Sex-Specific Effects of Nutritional Supplements for Infants Born Early or Small: An Individual Participant Data Meta-Analysis (ESSENCE IPD-MA) I—Cognitive Function and Metabolic Risk
Abstract
1. Introduction
2. Methods
2.1. Search Strategies
2.2. Criteria for Inclusion and Exclusion
2.3. Quality Assessment
2.4. Data Synthesis and Statistical Analysis
2.5. Planned Subgroup and Sensitivity Analyses
3. Results
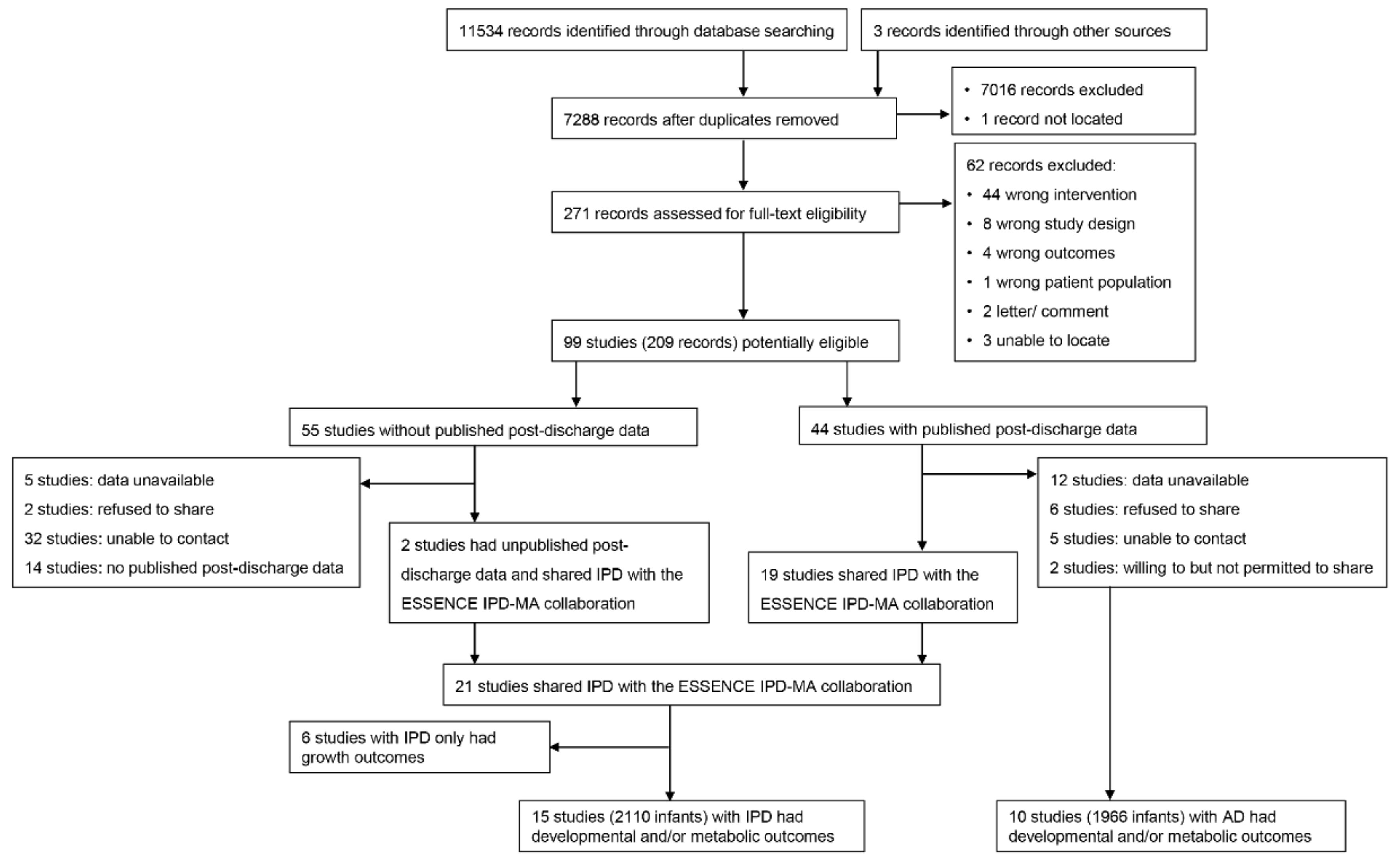
3.1. Search Results
3.2. Quality of the Included Studies
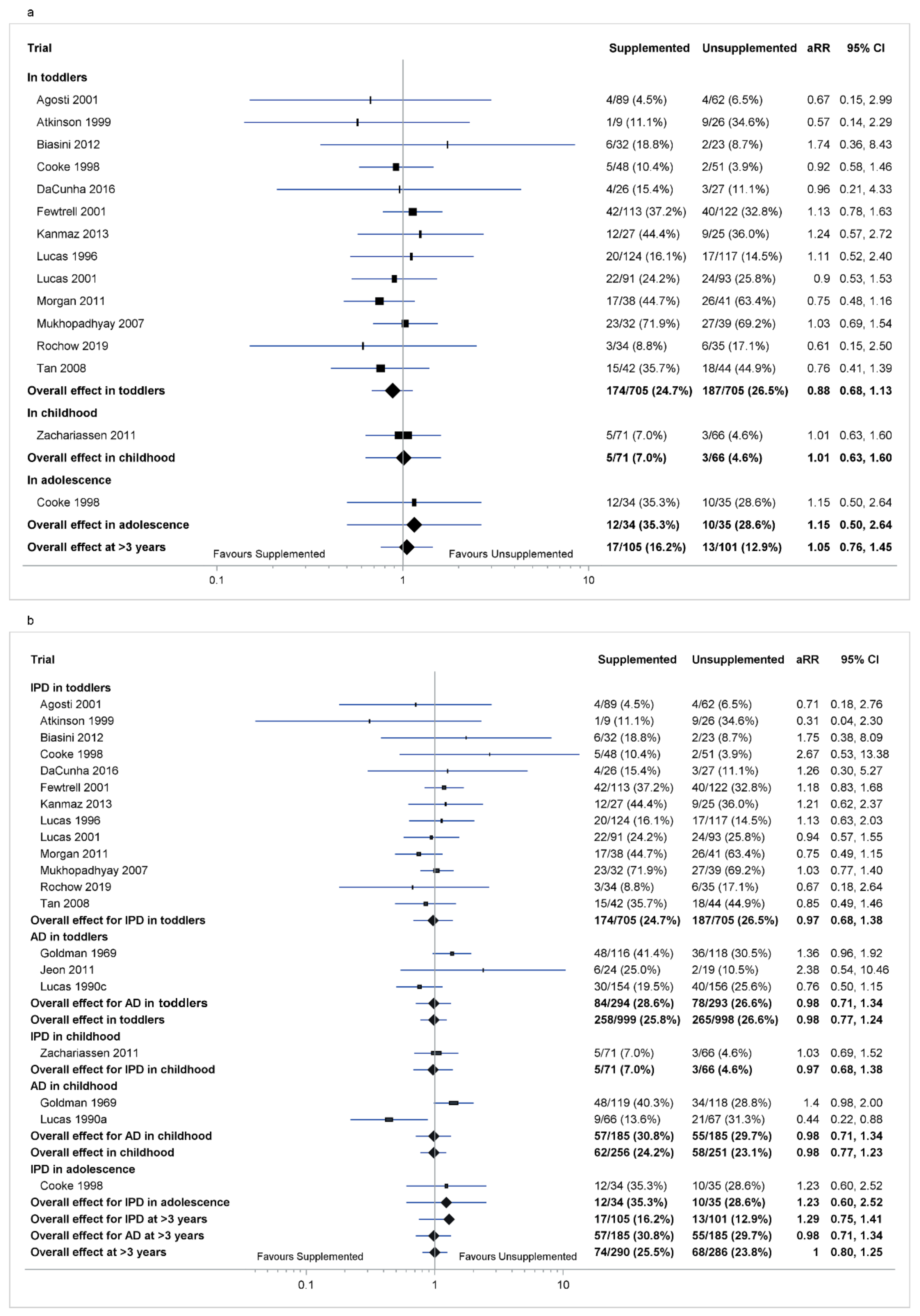
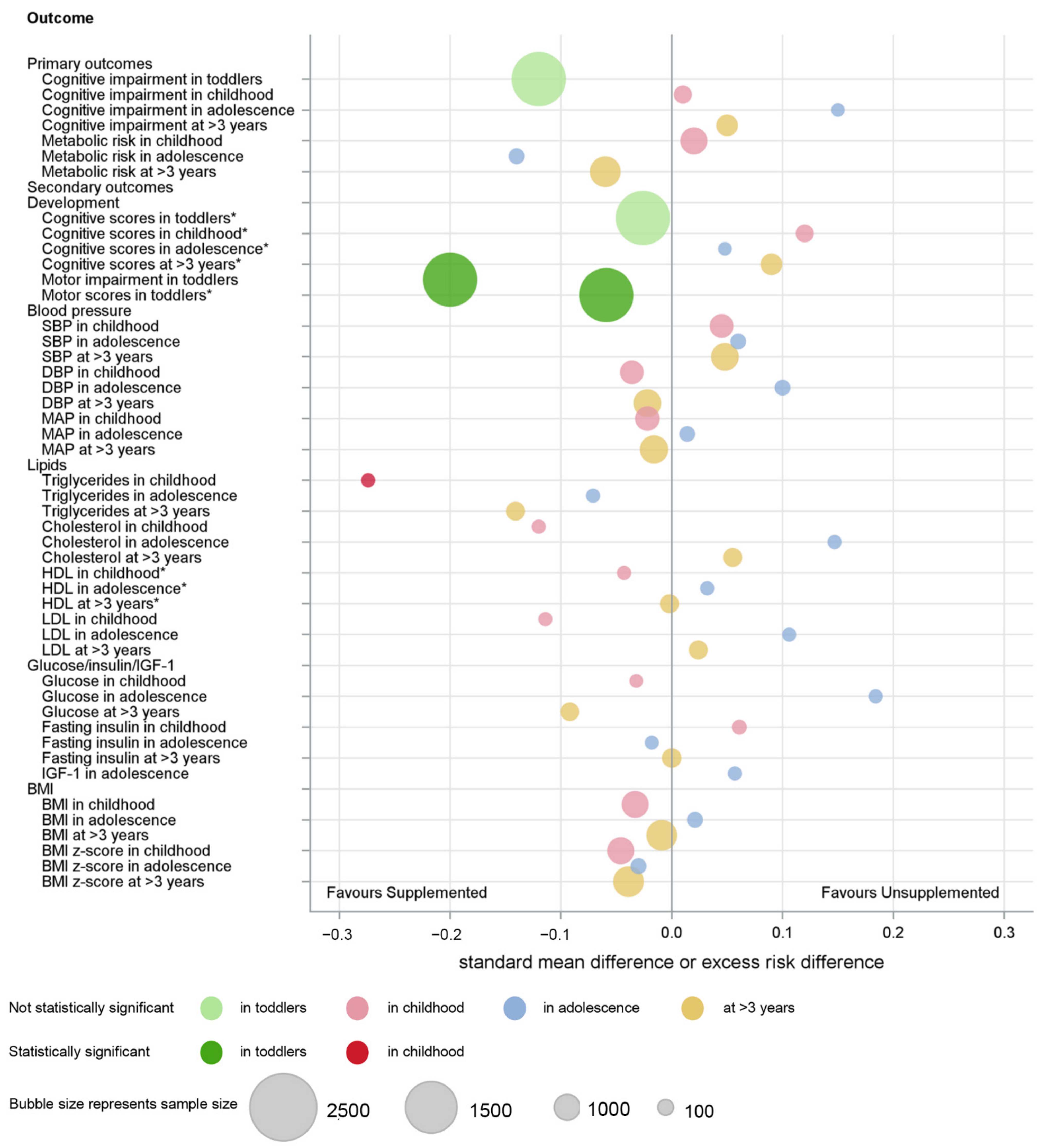
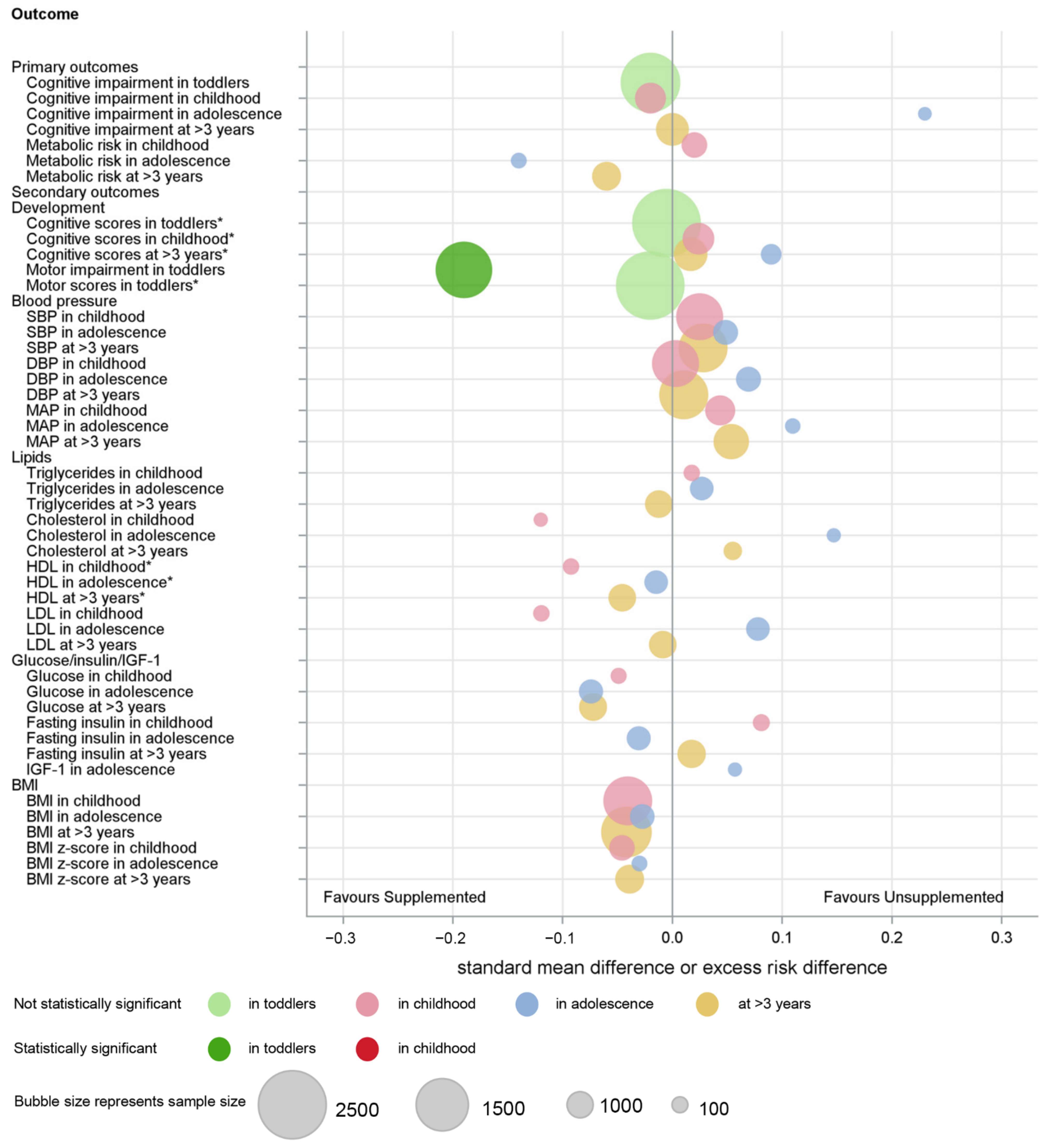
3.3. Co-Primary Outcome: Cognitive Impairment
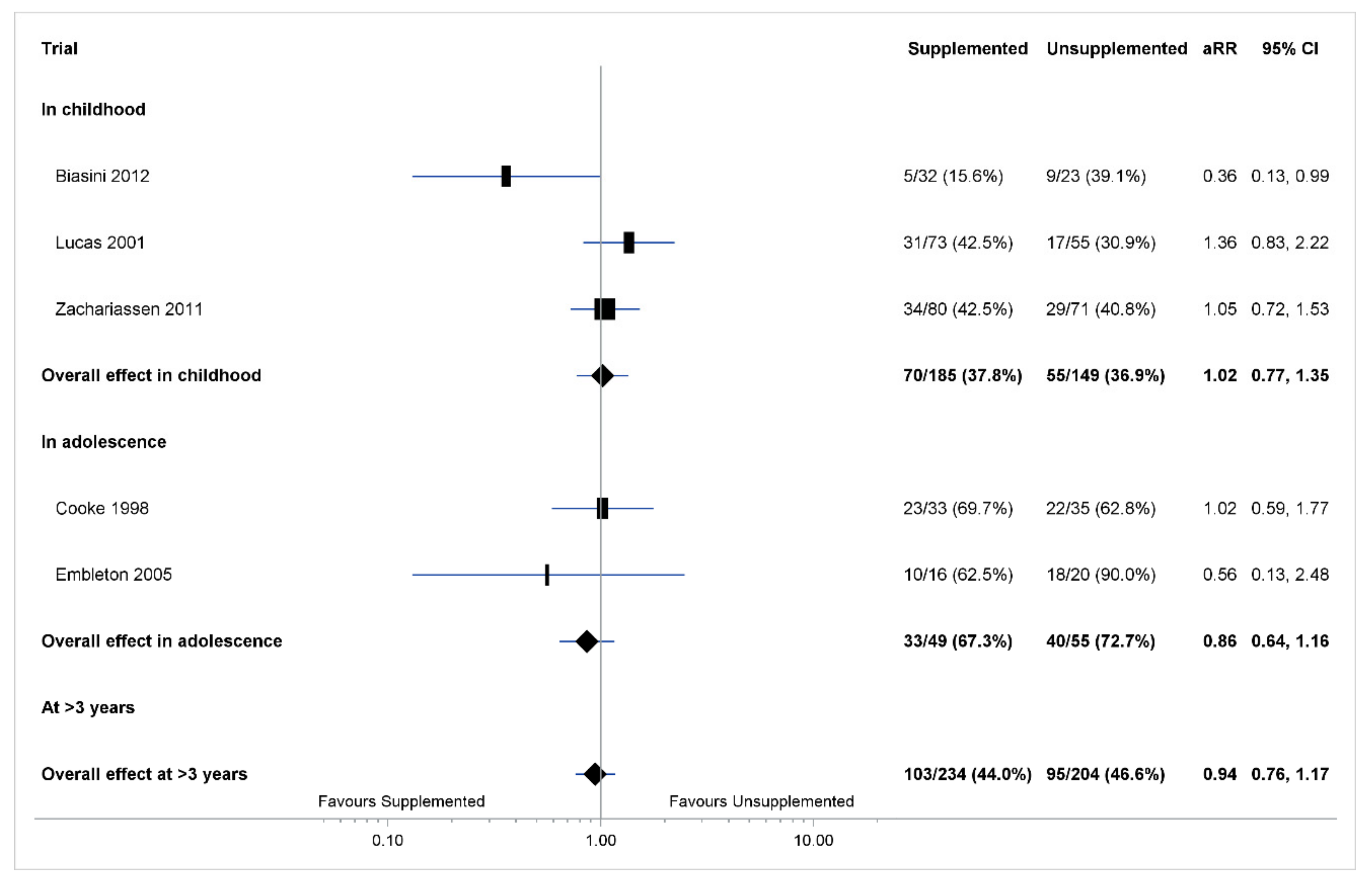
3.4. Co-Primary Outcome: Any Metabolic Risk
3.5. Secondary Developmental Outcomes
3.6. Secondary Metabolic Outcomes
3.7. Subgroup Analyses
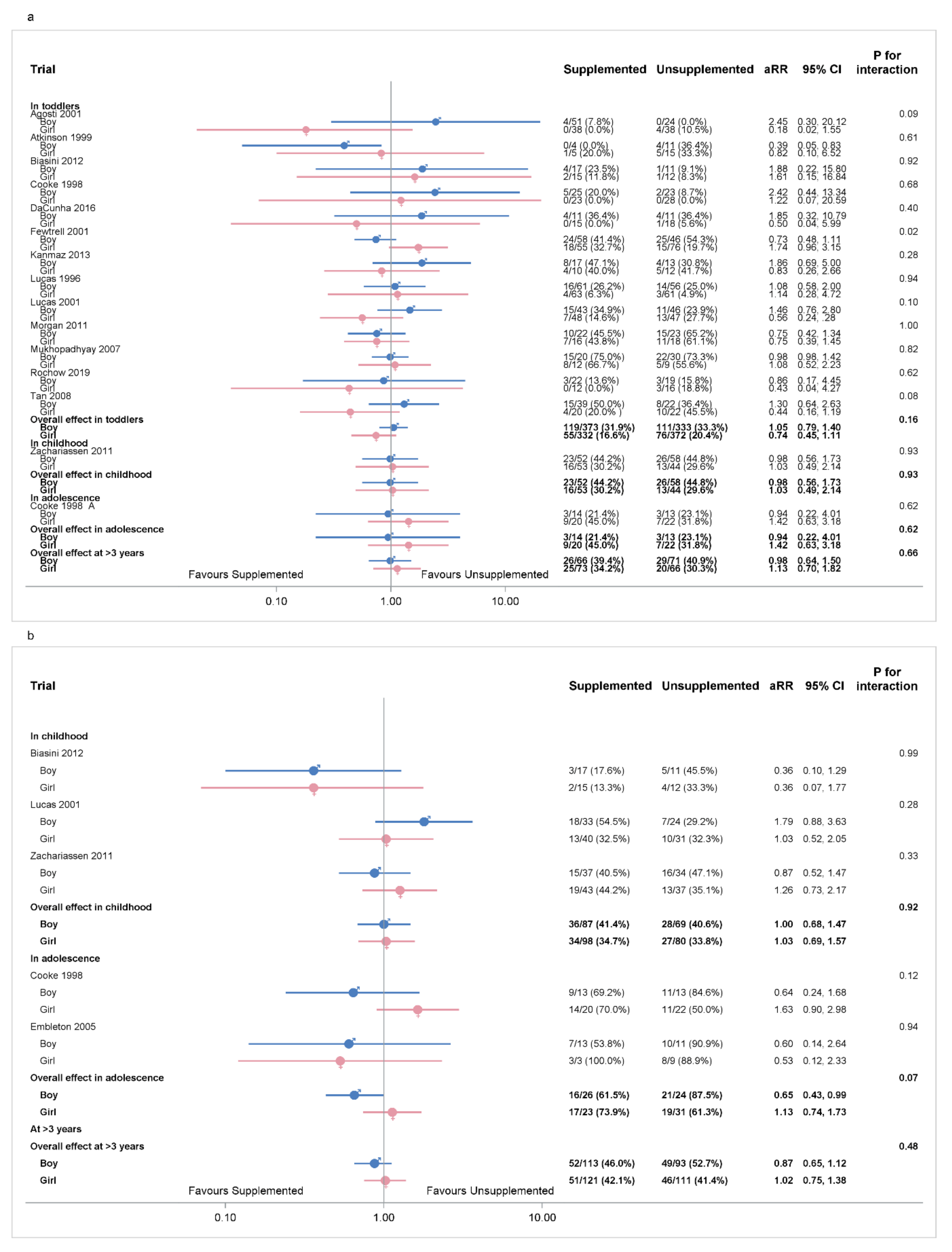
3.7.1. Sex of Infant: Primary Outcomes
3.7.2. Sex of Infant: Secondary Outcomes
3.7.3. Size for Gestation of the Infant
3.7.4. Size of Infant at Birth
3.7.5. Gestational Age of Infant at Birth
3.7.6. Timing of Supplement
3.7.7. Type of Supplement
3.7.8. Primary Feed
3.7.9. Different Trial Timing
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Embleton, N.E.; Pang, N.; Cooke, R.J. Postnatal malnutrition and growth retardation: An inevitable consequence of current recommendations in preterm infants? Pediatrics 2001, 107, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Hay, W.W., Jr. Nutritional requirements of extremely low birthweight infants. Acta Paediatr. Suppl. 1994, 402, 94–99. [Google Scholar] [PubMed]
- Cooke, R.W. Conventional birth weight standards obscure fetal growth restriction in preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 2007, 92, F189–F192. [Google Scholar] [CrossRef] [PubMed]
- Luu, T.M.; Katz, S.L.; Leeson, P.; Thebaud, B.; Nuyt, A.M. Preterm birth: Risk factor for early-onset chronic diseases. CMAJ Can. Med. Assoc. J. 2016, 188, 736–746. [Google Scholar] [CrossRef] [PubMed]
- Lucas, A.; Fewtrell, M.S.; Morley, R.; Lucas, P.J.; Baker, B.A.; Lister, G.; Bishop, N.J. Randomized outcome trial of human milk fortification and developmental outcome in preterm infants. Am. J. Clin. Nutr. 1996, 64, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Lucas, A.; Morley, R.; Cole, T.J. Randomised trial of early diet in preterm babies and later intelligence quotient. BMJ 1998, 317, 1481–1487. [Google Scholar] [CrossRef] [PubMed]
- Lucas, A.; Morley, R.; Cole, T.J.; Gore, S.M. A randomised multicentre study of human milk versus formula and later development in preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 1994, 70, F141–F146. [Google Scholar] [CrossRef]
- Peacock, J.L.; Marston, L.; Marlow, N.; Calvert, S.A.; Greenough, A. Neonatal and infant outcome in boys and girls born very prematurely. Pediatr. Res. 2012, 71, 305–310. [Google Scholar] [CrossRef]
- Leunissen, R.W.; Kerkhof, G.F.; Stijnen, T.; Hokken-Koelega, A. Timing and tempo of first-year rapid growth in relation to cardiovascular and metabolic risk profile in early adulthood. JAMA 2009, 301, 2234–2242. [Google Scholar] [CrossRef] [PubMed]
- Kerkhof, G.F.; Willemsen, R.H.; Leunissen, R.W.; Breukhoven, P.E.; Hokken-Koelega, A.C. Health profile of young adults born preterm: Negative effects of rapid weight gain in early life. J. Clin. Endocrinol. Metab. 2012, 97, 4498–4506. [Google Scholar] [CrossRef]
- Poindexter, B.B.; Langer, J.C.; Dusick, A.M.; Ehrenkranz, R.A.; National Institute of Child Health; Human Development Neonatal Research Network. Early provision of parenteral amino acids in extremely low birth weight infants: Relation to growth and neurodevelopmental outcome. J. Pediatr. 2006, 148, 300–305. [Google Scholar] [CrossRef]
- Lin, L.; Amissah, E.; Gamble, G.D.; Crowther, C.A.; Harding, J.E. Impact of macronutrient supplements for children born preterm or small for gestational age on developmental and metabolic outcomes: A systematic review and meta-analysis. PLoS Med. 2019, 16, e1002952. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Amissah, E.; Gamble, G.D.; Crowther, C.A.; Harding, J.E. Impact of macronutrient supplements on later growth of children born preterm or small for gestational age: A systematic review and meta-analysis of randomised and quasi-randomised controlled trials. PLoS Med. 2020, 17, e1003122. [Google Scholar] [CrossRef]
- Alur, P. Sex differences in nutrition, growth, and metabolism in preterm infants. Front. Pediatr. 2019, 7, 22. [Google Scholar] [CrossRef]
