrossRef] [Green Version]
- Refolo, M.G.; D’Alessandro, R.; Malerba, N.; Laezza, C.; Bifulco, M.; Messa, C.; Caruso, M.G.; Notarnicola, M.; Tutino, V. Anti Proliferative and Pro Apoptotic Effects of Flavonoid Quercetin Are Mediated by CB1 Receptor in Human Colon Cancer Cell Lines. J. Cell. Physiol. 2015, 230, 2973–2980. [Google Scholar] [CrossRef]
- Tutino, V.; DE Nunzio, V.; Tafaro, A.; Bianco, G.; Gigante, I.; Scavo, M.P.; D’Alessandro, R.; Refolo, M.G.; Messa, C.; Caruso, M.G.; et al. Cannabinoid Receptor-1 Up-regulation in Azoxymethane (AOM)-treated Mice After Dietary Treatment with Quercetin. Anticancer. Res. 2018, 38, 4485–4491. [Google Scholar] [CrossRef]
- Lin, R.; Piao, M.; Song, Y.; Liu, C. Quercetin Suppresses AOM/DSS-Induced Colon Carcinogenesis through Its Anti-Inflammation Effects in Mice. J. Immunol. Res. 2020, 2020, 9242601. [Google Scholar] [CrossRef]
- Wang, D.; Wang, H.; Ning, W.; Backlund, M.G.; Dey, S.K.; Dubois, R.N. Loss of Cannabinoid Receptor 1 Accelerates Intestinal Tumor Growth. Cancer Res. 2008, 68, 6468–6476. [Google Scholar] [CrossRef] [Green Version]
- Sun, A.Y.; Wang, Q.; Simonyi, A.; Sun, G.Y. Resveratrol as a Therapeutic Agent for Neurodegenerative Diseases. Mol. Neurobiol. 2010, 41, 375–383. [Google Scholar] [CrossRef] [Green Version]
- Bournival, J.; Quessy, P.; Martinoli, M.-G. Protective Effects of Resveratrol and Quercetin Against MPP+ -Induced Oxidative Stress Act by Modulating Markers of Apoptotic Death in Dopaminergic Neurons. Cell. Mol. Neurobiol. 2009, 29, 1169–1180. [Google Scholar] [CrossRef] [PubMed]
- Bertelli, A.; Bertelli, A.A.; Gozzini, A.; Giovannini, L. Plasma and Tissue Resveratrol Concentrations and Pharmacological Activity. Drugs Exp. Clin. Res. 1998, 24, 133–138. Available online: https://europepmc.org/article/med/9825229 (accessed on 3 September 2021).
- Walle, T.; Hsieh, F.; DeLegge, M.H.; Oatis, J.E., Jr.; Walle, U.K. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab. Dispos. 2004, 32, 1377–1382. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Hou, P.; Zhou, M.; Ren, Q.; Wang, X.; Huang, L.; Hui, S.; Yi, L.; Mi, M. Resveratrol attenuates high-fat diet-induced non-alcoholic steatohepatitis by maintaining gut barrier integrity and inhibiting gut inflammation through regulation of the endocannabinoid system. Clin. Nutr. 2019, 39, 1264–1275. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Plovier, H.; Van Hul, M.; Geurts, L.; Delzenne, N.M.; Druart, C.; Everard, A. Endocannabinoids—At the crossroads between the gut microbiota and host metabolism. Nat. Rev. Endocrinol. 2016, 12, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R.G.; Howlett, A.C.; Abood, M.E.; Alexander, S.P.; Di Marzo, V.; Elphick, M.R.; Greasley, P.J.; Hansen, H.S.; Kunos, G.; Mackie, K.; et al. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid Receptors and Their Ligands: Beyond CB1 and CB2. Pharmacol. Rev. 2010, 62, 588–631. [Google Scholar] [CrossRef] [Green Version]
