Association of Vasopressors Dose Trajectories with Enteral Nutrition Tolerance in Patients with Shock: A Prospective Observational Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patients
2.3. Nutrition Strategy
2.4. Outcomes Measurement
2.5. Statistical Analyses
3. Results
3.1. Participants
3.2. Baseline Characteristics of Patients in Each Trajectory Group
3.3. Clinical Outcomes
3.4. Cox Regression and Kaplan–Meier Survival Curve Analyses of Trajectories
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wischmeyer, P.E. Enteral Nutrition Can Be Given to Patients on Vasopressors. Crit. Care Med. 2020, 48, 122–125. [Google Scholar] [CrossRef]
- McClave, S.A.; Martindale, R.G.; Rice, T.W.; Heyland, D.K. Feeding the critically ill patient. Crit. Care Med. 2014, 42, 2600–2610. [Google Scholar] [CrossRef]
- Yang, S.; Wu, X.; Yu, W.; Li, J. Early enteral nutrition in critically ill patients with hemodynamic instability: An evidence-based review and practical advice. Nutr. Clin. Pract. 2014, 29, 90–96. [Google Scholar] [CrossRef]
- Arabi, Y.M.; McClave, S.A. Enteral Nutrition Should Not Be Given to Patients on Vasopressor Agents. Crit. Care Med. 2020, 48, 119–121. [Google Scholar] [CrossRef]
- Shukla, A.; Chapman, M.; Patel, J.J. Enteral nutrition in circulatory shock: Friend or foe? Curr. Opin. Clin. Nutr. Metab. Care 2021, 24, 159–164. [Google Scholar] [CrossRef]
- Mancl, E.E.; Muzevich, K.M. Tolerability and safety of enteral nutrition in critically ill patients receiving intravenous vasopressor therapy. JPEN J. Parenter. Enter. Nutr. 2013, 37, 641–651. [Google Scholar] [CrossRef]
- Merchan, C.; Altshuler, D.; Aberle, C.; Papadopoulos, J.; Schwartz, D. Tolerability of Enteral Nutrition in Mechanically Ventilated Patients With Septic Shock Who Require Vasopressors. J. Intensive Care Med. 2016, 32, 540–546. [Google Scholar] [CrossRef]
- Ohbe, H.; Jo, T.; Matsui, H.; Fushimi, K.; Yasunaga, H. Differences in effect of early enteral nutrition on mortality among ventilated adults with shock requiring low-, medium-, and high-dose noradrenaline: A propensity-matched analysis. Clin. Nutr. 2020, 39, 460–467. [Google Scholar] [CrossRef]
- Lasierra, J.L.F.; Pérez-Vela, J.L.; Makikado, L.D.U.; Sánchez, E.T.; Gómez, L.C.; Rodríguez, B.M.; López, P.A.; de la Camara, A.G.; González, J.C.M. Early Enteral Nutrition in Patients With Hemodynamic Failure Following Cardiac Surgery. J. Parenter. Enter. Nutr. 2013, 39, 154–162. [Google Scholar] [CrossRef]
