Influence of Consumption of Two Peruvian Cocoa Populations on Mucosal and Systemic Immune Response in an Allergic Asthma Rat Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Diets
2.2. Animals and Allergic Asthma Induction
2.3. Bronchoalveolar Lavage Fluid (BALF) Collection and Cellular Assessment
2.4. BALF Cytokine Determination
2.5. BALF and Fecal IgA Content Quantification
2.6. Serum Specific Anti-OVA Antibodies Quantification
2.7. Histopathological Examination
2.8. Statistical Analysis
3. Results
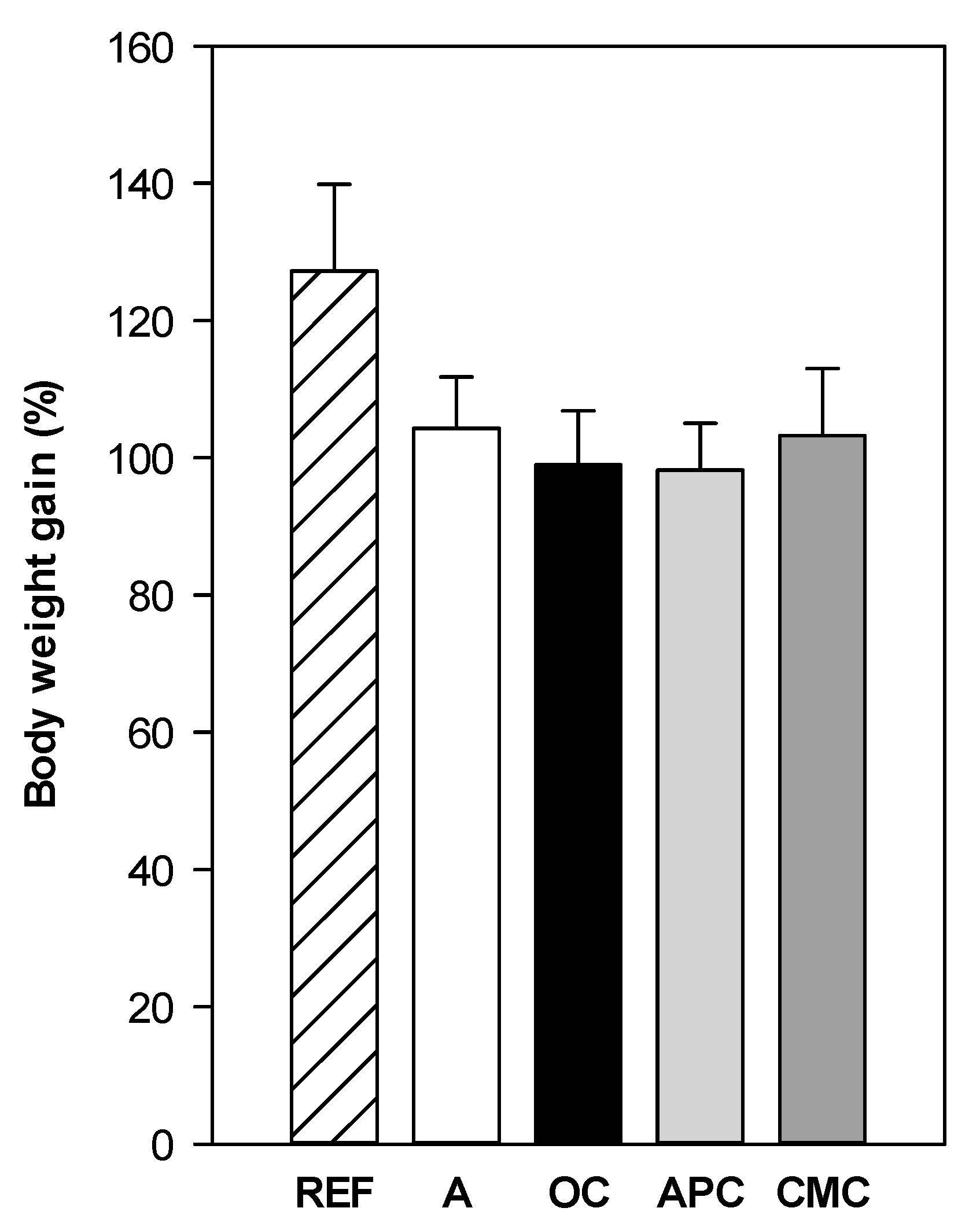
3.1. Effect of Allergic Asthma and Cocoa Diets on Body Weight
3.2. Influence of Allergic Asthma and Cocoa Diets on Serum Specific Antibodies
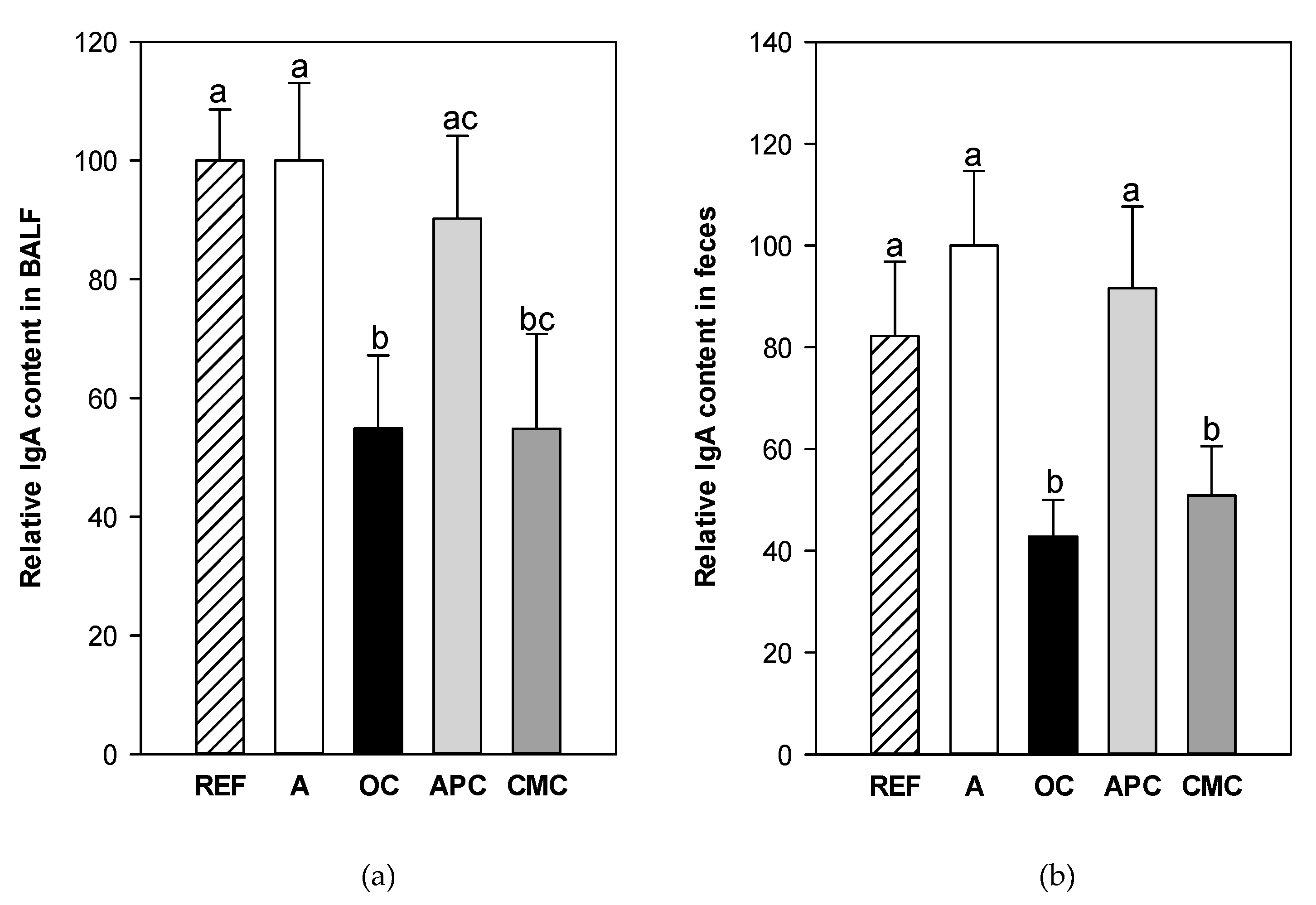
3.3. Effects of Allergic Asthma and Cocoa Diets on IgA Concentration in Mucosal Compartments
3.4. Influence of Allergic Asthma and Cocoa Diets on Blood Leukocyte Counts
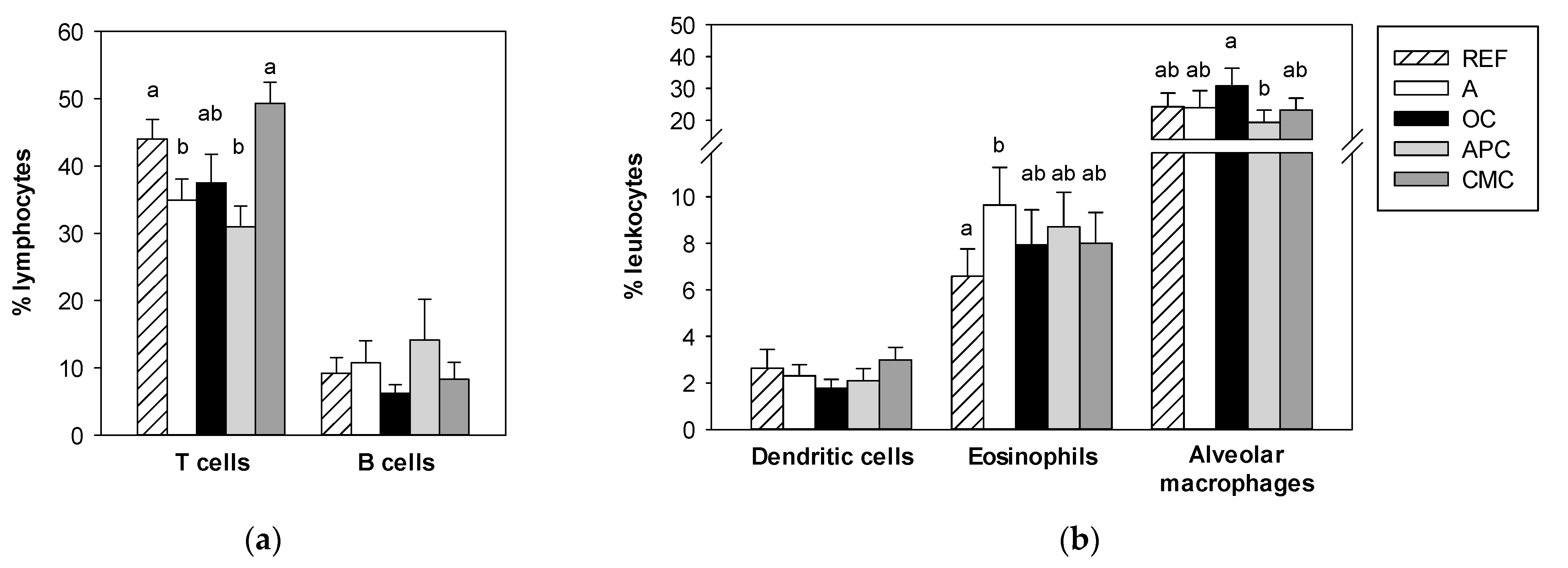
3.5. Effects of Allergic Asthma and Cocoa Diets on BALF Leukocytes
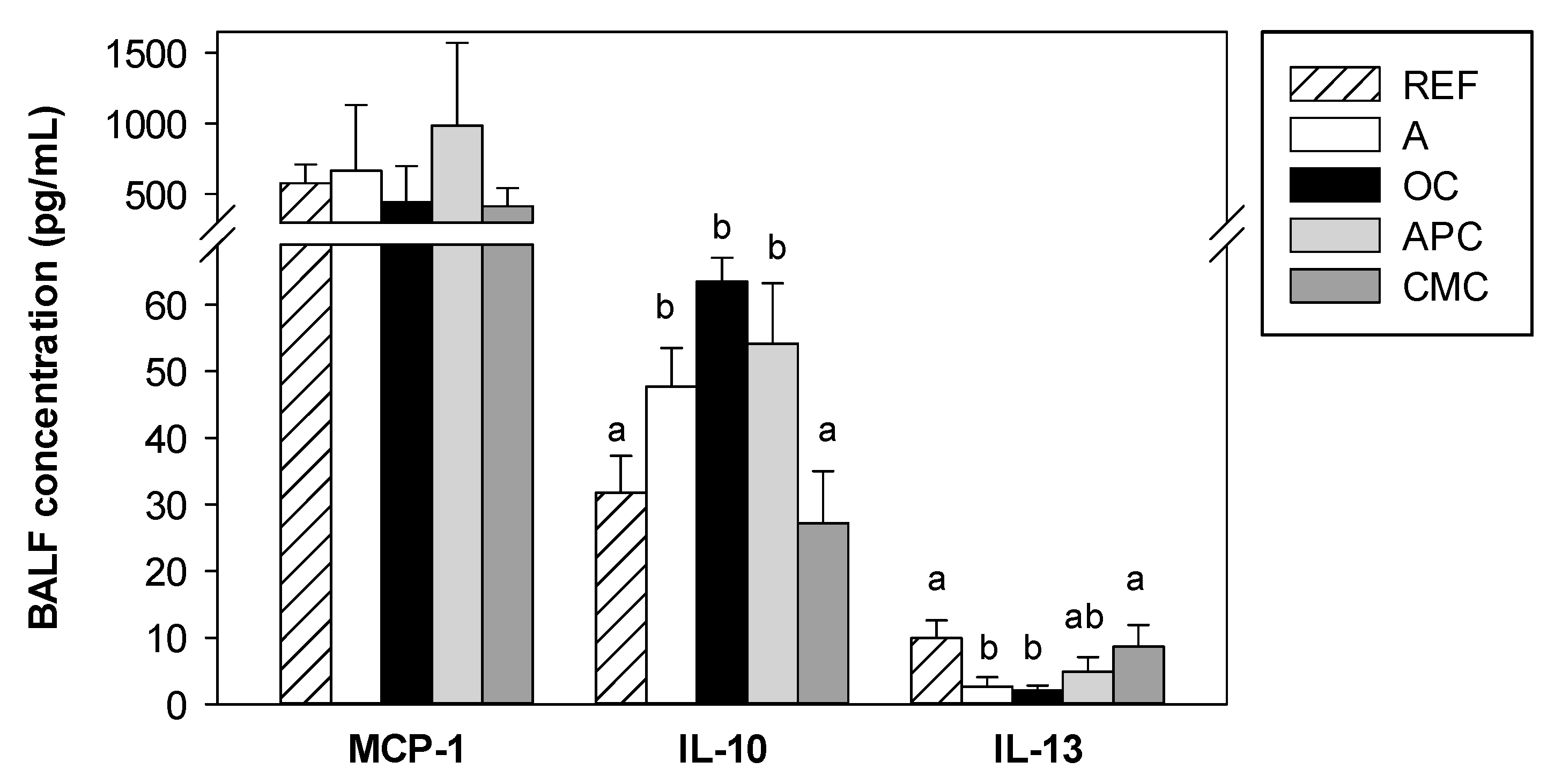
3.6. Effects of Allergic Asthma and Cocoa Diets on BALF Cytokines
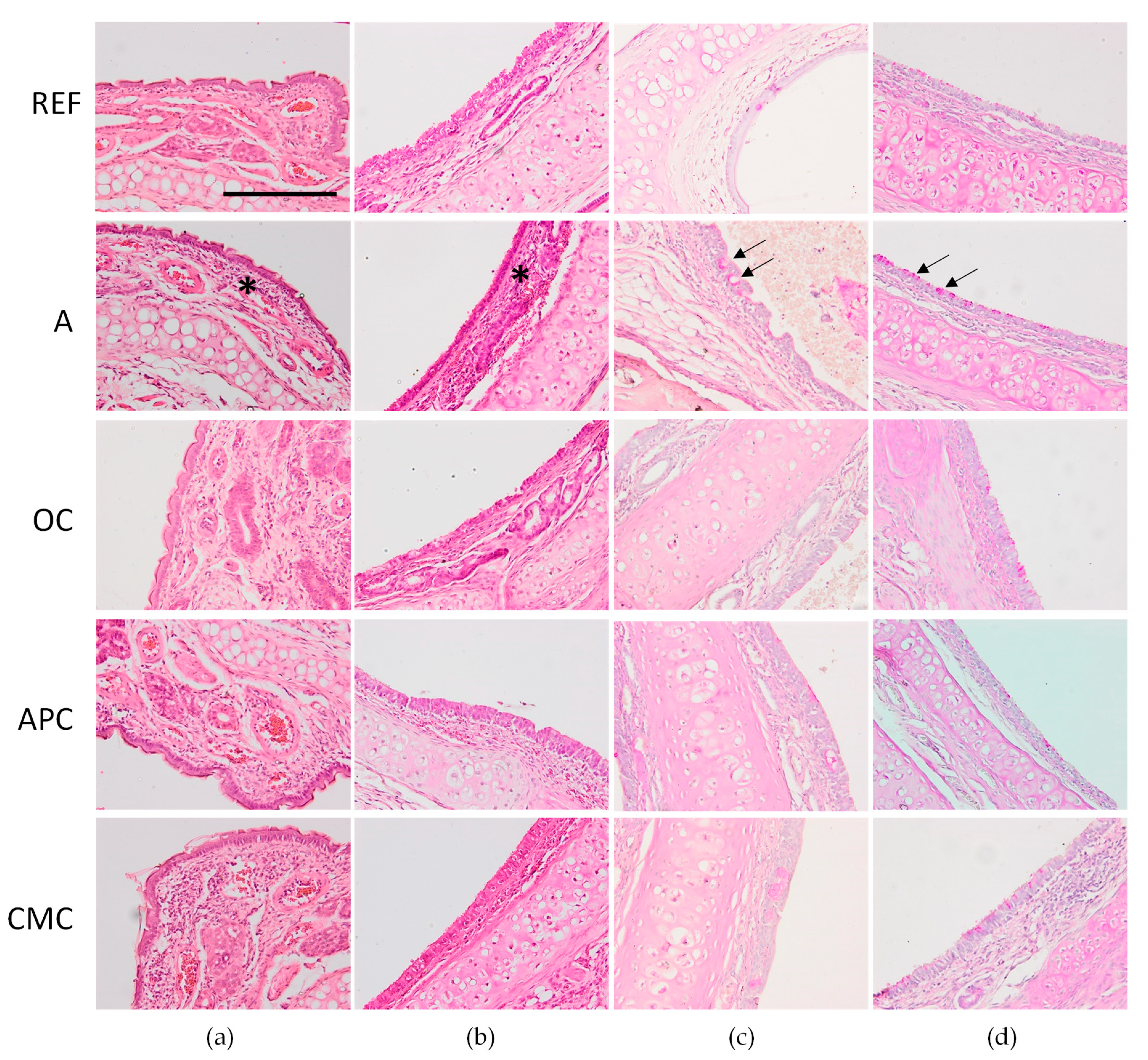
3.7. Effects of Allergic Asthma and Cocoa Diets on Nasal Tissue and Trachea
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seligson, F.H.; Krummel, D.A.; Apgar, J.L. Patterns of chocolate consumption. Am. J. Clin. Nutr. 1994, 60, 1060S–1064S. [Google Scholar] [CrossRef]
- Rusconi, M.; Conti, A. Theobroma cacao L., the Food of the Gods: A scientific approach beyond myths and claims. Pharmacol. Res. 2010, 61, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Dillinger, T.L.; Barriga, P.; Escárcega, S.; Jimenez, M.; Salazar Lowe, D.; Grivetti, L.E. Food of the Gods: Cure for Humanity? A Cultural History of the Medicinal and Ritual Use of Chocolate. J. Nutr. 2000, 130, 2057S–2072S. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E.; van Zonneveld, M.; Loo, J.; Hodgkin, T.; Galluzzi, G.; van Etten, J. Present spatial diversity patterns of Theobroma cacao L. in the neotropics reflect genetic differentiation in Pleistocene refugia followed by human-influenced dispersal. PLoS ONE 2012, 7, e47676. [Google Scholar] [CrossRef] [PubMed]
- Zarrillo, S.; Gaikwad, N.; Lanaud, C.; Powis, T.; Viot, C.; Lesur, I.; Fouet, O.; Argout, X.; Guichoux, E.; Salin, F.; et al. The use and domestication of Theobroma cacao during the mid-Holocene in the upper Amazon. Nat. Ecol. Evol. 2018, 2, 1879–1888. [Google Scholar] [CrossRef]