- Lin, L.; Crowther, C.; Gamble, G.; Bloomfield, F.; Harding, J.E.; ESSENCE IPD-MA Group. Sex-specific effects of nutritional supplements in infants born early or small: Protocol for an individual participant data meta-analysis (ESSENCE IPD-MA). BMJ Open 2020, 10, e033438. [Google Scholar] [CrossRef]
- Tierney, J.F.; Vale, C.; Riley, R.; Smith, C.T.; Stewart, L.; Clarke, M.; Rovers, M. Individual participant data (IPD) meta-analyses of randomised controlled trials: Guidance on their use. PLoS Med. 2015, 12, e1001855. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Green, S.E.; The Cochrane Collaboration. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [Updated March 2011]. 2011. Available online: www.cochrane-handbook.org (accessed on 15 June 2020).
- Review Manager (RevMan). Computer Program, Version 5.3; The Nordic Cochrane Centre, The Cochrane Collaboration: Copenhagen, Denmark, 2014. [Google Scholar]
- Agosti, M.; Vegni, C.; Calciolari, G.; Marini, A.; Group, G.S. Post-discharge nutrition of the very low-birthweight infant: Interim results of the multicentric GAMMA study. Acta Paediatr. Suppl. 2003, 91, 39–43. [Google Scholar] [CrossRef]
- Atkinson, S.A.; Randall-Simpson, J.; Chang, M.; Paes, B. Randomized trial of feeding nutrient-enriched vs standard formula to premature infants during the first year of life. Pediatr. Res. 1999, 45, 276. [Google Scholar] [CrossRef][Green Version]
- Biasini, A.; Marvulli, L.; Neri, E.; China, M.; Stella, M.; Monti, F. Growth and neurological outcome in ELBW preterms fed with human milk and extra-protein supplementation as routine practice: Do we need further evidence? J. Matern. Fetal Neonatal Med. 2012, 25 (Suppl. 4), 72–74. [Google Scholar] [CrossRef]
- Cooke, R.J.; Embleton, N.D.; Griffin, I.J.; Wells, J.C.; McCormick, K.P. Feeding preterm infants after hospital discharge: Growth and development at 18 months of age. Pediatr. Res. 2001, 49, 719–722. [Google Scholar] [CrossRef] [PubMed]
- Da Cunha, R.D.; Lamy Filho, F.; Rafael, E.V.; Lamy, Z.C.; de Queiroz, A.L. Breast milk supplementation and preterm infant development after hospital discharge: A randomized clinical trial. J. Pediatr. 2016, 92, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Embleton, N.D.; Cooke, R.J. Protein requirements in preterm infants: Effect of different levels of protein intake on growth and body composition. Pediatr. Res. 2005, 58, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Fewtrell, M.S.; Morley, R.; Abbott, R.A.; Singhal, A.; Stephenson, T.; MacFadyen, U.M.; Clements, H.; Lucas, A. Catch-up growth in small-for-gestational-age term infants: A randomized trial. Am. J. Clin. Nutr. 2001, 74, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Kanmaz, H.G.; Mutlu, B.; Canpolat, F.E.; Erdeve, O.; Oguz, S.S.; Uras, N.; Dilmen, U. Human milk fortification with differing amounts of fortifier and its association with growth and metabolic responses in preterm infants. J. Hum. Lact. 2013, 29, 400–405. [Google Scholar] [CrossRef]
- Lucas, A.; Fewtrell, M.S.; Morley, R.; Singhal, A.; Abbott, R.A.; Isaacs, E.; Stephenson, T.; MacFadyen, U.M.; Clements, H. Randomized trial of nutrient-enriched formula versus standard formula for postdischarge preterm infants. Pediatrics 2001, 108, 703–711. [Google Scholar] [CrossRef]
- Morgan, C.; McGowan, P.; Herwitker, S.; Hart, A.E.; Turner, M.A. Postnatal head growth in preterm infants: A randomized controlled parenteral nutrition study. Pediatrics 2014, 133, e120–e128. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, K.; Narnag, A.; Mahajan, R. Effect of human milk fortification in appropriate for gestation and small for gestation preterm babies: A randomized controlled trial. Indian Pediatr. 2007, 44, 286–290. [Google Scholar]
- Rochow, N.; Fusch, G.; Ali, A.; Bhatia, A.; So, H.Y.; Iskander, R.; Chessell, L.; Helou, S.; Fusch, C. Individualized target fortification of breast milk with protein, carbohydrates, and fat for preterm infants: A double-blind randomised controlled trial. Clin. Nutr. 2021, 40, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.J.; Cooke, R.W. Improving head growth in very preterm infants—A randomised controlled trial I: Neonatal outcomes. Arch. Dis. Child. Fetal Neonatal Ed. 2008, 93, F337–F341. [Google Scholar] [CrossRef]
- Zachariassen, G.; Faerk, J.; Grytter, C.; Esberg, B.H.; Hjelmborg, J.; Mortensen, S.; Thybo Christesen, H.; Halken, S. Nutrient enrichment of mother’s milk and growth of very preterm infants after hospital discharge. Pediatrics 2011, 127, e995–e1003. [Google Scholar] [CrossRef]
