Xenogeneic-Free Human Intestinal Organoids for Assessing Intestinal Nutrient Absorption
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Line and Media
2.2. Quantitative Reverse Transcription PCR
2.3. Immunofluorescence Staining
2.4. Alcian Blue Stain
2.5. Barrier Function of the Organoids
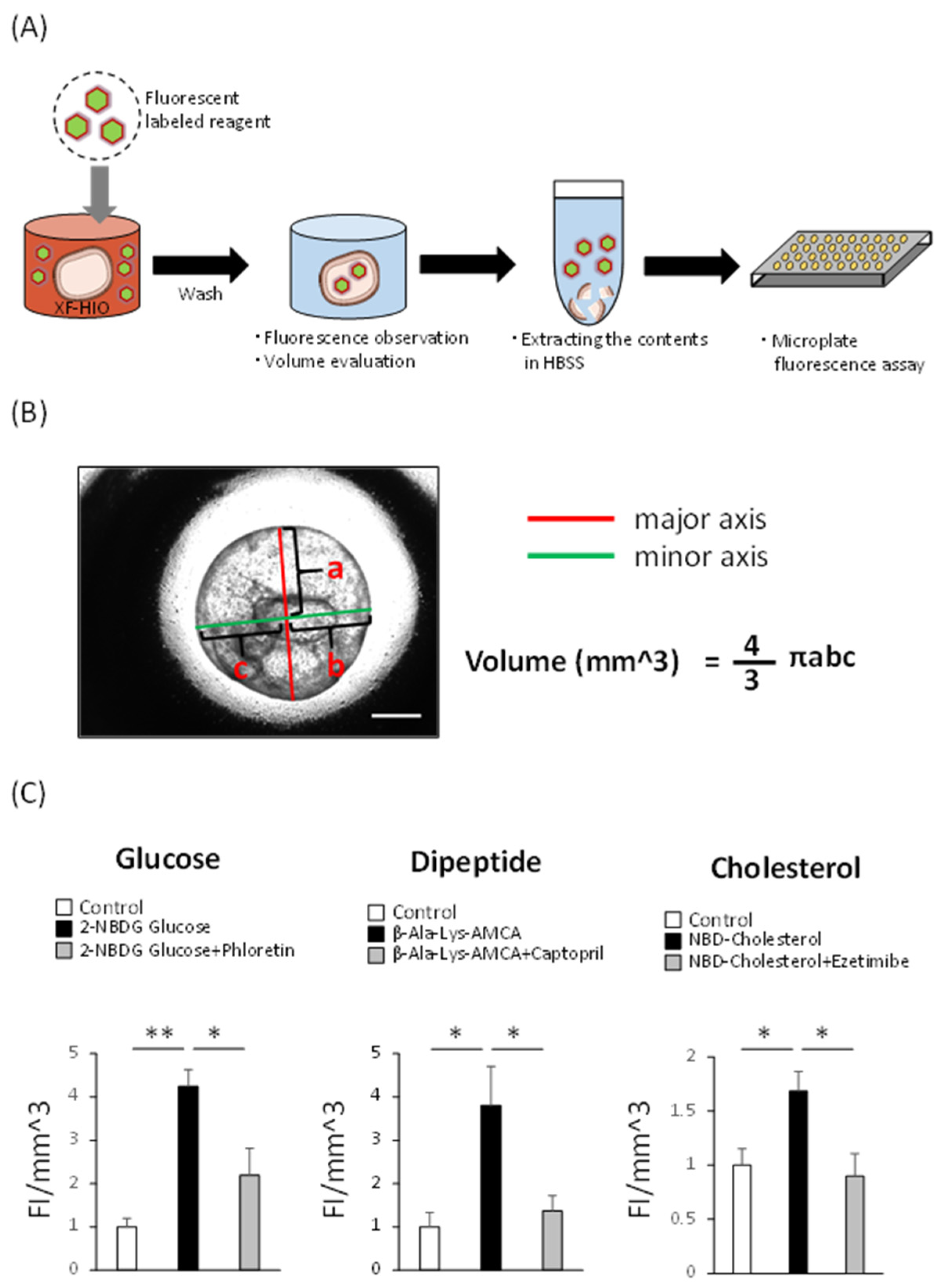
2.6. Fluorescent Labeling Reagent Absorption Assay of the Organoids
2.7. ELISA for Muc2 in the Organoids
2.8. Caco-2 Permeability Assay
2.9. Statistical Analysis
3. Results
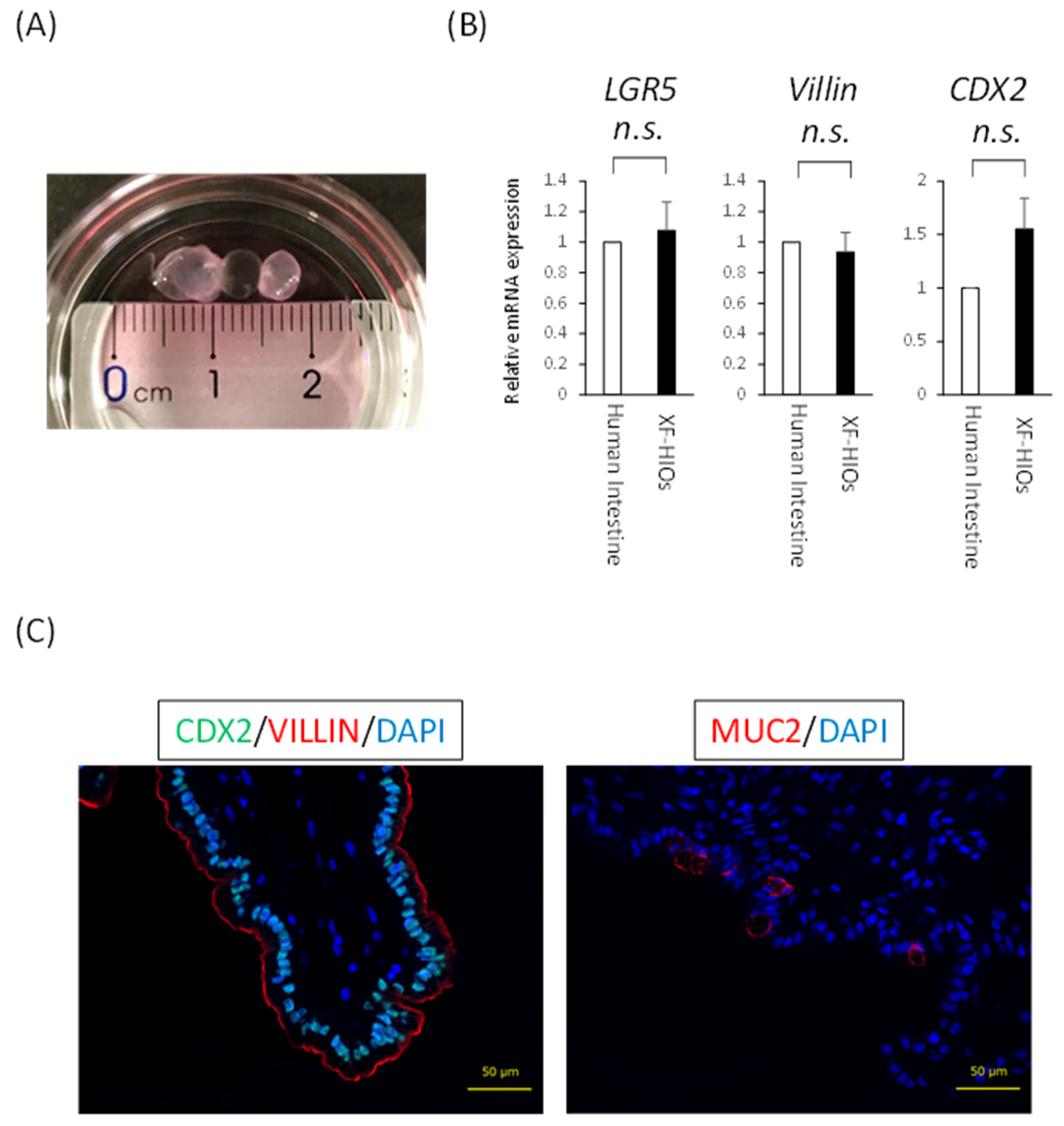
3.1. Structure, Properties and Nutritional Absorption of XF-HIOs
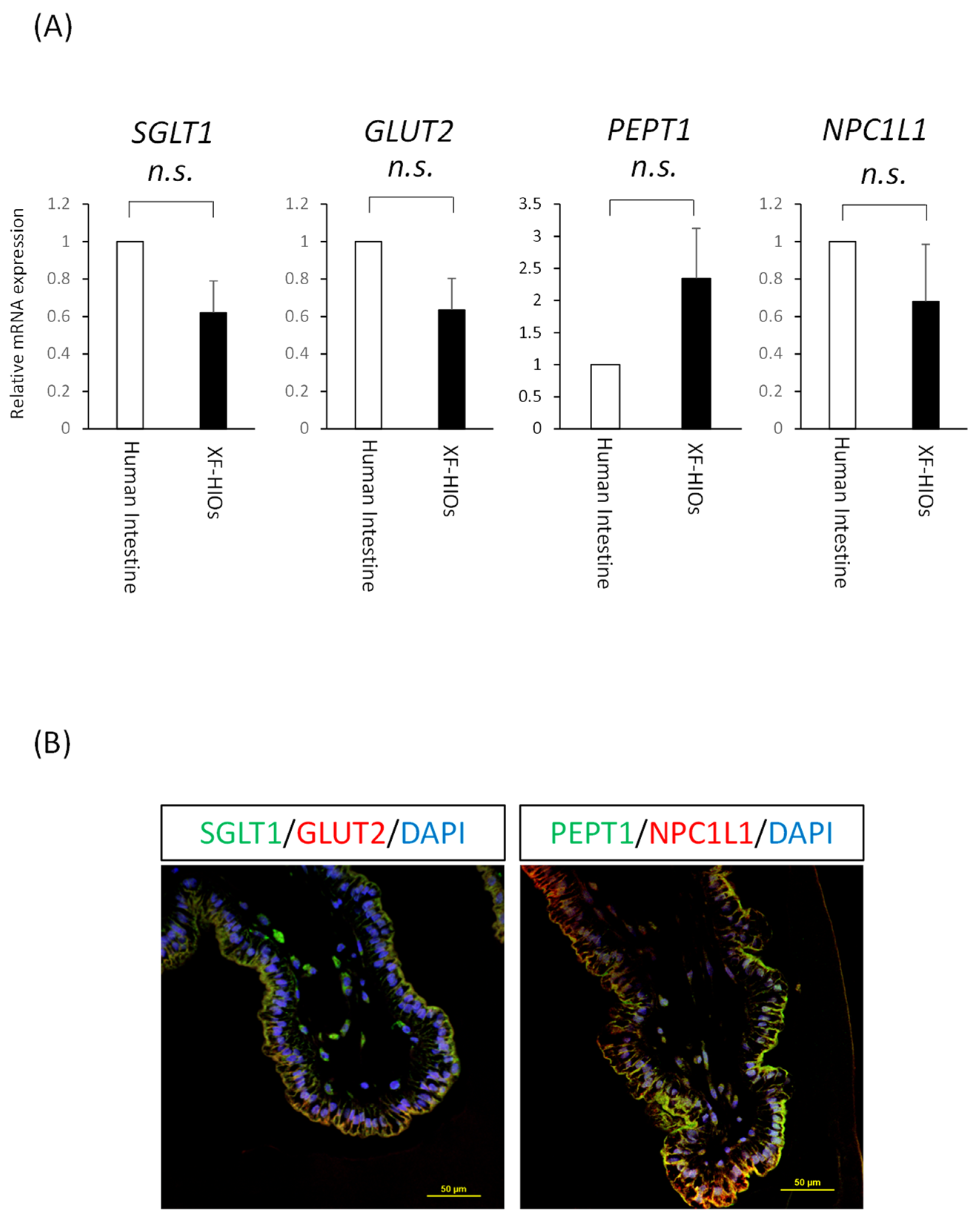
3.2. Nutrition-Related Transporters on Epithelial Cells
3.3. XF-HIOs Absorb Glucose, Dipeptide, and Cholesterol
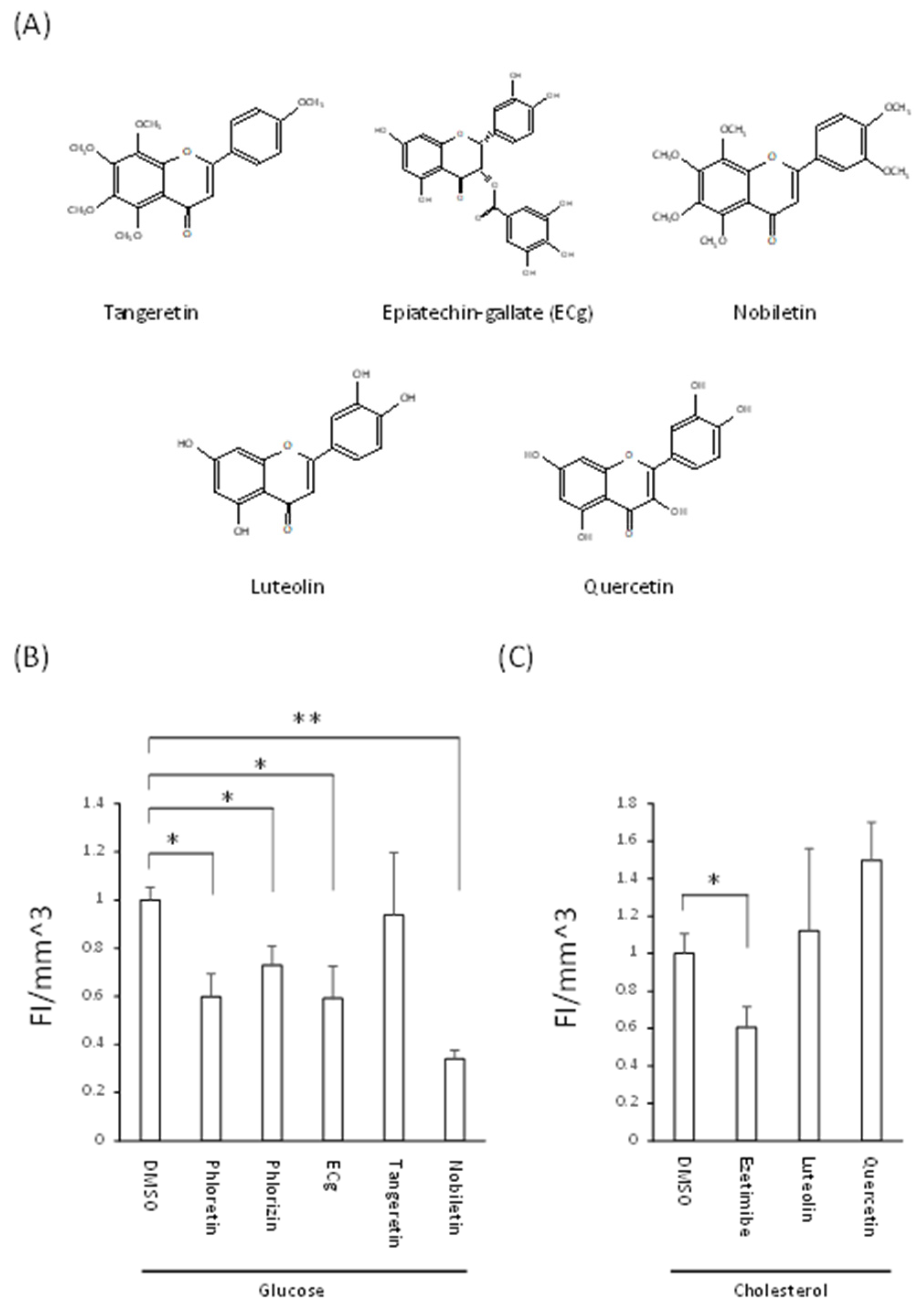
3.4. Decreased Nutrient Absorption Caused by Food Composition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zorn, A.M.; Wells, J.M. Vertebrate endoderm development and organ formation. Annu. Rev. Cell Dev. Biol. 2009, 25, 221–251. [Google Scholar] [CrossRef] [Green Version]
- Mowat, A.M.; Agace, W.W. Regional specialization within the intestinal immune system. Nat. Rev. Immunol. 2014, 14, 667–685. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Kaminsky, L.S. Human extrahepatic cytochromes P450: Function in xenobiotic metabolism and tissue-selective chemical toxicity in the respiratory and gastrointestinal tracts. Annu. Rev. Pharmacol. Toxicol. 2003, 43, 149–173. [Google Scholar] [CrossRef] [PubMed]