- Umezawa Makikado, L.D.; Flordelis Lasierra, J.L.; Perez-Vela, J.L.; Colino Gomez, L.; Torres Sanchez, E.; Maroto Rodriguez, B.; Montejo González, J.C. Early enteral nutrition in adults receiving venoarterial extracorporeal membrane oxygenation: An observational case series. JPEN J. Parenter. Enter. Nutr. 2013, 37, 281–284. [Google Scholar] [CrossRef]
- Patel, J.J.; Kozeniecki, M.; Peppard, W.J.; Peppard, S.R.; Zellner-Jones, S.; Graf, J.; Heyland, D.K. Phase 3 Pilot Randomized Controlled Trial Comparing Early Trophic Enteral Nutrition With “No Enteral Nutrition” in Mechanically Ventilated Patients With Septic Shock. J. Parenter. Enter. Nutr. 2019, 44, 866–873. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, H.; Cheng, Y.; Fu, X.; Yao, H.; Jin, X.; Kang, Y.; Wu, Q. Mean arterial pressure/norepinephrine equivalent dose index as an early measure of initiation time for enteral nutrition in patients with shock: A prospective observational study. Nutrition 2022, 96, 111586. [Google Scholar] [CrossRef] [PubMed]
- Khalid, I.; Doshi, P.; DiGiovine, B. Early Enteral Nutrition and Outcomes of Critically Ill Patients Treated With Vasopressors and Mechanical Ventilation. Am. J. Crit. Care 2010, 19, 261–268. [Google Scholar] [CrossRef]
- Ewy, M.; Aqeel, M.; Kozeniecki, M.; Patel, K.; Banerjee, A.; Heyland, D.K.; Patel, J.J. Impact of Enteral Feeding on Vasoactive Support in Septic Shock: A Retrospective Observational Study. Nutr. Clin. Pract. 2020, 35, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Lasierra, J.L.F.; González, J.C.M.; Delgado, J.C.L.; Chug, P.Z.; Lozano-Aranaga, F.M.; Cárdenas, C.L.; Laguna, M.L.B.; Maichle, S.; Almanza, L.J.T.; Martínez, M.V.T.; et al. Enteral nutrition in critically ill patients under vasoactive drug therapy: The NUTRIVAD study. JPEN J. Parenter. Enter. Nutr. 2022, 46, 1420–1430. [Google Scholar] [CrossRef]
- Ortiz-Reyes, L.; Patel, J.J.; Jiang, X.; Coz Yataco, A.; Day, A.G.; Shah, F.; Heyland, D.K. Early versus delayed enteral nutrition in mechanically ventilated patients with circulatory shock: A nested cohort analysis of an international multicenter, pragmatic clinical trial. Crit. Care 2022, 26, 173. [Google Scholar] [CrossRef]
