Trait Energy and Fatigue May Be Connected to Gut Bacteria among Young Physically Active Adults: An Exploratory Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Survey
2.3. Diet Recall
2.4. Microbial Community Analysis
2.5. Metabolomics
2.6. Statistical Analysis
3. Results
3.1. Subject Description
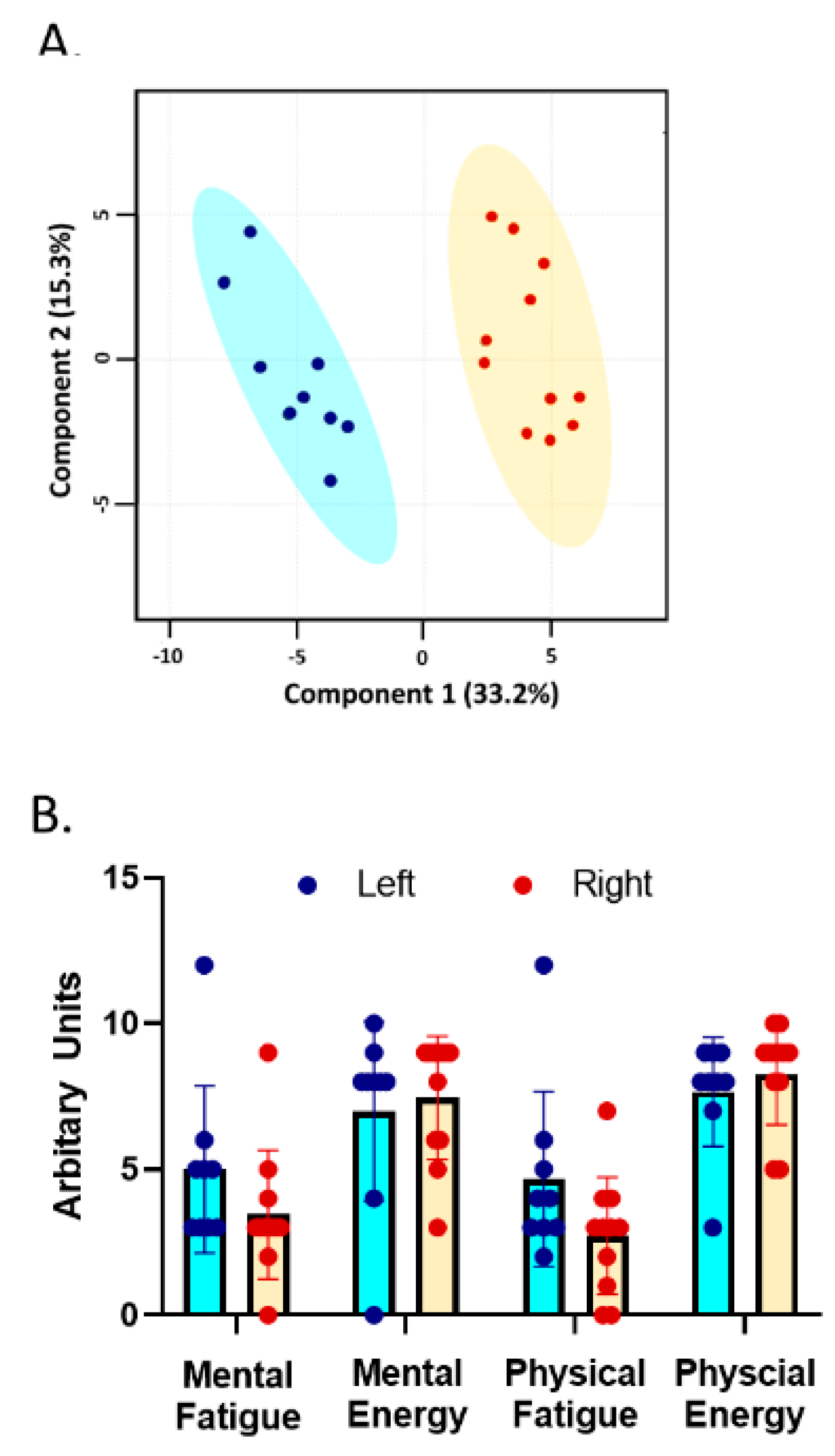
3.2. Trait Measures
3.3. Diversity Measures
3.4. Bacteria Taxa Correlated with Four Traits
3.5. Predicted Functional Pathways Correlated with Traits
3.6. Nutrients and Food Groups Correlated with the Four Traits
3.7. Nutrients and Food Groups Correlated with Bacteria Correlated with the Four Traits
3.8. Metabolomics
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Variable | Median | Interquartile Range |
|---|---|---|
| Age (years) | 31 | 7 |
| Sex (Males, Females) | 14, 6 | |
| Weight (lbs) | 180 | 54 |
| Height (inches) | 68 | 7 |
| BMI (kg/m2) | 25.3 | 5.1 |
| Total Walking MET-min/week | 1402.5 | 2883.4 |
| Total Moderate MET-min/week total | 1560.0 | 3532.5 |
| Total Vigorous MET-min/week | 2880.0 | 2520.0 |
| Total Physical Activity MET-min/week | 7939.5 | 5711.6 |
| Sitting Total Minutes/week | 2220.0 | 1245.0 |
| Average Sitting Total Minutes/day | 317.1 | 177.9 |
| Trait Physical Energy | 8.0 | 3.0 |
| Trait Physical Fatigue | 3.0 | 2.0 |
| Trait Mental Energy | 8.5 | 1 |
| Trait Mental Fatigue | 3.0 | 1.8 |
| Alpha Diversity Measure | Trait Mental Energy | Trait Mental Fatigue | Trait Physical Energy | Trait Physical Fatigue | |
|---|---|---|---|---|---|
| Evenness | Correlation Coefficient | 0.099 | −0.401 | 0.092 | −0.387 |
| Significance | 0.679 | 0.079 | 0.700 | 0.092 | |
| Shannon | Correlation Coefficient | 0.293 | −0.330 | 0.199 | −0.432 |
| Significance | 0.211 | 0.156 | 0.401 | 0.057 | |
| Observed Otus | Correlation Coefficient | 0.432 | −0.185 | 0.331 | −0.390 |
| Significance | 0.057 | 0.435 | 0.154 | 0.089 | |
| Faith PD | Correlation Coefficient | 0.293 | −0.367 | 0.247 | −0.509 |
| Significance | 0.209 | 0.111 | 0.294 | 0.022 |
References
- Chen, M.K. The epidemiology of self-perceived fatigue among adults. Prev. Med. 1986, 15, 74–81. [Google Scholar] [CrossRef]
- Cunningham, T.J.; Ford, E.S.; Chapman, D.P.; Liu, Y.; Croft, J.B. Independent and joint associations of race/ethnicity and educational attainment with sleep-related symptoms in a population-based US sample. Prev. Med. 2015, 77, 99–105. [Google Scholar] [CrossRef] [Green Version]
- Lewis, G.; Wessely, S. The epidemiology of fatigue: More questions than answers. J. Epidemiol. Community Health 1992, 46, 92–97. [Google Scholar] [CrossRef] [Green Version]
- Ricci, J.A.; Chee, E.; Lorandeau, A.L.; Berger, J. Fatigue in the U.S. workforce: Prevalence and implications for lost productive work time. J. Occup. Environ. Med. 2007, 49, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Taylor, A.H.; Dorn, L. Stress, fatigue, health, and risk of road traffic accidents among professional drivers: The contribution of physical inactivity. Annu. Rev. Public Health 2006, 27, 371–391. [Google Scholar] [CrossRef] [Green Version]
- Van Drongelen, A.; Boot, C.R.; Hlobil, H.; Smid, T.; van der Beek, A.J. Risk factors for fatigue among airline pilots. Int. Arch. Occup. Environ. Health 2017, 90, 39–47. [Google Scholar] [CrossRef]
- Samkoff, J.S.; Jacques, C.H. A review of studies concerning effects of sleep deprivation and fatigue on residents’ performance. Acad. Med. 1991, 66, 687–693. [Google Scholar] [CrossRef]
- Bakker, R.J.; van de Putte, E.M.; Kuis, W.; Sinnema, G. Risk factors for persistent fatigue with significant school absence in children and adolescents. Pediatrics 2009, 124, e89–e95. [Google Scholar] [CrossRef]