- Nezami, B.G.; Srinivasan, S. Enteric Nervous System in the Small Intestine: Pathophysiology and Clinical Implications. Curr. Gastroenterol. Rep. 2010, 12, 358–365. [Google Scholar] [CrossRef] [Green Version]
- Koepsell, H. Glucose transporters in the small intestine in health and disease. Pflug. Arch. 2020, 472, 1207–1248. [Google Scholar] [CrossRef]
- Spanier, B. Transcriptional and functional regulation of the intestinal peptide transporter PEPT1. J. Physiol. 2014, 592, 871–879. [Google Scholar] [CrossRef]
- Bröer, S.; Fairweather, S.J. Amino Acid Transport across the Mammalian Intestine. Compr. Physiol. 2018, 9, 343–373. [Google Scholar] [CrossRef] [PubMed]
- Altmann, S.W.; Davis, H.R.; Zhu, L.-J.; Yao, X.; Hoos, L.M.; Tetzloff, G.; Iyer, S.P.; Maguire, M.; Golovko, A.; Zeng, M.; et al. Niemann-Pick C1 Like 1 protein is critical for intestinal cholesterol absorption. Science 2004, 303, 1201–1204. [Google Scholar] [CrossRef] [Green Version]
- Abuajah, C.I.; Ogbonna, A.C.; Osuji, C.M. Functional components and medicinal properties of food: A review. J. Food Sci. Technol. 2015, 52, 2522–2529. [Google Scholar] [CrossRef] [Green Version]
- Harwood, M.D.; Achour, B.; Neuhoff, S.; Russell, M.R.; Carlson, G.; Warhurst, G.; Amin, R.-H. In Vitro-In Vivo Extrapolation Scaling Factors for Intestinal P-Glycoprotein and Breast Cancer Resistance Protein: Part I: A Cross-Laboratory Comparison of Transporter-Protein Abundances and Relative Expression Factors in Human Intestine and Caco-2 Cells. Drug Metab. Dispos. 2016, 44, 297–307. [Google Scholar] [CrossRef] [Green Version]