- Hassanzadeh, P.; Arbabi, E.; Atyabi, F.; Dinarvand, R. The endocannabinoid system and NGF are involved in the mechanism of action of resveratrol: A multi-target nutraceutical with therapeutic potential in neuropsychiatric disorders. Psychopharmacology 2016, 233, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- Carta, G.; Poddighe, L.; Serra, M.P.; Boi, M.; Melis, T.; Lisai, S.; Murru, E.; Muredda, L.; Collu, M.; Banni, S.; et al. Preventive Effects of Resveratrol on Endocannabinoid System and Synaptic Protein Modifications in Rat Cerebral Cortex Challenged by Bilateral Common Carotid Artery Occlusion and Reperfusion. Int. J. Mol. Sci. 2018, 19, 426. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, C.D.C.; e Castor, M.G.M.; e Castor, C.G.M.; Costa, D.F.; Ferreira, R.C.M.; Silva, J.; Navia-Pelaez, J.M.; Capettini, L.D.S.A.; Lemos, V.S.; Duarte, I.D.G.; et al. Evidence for the involvement of opioid and cannabinoid systems in the peripheral antinociception mediated by resveratrol. Toxicol. Appl. Pharmacol. 2019, 369, 30–38. [Google Scholar] [CrossRef]
- Hourani, W.; Alexander, S.P.H. Cannabinoid ligands, receptors and enzymes: Pharmacological tools and therapeutic potential. Brain Neurosci. Adv. 2018, 2. [Google Scholar] [CrossRef] [Green Version]
- Hill, M.N.; Gorzalka, B.B. Pharmacological enhancement of cannabinoid CB1 receptor activity elicits an antidepressant-like response in the rat forced swim test. Eur. Neuropsychopharmacol. 2005, 15, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Zanettini, C.; Panlilio, L.V.; Aliczki, M.; Goldberg, S.R.; Haller, J.; Yasar, S. Effects of endocannabinoid system modulation on cognitive and emotional behavior. Front. Behav. Neurosci. 2011, 5, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cebeci, F.; Şahin-Yeşilçubuk, N. The matrix effect of blueberry, oat meal and milk on polyphenols, antioxidant activity and potential bioavailability. Int. J. Food Sci. Nutr. 2013, 65, 69–78. [Google Scholar] [CrossRef]
- Ahmad, H.; Rauf, K.; Zada, W.; McCarthy, M.; Abbas, G.; Anwar, F.; Shah, A.J. Kaempferol Facilitated Extinction Learning in Contextual Fear Conditioned Rats via Inhibition of Fatty-Acid Amide Hydrolase. Molecules 2020, 25, 4683. [Google Scholar] [CrossRef] [PubMed]
- Haller, J.; Barna, I.; Barsvari, B.; Pelczer, K.G.; Yasar, S.; Panlilio, L.V.; Goldberg, S. Interactions between environmental aversiveness and the anxiolytic effects of enhanced cannabinoid signaling by FAAH inhibition in rats. Psychopharmacology 2009, 204, 607–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pamplona, F. Letters RT-N, 2006 Undefined. WIN 55212-2 Impairs Contextual Fear Conditioning through the Activation of CB1 Cannabinoid Receptors. Elsevier. Available online: https://www.sciencedirect.com/science/article/pii/S030439400501390X?casa_token=Z3rlu2a_d_UAAAAA:vpWV0cYjbyvluJzrLoOki0IXgoIo2PpfnsQUWTE8_Q6EY5gN9cjPwPzL4MAqaLeCUq21NkcE (accessed on 3 September 2021).
