Effects of Fish Oil Supplementation on Oxidative Stress Biomarkers and Liver Damage in Hypercholesterolemic Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Aspects
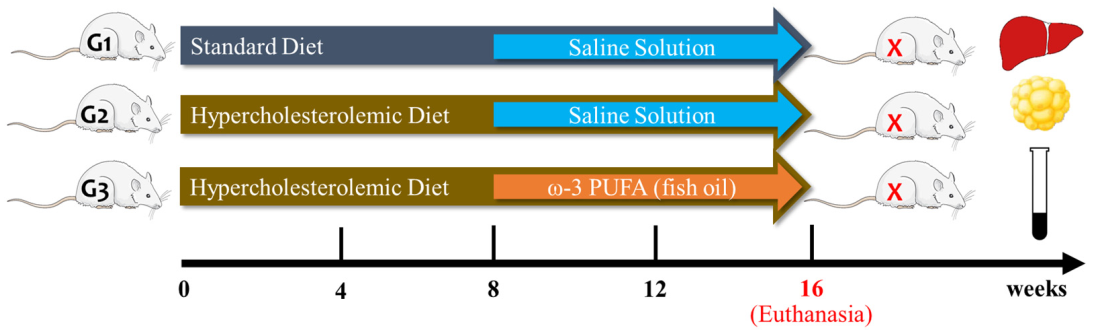
2.2. Experimental Protocol
2.3. Fish Oil Gel Capsules and Fatty Acids Composition
2.4. Determination of the Centesimal Chemical Composition of the Feed
2.5. Euthanasia, Organ Harvesting and Weighing
2.6. Determination of Glycemia and Lipid Profile
2.7. Determination of Albumin, Total Proteins, and Enzymes of Hepatic Cytolysis
2.8. Determination of Plasma and Hepatic Malondialdehyde (MDA) Concentrations
2.9. Determination of Plasma and Hepatic Myeloperoxidase (MPO) Activity
2.10. Determination of Erythrocyte and Hepatic Superoxide Dismutase (SOD) Activity
2.11. Determination of Hepatic Catalase (CAT) Activity
2.12. Determination of Hepatic Concentrations of Nonprotein Sulfhydryl Groups (SH-NP)
2.13. Histopathological Analysis of Liver Tissue
2.14. Hepatic Triglyceride and Total Cholesterol Content
2.15. Statistical Analysis
3. Results
3.1. Effect of Fish Oil Supplementation on Lipid Profile and Glycemia in Animals with Hypercholesterolemia
3.2. Effect of Fish Oil Supplementation on Biochemical Markers of Liver Function and on Macroscopic and Histological Aspects of the Liver of Hypercholesterolemic Animals
3.3. Effect of Fish Oil Supplementation on Biomarkers of Lipid Peroxidation in Animals with Hypercholesterolemia
3.4. Effect of Fish Oil Supplementation on Markers of Antioxidant Activity in Animals with Hypercholesterolemia
3.5. Effect of Fish Oil Supplementation on Hepatic Cholesterol and Triglyceride Concentrations in Hypercholesterolemic Animals
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Faludi, A.A.; Izar, M.; Saraiva, J.; Chacra, A.; Bianco, H.T.; Afiune, A.; Neto Bertolami, A.; Pereira, A.C.; Lottenberg, A.M.; Sposito, A.C.; et al. Atualização da Diretriz Brasileira de Dislipidemias e Prevenção da Aterosclerose—2017. Arq. Bras. Cardiol. 2017, 109, 1–76. [Google Scholar] [CrossRef]
- Monserrat-Mesquida, M.; Quetglas-Llabrés, M.; Capó, X.; Bouzas, C.; Mateos, D.; Pons, A.; Tur, J.A.; Sureda, A. Metabolic Syndrome Is Associated with Oxidative Stress and Proinflammatory State. Antioxidants 2020, 9, 236. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, A.M.; Francisco, P.; Motta, K.; Chagas, T.R.; Dos Santos, C.; Rafacho, A.; Nunes, E.A. Fish oil supplementation attenuates changes in plasma lipids caused by dexamethasone treatment in rats. Applied physiology, nutrition, and metabolism. Appl. Physiol. Nutr. Metab. 2015, 41, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Zock, P.L.; Blom, W.A.; Nettleton, J.A.; Hornstra, G. Progressing Insights into the Role of Dietary Fats in the Prevention of Cardiovascular Disease. Curr. Cardiol. Rep. 2016, 18, 111. [Google Scholar] [CrossRef] [PubMed]