- Jochems, P.G.M.; Garssen, J.; van Keulen, A.M.; Masereeuw, R.; Jeurink, P.V. Evaluating Human Intestinal Cell Lines for Studying Dietary Protein Absorption. Nutrients 2018, 10, 322. [Google Scholar] [CrossRef] [Green Version]
- Artegiani, B.; Clevers, H. Use and application of 3D-organoid technology. Hum. Mol. Genet. 2018, 27, R99–R107. [Google Scholar] [CrossRef] [Green Version]
- Spence, J.R.; Mayhew, C.N.; Rankin, S.A.; Kuhar, M.F.; Vallance, J.E.; Tolle, K.; Hoskins, E.E.; Kalinichenko, V.V.; Wells, S.I.; Zorn, A.M.; et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature 2011, 470, 105–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uchida, H.; Machida, M.; Miura, T.; Kawasaki, T.; Okazaki, T.; Sasaki, K.; Sakamoto, S.; Ohuchi, N.; Kasahara, M.; Umezawa, A.; et al. A xenogeneic-free system generating functional human gut organoids from pluripotent stem cells. JCI Insight 2017, 2, e86492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuruta, S.; Uchida, H.; Akutsu, H. Intestinal Organoids Generated from Human Pluripotent Stem Cells. JMA J. 2020, 3, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Okochi, N.; Okazaki, T.; Hattori, H. Encouraging effect of cadherin-mediated cell-cell junctions on transfer printing of micropatterned vascular endothelial cells. Langmuir 2009, 25, 6947–6953. [Google Scholar] [CrossRef]