- Fukuda, S.; Yamano, E.; Joudoi, T.; Mizuno, K.; Tanaka, M.; Kawatani, J.; Takano, M.; Tomoda, A.; Imai-Matsumura, K.; Miike, T.; et al. Effort-reward imbalance for learning is associated with fatigue in school children. Behav. Med. 2010, 36, 53–62. [Google Scholar] [CrossRef]
- Verbrugge, L.M.; Ascione, F.J. Exploring the iceberg. Common symptoms and how people care for them. Med. Care 1987, 25, 539–569. [Google Scholar] [CrossRef]
- Hjollund, N.H.; Andersen, J.H.; Bech, P. Assessment of fatigue in chronic disease: A bibliographic study of fatigue measurement scales. Health Qual. Life Outcomes 2007, 5, 12. [Google Scholar] [CrossRef] [Green Version]
- Loy, B.D.; Cameron, M.H.; O’Connor, P.J. Perceived fatigue and energy are independent unipolar states: Supporting evidence. Med. Hypotheses 2018, 113, 46–51. [Google Scholar] [CrossRef]
- Boolani, A.; O’Connor, P.J.; Reid, J.; Ma, S.; Mondal, S. Predictors of feelings of energy differ from predictors of fatigue. Fatigue Biomed. Health Behav. 2019, 7, 12–28. [Google Scholar] [CrossRef]
- Dupree, E.J.; Goodwin, A.; Darie, C.C.; Boolani, A. A Pilot Exploratory Proteomics Investigation of Mental Fatigue and Mental Energy. Adv. Exp. Med. Biol. 2019, 1140, 601–611. [Google Scholar]
- Manierre, M.; Jansen, E.; Boolani, A. Sleep quality and sex modify the relationships between trait energy and fatigue on state energy and fatigue. PLoS ONE 2020, 15, e0227511. [Google Scholar] [CrossRef]
- Boolani, A.; Manierre, M. An exploratory multivariate study examining correlates of trait mental and physical fatigue and energy. Fatigue Biomed. Health Behav. 2019, 7, 29–40. [Google Scholar] [CrossRef]
- Boolani, A.; Sur, S.; Yang, D.; Avolio, A.; Goodwin, A.; Mondal, S.; Fulk, G.; Towler, C.; Lee Smith, M. Six Minutes of Physical Activity Improves Mood in Older Adults: A Pilot Study. J. Geriatr. Phys. Ther. 2021, 44, 18–24. [Google Scholar] [CrossRef]
- O’Connor, P.J.; Puetz, T.W. Chronic physical activity and feelings of energy and fatigue. Med. Sci. Sports Exerc. 2005, 37, 299–305. [Google Scholar] [CrossRef]
- Loy, B.D.; O’Connor, P.J.; Dishman, R.K. The effect of a single bout of exercise on energy and fatigue states: A systematic review and meta-analysis. Fatigue Biomed. Health Behav. 2013, 1, 223–242. [Google Scholar] [CrossRef]
- Miller, M.; Lee-Chambers, J.; Cooper, B.; Boolani, A.; Jansen, E. Associations between physical activity and energy and fatigue depend on sleep quality. Fatigue Biomed. Health Behav. 2020, 8, 193–204. [Google Scholar] [CrossRef]
- Maridakis, V.; Herring, M.P.; O’Connor, P.J. Sensitivity to change in cognitive performance and mood measures of energy and fatigue in response to differing doses of caffeine or breakfast. Int. J. Neurosci. 2009, 119, 975–994. [Google Scholar] [CrossRef]
- Boolani, A.; Lindheimer, J.B.; Loy, B.D.; Crozier, S.; O’Connor, P.J. Acute effects of brewed cocoa consumption on attention, motivation to perform cognitive work and feelings of anxiety, energy and fatigue: A randomized, placebo-controlled crossover experiment. BMC Nutr. 2017, 3, 8. [Google Scholar] [CrossRef] [Green Version]
- Fuller, D.T.; Smith, M.L.; Boolani, A. Trait Energy and Fatigue Modify the Effects of Caffeine on Mood, Cognitive and Fine-Motor Task Performance: A Post-Hoc Study. Nutrients 2021, 13, 412. [Google Scholar] [CrossRef]
- Eshragh, J.; Dhruva, A.; Paul, S.M.; Cooper, B.A.; Mastick, J.; Hamolsky, D.; Levine, J.D.; Miaskowski, C.; Kober, K.M. Associations between Neurotransmitter Genes and Fatigue and Energy Levels in Women after Breast Cancer Surgery. J. Pain Symptom Manag. 2017, 53, 67–84.e7. [Google Scholar] [CrossRef]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the human intestinal microbial flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef] [Green Version]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef] [Green Version]