- Reintam Blaser, A.; Starkopf, J.; Alhazzani, W.; Berger, M.M.; Casaer, M.P.; Deane, A.M.; Oudemans-van Straaten, H.M. Early enteral nutrition in critically ill patients: ESICM clinical practice guidelines. Intensive Care Med. 2017, 43, 380–398. [Google Scholar] [CrossRef]
- Taylor, B.E.; McClave, S.A.; Martindale, R.G.; Warren, M.M.; Johnson, D.R.; Braunschweig, C.; McCarthy, M.S.; Davanos, E.; Rice, T.W.; Cresci, G.A.; et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient. Crit. Care Med. 2016, 44, 390–438. [Google Scholar] [CrossRef]
- Singer, P.; Blaser, A.R.; Berger, M.M.; Alhazzani, W.; Calder, P.C.; Casaer, M.P.; Hiesmayr, M.; Mayer, K.; Montejo, J.C.; Pichard, C.; et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin. Nutr. 2019, 38, 48–79. [Google Scholar] [CrossRef]
- Elke, G.; Hartl, W.H.; Kreymann, K.G.; Adolph, M.; Felbinger, T.W.; Graf, T.; de Heer, G.; Heller, A.R.; Kampa, U.; Mayer, K.; et al. Clinical Nutrition in Critical Care Medicine—Guideline of the German Society for Nutritional Medicine (DGEM). Clin. Nutr. ESPEN 2019, 33, 220–275. [Google Scholar] [CrossRef]
- Preiser, J.-C.; Arabi, Y.M.; Berger, M.M.; Casaer, M.; McClave, S.; Montejo-González, J.C.; Peake, S.; Blaser, A.R.; Berghe, G.V.D.; van Zanten, A.; et al. A guide to enteral nutrition in intensive care units: 10 expert tips for the daily practice. Crit. Care 2021, 25, 424. [Google Scholar] [CrossRef] [PubMed]
- Bechtold, M.L.; Brown, P.M.; Escuro, A.; Grenda, B.; Johnston, T.; Kozeniecki, M.; ASPEN Enteral Nutrition Committee. When is enteral nutrition indicated? JPEN J. Parenter. Enter. Nutr. 2022, 46, 1470–1496. [Google Scholar] [CrossRef] [PubMed]
- Simo Es Covello, L.H.; Gava-Brandolis, M.G.; Castro, M.G.; Dos Santos Netos, M.F.; Manzanares, W.; Toledo, D.O. Vasopressors and Nutrition Therapy: Safe Dose for the Outset of Enteral Nutrition? Crit. Care Res. Pract. 2020, 2020, 1095693. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.M. Vasoactive substances and their effects on nutrition in the critically ill patient. Nutr. Clin. Pract. 2012, 27, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Piton, G.; Cypriani, B.; Regnard, J.; Patry, C.; Puyraveau, M.; Capellier, G. Catecholamine use is associated with enterocyte damage in critically ill patients. Shock 2015, 43, 437–442. [Google Scholar] [CrossRef]