- Motamayor, J.C.; Lachenaud, P.; da Silva e Mota, J.W.; Loor, R.; Kuhn, D.N.; Brown, J.S.; Schnell, R.J. Geographic and genetic population differentiation of the Amazonian chocolate tree (Theobroma cacao L). PLoS ONE 2008, 3, e3311. [Google Scholar] [CrossRef]
- Lecumberri, E.; Mateos, R.; Izquierdo-Pulido, M.; Rupérez, P.; Goya, L.; Bravo, L. Dietary fibre composition, antioxidant capacity and physico-chemical properties of a fibre-rich product from cocoa (Theobroma cacao L.). Food Chem. 2007, 104, 948–954. [Google Scholar] [CrossRef]
- Shively, C.A.; Tarka, S.M.J. Methylxanthine composition and consumption patterns of cocoa and chocolate products. Prog. Clin. Biol Res. 1984, 158, 149–178. [Google Scholar]
- Calzavara-Pinton, P.; Calzavara-Pinton, I.; Arisi, M.; Rossi, M.T.; Scapagnini, G.; Davinelli, S.; Venturini, M. Cutaneous Photoprotective Activity of a Short-term Ingestion of High-Flavanol Cocoa: A Nutritional Intervention Study. Photochem. Photobiol. 2019, 95, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Ramiro-Puig, E.; Castell, M. Cocoa: Antioxidant and immunomodulator. Br. J. Nutr. 2009, 101, 931–940. [Google Scholar] [CrossRef]
- Périz, M.; Pérez-Cano, F.J.; Cambras, T.; Franch, À.; Best, I.; Pastor-Soplin, S.; Castell, M.; Massot-Cladera, M. Attenuating effect of Peruvian cocoa populations on the acute asthmatic response in Brown Norway rats. Nutrients 2020, 12, 2301. [Google Scholar] [CrossRef]
- Andújar, I.; Recio, M.C.; Giner, R.M.; Ríos, J.L. Cocoa polyphenols and their potential benefits for human health. Oxid. Med. Cell. Longev. 2012, 2012, 906252. [Google Scholar] [CrossRef]
- Vlachojannis, J.; Erne, P.; Zimmermann, B.; Chrubasik-Hausmann, S. The Impact of Cocoa Flavanols on Cardiovascular Health. Phyther. Res. 2016, 30, 1641–1657. [Google Scholar] [CrossRef] [PubMed]
- Ludovici, V.; Barthelmes, J.; Nägele, M.P.; Enseleit, F.; Ferri, C.; Flammer, A.J.; Ruschitzka, F.; Sudano, I. Cocoa, Blood Pressure, and Vascular Function. Front. Nutr. 2017, 4, 36. [Google Scholar] [CrossRef]
- Martins, T.F.; Palomino, O.M.; Álvarez-Cilleros, D.; Martín, M.A.; Ramos, S.; Goya, L. Cocoa Flavanols Protect Human Endothelial Cells from Oxidative Stress. Plant. Foods Hum. Nutr. 2020, 75, 161–168. [Google Scholar] [CrossRef]