- Conlon, M.A.; Bird, A.R. The impact of diet and lifestyle on gut microbiota and human health. Nutrients 2014, 7, 17–44. [Google Scholar] [CrossRef]
- Backhed, F.; Ley, R.E.; Sonnenburg, J.L.; Peterson, D.A.; Gordon, J.I. Host-bacterial mutualism in the human intestine. Science 2005, 307, 1915–1920. [Google Scholar] [CrossRef] [Green Version]
- Grenham, S.; Clarke, G.; Cryan, J.F.; Dinan, T.G. Brain-gut-microbe communication in health and disease. Front. Physiol. 2011, 2, 94. [Google Scholar] [CrossRef] [Green Version]
- Das, B.; Nair, G.B. Homeostasis and dysbiosis of the gut microbiome in health and disease. J. Biosci. 2019, 44, 1–8. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Mahony, S.M. The microbiome-gut-brain axis: From bowel to behavior. Neurogastroenterol. Motil. 2011, 23, 187–192. [Google Scholar] [CrossRef]
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef] [Green Version]
- Tremaroli, V.; Bäckhed, F. Functional interactions between the gut microbiota and host metabolism. Nature 2012, 489, 242–249. [Google Scholar] [CrossRef]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef] [Green Version]
- Koutsos, A.; Tuohy, K.M.; Lovegrove, J.A. Apples and cardiovascular health-is the gut microbiota a core consideration? Nutrients 2015, 7, 3959–3998. [Google Scholar] [CrossRef]
- O’Connor, P.J.; Kennedy, D.O.; Stahl, S. Mental energy: Plausible neurological mechanisms and emerging research on the effects of natural dietary compounds. Nutr. Neurosci. 2019, 24, 850–864. [Google Scholar] [CrossRef]
- Baguley, B.J.; Skinner, T.L.; Jenkins, D.G.; Wright, O.R.L. Mediterranean-style dietary pattern improves cancer-related fatigue and quality of life in men with prostate cancer treated with androgen deprivation therapy: A pilot randomised control trial. Clin. Nutr. 2021, 40, 245–254. [Google Scholar] [CrossRef]
- Hajjar, J.; Mendoza, T.; Zhang, L.; Fu, S.; Piha-Paul, S.A.; Hong, D.S.; Janku, F.; Karp, D.D.; Ballhausen, A.; Gong, J.; et al. Associations between the gut microbiome and fatigue in cancer patients. Sci. Rep. 2021, 11, 5847. [Google Scholar] [CrossRef]
- Giloteaux, L.; Goodrich, J.K.; Walters, W.A.; Levine, S.M.; Ley, R.E.; Hanson, M.R. Reduced diversity and altered composition of the gut microbiome in individuals with myalgic encephalomyelitis/chronic fatigue syndrome. Microbiome 2016, 4, 30. [Google Scholar] [CrossRef] [Green Version]
- Xiao, C.; Fedirko, V.; Beitler, J.; Bai, J.; Peng, G.; Zhou, C.; Gu, J.; Zhao, H.; Lin, I.H.; Chico, C.E.; et al. The role of the gut microbiome in cancer-related fatigue: Pilot study on epigenetic mechanisms. Support Care Cancer 2021, 29, 3173–3182. [Google Scholar] [CrossRef]
- Mandarano, A.H.; Giloteaux, L.; Keller, B.A.; Levine, S.M.; Hanson, M.R. Eukaryotes in the gut microbiota in myalgic encephalomyelitis/chronic fatigue syndrome. PeerJ 2018, 6, e4282. [Google Scholar] [CrossRef] [Green Version]
- Nuzzo, R. Scientific method: Statistical errors. Nature 2014, 506, 150–152. [Google Scholar] [CrossRef] [Green Version]
- Loy, B.D.; O’Connor, P.J. The effect of histamine on changes in mental energy and fatigue after a single bout of exercise. Physiol. Behav. 2016, 153, 7–18. [Google Scholar] [CrossRef]
- Bigelman, K.A.; Chapman, D.P.; Freese, E.C.; Trilk, J.L.; Cureton, K.J. Effects of 6 weeks of quercetin supplementation on energy, fatigue, and sleep in ROTC cadets. Mil. Med. 2011, 176, 565–572. [Google Scholar] [CrossRef] [Green Version]
- Bossie, H.M.; Willingham, T.B.; Schoick, R.A.V.; O’Connor, P.J.; McCully, K.K. Mitochondrial capacity, muscle endurance, and low energy in friedreich ataxia. Muscle Nerve 2017, 56, 773–779. [Google Scholar] [CrossRef]