- Thors, L.; Burston, J.; Alter, B.; McKinney, M.; Cravatt, B.; Ross, R.; Pertwee, R.; Th, R.G.; Wiley, J.; Fowler, C. Biochanin A, a naturally occurring inhibitor of fatty acid amide hydrolase. Br. J. Pharmacol. 2010, 160, 549–560. [Google Scholar] [CrossRef] [Green Version]
- Scholz; Williamson. Interactions Affecting the Bioavailability of Dietary Polyphenols in vivo. Int. J. Vitam. Nutr. Res. 2007, 77, 224–235. [Google Scholar] [CrossRef]
- Cocci, P.; Moruzzi, M.; Martinelli, I.; Maggi, F.; Di Bonaventura, M.V.M.; Cifani, C.; Mosconi, G.; Tayebati, S.K.; Damiano, S.; Lupidi, G.; et al. Tart cherry (Prunus cerasus L.) dietary supplement modulates visceral adipose tissue CB1 mRNA levels along with other adipogenesis-related genes in rat models of diet-induced obesity. Eur. J. Nutr. 2021, 60, 2695–2707. [Google Scholar] [CrossRef] [PubMed]
- Wojdyło, A.; Nowicka, P.; Laskowski, P.; Oszmiański, J. Evaluation of Sour Cherry (Prunus cerasus L.) Fruits for Their Polyphenol Content, Antioxidant Properties, and Nutritional Components. J. Agric. Food Chem. 2014, 62, 12332–12345. [Google Scholar] [CrossRef]
- Yılmaz, F.M.; Görgüç, A.; Karaaslan, M.; Vardin, H.; Bilek, S.E.; Uygun, Ö.; Bircan, C. Sour Cherry By-products: Compositions, Functional Properties and Recovery Potentials—A Review. Crit. Rev. Food Sci. Nutr. 2018, 59, 3549–3563. [Google Scholar] [CrossRef]
- Lee, Y.; Tharp, W.G.; Dixon, A.E.; Spaulding, L.; Trost, S.; Nair, S.; Permana, P.A.; Pratley, R.E. Dysregulation of cannabinoid CB1 receptor expression in subcutaneous adipocytes of obese individuals. Anim. Cells Syst. 2009, 13, 371–379. [Google Scholar] [CrossRef]
- Börner, C.; Bedini, A.; Höllt, V.; Kraus, J. Analysis of Promoter Regions Regulating Basal and Interleukin-4-Inducible Expression of the Human CB1 Receptor Gene in T Lymphocytes. Mol. Pharmacol. 2007, 73, 1013–1019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Börner, C.; Höllt, V.; Sebald, W.; Kraus, J. Transcriptional regulation of the cannabinoid receptor type 1 gene in T cells by cannabinoids. J. Leukoc. Biol. 2006, 81, 336–343. [Google Scholar] [CrossRef]
- Russo, G.L.; Siani, A.; Fogliano, V.; Geleijnse, J.M.; Giacco, R.; Giampaoli, S.; Iacoviello, L.; Kromhout, D.; Lionetti, L.; Naska, A.; et al. The Mediterranean diet from past to future: Key concepts from the second “Ancel Keys” International Seminar. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 717–732. [Google Scholar] [CrossRef]
- Bulló, M.; Casas, R.; Portillo, M.; Basora, J.; Estruch, R.; García-Arellano, A.; Lasa, A.; Juanola-Falgarona, M.; Arós, F.; Salas-Salvadó, J. Dietary glycemic index/load and peripheral adipokines and inflammatory markers in elderly subjects at high cardiovascular risk. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 443–450. [Google Scholar] [CrossRef]
- Babio, N.; Toledo, E.; Estruch, R.; Ros, E.; Martínez-González, M.A.; Castañer, O.; Bulló, M.; Corella, D.; Arós, F.; Gómez-Gracia, E.; et al. Mediterranean diets and metabolic syndrome status in the PREDIMED randomized trial. Can. Med Assoc. J. 2014, 186, E649–E657. [Google Scholar] [CrossRef] [Green Version]
- Toledo, E.; Salas-Salvadó, J.; Donat-Vargas, C.; Buil-Cosiales, P.; Estruch, R.; Ros, E.; Corella, D.; Fitó, M.; Hu, F.B.; Arós, F.; et al. Mediterranean Diet and Invasive Breast Cancer Risk Among Women at High Cardiovascular Risk in the PREDIMED Trial: A Randomized Clinical Trial. JAMA Intern. Med. 2015, 175, 1752–1760. [Google Scholar] [CrossRef] [PubMed]