- Reignier, J.; Boisramé-Helms, J.; Brisard, L.; Lascarrou, J.-B.; Ait Hssain, A.; Anguel, N.; Djibre, M.; Ganster, F.; Devaquet, J.; Canet, T.; et al. Enteral versus parenteral early nutrition in ventilated adults with shock: A randomised, controlled, multicentre, open-label, parallel-group study (NUTRIREA-2). Lancet 2018, 391, 133–143. [Google Scholar] [CrossRef]
- Cecconi, M.; De Backer, D.; Antonelli, M.; Beale, R.; Bakker, J.; Hofer, C.K.; Jaeschke, R.; Mebazaa, A.; Pinsky, M.R.; Teboul, J.-L.; et al. Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensive Care Med. 2014, 40, 1795–1815. [Google Scholar] [CrossRef]
- Khanna, A.; English, S.W.; Wang, X.S.; Ham, K.; Tumlin, J.; Szerlip, H.; Busse, L.W.; Altaweel, L.; Albertson, T.E.; Mackey, C.; et al. Angiotensin II for the Treatment of Vasodilatory Shock. N. Engl. J. Med. 2017, 377, 419–430. [Google Scholar] [CrossRef]
- Heyland, D.K.; Cahill, N.E.; Dhaliwal, R.; Wang, M.; Day, A.G.; Alenzi, A.; Aris, F.; Muscedere, J.; Drover, J.W.; McClave, S.A. Enhanced protein-energy provision via the enteral route in critically ill patients: A single center feasibility trial of the PEP uP protocol. Crit. Care 2010, 14, R78. [Google Scholar] [CrossRef]
- Schott, U.; Kander, T. NOMI after cardiac arrest. Could refined diagnostics improve outcome? Resuscitation 2020, 157, 266–268. [Google Scholar] [CrossRef]
- Lennon, H.; Kelly, S.; Sperrin, M.; Buchan, I.; Cross, A.J.; Leitzmann, M.; Cook, M.B.; Renehan, A.G. Framework to construct and interpret latent class trajectory modelling. BMJ Open 2018, 8, e020683. [Google Scholar] [CrossRef] [PubMed]
- Proust-Lima, C.; Philipps, V.; Liquet, B. Estimation of Extended Mixed Models Using Latent Classes and Latent Processes: The R Package lcmm. J. Stat. Softw. 2017, 78, 1–56. [Google Scholar] [CrossRef]
- Patel, J.J.; Rice, T.; Heyland, D.K. Safety and Outcomes of Early Enteral Nutrition in Circulatory Shock. JPEN J. Parenter. Enter. Nutr. 2020, 44, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Ram, N.; Grimm, K.J. Growth Mixture Modeling: A Method for Identifying Differences in Longitudinal Change Among Unobserved Groups. Int. J. Behav. Dev. 2009, 33, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Piton, G.; Le Gouge, A.; Boisramé-Helms, J.; Anguel, N.; Argaud, L.; Asfar, P.; Botoc, V.; Bretagnol, A.; Brisard, L.; Bui, H.-N.; et al. Factors associated with acute mesenteric ischemia among critically ill ventilated patients with shock: A post hoc analysis of the NUTRIREA2 trial. Intensive Care Med. 2022, 48, 458–466. [Google Scholar] [CrossRef] [PubMed]