- Boolani, A.; Fuller, D.T.; Mondal, S.; Wilkinson, T.; Darie, C.C.; Gumpricht, E. Caffeine-Containing, Adaptogenic-Rich Drink Modulates the Effects of Caffeine on Mental Performance and Cognitive Parameters: A Double-Blinded, Placebo-Controlled, Randomized Trial. Nutrients 2020, 12, 1922. [Google Scholar] [CrossRef]
- Boolani, A.A.A.; Barrios, N.; Sames, C. Association between trait energy and fatigue and aquatic functional tests: An exploratory study. J. Aquat. Phys. Ther. 2021, in press. [Google Scholar]
- Subar, A.F.; Kirkpatrick, S.I.; Mittl, B.; Zimmerman, T.P.; Thompson, F.E.; Bingley, C.; Willis, G.; Islam, N.G.; Baranowski, T.; McNutt, S.; et al. The Automated Self-Administered 24-hour dietary recall (ASA24): A resource for researchers, clinicians, and educators from the National Cancer Institute. J. Acad. Nutr. Diet. 2012, 112, 1134–1137. [Google Scholar] [CrossRef] [Green Version]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [Green Version]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [Green Version]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree 2-approximately maximum-likelihood trees for large alignments. PLoS ONE 2010, 5, e9490. [Google Scholar] [CrossRef]
- McDonald, D.; Price, M.N.; Goodrich, J.; Nawrocki, E.P.; DeSantis, T.Z.; Probst, A.; Andersen, G.L.; Knight, R.; Hugenholtz, P. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J. 2012, 6, 610–618. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Langille, M.G.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Vega Thurber, R.L.; Knight, R.; et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef]
- Parks, D.H.; Beiko, R.G. Identifying biologically relevant differences between metagenomic communities. Bioinformatics 2010, 26, 715–721. [Google Scholar] [CrossRef] [Green Version]
- Joshua, D.; Rabinowitz, E.K. Acidic acetonitrile for cellular metabolome extraction from Escherichia coli. Anal. Chem. 2007, 79, 6167–6173. [Google Scholar]
- Lu, W.; Clasquin, M.F.; Melamud, E.; Amador-Noguez, D.; Caudy, A.A.; Rabinowitz, J.D. Metabolomic Analysis via Reversed-Phase Ion-Pairing Liquid Chromatography Coupled to a Stand Alone Orbitrap Mass Spectrometer. Anal. Chem. 2010, 82, 3212–3221. [Google Scholar] [CrossRef] [Green Version]
- Stough, J.M.A.; Dearth, S.P.; Denny, J.E.; LeCleir, G.R.; Schmidt, N.W.; Campagna, S.R.; Wilhelm, S.W. Functional Characteristics of the Gut Microbiome in C57BL/6 Mice Differentially Susceptible to Plasmodium yoelii. Front. Microbiol. 2016, 7, 1520. [Google Scholar] [CrossRef] [Green Version]
- Martens, L.; Chambers, M.; Sturm, M.; Kessner, D.; Levander, F.; Shofstahl, J.; Tang, W.H.; Römpp, A.; Neumann, S.; Pizarro, A.D.; et al. mzML-a community standard for mass spectrometry data. Mol. Cell. Proteom. MCP 2011, 10, R110.000133. [Google Scholar] [CrossRef] [Green Version]
- Clasquin, M.F.; Melamud, E.; Rabinowitz, J.D. LC-MS data processing with MAVEN: A metabolomic analysis and visualization engine. Curr. Protoc. Bioinform. 2012, 37, 14.11.1–14.11.23. [Google Scholar]
- Melamud, E.; Vastag, L.; Rabinowitz, J.D. Metabolomic Analysis and Visualization Engine for LC−MS Data. Anal. Chem. 2010, 82, 9818–9826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Livera, A.M.; Olshansky, G.; Simpson, J.A.; Creek, D.J. NormalizeMets: Assessing, selecting and implementing statistical methods for normalizing metabolomics data. Metabolomics 2018, 14, 54. [Google Scholar] [CrossRef] [PubMed]
- Misra, B.B. Data normalization strategies in metabolomics: Current challenges, approaches, and tools. Eur. J. Mass Spectrom. 2020, 26, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.-É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef] [PubMed]
