Abstract
(1) Background: The florets of Carthamus tinctorius L. are traditionally used as a blood-activating drug and can be used for the treatment of atherosclerosis, but no compounds with anti-atherosclerotic activity have been reported. (2) Methods: This study investigated the chemical compounds from the florets of C. tinctorius. Comprehensive spectroscopic techniques revealed their structures, and ECD calculations established their absolute configurations. Nile Red staining, Oil Red O staining, and cholesterol assessment were performed on these compounds and their aglycones for the inhibitory activity against the formation of foam cells induced by oxidized low-density lipoprotein (ox-LDL) in RAW264.7 macrophages. In addition, RAW264.7 macrophages were tested for their anti-inflammatory activity by measuring the inhibition of NO production caused by LPS. (3) Results: Five new sesquiterpenoids (1–5) isolated from the florets of C. tinctorius were identified as (–)-(1R,4S,9S,11R)-caryophyll-8(13)-en-14-ol-5-one (1), (+)-(1R,4R,9S,11R)-caryophyll-8(13)-en-14-ol-5-one (2), (–)-(3Z,1R,5S,8S,9S,11R)-5,8-epoxycaryophyll-3-en-14-O-β-D-glucopyranoside (3), (+)-(1S,7R,10S)-guai-4-en-3-one-11-O-β-D-fucopyranoside (4), and (–)-(2R,5R,10R)-vetispir-6-en-8-one-11-O-β-D-fucopyranoside (5). All compounds except for compound 3 reduced the lipid content in ox-LDL-treated RAW264.7 cells. Compounds 3 and 4 and their aglycones were found to reduce the level of total cholesterol (TC) and free cholesterol (FC) in ox-LDL-treated RAW264.7 cells. However, no compounds showed anti-inflammatory activity. (4) Conclusion: Sesquiterpenoids from C. tinctorius help to decrease the content of lipids, TC and FC in RAW264.7 cells, but they cannot inhibit NO production, which implies that their anti-atherogenic effects do not involve the inhibition of inflammation.
1. Introduction
Cardiovascular diseases have recently topped the list of causes of diseases in general, posing an immediate danger to human health. Atherosclerosis (AS) is a major pathological basis for many cardiovascular and cerebrovascular diseases, and macrophages play a major role in the plaque formation process of AS. In the early stages of AS development, macrophages phagocytose oxidize more low-density lipoprotein (ox-LDL) than they can metabolize, causing their massive accumulation of lipids and turning into foam cells. Then, foam cells gather together under the vascular endothelium to form a raised plaque, which further narrows or blocks blood vessels, thereby promoting the development of AS [,]. Therefore, inhibition of macrophage foaminess can effectively treat AS.
Recently, it has been demonstrated that many traditional Chinese medicines (TCMs), especially the blood-activating and stasis-removing medicines, are effective in treating AS [,,]. The florets of Carthamus tinctorius L. (safflower), a well-known TCM widely cultivated in Xinjiang and Sichuan, are described as an “essential medicine for promoting blood circulation” in Ben Cao Gang Mu. Modern pharmacological studies have shown that safflower is effective in treating cardiovascular diseases and inhibiting the development of AS [,,,]. Additionally, safflower is utilized as food coloring, functional food, and feedstuff to supple fatty acids, improve hair health, extend endurance, and reduce wrinkles [,,,,]. Up to now, phytochemical studies on safflower have revealed more than 200 chemical compounds, mainly including terpenoids, flavonoids, alkaloids, organic acids, and polyacetylenes [,,,]. However, fewer than 10 sesquiterpenoids have been reported []. In this study, five new sesquiterpenoids (compounds 1–5) were identified from safflower, including caryophyllane-type, guaiane-type, and vetispirane-type sesquiterpenoids (Figure 1). Interestingly, compounds 4 and 5 are rare sesquiterpenoid fucopyranosides. To our knowledge, only a small number of fucopyranosides have been discovered in nature, and most of them exist as flavone glycosides [,], triterpenoid saponins [,], and steroidal saponins []. Their inhibitory actions on ox-LDL-induced lipid accumulation were explored by Nile Red staining, Oil Red O staining, and cholesterol ELISA testing to evaluate their anti-atherosclerotic activity.