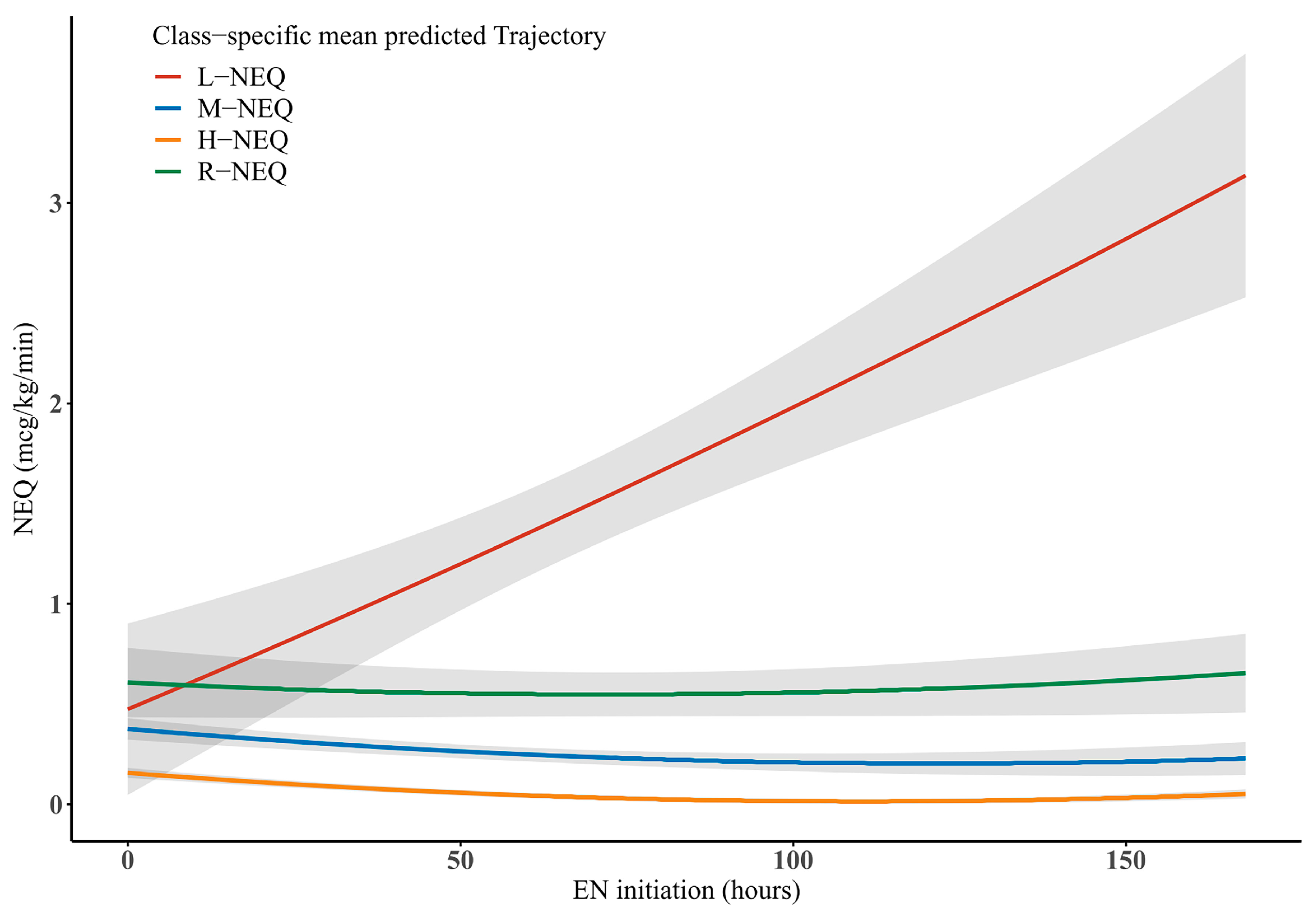

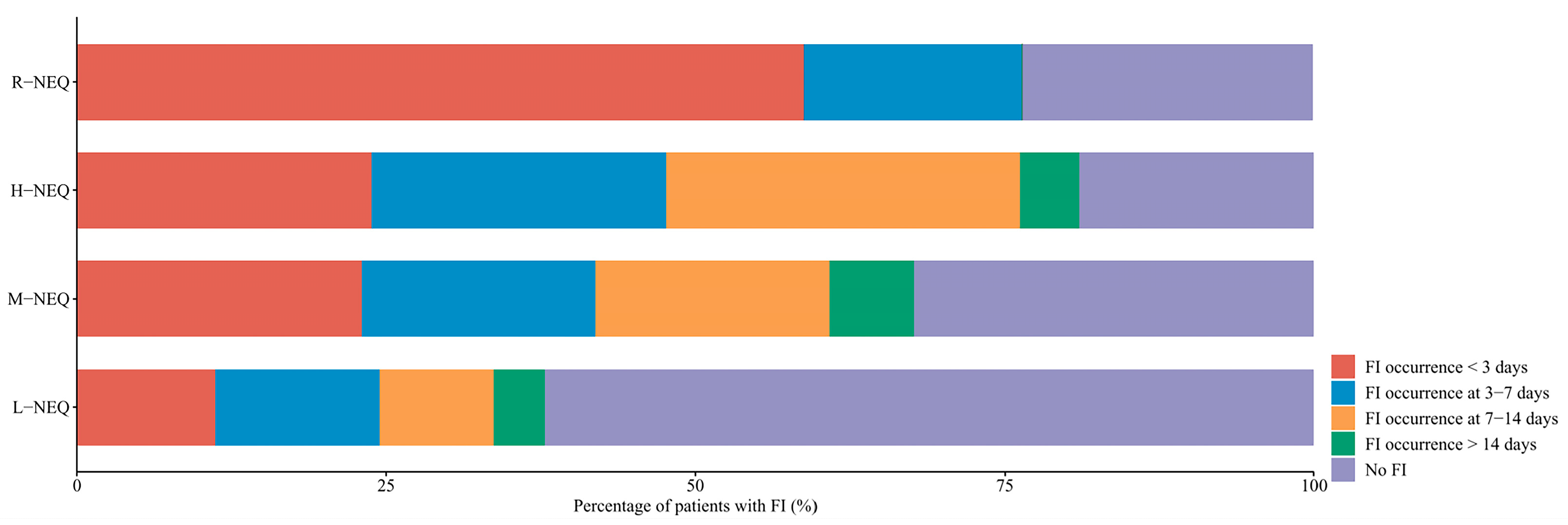
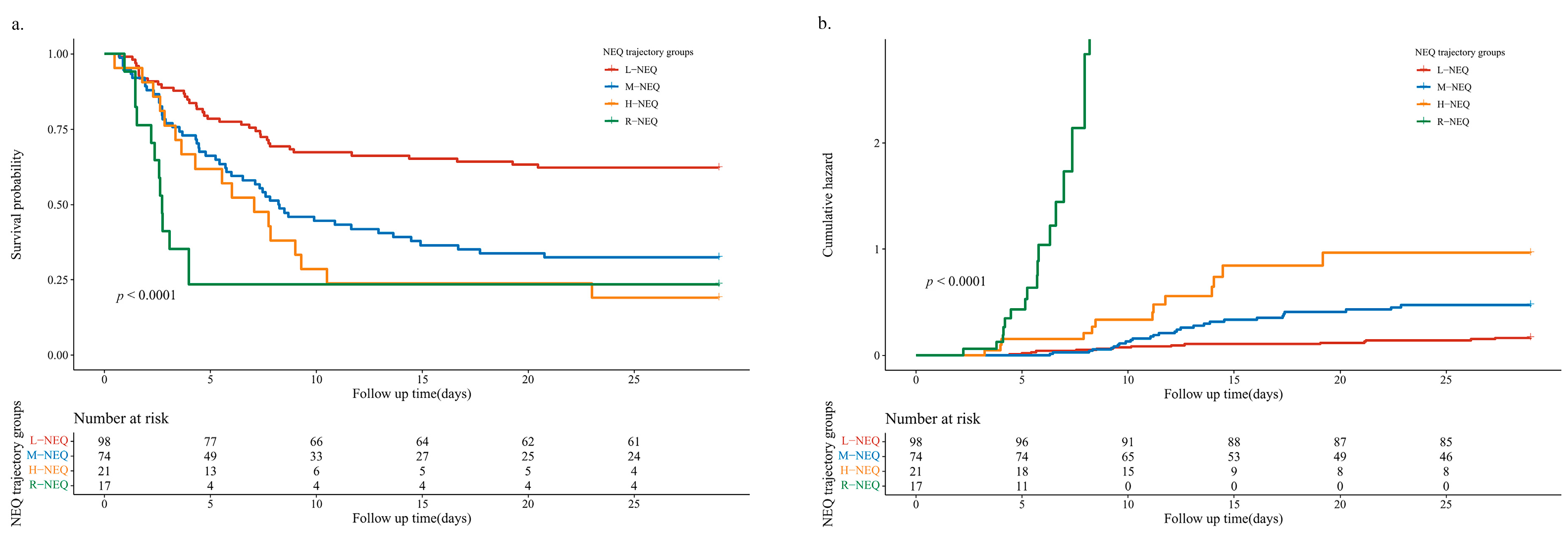
| Characteristics | Total (n = 210) | L-NEQ (n = 98) | M-NEQ (n = 74) | H-NEQ (n = 21) | R-NEQ (n = 17) | p Value |
|---|---|---|---|---|---|---|
| Age (years), median (IQR) | 64 (49.8–72) | 63 (48–71.3) | 64.5 (52–70) | 67 (53.5–76.5) | 62 (45.5–73) | 0.462 |
| Male Sex—No. (%) | 141 (67.14) | 66 (67.35) | 47 (63.51) | 14 (66.67) | 14 (82.35) | 0.526 |
| BMI, median (IQR) | 22.9 (20.2–24.8) | 23.9 (21.5–25.8) | 22.3 (19.9–24.7) | 22 (19.5–23.8) | 18.7 (17.2–23.5) | <0.001 |
| APACHE II score, median (IQR) | 20.9 (14–26) | 20.9 (15–26) | 20 (12.8–25.3) | 25 (15.5–27.5) | 24 (12–29) | 0.548 |
| NRS2002 score, median (IQR) | 3 (3–4) | 3 (3–4) | 3 (3–4.3) | 4 (3–5.5) | 4 (3–6) | 0.047 |
| Diagnose—No. (%) | ||||||
| Severe pneumonia | 92 (43.81) | 34 (34.69) | 38 (51.35) | 13 (61.9) | 7 (41.18) | 0.049 |
| ARDS | 20 (9.52) | 10 (10.2) | 4 (5.41) | 3 (14.29) | 3 (17.65) | 0.339 |
| MODS | 29 (13.81) | 14 (14.29) | 8 (10.81) | 2 (9.52) | 5 (29.41) | 0.223 |
| Cardiac arrest | 24 (11.43) | 14 (14.29) | 6 (8.11) | 2 (9.52) | 2 (11.76) | 0.643 |
| Cardiovascular disease 1 | 130 (61.9) | 57 (58.16) | 46 (62.16) | 14 (66.67) | 13 (76.47) | 0.51 |
| Neurological disease 2 | 85 (40.48) | 42 (42.86) | 30 (40.54) | 10 (47.62) | 3 (17.65) | 0.226 |
| Types of shock—No. (%) | ||||||
| Sepsis shock | 178 (84.76) | 80 (81.63) | 62 (83.78) | 19 (90.48) | 17 (100) | 0.223 |
| Cardiac shock | 22 (10.48) | 10 (10.2) | 10 (13.51) | 1 (4.76) | 1 (5.88) | 0.604 |
| Hemorrhagic shock | 25 (11.9) | 13 (13.27) | 10 (13.51) | 2 (9.52) | 0 (0) | 0.429 |
| Laboratory data, median (IQR) | ||||||
| White blood cell (×109/L) | 10.6 (8–14.3) | 10 (7.6–13.7) | 11.5 (8.2–14.3) | 13.7 (9.3–16.2) | 10.4 (4.5–18.8) | 0.124 |
| Platelet (×109/L) | 97.5 (52–166) | 95.5 (55.5–205.3) | 109 (61–171) | 87 (40–129.5) | 72 (22.5–130.5) | 0.122 |
| Hemoglobin (g/L) | 87 (76.8–100) | 83.5 (72–98.3) | 89 (78.8–100.3) | 91 (76.5–103.5) | 87 (74.5–118) | 0.197 |
| Albumin (g/L) | 32.4 (29.4–35.8) | 32.3 (29.3–36) | 31.8 (28.6–34.8) | 30.8 (28.8–37.3) | 34.4 (32.7–37) | 0.127 |
| Total bilirubin (μmol/L) | 17 (10.3–33.1) | 16.9 (10.3–32.1) | 17.9 (8.6–33.7) | 15.9 (12.3–21.2) | 27.6 (15.8–41.2) | 0.223 |