- de Pablos, R.M.; Espinosa-Oliva, A.M.; Hornedo-Ortega, R.; Cano, M.; Arguelles, S. Hydroxytyrosol protects from aging process via AMPK and autophagy; a review of its effects on cancer, metabolic syndrome, osteoporosis, immune-mediated and neurodegenerative diseases. Pharmacol. Res. 2019, 143, 58–72. [Google Scholar] [CrossRef] [PubMed]
- Castro-Barquero, S.; Lamuela-Raventós, R.M.; Doménech, M.; Estruch, R. Relationship between Mediterranean Dietary Polyphenol Intake and Obesity. Nutrients 2018, 10, 1523. [Google Scholar] [CrossRef] [Green Version]
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef] [PubMed]
- Hornedo-Ortega, R.; Cerezo, A.B.; De Pablos, R.M.; Krisa, S.; Richard, T.; García-Parrilla, M.C.; Troncoso, A.M. Phenolic Compounds Characteristic of the Mediterranean Diet in Mitigating Microglia-Mediated Neuroinflammation. Front. Cell. Neurosci. 2018, 12. [Google Scholar] [CrossRef] [PubMed]
- Notarnicola, M.; Pisanti, S.; Tutino, V.; Bocale, D.; Rotelli, M.T.; Gentile, A.; Memeo, V.; Bifulco, M.; Perri, E.; Caruso, M.G. Effects of olive oil polyphenols on fatty acid synthase gene expression and activity in human colorectal cancer cells. Genes Nutr. 2010, 6, 63–69. [Google Scholar] [CrossRef] [Green Version]
- Francesco, A.; Di Falconi, A.; Di Germanio, C.; Di Bonaventura, M.V.M.; Costa, A.; Caramuta, S.; Del Carlo, M.; Compagnone, D.; Dainese, E.; Cifani, C.; et al. CDG-TJ of Nutritional, 2015 Undefined. Extravirgin Olive Oil Up-Regulates CB1 Tumor Suppressor Gene in Human Colon Cancer Cells and in Rat Colon via Epigenetic Mechanisms. Elsevier. Available online: https://www.sciencedirect.com/science/article/pii/S0955286314002411?casa_token=bBNECjqYZBsAAAAA:w4ZuUKtZ6KQT3FbQZuW2y2ZnTTMRZ_e9RtbBKnzg46OLsjoF3N2eo3Z51Jo_Q3TEbrV4K3_E (accessed on 6 September 2021).
- Tutino, V.; Orlando, A.; Russo, F.; Notarnicola, M. Hydroxytyrosol Inhibits Cannabinoid CB1 Receptor Gene Expression in 3T3-L1 Preadipocyte Cell Line. J. Cell. Physiol. 2015, 231, 483–489. [Google Scholar] [CrossRef]
- You, T.; Disanzo, B.L.; Wang, X.; Yang, R.; Gong, D. Adipose tissue endocannabinoid system gene expression: Depot differences and effects of diet and exercise. Lipids Heal. Dis. 2011, 10, 194. [Google Scholar] [CrossRef] [Green Version]
- Roche, R.; Hoareau, L.; Bes-Houtmann, S.; Gonthier, M.-P.; Laborde, C.; Baron, J.-F.; Haffaf, Y.; Cesari, M.; Festy, F. Presence of the cannabinoid receptors, CB1 and CB2, in human omental and subcutaneous adipocytes. Histochem. Cell Biol. 2006, 126, 177–187. [Google Scholar] [CrossRef]
- Notarnicola, M.; Tutino, V.; Tafaro, A.; Bianco, G.; Guglielmi, E.; Caruso, M.G. Dietary olive oil induces cannabinoid CB2 receptor expression in adipose tissue of ApcMin/+ transgenic mice. Nutr. Health Aging 2016, 4, 73–80. [Google Scholar] [CrossRef] [Green Version]
- Hervera, A.; Negrete, R.; Leánez, S.; Martín-Campos, J.; Pol, O. The Role of Nitric Oxide in the Local Antiallodynic and Antihyperalgesic Effects and Expression of δ-Opioid and Cannabinoid-2 Receptors during Neuropathic Pain in Mice. ASPET. Available online: https://jpet.aspetjournals.org/content/334/3/887.short (accessed on 6 September 2021).