- McDonald, D.; Hyde, E.; Debelius, J.W.; Morton, J.T.; Gonzalez, A.; Ackermann, G.; Aksenov, A.A.; Behsaz, B.; Brennan, C.; Chen, Y.; et al. American Gut: An Open Platform for Citizen Science Microbiome Research. mSystems 2018, 3, e00031-18. [Google Scholar] [CrossRef] [Green Version]
- Meyers, L.D.; Hellwig, J.P.; Otten, J.J. (Eds.) Dietary Reference Intakes: The Essential Guide to Nutrient Requirements; The National Academies Press: Washington, DC, USA, 2006; p. 1344. [Google Scholar]
- Binda, C.; Lopetuso, L.R.; Rizzatti, G.; Gibiino, G.; Cennamo, V.; Gasbarrini, A. Actinobacteria: A relevant minority for the maintenance of gut homeostasis. Dig. Liver Dis. 2018, 50, 421–428. [Google Scholar] [CrossRef]
- Ilinskaya, O.N.; Ulyanova, V.V.; Yarullina, D.R.; Gataullin, I.G. Secretome of Intestinal Bacilli: A Natural Guard against Pathologies. Front. Microbiol. 2017, 8, 1666. [Google Scholar] [CrossRef]
- Bergstrom, A.; Skov, T.H.; Bahl, M.I.; Roager, H.M.; Christensen, L.B.; Ejlerskov, K.T.; Molgaard, C.; Michaelsen, K.F.; Licht, T.R. Establishment of intestinal microbiota during early life: A longitudinal, explorative study of a large cohort of Danish infants. Appl. Environ. Microbiol. 2014, 80, 2889–2900. [Google Scholar] [CrossRef] [Green Version]
- Faintuch, J.; Faintuch, S. Microbiome and Metabolome in Diagnosis, Therapy, and Other Strategic Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2019; p. 472. [Google Scholar]
- Gomez-Arango, L.F.; Barrett, H.L.; Wilkinson, S.A.; Callaway, L.K.; McIntyre, H.D.; Morrison, M.; Dekker Nitert, M. Low dietary fiber intake increases Collinsella abundance in the gut microbiota of overweight and obese pregnant women. Gut Microbes 2018, 9, 189–201. [Google Scholar] [CrossRef] [Green Version]
- Gomez-Arango, L.F.; Barrett, H.L.; McIntyre, H.D.; Callaway, L.K.; Morrison, M.; Dekker Nitert, M.; Group, S.T. Increased Systolic and Diastolic Blood Pressure Is Associated with Altered Gut Microbiota Composition and Butyrate Production in Early Pregnancy. Hypertension 2016, 68, 974–981. [Google Scholar] [CrossRef]
- Dinh, D.M.; Volpe, G.E.; Duffalo, C.; Bhalchandra, S.; Tai, A.K.; Kane, A.V.; Wanke, C.A.; Ward, H.D. Intestinal microbiota, microbial translocation, and systemic inflammation in chronic HIV infection. J. Infect. Dis. 2015, 211, 19–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frank, D.N.; St Amand, A.L.; Feldman, R.A.; Boedeker, E.C.; Harpaz, N.; Pace, N.R. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc. Natl. Acad. Sci. USA 2007, 104, 13780–13785. [Google Scholar] [CrossRef] [Green Version]
- Langgartner, D.; Peterlik, D.; Foertsch, S.; Fuchsl, A.M.; Brokmann, P.; Flor, P.J.; Shen, Z.; Fox, J.G.; Uschold-Schmidt, N.; Lowry, C.A.; et al. Individual differences in stress vulnerability: The role of gut pathobionts in stress-induced colitis. Brain Behav. Immun. 2017, 64, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Vacca, M.; Celano, G.; Calabrese, F.M.; Portincasa, P.; Gobbetti, M.; De Angelis, M. The Controversial Role of Human Gut Lachnospiraceae. Microorganisms 2020, 8, 573. [Google Scholar] [CrossRef]
- Hall, K.D.; Ayuketah, A.; Brychta, R.; Cai, H.; Cassimatis, T.; Chen, K.Y.; Chung, S.T.; Costa, E.; Courville, A.; Darcey, V.; et al. Ultra-Processed Diets Cause Excess Calorie Intake and Weight Gain: An Inpatient Randomized Controlled Trial of Ad Libitum Food Intake. Cell Metab. 2019, 30, 67–77.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wlodek, D.; Gonzales, M. Decreased energy levels can cause and sustain obesity. J. Theor. Biol. 2003, 225, 33–44. [Google Scholar] [CrossRef]