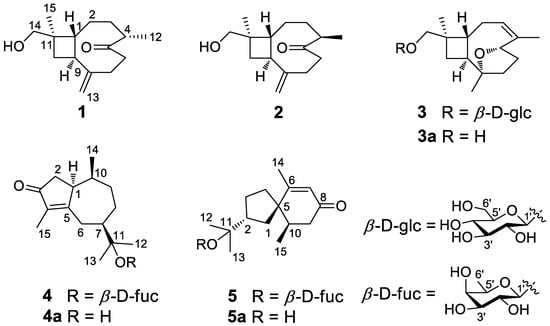
Figure 1.
Structures of compounds 1–5 and 3a–5a.
2. Results and Discussion
2.1. Structure Elucidation of Compounds 1–5
Compound 1 is a colorless oil, and its molecular formula was determined as C15H24O2 with four degrees of unsaturation according to HR-ESI-MS at m/z 259.1666 [M+Na]+ (calcd for C15H24O2Na, 259.1674). The 1H NMR data (Table 1) of compound 1 exhibit characteristic signals of a hydroxymethyl [δH 3.24 (2H, d, J = 11.4 Hz, H2-14)], two methyls [δH 0.97 (3H, s, H3-15), 1.03 (3H, d, J = 7.2 Hz, H3-12)], and a terminal double bond [δH 5.05 (1H, brs, H-13a), 5.01 (1H, brs, H-13b)]. The 13C NMR (Table 2) and DEPT data of compound 1 detected 15 carbon signals in total, including two methyls, seven methylenes (one oxygenated and one olefinic), three methines, and three quaternary carbons (one ketonic and one olefinic). Based on these data, compound 1 is a caryophyllane-type sesquiterpenoid similar to gibberosin P, but the hydroxymethyl functionality at C-11 in compound 1 takes the place of a 4-hydroxyvaleryl group in gibberosin P []. The planar structure of compound 1 was confirmed with the assistance of 1H–1H COSY and HMBC correlations as shown in Figure 2. In particular, the hydroxymethyl unit is located at C-11 based on the HMBC cross-peaks from H2-14 to C-1, C-10, C-11, and C-15. An NOESY experiment was applied to identify the relative configuration of compound 1. Correlations of H-9/H3-15 and H-1/H-4 and H2-14 (Figure 3) suggested that H-9 and Me-11 were co-facial, whereas H-1, H-4, and hydroxymethyl-11 occupied the opposite face. By comparing the computed electronic circular dichroism (ECD) data of compound 1 with the experimental ECD data (Figure 4), the absolute configuration of compound 1 was revealed to be 1R,4S,9S,11R. Therefore, compound 1 was determined to be (–)-(1R,4S,9S,11R)-caryophyll-8(13)-en-14-ol-5-one.

Table 1.
1H NMR data of compounds 1–3 and 3a in CD3OD (δ in ppm, J in Hz).

Table 2.
13C NMR data of compounds 1–5 in CD3OD (δ in ppm).
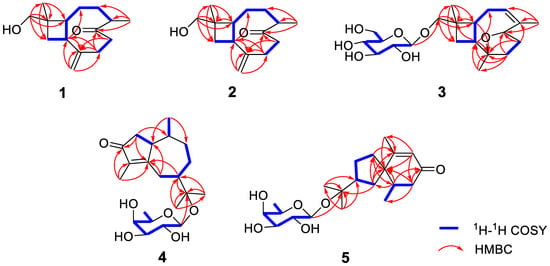
Figure 2.
Key 1H–1H COSY and HMBC correlations of compounds 1–5.
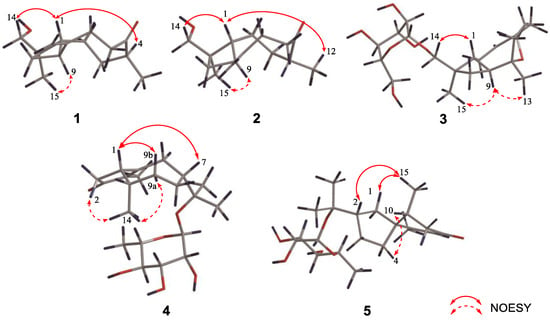
Figure 3.
Key NOESY correlations of compounds 1–5.
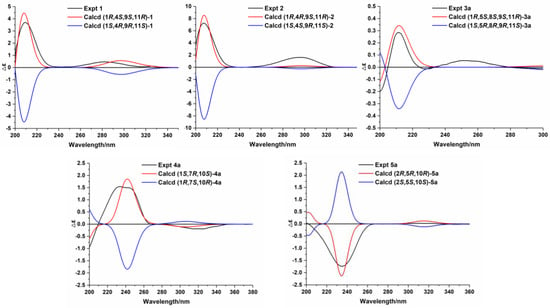
Figure 4.