| Serum creatinine (μmol/L) | 93 (60.8–152.3) | 93 (62.8–164.5) | 81.5 (57.8–150.8) | 100 (72–142.5) | 90 (63.5–124.5) | 0.659 |
| Glucose (mmol/L) | 9 (6.6–12.2) | 8.4 (6.5–11.4) | 9.3 (6.7–13.5) | 10.5 (8.7–12.6) | 8.9 (7–11.8) | 0.215 |
| C-reactive protein (mg/L) | 117.5 (65.2–167) | 93 (56.4–136.3) | 122.5 (69.1–172.3) | 162 (123–236.5) | 107 (39.3–178) | 0.005 |
| Procalcitonin (μg/L) | 1.8 (0.6–9.4) | 1.6 (0.4–6.9) | 1.8 (0.7–7.2) | 10.3 (0.8–21.7) | 1.3 (0.7–8.2) | 0.113 |
| Interleukin-6 (μg/L) | 74.5 (33.5–231.8) | 64.8 (26.5–151.5) | 101 (38.6–281.6) | 140 (43.9–523.7) | 64.9 (37–168.7) | 0.102 |
| Lactic acid (mmol/L) | 1.7 (1.3–2.2) | 1.5 (1.2–1.8) | 1.8 (1.4–2.3) | 1.8 (1.4–2.5) | 2.4 (1.4–3.6) | <0.001 |
| Arterial partial pressure of oxygen (mmHg) | 98.9 (72.9–127.3) | 104.4 (78.9–122.6) | 98.3 (70.7–126.1) | 93.5 (62.8–132.8) | 77.9 (69.2–124.8) | 0.569 |
| Arterial oxygen saturation (%) | 98.7 (95.6–99.4) | 98.8 (96.5–99.6) | 98.6 (96.2–99.3) | 98.5 (91–99.3) | 96.8 (93.8–99.3) | 0.456 |
| Sedation and analgesia, mean ± SD | ||||||
| Midazolam (mg/kg/day) | 1.1 ± 2.9 | 0.7 ± 1 | 0.7 ± 1.1 | 1.9 ± 2.6 | 3.8 ± 9.1 | <0.001 |
| Propofol (mg/kg/day) | 5.3 ± 6.4 | 5.5 ± 6.7 | 4.3 ± 5.6 | 5.4 ± 5.5 | 8 ± 8 | 0.195 |
| Dexmedetomidine (μg/kg/day) | 2.3 ± 4.4 | 1.9 ± 2.1 | 3.1 ± 6.7 | 1.4 ± 2.4 | 2.9 ± 3.6 | 0.278 |
| Remifentanil (μg/kg/day) | 37.6 ± 60.8 | 33.8 ± 62.6 | 40.4 ± 56.8 | 44.1 ± 70.9 | 39.3 ± 58.2 | 0.856 |
| Sufentanyl (μg/kg/day) | 1.3 ± 2 | 1 ± 1.7 | 1.2 ± 1.9 | 2.1 ± 2.2 | 2.6 ± 2.9 | 0.004 |
| Treatment—No.(%) | ||||||
| Norepinephrine at EN initiation (μg/kg/min), median (IQR) | 0.3 (0.1–0.5) | 0.2 (0.1–0.3) | 0.4 (0.2–0.6) | 0.4 (0.3–0.6) | 0.5 (0.4–0.7) | <0.001 |
| NEQ at EN initiation (μg/kg/min), median (IQR) | 0.3 (0.1–0.5) | 0.2 (0.1–0.2) | 0.4 (0.2–0.6) | 0.4 (0.3–0.6) | 0.5 (0.4–0.7) | <0.001 |
| Prokinetics | 94 (44.76) | 53 (54.08) | 28 (37.84) | 10 (47.62) | 3 (17.65) | 0.019 |
| CRRT | 76 (36.36) | 30 (30.93) | 29 (39.19) | 9 (42.86) | 8 (47.06) | 0.437 |
| ECMO | 13 (6.22) | 9 (9.28) | 2 (2.7) | 0 (0) | 2 (11.76) | 0.144 |
| Inotropic drugs use | 85 (40.48) | 22 (22.45) | 36 (48.65) | 16 (76.19) | 11 (64.71) | <0.001 |
| Dobutamine (daily dose) during the whole follow-up (μg/kg/day), mean ± SD | 0.1 ± 0.9 | 0.0 ± 0.0 | 0.1 ± 0.9 | 0.2 ± 0.7 | 0.6 ± 2.3 | 0.091 |
| Cumulative fluid balance in the first week (L), mean ± SD | 2.5 ± 5.7 | 0.8 ± 4.3 | 3.2 ± 5.9 | 5.4 ± 8.9 | 5.4 ± 3.9 | <0.001 |