- Selmi, C.; Mao, T.K.; Keen, C.L.; Schmitz, H.H.; Eric Gershwin, M. The anti-inflammatory properties of cocoa flavanols. J. Cardiovasc. Pharmacol. 2006, 47, S163–S176. [Google Scholar] [CrossRef] [PubMed]
- Socci, V.; Tempesta, D.; Desideri, G.; De Gennaro, L.; Ferrara, M. Enhancing Human Cognition with Cocoa Flavonoids. Front. Nutr. 2017, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- Camps-Bossacoma, M.; Massot-Cladera, M.; Abril-Gil, M.; Franch, A.; Pérez-Cano, F.J.; Castell, M. Cocoa Diet and Antibody Immune Response in Preclinical Studies. Front. Nutr. 2017, 4, 28. [Google Scholar] [CrossRef]
- Camps-Bossacoma, M.; Massot-Cladera, M.; Pérez-Cano, F.J.; Castell, M. Influence of a Cocoa-Enriched Diet on the Intestinal Immune System and Microbiota. In Dietary Interventions in Gastrointestinal Diseases: Foods, Nutrients, and Dietary Supplements; Watson, R.R., Preedy, V.R., Eds.; Academic Press (Elsevier Inc.): London, UK, 2019; pp. 213–225. ISBN 9780128144695. [Google Scholar]
- Ramiro, E.; Franch, A.; Castellote, C.; Pérez-Cano, F.; Permanyer, J.; Izquierdo-Pulido, M.; Castell, M. Flavonoids from Theobroma cacao down-regulate inflammatory mediators. J. Agric. Food Chem. 2005, 53, 8506–8511. [Google Scholar] [CrossRef]
- Castell, M.; Franch, A.; Ramos-Romero, S.; Ramiro-Puig, E.; Pérez-Cabo, F.J.; Castellote, C. Effect of a diet rich in cocoa flavonoids on experimental acute inflammation. In Flavonoids: Biosynthesis, Biological effects and Dietary Sources; Keller, R.B., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2009; Volume 6, pp. 213–229. ISBN 9781607416227. [Google Scholar]
- Ramos-Romero, S.; Pérez-Cano, F.J.; Castellote, C.; Castell, M.; Franch, À. Effect of cocoa-enriched diets on lymphocytes involved in adjuvant arthritis in rats. Br. J. Nutr. 2012, 107, 378–387. [Google Scholar] [CrossRef]
- Pérez-Berezo, T.; Ramiro-Puig, E.; Pérez-Cano, F.J.; Castellote, C.; Permanyer, J.; Franch, À.; Castell, M. Influence of a cocoa-enriched diet on specific immune response in ovalbumin-sensitized rats. Mol. Nutr. Food Res. 2009, 53, 389–397. [Google Scholar] [CrossRef]
- Ramiro-Puig, E.; Pérez-Cano, F.J.; Ramírez-Santana, C.; Castellote, C.; Izquierdo-Pulido, M.; Permanyer, J.; Franch, A.; Castell, M. Spleen lymphocyte function modulated by a cocoa-enriched diet. Clin. Exp. Immunol. 2007, 149, 535–542. [Google Scholar] [CrossRef]