- Pastier, N.; Jansen, E.; Boolani, A. Sleep quality in relation to trait energy and fatigue: An exploratory study of healthy young adults. Sleep Sci. 2022. e-pub ahead of print. [Google Scholar]
- Jeffery, I.B.; Lynch, D.B.; O’Toole, P.W. Composition and temporal stability of the gut microbiota in older persons. ISME J. 2016, 10, 170–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fassarella, M.; Blaak, E.E.; Penders, J.; Nauta, A.; Smidt, H.; Zoetendal, E.G. Gut microbiome stability and resilience: Elucidating the response to perturbations in order to modulate gut health. Gut 2021, 70, 595–605. [Google Scholar] [CrossRef]
- Rigottier-Gois, L. Dysbiosis in inflammatory bowel diseases: The oxygen hypothesis. ISME J. 2013, 7, 1256–1261. [Google Scholar] [CrossRef]
- Gevers, D.; Kugathasan, S.; Denson, L.A.; Vazquez-Baeza, Y.; Van Treuren, W.; Ren, B.; Schwager, E.; Knights, D.; Song, S.J.; Yassour, M.; et al. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe 2014, 15, 382–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology: Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef]
- Parekh, P.J.; Arusi, E.; Vinik, A.I.; Johnson, D.A. The role and influence of gut microbiota in pathogenesis and management of obesity and metabolic syndrome. Front. Endocrinol. 2014, 5, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeffery, I.B.; O’Toole, P.W.; Ohman, L.; Claesson, M.J.; Deane, J.; Quigley, E.M.; Simren, M. An irritable bowel syndrome subtype defined by species-specific alterations in faecal microbiota. Gut 2012, 61, 997–1006. [Google Scholar] [CrossRef]
- Dupont, H.L. Review article: Evidence for the role of gut microbiota in irritable bowel syndrome and its potential influence on therapeutic targets. Aliment. Pharmacol. Ther. 2014, 39, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Larsen, N.; Vogensen, F.K.; van den Berg, F.W.; Nielsen, D.S.; Andreasen, A.S.; Pedersen, B.K.; Al-Soud, W.A.; Sorensen, S.J.; Hansen, L.H.; Jakobsen, M. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS ONE 2010, 5, e9085. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.C.; Randolph, T.W.; Lim, U.; Monroe, K.R.; Cheng, I.; Wilkens, L.R.; Le Marchand, L.; Lampe, J.W.; Hullar, M.A.J. Temporal Variability and Stability of the Fecal Microbiome: The Multiethnic Cohort Study. Cancer Epidemiol. Biomark. Prev. 2019, 28, 154–162. [Google Scholar] [CrossRef] [Green Version]
| Correlation Coefficient | Significance | |
|---|---|---|
| Trait Mental Energy | ||
| p__Actinobacteria | 0.469 | 0.037 |
| p__Firmicutes | 0.520 | 0.019 |
| p__Firmicutes;c__Bacilli;o__Turicibacterales | 0.470 | 0.037 |
| p__Firmicutes;c__Bacilli;o__Turicibacterales;f__Turicibacteraceae | 0.470 | 0.037 |
| p__Firmicutes;c__Bacilli;o__Turicibacterales;f__Turicibacteraceae;g__Turicibacter | 0.470 | 0.037 |
| p__Firmicutes;c__Clostridia;o__Clostridiales;f__Lachnospiraceae;g__;s__ | 0.461 | 0.041 |
| p__Firmicutes;c__Clostridia;o__Clostridiales;f__Lachnospiraceae;g__[Ruminococcus];s__gnavus | 0.478 | 0.033 |
| p__Firmicutes;c__Clostridia;o__Clostridiales;f__Lachnospiraceae;g__Anaerostipes;s__ * | 0.480 | 0.032 |
| p__Firmicutes;c__Clostridia;o__Clostridiales;f__Ruminococcaceae;g__;s__ | 0.454 | 0.044 |
| p__Firmicutes;c__Clostridia;o__Clostridiales;f__Lachnospiraceae;g__Coprococcus;s__catus | 0.479 | 0.032 |
| p__Firmicutes;c__Clostridia;o__Clostridiales;f__Lachnospiraceae;g__Roseburia;s__faecis | 0.558 | 0.011 |
| p__Verrucomicrobia;c__Verrucomicrobiae | 0.475 | 0.034 |
| p__Verrucomicrobia;c__Verrucomicrobiae;o__Verrucomicrobiales | 0.475 | 0.034 |
| p__Verrucomicrobia;c__Verrucomicrobiae;o__Verrucomicrobiales;f__Verrucomicrobiaceae | 0.475 | 0.034 |
| p__Verrucomicrobia;c__Verrucomicrobiae;o__Verrucomicrobiales;f__Verrucomicrobiaceae; g__Akkermansia | 0.475 | 0.034 |