- Sakkas, H.; Bozidis, P.; Touzios, C.; Kolios, D.; Athanasiou, G.; Athanasopoulou, E.; Gerou, I.; Gartzonika, C. Nutritional Status and the Influence of the Vegan Diet on the Gut Microbiota and Human Health. Medicina 2020, 56, 88. [Google Scholar] [CrossRef] [Green Version]
- Paoli, A.; Mancin, L.; Bianco, A.; Thomas, E.; Mota, J.F.; Piccini, F. Ketogenic Diet and Microbiota: Friends or Enemies? Genes 2019, 10, 534. [Google Scholar] [CrossRef] [Green Version]
- Zmora, N.; Suez, J.; Elinav, E. You are what you eat: Diet, health and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2018, 16, 35–56. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, R.; Shively, C.A.; Register, T.C.; Craft, S.; Yadav, H. Gut microbiome-mediterranean diet interactions in improving host health [version 1; peer review: 1 approved]. F1000Research 2019, 8, 699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tosti, V.; Bertozzi, B.; Fontana, L. Health Benefits of the Mediterranean Diet: Metabolic and Molecular Mechanisms. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 318–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cani, P.D. Crosstalk between the gut microbiota and the endocannabinoid system: Impact on the gut barrier function and the adipose tissue. Clin. Microbiol. Infect. 2012, 18, 50–53. [Google Scholar] [CrossRef] [Green Version]
- Mehrpouya-Bahrami, P.; Chitrala, K.N.; Ganewatta, M.S.; Tang, C.; Murphy, E.A.; Enos, R.T.; Velazquez, K.T.; McCellan, J.; Nagarkatti, M.; Nagarkatti, P. Blockade of CB1 cannabinoid receptor alters gut microbiota and attenuates inflammation and diet-induced obesity. Sci. Rep. 2017, 7, 15645. [Google Scholar] [CrossRef]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cani, P.; Possemiers, S.; Wiele, T.; Van de Gut, Y.G. 2009 undefined. Changes in Gut Microbiota Control Inflammation in Obese Mice through a Mechanism Involving GLP-2-Driven Improvement of gut Permeability. gut.bmj.com. Available online: https://gut.bmj.com/content/58/8/1091.short (accessed on 6 September 2021).
- Kameyama, K.; Itoh, K. Intestinal Colonization by a Lachnospiraceae Bacterium Contributes to the Development of Diabetes in Obese Mice. Microbes Environ. 2014, 29, 427–430. [Google Scholar] [CrossRef] [Green Version]
- Kaakoush, N.O. Insights into the Role of Erysipelotrichaceae in the Human Host. Front. Cell. Infect. Microbiol. 2015, 5, 84. [Google Scholar] [CrossRef] [Green Version]
- Iannotti, F.; Piscitelli, F.; Martella, A.; Mazzarella, E.; Allarà, M.; Palmieri, V.; Parrella, C.; Capasso, R.; Di Marzo, V. Analysis of the “endocannabinoidome” in peripheral tissues of obese Zucker rats. Prostaglandins Leukot. Essent. Fat. Acids 2013, 89, 127–135. [Google Scholar] [CrossRef]
- Syed, S.K.; Bui, H.H.; Beavers, L.S.; Farb, T.B.; Ficorilli, J.; Chesterfield, A.K.; Kuo, M.-S.; Bokvist, K.; Barrett, D.G.; Efanov, A. Regulation of GPR119 receptor activity with endocannabinoid-like lipids. Am. J. Physiol. Metab. 2012, 303, E1469–E1478. [Google Scholar] [CrossRef]
- Muccioli, G.G.; Naslain, D.; Bäckhed, F.; Reigstad, C.S.; Lambert, D.M.; Delzenne, N.M.; Cani, P.D. The endocannabinoid system links gut microbiota to adipogenesis. Mol. Syst. Biol. 2010, 6, 392. [Google Scholar] [CrossRef] [PubMed]
- Geurts, L.; Everard, A.; Van Hul, M.; Essaghir, A.; Duparc, T.; Matamoros, S.; Plovier, H.; Castel, J.; Denis, R.; Bergiers, M.; et al. Adipose tissue NAPE-PLD controls fat mass development by altering the browning process and gut microbiota. Nat. Commun. 2015, 6, 6495. [Google Scholar] [CrossRef] [Green Version]