- Nassir, F.; Rector, R.S.; Hammoud, G.M.; Ibdah, J.A. Pathogenesis and Prevention of Hepatic Steatosis. Gastroenterol. Hepatol. 2015, 11, 167–175. [Google Scholar]
- Younossi, Z.M. Non-alcoholic fatty liver disease—A global public health perspective. J. Hepatol. 2019, 70, 531–544. [Google Scholar] [CrossRef]
- Seko, Y.; Yamaguchi, K.; Itoh, Y. The genetic backgrounds in nonalcoholic fatty liver disease. Clin. J. Gastroenterol. 2018, 11, 97–102. [Google Scholar] [CrossRef]
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef]
- Manne, V.; Handa, P.; Kowdley, K.V. Pathophysiology of Nonalcoholic Fatty Liver Disease/Nonalcoholic Steatohepatitis. Int. J. Mol. Sci. 2016, 17, 2082. [Google Scholar] [CrossRef]
- Smith, S.C.; Allen, J.; Blair, S.N.; Bonow, R.O.; Brass, L.M.; Fonarow, G.C.; Grundy, S.M.; Hiratzka, L.; Jones, D.; Krumholz, H.M.; et al. AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update: Endorsed by the National Heart, Lung, and Blood Institute. Circulation 2006, 113, 2363–2372. [Google Scholar] [CrossRef]
- Santos, R.D.; Gagliardi, A.C.; Xavier, H.T.; Magnoni, C.D.; Cassani, R.; Lottenberg, A.M.; Sociedade Brasileira de Cardiologia. I Diretriz sobre o consumo de gorduras e saúde cardiovascular [First guidelines on fat consumption and cardiovascular health]. Arq. Bras. Cardiol. 2013, 100 (Suppl. 3), 1–40. [Google Scholar] [CrossRef] [PubMed]
- De Backer, G.; Ambrosioni, E.; Borch-Johnsen, K.; Brotons, C.; Cifkova, R.; Dallongeville, J.; Ebrahim, S.; Faergeman, O.; Graham, I.; Mancia, G.; et al. European guidelines on cardiovascular disease prevention in clinical practice: Third Joint Task Force of European and Other Societies on Cardiovascular Disease Prevention in Clinical Practice. Eur. Heart J. 2003, 24, 1601–1610. [Google Scholar] [CrossRef]
- Innes, J.K.; Calder, P.C. The Differential Effects of Eicosapentaenoic Acid and Docosahexaenoic Acid on Cardiometabolic Risk Factors: A Systematic Review. Int. J. Mol. Sci. 2018, 19, 532. [Google Scholar] [CrossRef] [PubMed]
- Cholewski, M.; Tomczykowa, M.; Tomczyk, M. A Comprehensive Review of Chemistry, Sources and Bioavailability of Omega-3 Fatty Acids. Nutrients 2018, 10, 1662. [Google Scholar] [CrossRef] [PubMed]
- Jardim, T.V.; Lima Sousa, A.L.; Povoa, T.R.; Barroso, W.S.; Chinem, B.; Veiga Jardim, P.C. Comparação entre Fatores de Risco Cardiovascular em Diferentes Áreas da Saúde num Intervalo de Vinte Anos. Arq. Bras. Cardiol. 2014, 103, 493–501. [Google Scholar] [PubMed]
- Précoma, D.B.; Oliveira, G.M.M.D.; Simão, A.F.; Dutra, O.P.; Coelho, O.R.; Izar, M.C.D.O.; Póvoa, R.M.D.S.; Giuliano, I.D.C.B.; Alencar Filho, A.C.D.; Machado, C.A.; et al. Vitaminas e Ácidos Graxos Ômega-3. Atualização da Diretriz de Prevenção Cardiovascular da Sociedade Brasileira de Cardiologia—2019. Arq. Bras. Cardiol. 2019, 113, 787–891. [Google Scholar] [CrossRef]