- Ramiro-Puig, E.; Pérez-Cano, F.J.; Ramos-Romero, S.; Pérez-Berezo, T.; Castellote, C.; Permanyer, J.; Franch, À.; Izquierdo-Pulido, M.; Castell, M. Intestinal immune system of young rats influenced by cocoa-enriched diet. J. Nutr. Biochem. 2008, 19, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Camps-Bossacoma, M.; Abril-Gil, M.; Saldaña-Ruiz, S.; Franch, À.; Pérez-Cano, F.J.; Castell, M. Cocoa diet prevents antibody synthesis and modifies lymph node composition and functionality in a rat oral sensitization model. Nutrients 2016, 8, 242. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Getahun, A.; Heiser, R.A.; Detanico, T.O.; Aviszus, K.; Kirchenbaum, G.A.; Casper, T.L.; Huang, C.; Aydintug, M.K.; Carding, S.R.; et al. γδ T Cells Shape Preimmune Peripheral B Cell Populations. J. Immunol. 2016, 196, 217–231. [Google Scholar] [CrossRef]
- Deniz, G.; Erten, G.; Kücüksezer, U.C.; Kocacik, D.; Karagiannidis, C.; Aktas, E.; Akdis, C.A.; Akdis, M. Regulatory NK cells suppress antigen-specific T cell responses. J. Immunol. 2008, 180, 850–857. [Google Scholar] [CrossRef]
- Steinke, J.W.; Borish, L. Th2 cytokines and asthma. Interleukin-4: Its role in the pathogenesis of asthma, and targeting it for asthma treatment with interleukin-4 receptor antagonists. Respir. Res. 2001, 2, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Abril-Gil, M.; Pérez-Cano, F.J.; Franch, À.; Castell, M. Effect of a cocoa-enriched diet on immune response and anaphylaxis in a food allergy model in Brown Norway rats. J. Nutr. Biochem. 2016, 27, 317–326. [Google Scholar] [CrossRef]
- Kang, H.; Lee, C.H.; Kim, J.-E.R.E.; Kwon, J.Y.; Son, M.-J.J.; Kim, J.-E.R.E.; Lee, K.W. Theobroma cacao extract attenuates the development of Dermatophagoides farinae-induced atopic dermatitis-like symptoms in NC/Nga mice. Food Chem. 2017, 216, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Lagunas, M.J.; Vicente, F.; Pereira, P.; Castell, M.; Pérez-Cano, F.J. Relationship between cocoa intake and healthy status: A pilot study in university students. Molecules 2019, 24, 812. [Google Scholar] [CrossRef] [PubMed]
- Papi, A.; Brightling, C.; Pedersen, S.E.; Reddel, H.K. Asthma. Lancet 2018, 391, 783–800. [Google Scholar] [CrossRef]