| Characteristics | Total (n = 210) | L-NEQ (n = 98) | M-NEQ (n = 74) | H-NEQ (n = 21) | R-NEQ (n = 17) | p Value |
|---|---|---|---|---|---|---|
| EN starting time from ICU admission (hours), median (IQR) | 30.6 (18.1–54.2) | 25.1 (17.8–46.4) | 34.8 (17.1–60.1) | 35.6 (18.7–72.3) | 36.7 (20.2–71.8) | 0.457 |
| The EN intake at first week after EN initiation, median (IQR) | ||||||
| Energy (kcal/kg) | 8.6 (5.2–12.4) | 8 (4.8–11.9) | 9.9 (6.8–14.2) | 7.7 (4.1–10.8) | 5.5 (3.4–11) | 0.017 |
| Protein (g/kg) | 0.3 (0.2–0.5) | 0.3 (0.2–0.5) | 0.4 (0.3–0.6) | 0.3 (0.2–0.4) | 0.2 (0.1–0.5) | 0.014 |
| Fat (g/kg) | 0.2 (0.1–0.4) | 0.2 (0.1–0.4) | 0.3 (0.1–0.4) | 0.2 (0–0.3) | 0.2 (0–0.4) | 0.465 |
| The total nutrition intake at first week after EN initiation, median (IQR) | ||||||
| Energy (kcal) | 19 (13.6–24.7) | 19 (13.9–24.8) | 20.1 (14.2–25.1) | 18.6 (10.9–23.9) | 16.8 (10.6–23.1) | 0.494 |
| Protein (g) | 0.6 (0.4–0.9) | 0.7 (0.5–0.9) | 0.6 (0.5–1) | 0.6 (0.4–0.9) | 0.3 (0.2–0.8) | 0.043 |
| Fat (g) | 0.6 (0.4–0.9) | 0.7 (0.4–0.9) | 0.6 (0.4–0.9) | 0.7 (0.3–0.9) | 0.4 (0.2–0.9) | 0.254 |
| FI—no. (%) | 117 (55.71) | 37 (37.76) | 50 (67.57) | 17 (80.95) | 13 (76.47) | <0.001 |
| Diarrhea | 34 (16.19) | 9 (9.18) | 17 (22.97) | 6 (28.57) | 2 (11.76) | |
| Vomiting/regurgitation | 42 (20) | 15 (15.31) | 16 (21.62) | 8 (38.1) | 3 (17.65) | |
| Abdominal distension | 46 (21.9) | 17 (17.35) | 17 (22.97) | 4 (19.05) | 8 (47.06) | |
| Ileus | 8 (3.81) | 3 (3.06) | 4 (5.41) | 1 (4.76) | 0 (0) | |
| Suspected mesenteric ischemia | 4 (1.9) | 0 (0) | 1 (1.35) | 1 (4.76) | 2 (11.76) | |
| Confirmed mesenteric ischemia | 2 (1.0) | 0 (0) | 1 (1.35) | 1 (4.76) | 0 (0) | |
| Hospital infections—no. (%) | 52 (24.88) | 17 (17.53) | 23 (31.08) | 7 (33.33) | 5 (29.41) | 0.150 |
| MV (day), median (IQR) | 12 (6.5–21.1) | 12.5 (5.5–23.1) | 15 (9.9–25.3) | 10.9 (5.4–17.6) | 6.6 (5.1–7.7) | <0.001 |
| ICU Length of stay (day), median (IQR) | 15.8 (10.6–24) | 17.5 (11.8–25.3) | 17 (12–27.1) | 12 (7–18.2) | 7.6 (6–9) | <0.001 |
| 28-days mortality—no. (%) | 73 (34.76) | 15 (17.35) | 28 (37.83) | 13 (61.9) | 17 (100) | <0.001 |
| FI | Model 1 | Model 2 | Model 3 | Model 4 | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Baseline joint groups | ||||||||
| L-NEQ | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | ||||