- Amesz, E.M.; Schaafsma, A.; Cranendonk, A.; Lafeber, H.N. Optimal growth and lower fat mass in preterm infants fed a protein-enriched postdischarge formula. J. Pediatr. Gastroenterol. Nutr. 2010, 50, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Bellagamba, M.P.; Carmenati, E.; D’Ascenzo, R.; Malatesta, M.; Spagnoli, C.; Biagetti, C.; Burattini, I.; Carnielli, V.P. One extra gram of protein to preterm infants from birth to 1800 g: A single-blinded randomized clinical trial. J. Pediatr. Gastroenterol. Nutr. 2016, 62, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Dogra, S.; Thakur, A.; Garg, P.; Kler, N. Effect of differential enteral protein on growth and nurodevelopment in infants <1500 g: A randomized controlled trial. J. Pediatr. 2017, 64, e126–e132. [Google Scholar] [CrossRef]
- Goldman, H.I.; Freudenthal, R.; Holland, B.; Karelitz, S. Clinical effects of two different levels of protein intake on low-birth-weight infants. J. Pediatr. 1969, 74, 881–889. [Google Scholar] [CrossRef]
- Jeon, G.W.; Jung, Y.J.; Koh, S.Y.; Lee, Y.K.; Kim, K.A.; Shin, S.M.; Kim, S.S.; Shim, J.W.; Chang, Y.S.; Park, W.S. Preterm infants fed nutrient-enriched formula until 6 months show improved growth and development. Pediatr. Int. 2011, 53, 683–688. [Google Scholar] [CrossRef]
- Lucas, A.; Morley, R.; Cole, T.J.; Gore, S.M.; Davis, J.A.; Bamford, M.F.; Dossetor, J.F. Early diet in preterm babies and developmental status in infancy. Arch. Dis. Child. 1989, 64, 1570–1578. [Google Scholar] [CrossRef]
- Lucas, A.; Morley, R.; Cole, T.J.; Gore, S.M.; Lucas, P.J.; Crowle, P.; Pearse, R.; Boon, A.J.; Powell, R. Early diet in preterm babies and developmental status at 18 months. Lancet 1990, 335, 1477–1481. [Google Scholar] [CrossRef]
- O’Connor, D.L.; Khan, S.; Weishuhn, K.; Vaughan, J.; Jefferies, A.; Campbell, D.M.; Asztalos, E.; Feldman, M.; Rovet, J.; Westall, C.; et al. Growth and nutrient intakes of human milk-fed preterm infants provided with extra energy and nutrients after hospital discharge. Pediatrics 2008, 121, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Roggero, P.; Gianni, M.L.; Amato, O.; Liotto, N.; Morlacchi, L.; Orsi, A.; Piemontese, P.; Taroni, F.; Morniroli, D.; Bracco, B.; et al. Growth and fat-free mass gain in preterm infants after discharge: A randomized controlled trial. Pediatrics 2012, 130, e1215–e1221. [Google Scholar] [CrossRef]
- Svenningsen, N.W.; Lindroth, M.; Lindquist, B. A comparative study of varying protein intake in low birthweight infant feeding. Acta Paediatr. Suppl. 1982, 296, 28–31. [Google Scholar] [CrossRef]
- Williams, J.; Lee, K.J.; Anderson, P.J. Prevalence of motor-skill impairment in preterm children who do not develop cerebral palsy: A systematic review. Dev. Med. Child Neurol. 2010, 52, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Dewey, D.; Thompson, D.K.; Kelly, C.E.; Spittle, A.J.; Cheong, J.L.Y.; Doyle, L.W.; Anderson, P.J. Very preterm children at risk for developmental coordination disorder have brain alterations in motor areas. Acta Paediatr. 2019, 108, 1649–1660. [Google Scholar] [CrossRef] [PubMed]
- Bolk, J.; Farooqi, A.; Hafstrom, M.; Aden, U.; Serenius, F. Developmental coordination disorder and its association with developmental comorbidities at 6.5 years in apparently healthy children born extremely preterm. JAMA Pediatr. 2018, 172, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.; Berube, M.; Erlandson, K.; Haug, S.; Johnstone, H.; Meagher, M.; Sarkodee-Adoo, S.; Zwicker, J.G. Developmental coordination disorder in school-aged children born very preterm and/or at very low birth weight: A systematic review. J. Dev. Behav. Pediatr. 2011, 32, 678–687. [Google Scholar] [CrossRef]
- Spittle, A.J.; Orton, J. Cerebral palsy and developmental coordination disorder in children born preterm. Semin. Fetal Neonatal Med. 2014, 19, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Singhal, A. Long-term adverse effects of early growth acceleration or catch-up growth. Ann. Nutr. Metab. 2017, 70, 236–240. [Google Scholar] [CrossRef]
- Belfort, M.B.; Gillman, M.W.; Buka, S.L.; Casey, P.H.; McCormick, M.C. Preterm infant linear growth and adiposity gain: Trade-offs for later weight status and intelligence quotient. J. Pediatr. 2013, 163, U1564–U1571. [Google Scholar] [CrossRef]
- Woo Baidal, J.A.; Locks, L.M.; Cheng, E.R.; Blake-Lamb, T.L.; Perkins, M.E.; Taveras, E.M. Risk factors for childhood obesity in the first 1000 days: A systematic review. Am. J. Prev. Med. 2016, 50, 761–779. [Google Scholar] [CrossRef]
- Castanys-Munoz, E.; Kennedy, K.; Castaneda-Gutierrez, E.; Forsyth, S.; Godfrey, K.M.; Koletzko, B.; Ozanne, S.E.; Rueda, R.; Schoemaker, M.; van der Beek, E.M.; et al. Systematic review indicates postnatal growth in term infants born small-for-gestational-age being associated with later neurocognitive and metabolic outcomes. Acta Paediatr. 2017, 106, 1230–1238. [Google Scholar] [CrossRef]