- Boonpiyathad, T.; Celebi Sözener, Z.; Satitsuksonoa, P.; Akdis, C.A. Immunological mechanisms in asthma. Semin. Immunol. 2019, 46, 101333. [Google Scholar] [CrossRef] [PubMed]
- Nauta, A.J.; Engels, F.; Knippels, L.M.; Garssen, J.; Nijkamp, F.P.; Redegeld, F.A. Mechanisms of allergy and asthma. Eur. J. Pharmacol. 2008, 585, 354–360. [Google Scholar] [CrossRef]
- Nakagome, K.; Nagata, M. Involvement and Possible Role of Eosinophils in Asthma Exacerbation. Front. Immunol. 2018, 9, 2220. [Google Scholar] [CrossRef] [PubMed]
- Périz, M.; Pérez-Cano, F.J.; Rodríguez-Lagunas, M.J.; Cambras, T.; Pastor-Soplin, S.; Best, I.; Castell, M.; Massot-Cladera, M. Development and characterization of an allergic asthma rat model for interventional studies. Int. J. Mol. Sci. 2020, 21, 3841. [Google Scholar] [CrossRef]
- Estruel-Amades, S.; Ruiz-Iglesias, P.; Périz, M.; Franch, À.; Pérez-Cano, F.J.; Camps-Bossacoma, M.; Castell, M. Changes in Lymphocyte Composition and Functionality After Intensive Training and Exhausting Exercise in Rats. Front. Physiol. 2019, 10, 1491. [Google Scholar] [CrossRef]
- Massot-Cladera, M.; Franch, À.; Pérez-Cano, F.J.; Castell, M. Cocoa and cocoa fibre differentially modulate IgA and IgM production at mucosal sites. Br. J. Nutr. 2016, 115, 1539–1546. [Google Scholar] [CrossRef][Green Version]
- Abril-Gil, M.; Garcia-Just, A.; Pérez-Cano, F.J.; Franch, À.; Castell, M. Development and characterization of an effective food allergy model in Brown Norway rats. PLoS ONE 2015, 10, e0125314. [Google Scholar] [CrossRef]
- Abril-Gil, M.; Massot-Cladera, M.; Pérez-Cano, F.J.; Castellote, C.; Franch, A.; Castell, M. A diet enriched with cocoa prevents IgE synthesis in a rat allergy model. Pharmacol. Res. 2012, 65, 603–608. [Google Scholar] [CrossRef]
- Thakur, V.R.; Khuman, V.; Beladiya, J.V.; Chaudagar, K.K.; Mehta, A.A. An experimental model of asthma in rats using ovalbumin and lipopolysaccharide allergens. Heliyon 2019, 5, e02864. [Google Scholar] [CrossRef]
- Bui, T.T.; Piao, C.H.; Song, C.H.; Lee, C.H.; Shin, H.S.; Chai, O.H. Baicalein, wogonin, and Scutellaria baicalensis ethanol extract alleviate ovalbumin-induced allergic airway inflammation and mast cell-mediated anaphylactic shock by regulation of Th1/Th2 imbalance and histamine release. Anat. Cell Biol. 2017, 50, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Zemmouri, H.; Sekiou, O.; Ammar, S.; El Feki, A.; Bouaziz, M.; Messarah, M.; Boumendjel, A. Urtica dioica attenuates ovalbumin-induced inflammation and lipid peroxidation of lung tissues in rat asthma model. Pharm. Biol. 2017, 55, 1561–1568. [Google Scholar] [CrossRef] [PubMed]