| M-NEQ | 2.226 (1.453, 3.409) | <0.001 | 1.895 (1.182, 3.038) | 0.008 | 1.96 (1.214, 3.163) | 0.006 | 1.963 (1.214, 3.177) | 0.006 |
| H-NEQ | 2.974 (1.67, 5.296) | <0.001 | 2.384 (1.25, 4.545) | 0.008 | 2.444 (1.279, 4.67) | 0.007 | 2.317 (1.197, 4.484) | 0.013 |
| R-NEQ | 4.258 (2.252, 8.053) | <0.001 | 3.374 (1.666, 6.833) | 0.001 | 3.344 (1.653, 6.764) | 0.001 | 3.146 (1.51, 6.554) | 0.002 |
| p for trend | <0.001 | <0.001 | <0.001 | <0.001 | ||||
| Covariates | ||||||||
| Age (years) | 0.995 (0.983, 1.006) | 0.369 | 0.992 (0.98, 1.005) | 0.246 | 0.992 (0.979, 1.005) | 0.238 | ||
| BMI | 0.983 (0.931, 1.036) | 0.518 | 0.98 (0.929, 1.034) | 0.463 | 0.984 (0.932, 1.039) | 0.569 | ||
| NEQ at EN initiation (μg/kg/min) | 1.72 (0.794, 3.723) | 0.169 | 1.632 (0.75, 3.554) | 0.217 | 1.594 (0.729, 3.486) | 0.243 | ||
| APACHE II score | 1.011 (0.987, 1.035) | 0.389 | 1.01 (0.986, 1.035) | 0.397 | ||||
| Dobutamine (μg/kg/day) | 1.089 (0.926, 1.282) | 0.302 | ||||||
| Cumulative fluid balance at first week (L) | 1.019 (0.986, 1.052) | 0.258 | ||||||
| Lactic acid (mmol/L) | 0.944 (0.802, 1.111) | 0.489 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Zhang, T.; Yao, H.; Xu, Q.; Fu, X.; Yang, J.; Wang, B.; Zhang, Z.; Jin, X.; Kang, Y.; et al. Association of Vasopressors Dose Trajectories with Enteral Nutrition Tolerance in Patients with Shock: A Prospective Observational Study. Nutrients 2022, 14, 5393. https://doi.org/10.3390/nu14245393
Wang L, Zhang T, Yao H, Xu Q, Fu X, Yang J, Wang B, Zhang Z, Jin X, Kang Y, et al. Association of Vasopressors Dose Trajectories with Enteral Nutrition Tolerance in Patients with Shock: A Prospective Observational Study. Nutrients. 2022; 14(24):5393. https://doi.org/10.3390/nu14245393
Chicago/Turabian StyleWang, Luping, Tao Zhang, Hua Yao, Qian Xu, Xin Fu, Jing Yang, Bo Wang, Zhongwei Zhang, Xiaodong Jin, Yan Kang, and et al. 2022. "Association of Vasopressors Dose Trajectories with Enteral Nutrition Tolerance in Patients with Shock: A Prospective Observational Study" Nutrients 14, no. 24: 5393. https://doi.org/10.3390/nu14245393
APA StyleWang, L., Zhang, T., Yao, H., Xu, Q., Fu, X., Yang, J., Wang, B., Zhang, Z., Jin, X., Kang, Y., & Wu, Q. (2022). Association of Vasopressors Dose Trajectories with Enteral Nutrition Tolerance in Patients with Shock: A Prospective Observational Study. Nutrients, 14(24), 5393. https://doi.org/10.3390/nu14245393