- Ong, K.K.; Kennedy, K.; Castaneda-Gutierrez, E.; Forsyth, S.; Godfrey, K.M.; Koletzko, B.; Latulippe, M.E.; Ozanne, S.E.; Rueda, R.; Schoemaker, M.H.; et al. Postnatal growth in preterm infants and later health outcomes: A systematic review. Acta Paediatr. 2015, 104, 974–986. [Google Scholar] [CrossRef]
- Grigore, D.; Ojeda, N.B.; Alexander, B.T. Sex differences in the fetal programming of hypertension. Gend. Med. 2008, 5 (Suppl. A), S121–S132. [Google Scholar] [CrossRef] [PubMed]
- Ingemarsson, I. Gender aspects of preterm birth. BJOG Int. J. Obstet. Gynaecol. 2003, 110 (Suppl. 20), 34–38. [Google Scholar] [CrossRef]
- Reynolds, C.M.; O’Sullivan, J.M.; Segovia, S.A.; Vickers, M.H. Chapter 10—Early-Life nutrition, epigenetics, and altered energy balance later in life. In Epigenetics of Aging and Longevity; Moskalev, A., Vaiserman, A.M., Eds.; Academic Press: Boston, MA, USA, 2018; Volume 4, pp. 213–227. [Google Scholar]
- Wang, G.; Johnson, S.; Gong, Y.; Polk, S.; Divall, S.; Radovick, S.; Moon, M.; Paige, D.; Hong, X.; Caruso, D.; et al. Weight gain in infancy and overweight or obesity in childhood across the gestational spectrum: A prospective birth cohort study. Sci. Rep. 2016, 6, 29867. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E.L.; Al Saud, N.B.; Durighel, G.; Frost, G.; Bell, J.D. The effect of preterm birth on adiposity and metabolic pathways and the implications for later life. J. Clin. Lipidol. 2012, 7, 275–288. [Google Scholar] [CrossRef]
- Olsen, I.E.; Harris, C.L.; Lawson, M.L.; Berseth, C.L. Higher protein intake improves length, not weight, z scores in preterm infants. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 409–416. [Google Scholar] [CrossRef]
- Cormack, B.E.; Jiang, Y.; Harding, J.E.; Crowther, C.A.; Bloomfield, F.H. Relationships between neonatal nutrition and growth to 36 weeks’ corrected age in ELBW babies-secondary cohort analysis from the provide trial. Nutrients 2020, 12, 760. [Google Scholar] [CrossRef]
- Pinelli, J.; Saigal, S.; Atkinson, S.A. Effect of breastmilk consumption on neurodevelopmental outcomes at 6 and 12 months of age in VLBW infants. Adv. Neonatal Care 2003, 3, 76–87. [Google Scholar] [CrossRef]
- Bier, J.A.; Oliver, T.; Ferguson, A.E.; Vohr, B.R. Human milk improves cognitive and motor development of premature infants during infancy. J. Hum. Lact. 2002, 18, 361–367. [Google Scholar] [CrossRef]
- O’Connor, D.L.; Jacobs, F.; Hall, R.; Adamkin, D.; Auestad, T.; Castillo, I.; Connor, W.E.; Connor, J.L.; Fitzgerald, K.; Groh-Wargo, S.; et al. Growth and development of premature infants fed predominantly human milk, predominantly premature infant formula, or a combination of human milk and premature formula. J. Pediatr. Gastroenterol. Nutr. 2003, 37, 437–446. [Google Scholar] [CrossRef]
- Coviello, C.; Keunen, K.; Kersbergen, K.J.; Groenendaal, F.; Leemans, A.; Peels, B.; Isgum, I.; Viergever, M.A.; de Vries, L.S.; Buonocore, G.; et al. Effects of early nutrition and growth on brain volumes, white matter microstructure, and neurodevelopmental outcome in preterm newborns. Pediatr. Res. 2018, 83, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Klein, C.J. Nutrient requirements for preterm infant formulas. J. Nutr. 2002, 132 (Suppl. 1), 1395S–1577S. [Google Scholar] [CrossRef] [PubMed]
- Boyce, C.; Watson, M.; Lazidis, G.; Reeve, S.; Dods, K.; Simmer, K.; McLeod, G. Preterm human milk composition: A systematic literature review. Br. J. Nutr. 2016, 116, 1033–1045. [Google Scholar] [CrossRef] [PubMed]
- Young, L.; Embleton, N.D.; McGuire, W. Nutrient-enriched formula versus standard formula for preterm infants following hospital discharge. Cochrane Database Syst. Rev. 2016, 12, CD004696. [Google Scholar] [CrossRef] [PubMed]
- Young, L.; Embleton, N.D.; McCormick, F.M.; McGuire, W. Multinutrient fortification of human breast milk for preterm infants following hospital discharge. Cochrane Database Syst. Rev. 2013, 2, CD004866. [Google Scholar] [CrossRef]
- Brown, J.V.; Lin, L.; Embleton, N.D.; Harding, J.E.; McGuire, W. Multi-nutrient fortification of human milk for preterm infants. Cochrane Database Syst. Rev. 2020, 6, CD000343. [Google Scholar] [CrossRef]
- Stewart, L.; Tierney, J.F. To IPD or not to IPD? Advantages and disadvantages of systematic reviews using individual patient data. Eval. Health Prof. 2002, 25, 76–97. [Google Scholar] [CrossRef] [PubMed]