- Tadic, M.; Sala, C.; Grassi, G.; Mancia, G.; Taddei, S.; Rottbauer, W.; Cuspidi, C. Omega-3 Fatty Acids and Coronary Artery Disease: More Questions Than Answers. J. Clin. Med. 2021, 10, 2495. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Ann. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef]
- Ratziu, V.; Ghabril, M.; Romero-Gomez, M.; Svegliati-Baroni, G. Recommendations for Management and Treatment of Nonalcoholic Steatohepatitis. Transplantation 2019, 103, 28–38. [Google Scholar] [CrossRef]
- Marchisello, S.; Di Pino, A.; Scicali, R.; Urbano, F.; Piro, S.; Purrello, F.; Rabuazzo, A.M. Pathophysiological, Molecular and Therapeutic Issues of Nonalcoholic Fatty Liver Disease: An Overview. Int. J. Mol. Sci. 2019, 20, 1948. [Google Scholar] [CrossRef] [PubMed]
- Baracho, N.C.D.V.; Nunes, L.A.S.; de Paula e Silva, K.T.; Marques, T.F.; Dos Santos, A.L.R.; Marcelino, A.R. Desenvolvimento de um Modelo Experimental de Dislipidemia de Baixo Custo/Development of low Cost Dislipidemia Experimental Model. Desenvolvimento de um modelo experimental de dislipidemia de baixo custo. Rev. Cienc. Saude 2014, 4, 21–22. [Google Scholar] [CrossRef][Green Version]
- Krauss, R.M.; Eckel, R.H.; Howard, B.; Appel, L.J.; Daniels, S.R.; Deckelbaum, R.J.; Erdman, J.W., Jr.; Kris-Etherton, P.; Goldberg, I.J.; Kotchen, T.A.; et al. AHA Dietary Guidelines: Revision 2000: A statement for healthcare professionals from the Nutrition Committee of the American Heart Association. Circulation 2000, 102, 2284–2299. [Google Scholar] [CrossRef] [PubMed]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2007, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Nevigato, T.; Masci, M.; Caproni, R. Quality of Fish-Oil-Based Dietary Supplements Available on the Italian Market: A Preliminary Study. Molecules 2021, 26, 5015. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Pyles, L.A.; Stejskal, E.; Einzing, S. Spectrophotometric measurement of plasma 2 thiobarbituric acid-reactive substances in the presence of hemoglobin and bilirubin interfernce. Proc. Soc. Exp. Biol. Med. 1993, 202, 407–419. [Google Scholar] [CrossRef]
- Bradley, P.P.; Priebat, D.A.; Christensen, R.D.; Rothstein, G. Measurement of cutaneous inflammation: Estimation of neutrophil content with an enzyme marker. J. Investig. Dermatol. 1982, 78, 206–209. [Google Scholar] [CrossRef]
- Das, K.; Samanta, L.; Chainy, G.B.D. A modified spectrophotometric assay of superoxide dismutase using nitrite formation by superoxide radicals. IJBB 2000, 37, 201–204. [Google Scholar]
- Beutler, E. Metabolismo das Células Vermelhas: Um Manual de Métodos Bioquímicos; Grune & Stratton: New York, NY, USA, 1975. [Google Scholar]
- Habeeb, A.F.S.A. [37] Reaction of protein sulfhydryl groups with Ellman’s reagent. Methods Enzymol. 1972, 25, 457–464. [Google Scholar] [CrossRef]
- Trinh, K.Y.; O’Doherty, R.M.; Anderson, P.; Lange, A.J.; Newgard, C.B. Perturbation of fuel homeostasis caused by overexpression of the glucose-6-phosphatase catalytic subunit in liver of normal rats. J. Biol. Chem. 1998, 273, 31615–31620. [Google Scholar] [CrossRef]