- Sasaki, K.; Inoue, M.; Machida, M.; Kawasaki, T.; Tsuruta, S.; Uchida, H.; Sakamoto, S.; Kasahara, M.; Umezawa, A.; Akutsu, H. Human Pluripotent Stem Cell-Derived Organoids as a Model of Intestinal Xenobiotic Metabolism. StemJournal 2021, 3, 1–10. [Google Scholar] [CrossRef]
- Nishino, K.; Arai, Y.; Takasawa, K.; Toyoda, M.; Yamazaki-Inoue, M.; Sugawara, T.; Akutsu, H.; Nishimura, K.; Ohtaka, M.; Nakanishi, M.; et al. Epigenetic-scale comparison of human iPSCs generated by retrovirus, Sendai virus or episomal vectors. Regen. Ther. 2018, 9, 71–78. [Google Scholar] [CrossRef]
- Iseoka, H.; Sasai, M.; Miyagawa, S.; Takekita, K.; Date, S.; Ayame, H.; Nishida, A.; Sanami, S.; Hayakawa, T.; Sawa, Y. Rapid and sensitive mycoplasma detection system using image-based deep learning. J. Artif. Organs 2021. [Google Scholar] [CrossRef] [PubMed]
- Onozato, D.; Akagawa, T.; Kida, Y.; Ogawa, I.; Hashita, T.; Iwao, T.; Matsunaga, T. Application of Human Induced Pluripotent Stem Cell-Derived Intestinal Organoids as a Model of Epithelial Damage and Fibrosis in Inflammatory Bowel Disease. Biol. Pharm. Bull. 2020, 43, 1088–1095. [Google Scholar] [CrossRef]
- Chan, L.K.; Leung, P.S. Multifaceted interplay among mediators and regulators of intestinal glucose absorption: Potential impacts on diabetes research and treatment. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E887–E899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howles, P.N. Cholesterol Absorption and Metabolism. Methods Mol. Biol. 2016, 1438, 177–197. [Google Scholar] [CrossRef]