- Bender, R.; Bunce, C.; Clarke, M.; Gates, S.; Lange, S.; Pace, N.L.; Thorlund, K. Attention should be given to multiplicity issues in systematic reviews. J. Clin. Epidemiol. 2008, 61, 857–865. [Google Scholar] [CrossRef]
| Author/Year | Country | Participants | Participants, n | Intervention | Control | Duration | Outcomes |
|---|---|---|---|---|---|---|---|
| Studies with IPD Available | |||||||
| Agosti 2003 [19] | Italy | Inclusion criteria: preterm BW < 1500 g and previously fed with a preterm formula. Exclusion criteria: malformations intraventricular haemorrhage, periventricular leukomalacia, chronic lung disease, necrotising enterocolitis grade >1, total parenteral nutrition >2 weeks, sepsis, retinopathy of prematurity grade >1. | Intervention: 89 Control: 67 | Preterm formula (protein 2.4 g/100 mL, energy 80 kcal/100 mL) | Standard term formula (protein 1.7 g/100 mL, energy 70 kcal/100 mL) | Started from 40 weeks PMA, stopped at 55 weeks PMA. | GMDS at 6, 9, 12 and 18 months’ CA |
| Atkinson 1999 [20] | Canada | Inclusion criteria: BW < 2500 g; GA < 42 weeks; birthweight <5th percentile and fed only formula at entry into the study. | Intervention: 22 Control: 28 | Ross Discharge formula (protein 1.8 g/100 mL, energy 74 kcal/100 mL) | Similac with Iron formula (energy 68 kcal/100 mL) | Started from discharge, stopped at 1 year CA | Bayley II at 6 and 12 months’ CA. |
| Biasini 2012 [21] | Italy | Inclusion criteria: BW 580–1250 g and GA < 32 weeks. | Intervention: 34 Control:27 | Protein supplemented (protein 4.8 g/kg/day, energy 141 kcal/day) | Control (protein 3.5 g/kg/day, energy 135 kcal/day) | Started from the first day of full enteral feeding, stopped at discharge. | GMDS at 3, 6, 9, 12, 15, 18 and 24 months’ CA. |
| Cooke 1998 [22] | UK | Inclusion criteria: GA ≤ 34 weeks and BW ≤ 1750 g, and growing normally at the time of hospital discharge, i.e., ≥25 g/day. Exclusion criteria: systemic disease or require medication. | Intervention: 56 Control: 57 | Preterm formula (protein 2.2 g/100 mL, fat 4.4 g/100 mL, carbohydrate 8.5 g/100 mL, energy 80 kcal/100 mL) | Term formula (protein 1.4 g/100 mL, fat 3.6 g/100 mL, carbohydrate 7.5 g/100 mL, energy 66 kcal/100 mL) | Started from discharge, stopped at 6 months’ CA. | Bayley II at 18 months’ CA; WISC at 10 years’ CA; blood pressure, triglyceride, cholesterol, HDL, LDL, fasting blood glucose concentration, fasting insulin concentration IGF-I at 13 years’ CA. |
| da Cunha 2016 [23] | Brazil | Inclusion criteria: GA < 37 weeks and BW < 1500 g, and discharged exclusively breastfeeding. Exclusion criteria: major malformations; hydrocephalus; chromosomal abnormalities; fetal hydrops; congenital infections; maternal use of illicit drugs, tobacco, alcohol and continuous use of corticosteroids; twin pregnancy; necrotising enterocolitis sequelae; cerebral palsy. | Intervention: 26 Control: 27 | Breast milk supplementation (daily increase of 0.56 g of protein, 1.04 g of total fat and 2.12 g of carbohydrates) | Breast milk without supplementation | Started 7–10 days after discharge, stopped at four to six months. | Bayley III at 12 months’ CA. |
| Embleton 2005 [24] | UK | Inclusion criteria: GA ≤ 34 weeks and BW ≤ 1750 g, tolerating enteral intake ≥150 mL/kg/day for ≥48 h and current weight ≥1000 g. | Intervention: 25 Control: 26 | Formula A (protein 2.6 g/100 mL, fat 4.3 g/100 mL, carbohydrate 7.9 g/100 mL, energy 80 kcal/100 mL) | Formula C (protein 2.2 g/100 mL, fat 4.5 g/100 mL, carbohydrate 7.9 g/100 mL, energy 80 kcal/100 mL) | Started when full enteral feeding 150 mL/kg/day, stopped at 12 week’ CA. | Blood pressure, triglyceride, cholesterol, HDL, LDL, fasting blood glucose concentration, fasting insulin concentration, IGF-I at 10 years’ CA. |
| Fewtrell 2001 [25] | UK | Inclusion criteria: GA ≥ 37 weeks and BW below the 10th centile for gestation and sex according to UK growth charts. | Intervention: 152 Control: 147 | Enriched formula (protein 1.85 g/100 mL, fat 3.96 g/100 mL, carbohydrate 7.24 g/100 mL, energy 72 kcal/100 mL) | Term formula (protein 1.45 g/100 mL, fat 3.85 g/100 mL, carbohydrate 6.96 g/100 mL, energy 68 kcal/100 mL) | Started within the first week, stopped at 9 months’ CA. | Bayley II at 18 months’ corrected age. |
| Kanmaz 2013 [26] | Turkey | Inclusion criteria: GA ≤ 32 weeks and BW ≤ 1500 g; fed with human milk. Exclusion criteria: major congenital anomalies, chronic illnesses, respiratory support requirements, or sepsis and those who were receiving mixed feeding. | Intervention: 29 Control: 26 | Aggressive fortification: 1.2 g of human milk fortifier added to each 20 mL human milk (protein: 3.6 g/kg/day) | Standard fortification: 1.2 g of human milk fortifier added to each 30 mL human milk (protein: 3.0 g/kg/day) | Started when infants reached 90–100 mL/kg enteral feeding, stopped at discharge. | Bayley II at 2 years’ corrected age. |