- Godea Lupei, S.; Ciubotariu, D.; Danciu, M.; Lupușoru, R.V.; Ghiciuc, C.M.; Cernescu, I.; Gheţu, N.; Lupei, M.; Lupușoru, C.E. Improvement in serum lipids and liver morphology after fish oil supplementation of the diet is more evident under regular feeding conditions than under high-fat or mixed diets in rats. Lipids Health Dis. 2020, 19, 162. [Google Scholar] [CrossRef] [PubMed]
- Bays, H.E.; Tighe, A.P.; Sadovsky, R.; Davidson, M.H. Prescription omega-3 fatty acids and their lipid effects: Physiologic mechanisms of action and clinical implications. Expert. Rev. Cardiovasc. Ther. 2008, 6, 391–409. [Google Scholar] [CrossRef] [PubMed]
- Haimeur, A.; Meskini, N.; Mimouni, V.; Ulmann, L.; Messaouri, H.; Pineau-Vincent, F.; Abouakil, N.; Tremblin, G. A comparative study on the effect of argan oil versus fish oil on risk factors for cardio-vascular disease in high-fat-fed rats. Nutrition 2019, 57, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Wang, H.; Tian, Y.; Li, Q.; He, L.; Li, N.; Liu, Z. Fish oil alleviated high-fat diet-induced non-alcoholic fatty liver disease via regulating hepatic lipids metabolism and metaflammation: A transcriptomic study. Lipids Health Dis. 2016, 15, 20. [Google Scholar] [CrossRef] [PubMed]
- Papatheodoridi, M.; Cholongitas, E. Diagnosis of Non-alcoholic Fatty Liver Disease (NAFLD): Current Concepts. CPD 2019, 24, 4574–4586. [Google Scholar] [CrossRef]
- Khadge, S.; Sharp, J.G.; Thiele, G.M.; McGuire, T.R.; Klassen, L.W.; Duryee, M.J.; Britton, H.C.; Dafferner, A.J.; Beck, J.; Black, P.N.; et al. Dietary omega-3 and omega-6 polyunsaturated fatty acids modulate hepatic pathology. J. Nutr. Biochem. 2018, 52, 92–102. [Google Scholar] [CrossRef]
- Botros, M.; Sikaris, K.A. The de ritis ratio: The test of time. Clin. Biochem Rev. 2013, 34, 117–130. [Google Scholar]
- Tsikas, D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef]
- Heshmati, J.; Morvaridzadeh, M.; Maroufizadeh, S.; Akbari, A.; Yavari, M.; Amirinejad, A.; Maleki-Hajiagha, A.; Sepidarkish, M. Omega-3 fatty acids supplementation and oxidative stress parameters: A systematic review and meta-analysis of clinical trials. Pharmacol. Res. 2019, 149, 104462. [Google Scholar] [CrossRef]
- Stocker, R.; Keaney, J.F. Role of Oxidative Modifications in Atherosclerosis. Physiol. Rev. 2004, 84, 1381–1478. [Google Scholar] [CrossRef]
- Drouin, G.; Catheline, D.; Guillocheau, E.; Gueret, P.; Baudry, C.; Le Ruyet, P.; Rioux, V.; Legrand, P. Comparative effects of dietary n-3 docosapentaenoic acid (DPA), DHA and EPA on plasma lipid parameters, oxidative status and fatty acid tissue composition. J. Nutr. Biochem. 2019, 63, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Richard, D.; Kefi, K.; Barbe, U.; Bausero, P.; Visioli, F. Polyunsaturated fatty acids as antioxidants. Pharmacol. Res. 2008, 57, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.H.; Lim, S.J.; Ryu, Y.M.; Park, H.R.; Suh, H.J.; Han, S.H. Absorption rate of krill oil and fish oil in blood and brain of rats. Lipids Health Dis. 2018, 17, 162. [Google Scholar] [CrossRef] [PubMed]