Experimental and calculated ECD spectra of compounds 1–2 and 3a–5a. Based on the HR-ESI-MS data of compound 2, its chemical formula is similar to that of compound 1. The 1H, 13C, and 2D NMR spectra of compounds 2 and 1 showed that the planar structures of these two compounds are identical. Further analysis revealed that compound 2 was a C-4 epimer of compound 1 based on the NOESY correlations of H2-14/H-1, H-1/H3-12, and H-9/H3-15 (Figure 3). Moreover, ECD calculations showed that the absolute configuration of compound 2 is 1R,4R,9S,11R (Figure 4). Therefore, compound 2 was identified as (+)-(1R,4R,9S,11R)-caryophyll-8(13)-en-14-ol-5-one.
Compound 3 has a molecular formula of C21H34O7, as determined by the HR-ESI-MS. The 1H, 13C, and DEPT NMR data of compound 3 suggested that it is also a caryophyllane-type sesquiterpenoid. The signals for an anomeric proton [δH 4.24 (d, J = 7.8 Hz)] and an anomeric carbon (δC 104.7) revealed the existence of a β-glucosyl [], indicating that compound 3 is a caryophyllane glycoside. In addition, the 1H and 13C NMR data (Table 1 and Table 2) exhibited signals attributed to an olefinic methine [δH 5.38 (1H, m, H-3), δC 124.1], an oxygenated methine [δH 4.50 (1H, dd, J = 9.6, 5.4 Hz, H-5), δC 82.0], an oxygenated quaternary carbon (δC 87.3), and an olefinic quaternary carbon (δC 140.8) in the aglycone unit. Thus, the existence of an epoxy moiety and a trisubstituted double bond was deduced based on the molecular formula. Comparison of the NMR data between compound 3 and 5α,8α-epoxycaryophyll-3-ene [] suggested that an additional β-glucose moiety and an oxygenated methene group in compound 3 replaced a methyl group (C-14) in 5α,8α-epoxycaryophyll-3-ene, and this deduction was confirmed by the HMBC correlation from H-1′ to C-14.
In the NOESY spectrum of compound 3, cross-peaks of H2-14/H-1 and H-9/H3-15 demonstrated the same orientation of H2-14 and H-1, whereas H-9 and H3-15 had the opposite orientation. The orientation of the 5-O-8 bridge was established by an NOE signal of H-9/H3-13. In addition, an NOE interaction between H-3 and H3-12 disclosed a Z-geometry for Δ3. To verify the absolute configuration of compound 3, it was subjected to enzymatic hydrolysis, which yielded an aglycone 3a and a D-glucose. Then, ECD calculation was carried out to resolve the absolute configuration of 3a. As depicted in Figure 4, the calculated ECD spectrum of (1R,5S,8S,9S,11R)-3a and the experimental ECD spectrum of 3a were in good agreement. Therefore, it was concluded that compound 3 is (–)-(3Z,1R,5S,8S,9S,11R)-5,8-epoxycaryophyll-3-en-14-O-β-D-glucopyranoside.
The molecular formula of compound 4 was identified as C21H34O6 by HR-ESI-MS at m/z 405.2244 [M+Na]+ (calcd for 405.2253). The 1H NMR spectrum (Table 3) exhibited distinguishable signals of five oxygenated methines [δH 4.42 (1H, d, J = 7.8 Hz, H-1′), 3.58 (1H, q, J = 6.6 Hz, H-5′), 3.56 (1H, d, J = 3.6 Hz, H-4′), 3.46 (1H, dd, J = 9.6, 3.6 Hz, H-3′), and 3.42 (1H, dd, J = 9.6, 7.8 Hz, H-2′)], which might be attributable to a β-glycosyl moiety. Additionally, five methyl groups [δH 0.64 (3H, d, J = 7.2 Hz, H3-14), 1.09 (3H, d, J = 6.6 Hz, H3-6′), 1.21 (3H, s, H3-12), 1.33 (3H, s, H3-13), and 1.66 (3H, s, H3-15)] were deducible from the 1H NMR data. The 13C NMR (Table 2) and the DEPT data revealed 21 carbon resonances attributed to the above units, four aliphatic methylenes, three aliphatic methines, and four quaternary carbons [an α,β-unsaturated ketone unit (δC 211.3, 138.3, and 181.1) and an oxygenated quaternary carbon (δC 81.3)]. Comprehensive analysis of the 1H-1H COSY, HSQC, and HMBC spectra indicated that the planar structure of compound 4 is similar to (1R,7R,10S)-11-O-β-D-glucopyranosyl-4-guaien-3-one [], except for the glycosyl unit C-11. The glycosyl unit in compound 4 was further determined to be β-D-fucosyl due to the coupling constants (J1′,2′ = 7.8 Hz, J2′,3′ = 9.6 Hz, J3′,4′ = 3.6 Hz, and J4′,5′ ≈ 0 Hz), together with the 13C NMR data (δC 98.9, 72.5, 75.3, 73.1, 71.4, and 16.8) [,]. The NOESY correlations of H-1/H-7, H-1/H-9b, H3-14/H-9a, and H3-14/H-2b (Figure 3) were used to ascertain the relative structure of the aglycone moiety in compound 4. Enzymatic hydrolysis of compound 4 provided the aglycone 4a. Comparison of the experimental and calculated ECD spectra of 4a (Figure 4) assigned 1S,7R,10S configuration to 4a. Consequently, compound 4 was determined to be (+)-(1S,7R,10S)-guai-4-en-3-one-11-O-β-D-fucopyranoside.