| Trait Mental Fatigue | ||
| p__Firmicutes;c__Erysipelotrichi | 0.451 | 0.046 |
| p__Firmicutes;c__Erysipelotrichi;o__Erysipelotrichales | 0.451 | 0.046 |
| p__Firmicutes;c__Erysipelotrichi;o__Erysipelotrichales;f__Erysipelotrichaceae | 0.451 | 0.046 |
| p__Firmicutes;c__Clostridia;o__Clostridiales;f__Lachnospiraceae;g__Anaerostipes;s__ | −0.532 | 0.016 |
| Trait Physical Energy | ||
| p__Firmicutes;c__Erysipelotrichi;o__Erysipelotrichales;f__Erysipelotrichaceae;g__Holdemania | −0.533 | 0.015 |
| p__Firmicutes;c__Clostridia;o__Clostridiales;f__Lachnospiraceae;g__Dorea;s__ | −0.463 | 0.040 |
| p__Firmicutes;c__Clostridia;o__Clostridiales;f__Peptostreptococcaceae;g__;s__ | −0.461 | 0.041 |
| Trait Physical Fatigue | ||
| p__Firmicutes;c__Clostridia;o__Clostridiales;f__Christensenellaceae;g__;s__ | −0.630 | 0.003 |
| p__Firmicutes;c__Clostridia;o__Clostridiales;f__Lachnospiraceae;g__Anaerostipes;s__ | −0.448 | 0.048 |
| p__Proteobacteria;c__Gammaproteobacteria;o__Pasteurellales | 0.445 | 0.049 |
| p__Proteobacteria;c__Gammaproteobacteria;o__Pasteurellales;f__Pasteurellaceae | 0.445 | 0.049 |
| p__Proteobacteria;c__Gammaproteobacteria;o__Pasteurellales;f__Pasteurellaceae;g__Haemophilus | 0.512 | 0.021 |
| p__Bacteroidetes;c__Bacteroidia;o__Bacteroidales;f__Bacteroidaceae;g__Bacteroides;s__ | −0.451 | 0.046 |
| Correlation Coefficient | Significance | |
|---|---|---|
| Trait Mental Energy | ||
| Cellular Processes; Cell Motility; Bacterial motility proteins | 0.494 | 0.027 |
| Genetic Information Processing; Replication and Repair; Non-homologous end-joining | 0.523 | 0.018 |
| Human Diseases; Infectious Diseases; African trypanosomiasis | 0.501 | 0.025 |
| Metabolism; Biosynthesis of Other Secondary Metabolites; Butirosin and neomycin biosynthesis | 0.445 | 0.049 |
| Metabolism; Biosynthesis of Other Secondary Metabolites; Flavonoid biosynthesis | 0.531 | 0.016 |
| Metabolism; Lipid Metabolism; Biosynthesis of unsaturated fatty acids | 0.470 | 0.037 |
| Metabolism; Metabolism of Terpenoids and Polyketides Biosynthesis of siderophore group nonribosomal peptides | 0.450 | 0.046 |
| Metabolism; Metabolism of Terpenoids and Polyketides; Carotenoid biosynthesis | 0.621 | 0.003 |
| Metabolism; Xenobiotics Biodegradation and Metabolism; Benzoate degradation | 0.461 | 0.041 |
| Metabolism; Xenobiotics Biodegradation and Metabolism; Chloroalkane and chloroalkene degradation | 0.470 | 0.037 |
| Metabolism; Xenobiotics Biodegradation and Metabolism; Dioxin degradation | 0.464 | 0.039 |
| Metabolism; Xenobiotics Biodegradation and Metabolism; Metabolism of xenobiotics by cytochrome P450 | 0.446 | 0.049 |
| Metabolism; Xenobiotics Biodegradation and Metabolism; Naphthalene degradation | 0.451 | 0.046 |
| Metabolism; Xenobiotics Biodegradation and Metabolism; Xylene degradation | 0.453 | 0.045 |
| Organismal Systems; Digestive System; Carbohydrate digestion and absorption | 0.511 | 0.021 |
| Organismal Systems; Endocrine System; Insulin signaling pathway | 0.447 | 0.048 |
| Organismal Systems; Immune System; NOD-like receptor signaling pathway | 0.446 | 0.049 |
| Unclassified; Cellular Processes and Signaling; Electron transfer carriers | 0.484 | 0.031 |
| Unclassified; Metabolism; Lipid metabolism | 0.448 | 0.048 |
| Trait Physical Energy | ||
| Human Diseases; Infectious Diseases; Bacterial invasion of epithelial cells | −0.604 | 0.005 |
| Trait Mental Energy | Trait Mental Fatigue | Trait Physical Energy | Trait Physical Fatigue | ||
|---|---|---|---|---|---|
| Folate, food (mcg) | Correlation | 0.465 * | 0.021 | 0.313 | 0.129 |
| Sig. (2-tailed) | 0.039 | 0.931 | 0.178 | 0.588 | |
| Lycopene (mcg) | Correlation | −0.399 | 0.505 | −0.438 | 0.503 |