| Lucas 1996 [5] | UK | Inclusion criteria: BW < 1850 g, GA < 37 weeks, and survived to be assigned to a study group between 48 and 72 h of age. Exclusion criteria: major congenital anomalies. | Intervention: 137 Control: 138 | Fortified human breast milk; fortifier containing protein 0.7 g/100 mL, fat 0.05 g/100 mL, carbohydrate 2.73 g/100 mL, energy 14 kcal/100 mL | Human breast milk | Started within 48 h, stopped at discharge or when the infants reached 2000 g. | Bayley II at 18 months’ CA. |
| Lucas 2001 [27] | UK | Inclusion criteria: GA < 37 weeks and BW < 1750 g. Exclusion criteria: congenital malformations or conditions known to affect growth or development. | Intervention: 113 Control: 116 | Post-discharge formula (protein 1.85 g/100 mL, fat 3.96 g/100 mL, carbohydrate 7.24 g/100 mL, energy 72 kcal/100 mL) | Term formula (protein 1.45 g/100 mL, fat 3.82 g/100 mL, carbohydrate 6.96 g/100 mL, energy 68 kcal/100 mL) | Started one week before discharge, stopped at 9 months post-term. | Bayley II at 18 months’ CA. Blood pressure at 5 years, body composition at 5 years. |
| Morgan 2014 [28] | UK | Inclusion criteria: GA 24–28 weeks and BW < 1200 g. Exclusion criteria: unlikely to survive the first week after birth; diagnosed with major congenital or chromosomal abnormalities known to affect gastrointestinal function or head growth, including definite parenchymal lesions on cranial ultrasound scan in first 48 h. | Intervention: 74 Control: 76 | Higher macronutrient content (parenteral intake with protein 2.8 g/kg/day, fat 2.8 g/kg/day, carbohydrate 13.5 g/kg/day, energy 85 kcal/kg/day) | Standard macronutrient content (parenteral intake with protein 3.8 g/kg/day, fat 3.8 g/kg/day, carbohydrate 15.6 g/kg/day, energy 103 kcal/kg/day) | Started within 120 h of birth, stopped at 28 days. | Bayley III at 2 to 3.5 years of CA. |
| Mukhopadhyay 2007 [29] | India | Inclusion criteria: GA ≤ 34 weeks and BW ≤ 1500 g, reached feed volume of 150 mL/kg/day, feed constituted at least 80% breast milk. Exclusion criteria: major congenital malformation, gastrointestinal abnormalities. | Intervention: 84 Control: 82 | Fortified human milk: (fortifier contained protein 0.4 g/100 mL; fat 0.2 g/100 mL; carbohydrate 2.4 g/100 mL; energy 13 kcal/100 mL) | Exclusive human milk. | Started when feed volume reached 150 mL/kg/day, stopped when reached 2 kg or full breastfeeds. | Bayley II at 12 months’ CA. |
| Rochow 2019 [30] | Canada | Inclusion criteria: GA < 30 weeks, and length of stay > 21 days and receiving fortified BM. Exclusion criteria: gastrointestinal malformation, major congenital anomalies, necrotising enterocolitis abdominal surgery, and gram-negative sepsis. | Intervention: 52 Control: 51 | Target fortified human milk:(protein 3.0 g/100 mL, fat 4.4 g/100 mL, carbohydrates 8.5 g/100 mL) | Standard fortified human milk | Started when enteral intake was ≥100 mL/kg/day, stopped at 36 weeks’ PMA. | Bayley III at 18 months’ CA. |
| Tan 2008 [31] | UK | Inclusion criteria: GA < 29 weeks. Exclusion criteria: triplets and higher multiplicity, admitted after 7 days of age, major congenital abnormalities | Intervention: 68 Control: 74 | Parenteral protein 4 g/kg/day, fat 4 g/kg/day, carbohydrate 16.3 g/kg/day, energy 117 kcal/kg/day; enteral breast milk or formula with target protein 4 g/kg/day, energy 133–150 kcal/kg/day | Parenteral protein 3 g/kg/day, fat 3 g/kg/day, carbohydrate 13.5 g/kg/day, energy 93 kcal/kg/day; enteral breast milk or formula with target protein 3.3 g/kg/day, energy 133 kcal/kg/day | Started when infants received parenteral and enteral nutrition from the first week, stopped at 34 weeks’ PMA. | Bayley II at 3 and 9 months’ CA. |
| Zachariassen 2011 [32] | Denmark | Inclusion criteria: GA ≤ 32 weeks, breastfeeding. Exclusion criteria: severe diseases or circumstances influencing eating and feeding ability at discharge. | Intervention: 105 Control: 102 | Fortified mother’s milk (protein 1.375 g/day, energy 17.5 kcal/day) | Unfortified mother’s milk | Started from shortly before discharge, stopped at 4 months’ CA. | WISC at 6 years’ CA; Blood pressure, triglyceride, HDL, LDL, fasting blood glucose concentration, fasting insulin concentration, at 6 years’ CA. |
| Studies with AD available | |||||||