- Satsu, H.; Awara, S.; Unno, T.; Shimizu, M. Suppressive effect of nobiletin and epicatechin gallate on fructose uptake in human intestinal epithelial Caco-2 cells. Biosci. Biotechnol. Biochem. 2018, 82, 636–646. [Google Scholar] [CrossRef] [Green Version]
- Nekohashi, M.; Ogawa, M.; Ogihara, T.; Nakazawa, K.; Kato, H.; Misaka, T.; Abe, K.; Kobayashi, S. Luteolin and Quercetin Affect the Cholesterol Absorption Mediated by Epithelial Cholesterol Transporter Niemann–Pick C1-Like 1 in Caco-2 Cells and Rats. PLoS ONE 2014, 9, e97901. [Google Scholar] [CrossRef] [Green Version]
- Sugimoto, S.; Kobayashi, E.; Fujii, M.; Ohta, Y.; Arai, K.; Matano, M.; Ishikawa, K.; Miyamoto, K.; Toshimitsu, K.; Takahashi, S.; et al. An organoid-based organ-repurposing approach to treat short bowel syndrome. Nature 2021, 592, 99–104. [Google Scholar] [CrossRef]
- Zietek, T.; Giesbertz, P.; Ewers, M.; Reichart, F.; Weinmüller, M.; Urbauer, E.; Haller, D.; Demir, I.E.; Ceyhan, G.O.; Kessler, H.; et al. Organoids to Study Intestinal Nutrient Transport, Drug Uptake and Metabolism—Update to the Human Model and Expansion of Applications. Front. Bioeng. Biotechnol. 2020, 8, 577656. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, R.P.; Choe, J.Y.; Patel, C.R. Intestinal Absorption of Fructose. Annu. Rev. Nutr. 2018, 38, 41–67. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S. The Effect of Polyphenols on Hypercholesterolemia through Inhibiting the Transport and Expression of Niemann-Pick C1-Like 1. Int. J. Mol. Sci. 2019, 20, 4939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williamson, G. The