- Rosa, S.I.G.; Rios-Santos, F.; Balogun, S.O.; de Almeida, D.A.T.; Damazo, A.S.; da Cruz, T.C.D.; Pavan, E.; Barbosa, R.d.S.; Alvim, T.d.C.; Soares, I.M.; et al. Hydroethanolic extract from Echinodorus scaber Rataj leaves inhibits inflammation in ovalbumin-induced allergic asthma. J. Ethnopharmacol. 2017, 203, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Huang, S.; Wang, C.; Zhang, H.; Fang, S.; Zhang, Y. Anti-inflammatory effects of dihydromyricetin in a mouse model of asthma. Mol. Med. Rep. 2017, 15, 3674–3680. [Google Scholar] [CrossRef]
- Dogan, M.F.; Parlar, A.; Cam, S.A.; Tosun, E.M.; Uysal, F.; Arslan, S.O. Glabridin attenuates airway inflammation and hyperresponsiveness in a mice model of ovalbumin-induced asthma. Pulm. Pharmacol. Ther. 2020, 63, 101936. [Google Scholar] [CrossRef]
- Yuan, W.Y.; Li, L.Q.; Chen, Y.Y.; Zhou, Y.J.; Bao, K.F.; Zheng, J.; Hua, Y.Q.; Jiang, G.R.; Hong, M. Frontline Science: Two flavonoid compounds attenuate allergic asthma by regulating epithelial barrier via G protein-coupled estrogen receptor: Probing a possible target for allergic inflammation. J. Leukoc. Biol. 2020, 108, 59–71. [Google Scholar] [CrossRef]
- Roy, S.; Manna, K.; Jha, T.; Saha, K.D. Chrysin-loaded PLGA attenuates OVA-induced allergic asthma by modulating TLR/NF-κB/NLRP3 axis. Nanomed. Nanotechnol. Biol. Med. 2020, 30, 102292. [Google Scholar] [CrossRef]
- Gloudemans, A.K.; Lambrecht, B.N.; Smits, H.H. Potential of immunoglobulin A to prevent allergic asthma. Clin. Dev. Immunol. 2013, 2013, 542091. [Google Scholar] [CrossRef]
- Kumar, R.K.; Temelkovski, J.; McNeil, H.P.; Hunter, N. Airway inflammation in a murine model of chronic asthma: Evidence for a local humoral immune response. Clin. Exp. Allergy 2000, 30, 1486–1492. [Google Scholar] [CrossRef]
- Ward, M.D.W.; Chung, Y.J.; Haykal-Coates, N.; Copeland, L.B. Differential allergy responses to Metarhizium anisopliae fungal component extracts in BALB/c mice Allergy-like responses to fungal components. J. Immunotoxicol. 2009, 6, 62–73. [Google Scholar] [CrossRef]