| Amesz 2010 [33] | Netherlands | Inclusion criteria: GA ≤ 32 weeks or BW ≤ 1500 g. Exclusion criteria: congenital malformations or conditions known to affect growth or body composition (e.g., severe bronchopulmonary dysplasia, an inborn error of metabolism, cardiac or renal disease, necrotising enterocolitis with substantial gut loss, grade IV intraventricular haemorrhage). | Intervention: 52 Control:50 | Post-discharge formula (protein 1.7 g/100 mL, fat 3.5 g/100 mL, carbohydrate 7.0 g/100 mL, energy 67 kcal/100 mL) | Term formula (protein 1.47 g/100 mL, fat 3.5 g/100 mL, carbohydrate 7.2 g/100 mL, energy 70 kcal/100 mL) | Started from term, stopped at 6 months’ CA. | Blood pressure, triglyceride, HDL, LDL, fasting blood glucose concentration, insulin sensitivity, insulin resistance (HOMA-IR), fasting leptin at 8 years’ CA. |
| Bellagamba 2016 [34] | Italy | Inclusion criteria: preterm BW 500–1249 g. | Intervention: 82 Control: 82 | High protein (protein supplementation started at 1.5 g/kg/day and increased by 0.5 g/kg/day to a maximum of 3.5 g/kg/day on the fifth day after birth) | Standard protein (protein supplementation started at 1.5 g/kg/day and increased by 0.5 g/kg/day to a maximum of 2.5 g/kg/day on the third day after birth) | Started from birth, stopped at discharge. | Bayley III at 2 years’ corrected age. |
| Dogra 2017 [35] | India | Inclusion criteria: GA < 32 weeks. Exclusion criteria: lethal congenital malformations. | Intervention: 59 Control: 56 | Fortified breast milk with higher protein (fortifier containing protein 1.0 g/100 mL, fat 0.01 g/100 mL, carbohydrate 3.6 g/100 mL, energy 17.2 kcal/100 mL) | Fortified breast milk with standard protein (fortifier containing protein 0.4 g/100 mL, fat 0.2 g/100 mL, carbohydrate 2.4 g/100 mL, energy 13 kcal/100 mL) | Started when infants reached a feed volume of 100 mL/kg/day, stopped at discharge or full breast-feeds, whichever was earlier. | DASII at 12 to 18 months’ CA |
| Goldman 1969 [36] | USA | Inclusion criteria: BW < 2000 g. Exclusion criteria: major congenital malformations, intestinal obstruction, Rhesus disease, > 3 days old on admission, or died during the first few days generally received no milk feedings. | Intervention: 152 Control: 152 | Enriched formula (protein 4.0 g/100 mL, fat 3.9 g/100 mL, carbohydrate 7.6 g/10 mL, 80 kcal/100 mL) | Standard formula (protein 2.0 g/100 mL, fat 3.9 g/100 mL, carbohydrate 9.6 g/100 mL, energy 80 kcal/100 mL) | Started from 24 to 72 h, stopped when the infants reached 2200 g (at discharge). | Cognitive impairment (Stanford–Binet scores) at 3 years’ CA. |
| Jeon 2011 [37] | Korea | Inclusion criteria: GA < 33 weeks and BW < 1500 g, formula as the primary food source. Exclusion criteria: chromosomal disorders or serious congenital malformations at discharge that would affect growth and development. | Intervention: 35 Control: 34 | Preterm formula (protein 2.3 g/100 mL, fat 4.1 g/100 mL, carbohydrate 8.5 g/100 mL, energy 80 kcal/100 mL) | Term formula (protein 1.6 g/100 mL, fat 3.5 g/100 mL, carbohydrate 7.2 g/100 mL, energy 67 kcal/100 mL) | Started at term, stopped at 6 months’ corrected age. | Bayley II at 18 months’ CA. |
| Lucas 1989 [38] | UK | Inclusion criteria: GA < 37 weeks and BW < 1850 g. Exclusion criteria: major congenital abnormality known to impair growth or development, or died before randomisation within the first 48 h. | (1) Lucas 1989a: Intervention: 76 Control: 83 (2) Lucas 1989b: Intervention: 173 Control: 170 (3) Lucas 1989c: combined Lucas 1989a and Lucas 1989b: Intervention: 249 Control: 253 | (1) Lucas 1989a Preterm formula as sole diet (protein 2.0 g/100 mL, fat 4.9 g/100 mL, carbohydrate 7.0 g/100 mL, energy 80 kcal/100 mL) (2) Lucas 1989b Preterm formula as supplement (3) Lucas 1989c: combined Lucas 1989a and Lucas 1989b | (1) Lucas 1989a: Banked breast milk as sole diet (protein 1.1 g/100 mL, fat 1.7 g/100 mL, carbohydrate 7.1 g/100 mL, energy 46 kcal/100 mL); (2) Lucas 1989b: banked breast milk as supplement; (3) Lucas 1989c: combined Lucas 1989a and Lucas 1989b | Started within 48 h, stopped at discharge or when the infants reached 2000 g. | Bayley II at 9, 18 months’ CA; Blood pressure at 7.5 to 8 years’ and 13 to 16 years’ CA; Triglyceride, HDL, LDL, fasting blood glucose concentration, fasting insulin concentration, insulin resistance (fasting 32–33 split proinsulin) at 13 to 16 years’ CA. |