role of polyphenols in modern nutrition. Nutr. Bull. 2017, 42, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Satsu, H.; Shibata, R.; Suzuki, H.; Kimura, S.; Shimizu, M. Inhibitory Effect of Tangeretin and Cardamonin on Human Intestinal SGLT1 Activity In Vitro and Blood Glucose Levels in Mice In Vivo. Nutrients 2021, 13, 3382. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Suzuki, M.; Satsu, H.; Arai, S.; Hara, Y.; Suzuki, K.; Miyamoto, Y.; Shimizu, M. Green tea polyphenols inhibit the sodium-dependent glucose transporter of intestinal epithelial cells by a competitive mechanism. J. Agric. Food Chem. 2000, 48, 5618–5623. [Google Scholar] [CrossRef]
- Shimizu, M.; Kobayashi, Y.; Suzuki, M.; Satsu, H.; Miyamoto, Y. Regulation of intestinal glucose transport by tea catechins. Biofactors 2000, 13, 61–65. [Google Scholar] [CrossRef]
- Satsu, H. Molecular and cellular studies on the absorption, function, and safety of food components in intestinal epithelial cells. Biosci. Biotechnol. Biochem. 2017, 81, 419–425. [Google Scholar] [CrossRef] [Green Version]
- Ni, D.; Ai, Z.; Munoz-Sandoval, D.; Suresh, R.; Ellis, P.R.; Yuqiong, C.; Sharp, P.A.; Butterworth, P.J.; Yu, Z.; Corpe, C.P. Inhibition of the facilitative sugar transporters (GLUTs) by tea extracts and catechins. FASEB J. 2020, 34, 9995–10010. [Google Scholar] [CrossRef]