- Martorell, M.; Capó, X.; Sureda, A.; Batle, J.M.; Llompart, I.; Argelich, E.; Tur, J.A.; Pons, A. Effect of DHA on plasma fatty acid availability and oxidative stress during training season and football exercise. Food Funct. 2014, 5, 1920–1931. [Google Scholar] [CrossRef] [PubMed]








| Components | Standard Feed Mean ± SD | HC diet Mean ± SD | * p Value |
|---|---|---|---|
| Carbohydrates (%) | 40.64 ± 0.83 | 38.53 ± 0.35 | 0.016 |
| Proteins (%) | 39.53 ± 0.97 | 28.16 ± 0.01 | 0.002 |
| Lipids (%) | 2.12 ± 0.08 | 16.08 ± 0.06 | 0.000 |
| Humidity (%) | 8.46 ± 0.16 | 9.65 ± 0.21 | 0.002 |
| Ash (%) | 9.24 ± 0.29 | 7.55 ± 0.08 | 0.001 |
| VET (kcal) | 339.79 ± 7.97 | 411.59 ± 0.88 | 0.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima Rocha, J.É.; Mendes Furtado, M.; Mello Neto, R.S.; da Silva Mendes, A.V.; Brito, A.K.d.S.; Sena de Almeida, J.O.C.; Rodrigues Queiroz, E.I.; de Sousa França, J.V.; Silva Primo, M.G.; Cunha Sales, A.L.d.C.; et al. Effects of Fish Oil Supplementation on Oxidative Stress Biomarkers and Liver Damage in Hypercholesterolemic Rats. Nutrients 2022, 14, 426. https://doi.org/10.3390/nu14030426
Lima Rocha JÉ, Mendes Furtado M, Mello Neto RS, da Silva Mendes AV, Brito AKdS, Sena de Almeida JOC, Rodrigues Queiroz EI, de Sousa França JV, Silva Primo MG, Cunha Sales ALdC, et al. Effects of Fish Oil Supplementation on Oxidative Stress Biomarkers and Liver Damage in Hypercholesterolemic Rats. Nutrients. 2022; 14(3):426. https://doi.org/10.3390/nu14030426
Chicago/Turabian StyleLima Rocha, Joana Érica, Mariely Mendes Furtado, Renato Sampaio Mello Neto, Ana Victória da Silva Mendes, Ana Karolinne da Silva Brito, José Otávio Carvalho Sena de Almeida, Emerson Iuri Rodrigues Queiroz, José Vinícius de Sousa França, Maísa Guimarães Silva Primo, Ana Lina de Carvalho Cunha Sales, and et al. 2022. "Effects of Fish Oil Supplementation on Oxidative Stress Biomarkers and Liver Damage in Hypercholesterolemic Rats" Nutrients 14, no. 3: 426. https://doi.org/10.3390/nu14030426
APA StyleLima Rocha, J. É., Mendes Furtado, M., Mello Neto, R. S., da Silva Mendes, A. V., Brito, A. K. d. S., Sena de Almeida, J. O. C., Rodrigues Queiroz, E. I., de Sousa França, J. V., Silva Primo, M. G., Cunha Sales, A. L. d. C., Gomes Vasconcelos, A., Felix Cabral, W., Souza Kückelhaus, S. A., de Souza de Almeida Leite, J. R., Fortes Lustosa, A. K. M., Lucarini, M., Durazzo, A., Arcanjo, D. D. R., & Martins, M. d. C. d. C. e. (2022). Effects of Fish Oil Supplementation on Oxidative Stress Biomarkers and Liver Damage in Hypercholesterolemic Rats. Nutrients, 14(3), 426. https://doi.org/10.3390/nu14030426