- Pérez-Berezo, T.; Franch, A.; Ramos-Romero, S.; Castellote, C.; Pérez-Cano, F.J.; Castell, M. Cocoa-enriched diets modulate intestinal and systemic humoral immune response in young adult rats. Mol. Nutr. Food Res. 2011, 55, S56–S66. [Google Scholar] [CrossRef]
- Pérez-Berezo, T.; Franch, A.; Castellote, C.; Castell, M.; Pérez-Cano, F.J. Mechanisms involved in down-regulation of intestinal IgA in rats by high cocoa intake. J. Nutr. Biochem. 2012, 23, 838–844. [Google Scholar] [CrossRef]
- Massot-Cladera, M.; Mayneris-Perxachs, J.; Costabile, A.; Swann, J.R.; Franch, À.; Pérez-Cano, F.J.; Castell, M. Association between urinary metabolic profile and the intestinal effects of cocoa in rats. Br. J. Nutr. 2017, 117, 623–634. [Google Scholar] [CrossRef][Green Version]
- Massot-Cladera, M.; Franch, A.; Castellote, C.; Castell, M.; Pérez-Cano, F.J. Cocoa flavonoid-enriched diet modulates systemic and intestinal immunoglobulin synthesis in adult Lewis rats. Nutrients 2013, 5, 3272–3286. [Google Scholar] [CrossRef] [PubMed]
- Massot-Cladera, M.; Abril-Gil, M.; Torres, S.; Franch, A.; Castell, M.; Pérez-Cano, F.J. Impact of cocoa polyphenol extracts on the immune system and microbiota in two strains of young rats. Br. J. Nutr. 2014, 112, 1944–1954. [Google Scholar] [CrossRef] [PubMed]
- Camps-Bossacoma, M.; Pérez-Cano, F.J.; Franch, À.; Castell, M. Theobromine is responsible for the effects of cocoa on the antibody immune status of rats. J. Nutr. 2018, 148, 464–471. [Google Scholar] [CrossRef] [PubMed]

| APC Diet | CMC Diet | OC Diet | REF Diet | |
|---|---|---|---|---|
| Casein (g/kg) | 126 | 126 | 126 | 140 |
| L-Cystine (g/kg) | 1.62 | 1.62 | 1.62 | 1.8 |
| Corn Starch (g/kg) | 419.12 | 419.12 | 419.12 | 466 |
| Maltodextrin (g/kg) | 139.5 | 139.5 | 139.5 | 155 |
| Sucrose (g/kg) | 90 | 90 | 90 | 100 |
| Soybean oil (g/kg) | 36 | 36 | 36 | 40 |
| Cellulose (g/kg) | 45 | 45 | 45 | 50 |
| Mineral mix (g/kg) | 31.5 | 31.5 | 31.5 | 35.0 |
| Vitamin mix (g/kg) | 9 | 9 | 9 | 10 |
| Choline bitartrate (g/kg) | 2.25 | 2.25 | 2.25 | 2.50 |
| TBHQ (antioxidant) (g/kg) | 0.007 | 0.007 | 0.007 | 0.008 |
| Cocoa paste (g/kg): | 100 | 100 | 100 | - |
| Total phenolics (g gallic acid equivalents/kg) | 2.87 | 3.04 | 2.41 | - |
| Total flavonoids (g gallic acid equivalents/kg) | 5.04 | 5.66 | 3.48 | - |
| Total methylxanthines (g/kg) | 0.841 | 0.966 | 0.799 | - |