- Wanner, C.; Inzucchi, S.E.; Lachin, J.M.; Fitchett, D.; von Eynatten, M.; Mattheus, M.; Johansen, O.E.; Woerle, H.J.; Broedl, U.C.; Zinman, B.; et al. Empagliflozin and Progression of Kidney Disease in Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Neal, B.; Perkovic, V.; Mahaffey, K.W.; de Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Law, G.; Desai, M.; Matthews, D.R.; et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 644–657. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, M.; Yamanashi, Y.; Takada, T.; Abe, K.; Kobayashi, S. Effect of luteolin on the expression of intestinal cholesterol transporters. J. Funct. Foods 2017, 36, 274–279. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inoue, M.; Tanaka, Y.; Matsushita, S.; Shimozaki, Y.; Ayame, H.; Akutsu, H. Xenogeneic-Free Human Intestinal Organoids for Assessing Intestinal Nutrient Absorption. Nutrients 2022, 14, 438. https://doi.org/10.3390/nu14030438
Inoue M, Tanaka Y, Matsushita S, Shimozaki Y, Ayame H, Akutsu H. Xenogeneic-Free Human Intestinal Organoids for Assessing Intestinal Nutrient Absorption. Nutrients. 2022; 14(3):438. https://doi.org/10.3390/nu14030438
Chicago/Turabian StyleInoue, Makoto, Yuichi Tanaka, Sakiko Matsushita, Yuri Shimozaki, Hirohito Ayame, and Hidenori Akutsu. 2022. "Xenogeneic-Free Human Intestinal Organoids for Assessing Intestinal Nutrient Absorption" Nutrients 14, no. 3: 438. https://doi.org/10.3390/nu14030438
APA StyleInoue, M., Tanaka, Y., Matsushita, S., Shimozaki, Y., Ayame, H., & Akutsu, H. (2022). Xenogeneic-Free Human Intestinal Organoids for Assessing Intestinal Nutrient Absorption. Nutrients, 14(3), 438. https://doi.org/10.3390/nu14030438