| Lucas 1990 [39] | UK | Inclusion criteria: BW < 1850 g and GA < 37 weeks; Exclusion criteria: major congenital abnormality known to impair growth or development or died before randomisation within the first 48 h. | (1) Lucas 1990a:Intervention: 81 Control: 79 (2) Lucas 1990b: Intervention: 132 Control: 132 (3) Lucas 1990c: combined Lucas 1990a and Lucas 1990b: Intervention: 213 Control: 211 | (1) Lucas 1990a: Preterm formula as sole diet (protein 2.0 g/100 mL, fat 4.9 g/100 mL, carbohydrate 7.0 g/100 mL, energy 80 kcal/100 mL) (2) Lucas 1990b Preterm formula as supplement (3) Lucas 1990c: combined Lucas 1990a and Lucas 1990b | (1) Lucas 1990a: term formula as sole diet (protein 1.5 g/100 mL, fat 3.8 g/100 mL, carbohydrate 7.0 g/100 mL, energy 68 kcal/100 mL) (2) Lucas 1990b: term formula as supplement (3) Lucas 1990c: combined Lucas 1990a and Lucas 1990b | Started within 48 h, stopped at discharge or when the infants reached 2000 g. | Bayley II at 9, 18 months’ CA; Wechsler Intelligence Scale for Children (WISC) at 7.5 to 8 years’ CA; Blood pressure at 7.5 to 8 years’ and 13 to 16 years’ CA; Triglyceride, HDL, LDL, fasting blood glucose concentration, fasting insulin concentration, insulin resistance (fasting 32–33 split proinsulin) at 13 to 16 years’ CA. |
| O’Connor 2008 [40] | Canada | Inclusion criteria: GA < 33 weeks and BW between 750 and 1800 g who received ≥80% of their total feedings as human milk 3 days before hospital discharge; Exclusion criteria: serious congenital or chromosomal anomalies that could affect growth, grade 3 or 4 periventricular or intraventricular haemorrhage, oral steroids within 14 days of randomisation, severe asphyxia and known maternal alcohol or drug abuse. | Intervention: 19 Control: 20 | Human milk with a multi-nutrient fortifier (protein 2.0 g/100 mL, fat 4.2 g/100 mL, carbohydrate 8.8 g/100 mL, energy 81 kcal/100 mL) | Unfortified human milk (protein 1.3 g/100 mL, fat 3.9 g/100 mL, carbohydrate 7.2 g/100 mL, energy 68 kcal/100 mL | Started from discharge, stopped at 12 weeks after discharge. | Bayley II at 18 months’ CA. |
| Roggero 2012 [41] | Italy | Inclusion criteria: GA ≤ 32 weeks or BW ≤ 1500 g and being fed human milk for 20% of the total milk intake; Exclusion criteria: congenital malformations or conditions that interfere with growth or body composition. | Intervention: 110 Control: 107 | Nutrient-enriched formula (protein 2.0 g/100 mL, fat 4.1 g/100 mL, carbohydrate 7.5 g/100 mL, energy 75 kcal/100 mL) | Term formula (protein 1.4 g/100 mL, fat 3.7 g/100 mL, carbohydrate 7.4 g/100 mL, energy 68 kcal/100 mL) | Started from term CA, stopped at 6 months. | GMDS at 24 months’ CA. |
| Svenningsen 1982 [42] | Sweden | Inclusion criteria: Very low birthweight preterm with mean BW 1385 ± 343 g and GA 30.8 ± 2.9 weeks. | Intervention: 16 Control: 14 | Nutrition enriched formula (protein 2.1 g/100 mL, energy 69.5 kcal/100 mL) | Standard formula (protein 1.6 g/100 mL, energy 68.5 kcal/100 mL) | Started from the third week after birth, stopped at the seventh week after birth. | Development impairments at 6 months, 1 and 2 years of age. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, L.; Gamble, G.D.; Crowther, C.A.; Bloomfield, F.H.; Agosti, M.; Atkinson, S.A.; Biasini, A.; Embleton, N.D.; Fewtrell, M.S.; Lamy-Filho, F.; et al. Sex-Specific Effects of Nutritional Supplements for Infants Born Early or Small: An Individual Participant Data Meta-Analysis (ESSENCE IPD-MA) I—Cognitive Function and Metabolic Risk. Nutrients 2022, 14, 418. https://doi.org/10.3390/nu14030418
Lin L, Gamble GD, Crowther CA, Bloomfield FH, Agosti M, Atkinson SA, Biasini A, Embleton ND, Fewtrell MS, Lamy-Filho F, et al. Sex-Specific Effects of Nutritional Supplements for Infants Born Early or Small: An Individual Participant Data Meta-Analysis (ESSENCE IPD-MA) I—Cognitive Function and Metabolic Risk. Nutrients. 2022; 14(3):418. https://doi.org/10.3390/nu14030418
Chicago/Turabian StyleLin, Luling, Greg D. Gamble, Caroline A. Crowther, Frank H. Bloomfield, Massimo Agosti, Stephanie A. Atkinson, Augusto Biasini, Nicholas D. Embleton, Mary S. Fewtrell, Fernando Lamy-Filho, and et al. 2022. "Sex-Specific Effects of Nutritional Supplements for Infants Born Early or Small: An Individual Participant Data Meta-Analysis (ESSENCE IPD-MA) I—Cognitive Function and Metabolic Risk" Nutrients 14, no. 3: 418. https://doi.org/10.3390/nu14030418
APA StyleLin, L., Gamble, G. D., Crowther, C. A., Bloomfield, F. H., Agosti, M., Atkinson, S. A., Biasini, A., Embleton, N. D., Fewtrell, M. S., Lamy-Filho, F., Fusch, C., Gianni, M. L., Kanmaz Kutman, H. G., Koo, W., Litmanovitz, I., Morgan, C., Mukhopadhyay, K., Neri, E., Picaud, J.-C., ... Harding, J. E. (2022). Sex-Specific Effects of Nutritional Supplements for Infants Born Early or Small: An Individual Participant Data Meta-Analysis (ESSENCE IPD-MA) I—Cognitive Function and Metabolic Risk. Nutrients, 14(3), 418. https://doi.org/10.3390/nu14030418