- Alhouayek, M.; Lambert, D.M.; Delzenne, N.M.; Cani, P.D.; Muccioli, G.G. Increasing endogenous 2-arachidonoylglycerol levels counteracts colitis and related systemic inflammation. FASEB J. 2011, 25, 2711–2721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murataeva, N.; Dhopeshwarkar, A.; Yin, D.; Mitjavila, J.; Bradshaw, H.; Straiker, A.; Mackie, K. Where’s my entourage? The curious case of 2-oleoylglycerol, 2-linolenoylglycerol, and 2-palmitoylglycerol. Pharmacol. Res. 2016, 110, 173–180. [Google Scholar] [CrossRef] [Green Version]
- Kang, C.; Wang, B.; Kaliannan, K.; Wang, X.; Lang, H.; Hui, S.; Huang, L.; Zhang, Y.; Zhou, M.; Chen, M.; et al. Gut Microbiota Mediates the Protective Effects of Dietary Capsaicin against Chronic Low-Grade Inflammation and Associated Obesity Induced by High-Fat Diet. mBio 2017, 8, e00470-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cantu-Jungles, T.M.; Rasmussen, H.E.; Hamaker, B.R. Potential of Prebiotic Butyrogenic Fibers in Parkinson’s Disease. Front. Neurol. 2019, 10, 663. [Google Scholar] [CrossRef] [Green Version]
- Scott, K.P.; Martin, J.C.; Duncan, S.; Flint, H.J. Prebiotic stimulation of human colonic butyrate-producing bacteria and bifidobacteria, in vitro. FEMS Microbiol. Ecol. 2013, 87, 30–40. [Google Scholar] [CrossRef] [Green Version]
- La Rosa, S.L.; Kachrimanidou, V.; Buffetto, F.; Pope, P.B.; Pudlo, N.A.; Martens, E.C.; Rastall, R.A.; Gibson, G.R.; Westereng, B. Wood-Derived Dietary Fibers Promote Beneficial Human Gut Microbiota. mSphere 2019, 4, e00554-18. [Google Scholar] [CrossRef] [Green Version]
- Markey, L.; Hooper, A.; Melon, L.C.; Baglot, S.; Hill, M.N.; Maguire, J.; Kumamoto, C.A. Colonization with the commensal fungus Candida albicans perturbs the gut-brain axis through dysregulation of endocannabinoid signaling. Psychoneuroendocrinology 2020, 121, 104808. [Google Scholar] [CrossRef]
- Lacroix, S.; Pechereau, F.; Leblanc, N.; Boubertakh, B.; Houde, A.; Martin, C.; Flamand, N.; Silvestri, C.; Raymond, F.; Di Marzo, V.; et al. Rapid and Concomitant Gut Microbiota and Endocannabinoidome Response to Diet-Induced Obesity in Mice. mSystems 2019, 4, e00407-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barutta, F.; Piscitelli, F.; Pinach, S.; Bruno, G.; Gambino, R.; Rastaldi, M.P.; Salvidio, G.; Di Marzo, V.; Perin, P.C.; Gruden, G. Protective Role of Cannabinoid Receptor Type 2 in a Mouse Model of Diabetic Nephropathy. Diabetes 2011, 60, 2386–2396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barutta, F.; Grimaldi, S.; Franco, I.; Bellini, S.; Gambino, R.; Pinach, S.; Corbelli, A.; Bruno, G.; Rastaldi, M.P.; Aveta, T.; et al. Deficiency of cannabinoid receptor of type 2 worsens renal functional and structural abnormalities in streptozotocin-induced diabetic mice. Kidney Int. 2014, 86, 979–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vago, R.; Fiorio, F.; Trevisani, F.; Salonia, A.; Montorsi, F.; Bettiga, A. The Mediterranean Diet as a Source of Bioactive Molecules with Cannabinomimetic Activity in Prevention and Therapy Strategy. Nutrients 2022, 14, 468. https://doi.org/10.3390/nu14030468
Vago R, Fiorio F, Trevisani F, Salonia A, Montorsi F, Bettiga A. The Mediterranean Diet as a Source of Bioactive Molecules with Cannabinomimetic Activity in Prevention and Therapy Strategy. Nutrients. 2022; 14(3):468. https://doi.org/10.3390/nu14030468
Chicago/Turabian StyleVago, Riccardo, Francesco Fiorio, Francesco Trevisani, Andrea Salonia, Francesco Montorsi, and Arianna Bettiga. 2022. "The Mediterranean Diet as a Source of Bioactive Molecules with Cannabinomimetic Activity in Prevention and Therapy Strategy" Nutrients 14, no. 3: 468. https://doi.org/10.3390/nu14030468
APA StyleVago, R., Fiorio, F., Trevisani, F., Salonia, A., Montorsi, F., & Bettiga, A. (2022). The Mediterranean Diet as a Source of Bioactive Molecules with Cannabinomimetic Activity in Prevention and Therapy Strategy. Nutrients, 14(3), 468. https://doi.org/10.3390/nu14030468