| REF Group | A Group | OC Group | APC Group | CMC Group | |
|---|---|---|---|---|---|
| Total leukocyte counts (×109/L) | 11.22 ± 0.86 | 10.23 ± 1.69 | 10.76 ± 1.40 | 9.92 ± 1.08 | 10.08 ± 1.38 |
| Lymphocyte (%) | 70.79 ± 2.08 a | 64.03 ± 1.56 b | 63.07 ± 8.70 b | 64.26 ± 7.72 b | 59.03 ± 3.93 b |
| Monocyte (%) | 6.36 ± 0.15 a | 6.24 ± 0.21 ab | 5.96 ± 0.08 b | 5.84 ± 0.70 b | 5.91 ± 0.23 ab |
| Granulocyte (%) | 22.86 ± 2.07 a | 29.72 ± 1.72 b | 31.01 ± 4.68 b | 29.90 ± 3.85 b | 35.06 ± 3.98 b |
| REF Group | A Group | OC Group | APC Group | CMC Group | |
|---|---|---|---|---|---|
| Total leukocyte counts (×108/L) | 1.31 ± 0.10 a | 2.07 ± 0.31 b | 1.60 ± 0.15 ab | 2.47 ± 0.42 b | 2.42 ± 0.36 b |
| Lymphocyte (%) | 32.65 ± 2.43 | 32.43 ± 2.98 | 35.06 ± 3.85 | 46.35 ± 6.47 | 42.11 ± 4.58 |
| Monocyte (%) | 14.63 ± 0.77 | 12.39 ± 0.58 | 12.81 ± 0.56 | 12.32 ± 0.93 | 12.75 ± 1.43 |
| Granulocyte (%) | 52.72 ± 2.85 | 55.17 ± 2.88 | 52.13 ±3.97 | 41.33 ± 6.60 | 45.13 ± 4.77 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Périz, M.; Rodríguez-Lagunas, M.J.; Pérez-Cano, F.J.; Best, I.; Pastor-Soplin, S.; Castell, M.; Massot-Cladera, M. Influence of Consumption of Two Peruvian Cocoa Populations on Mucosal and Systemic Immune Response in an Allergic Asthma Rat Model. Nutrients 2022, 14, 410. https://doi.org/10.3390/nu14030410
Périz M, Rodríguez-Lagunas MJ, Pérez-Cano FJ, Best I, Pastor-Soplin S, Castell M, Massot-Cladera M. Influence of Consumption of Two Peruvian Cocoa Populations on Mucosal and Systemic Immune Response in an Allergic Asthma Rat Model. Nutrients. 2022; 14(3):410. https://doi.org/10.3390/nu14030410
Chicago/Turabian StylePériz, Marta, Maria J. Rodríguez-Lagunas, Francisco J. Pérez-Cano, Ivan Best, Santiago Pastor-Soplin, Margarida Castell, and Malén Massot-Cladera. 2022. "Influence of Consumption of Two Peruvian Cocoa Populations on Mucosal and Systemic Immune Response in an Allergic Asthma Rat Model" Nutrients 14, no. 3: 410. https://doi.org/10.3390/nu14030410
APA StylePériz, M., Rodríguez-Lagunas, M. J., Pérez-Cano, F. J., Best, I., Pastor-Soplin, S., Castell, M., & Massot-Cladera, M. (2022). Influence of Consumption of Two Peruvian Cocoa Populations on Mucosal and Systemic Immune Response in an Allergic Asthma Rat Model. Nutrients, 14(3), 410. https://doi.org/10.3390/nu14030410