| Sig. (2-tailed) | 0.081 | 0.023 | 0.053 | 0.024 | |
| Total dark green, red and orange, starchy, and other vegetables; excludes legumes (cup eq.) | Correlation | 0.500 | −0.018 | 0.221 | 0.036 |
| Sig. (2-tailed) | 0.025 | 0.940 | 0.350 | 0.880 | |
| Dark green vegetables (cup eq.) | P Correlation | 0.456 | 0.052 | 0.322 | 0.187 |
| Sig. (2-tailed) | 0.043 | 0.829 | 0.166 | 0.429 | |
| Grains defined as whole grains and which contain the entire grain kernel: bran, germ, and endosperm (oz. eq.) | Correlation | −0.609 | 0.383 | −0.442 | 0.466 |
| Sig. (2-tailed) | 0.004 | 0.095 | 0.051 | 0.038 | |
| Frankfurters, sausages, corned beef, and luncheon meat that are made from beef, pork, or poultry (oz. eq.) | Correlation | −0.790 | 0.538 * | −0.478 | 0.513 |
| Sig. (2-tailed) | <0.0001 | 0.014 | 0.033 | 0.021 |
| Bacteria (All Belong to Firmicutes Phylum) | Folate, Food (mcg) | Lycopene (mcg) | Total Dark Green, Red and Orange, Starchy, and Other Vegetables; Excludes Legumes (Cup Eq.) | Dark Green Vegetables (Cup Eq.) | Grains Defined as Whole Grains and Which Contain the Entire Grain Kernel: Bran, Germ, and Endosperm (Oz. Eq.) | Frankfurters, Sausages, Corned Beef, and Luncheon Meat That Are Made from Beef, Pork, or Poultry (Oz. Eq.) | |
|---|---|---|---|---|---|---|---|
| c__Clostridia;o__Clostridiales;f__Lachnospiraceae;g__Coprococcus;s__catus | Correlation | 0.429 | −0.354 | 0.391 | 0.491 * | 0.209 | −0.075 |
| Sig. (2-tailed) | 0.059 | 0.126 | 0.088 | 0.028 | 0.376 | 0.753 | |
| c__Erysipelotrichi | Correlation | −0.281 | 0.470 | −0.277 | −0.212 | 0.344 | 0.262 |
| Sig. (2-tailed) | 0.230 | 0.037 | 0.238 | 0.370 | 0.137 | 0.264 | |
| c__Erysipelotrichi;o__Erysipelotrichales | Correlation | −0.281 | 0.470 | −0.277 | −0.212 | 0.344 | 0.262 |
| Sig. (2-tailed) | 0.230 | 0.037 | 0.238 | 0.370 | 0.137 | 0.264 | |
| c__Erysipelotrichi;o__Erysipelotrichales;f__Erysipelotrichaceae | Correlation | −0.281 | 0.470 | −0.277 | −0.212 | 0.344 | 0.262 |
| Sig. (2-tailed) | 0.230 | 0.037 | 0.238 | 0.370 | 0.137 | 0.264 | |
| c__Erysipelotrichi;o__Erysipelotrichales;f__Erysipelotrichaceae;g__Holdemania | Correlation | −0.268 | 0.088 | −0.339 | −0.330 | 0.455 | 0.488 |
| Sig. (2-tailed) | 0.254 | 0.713 | 0.143 | 0.155 | 0.044 | 0.029 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boolani, A.; Gallivan, K.M.; Ondrak, K.S.; Christopher, C.J.; Castro, H.F.; Campagna, S.R.; Taylor, C.M.; Luo, M.; Dowd, S.E.; Smith, M.L.; et al. Trait Energy and Fatigue May Be Connected to Gut Bacteria among Young Physically Active Adults: An Exploratory Study. Nutrients 2022, 14, 466. https://doi.org/10.3390/nu14030466
Boolani A, Gallivan KM, Ondrak KS, Christopher CJ, Castro HF, Campagna SR, Taylor CM, Luo M, Dowd SE, Smith ML, et al. Trait Energy and Fatigue May Be Connected to Gut Bacteria among Young Physically Active Adults: An Exploratory Study. Nutrients. 2022; 14(3):466. https://doi.org/10.3390/nu14030466
Chicago/Turabian StyleBoolani, Ali, Karyn M. Gallivan, Kristin S. Ondrak, Courtney J. Christopher, Hector F. Castro, Shawn R. Campagna, Christopher M. Taylor, Meng Luo, Scot E. Dowd, Matthew Lee Smith, and et al. 2022. "Trait Energy and Fatigue May Be Connected to Gut Bacteria among Young Physically Active Adults: An Exploratory Study" Nutrients 14, no. 3: 466. https://doi.org/10.3390/nu14030466
APA StyleBoolani, A., Gallivan, K. M., Ondrak, K. S., Christopher, C. J., Castro, H. F., Campagna, S. R., Taylor, C. M., Luo, M., Dowd, S. E., Smith, M. L., & Byerley, L. O. (2022). Trait Energy and Fatigue May Be Connected to Gut Bacteria among Young Physically Active Adults: An Exploratory Study. Nutrients, 14(3), 466. https://doi.org/10.3390/nu14030466