Table 3.
1H NMR data of compounds 4, 4a, 5, and 5a in CD3OD (δ in ppm, J in Hz).
The HR-ESI-MS of compound 5 revealed that it is a structural isomer of compound 4. Comparative analysis of their 1H and 13C NMR data exhibited that they share similar functional groups such as an α,β-unsaturated ketone unit, four methyl groups, and a β-D-fucosyl moiety. However, as shown in Figure 2, 2D NMR data analysis pointed out that compound 5 is a vetispirane-type sesquiterpenoid []. The NOESY correlations of H3-15/H-1a, H-10/H2-4, and H-2/H3-15 (Figure 3) determined the relative structure of compound 5. Similar to compound 4, the aglycone 5a was produced by enzymatic hydrolysis of compound 5, and 2R,5R,10R configuration was assigned to 5a according to ECD calculations (Figure 4). Therefore, the structure of compound 5 was established as (−)-(2R,5R,10R)-vetispir-6-en-8-one-11-O-β-D-fucopyranoside.
2.2. Anti-Atherosclerotic Activity of Compounds 1–5 and Aglycones 3a–5a
The anti-atherosclerotic activity of the isolates (1–5) and their aglycones (3a–5a) was evaluated by detecting their inhibitory effects against RAW264.7 cell foaminess induced by ox-LDL (Yiyuan Biotechnologies, Guangzhou, China) [,]. First, a CCK-8 assay was applied to evaluate the effects of the eight compounds on RAW264.7 cell viability, and all of the compounds were found to be noncytotoxic. Then, they were tested for anti-macrophage foaming activity by reducing the content of lipid in RAW264.7 cells treated by ox-LDL. As depicted in Figure 5, all compounds, except for compound 3, showed significant activity by Nile Red staining. In particular, the effects of compounds 1, 2, 3a, 4a, 5, and 5a were equal to or superior to the effect of the positive control (25 μM of simvastatin). Interestingly, all of the aglycones (3a–5a) had better effects than their glycosides (3–5), which suggests that glycosidation of the sesquiterpenoids of safflower may result in a decrease in their anti-atherosclerotic activity. Although compound 4 showed weak activity in Nile Red staining, a significant effect was observed for compound 4 in Oil Red O staining (Figure 6). Specifically, compared with the control group, the model group collected more Oil Red O-stained lipid. In the drug group, compound 4 significantly decreased Oil Red O-stained lipid accretion.
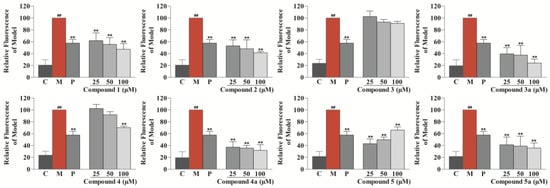
Figure 5.
Effects of compounds 1–5 and 3a–5a to inhibit lipid drops accumulation in ox-LDL-treated RAW264.7 cells determined by Nile Red staining. Results are expressed as the mean ± SD (n = 3). Model group was subject to 75 μg/mL ox-LDL. Positive group was subject to 25 μM simvastatin. ## p < 0.01 vs. control group; ** p < 0.01 vs. model group. C: control group, M: model group, and P: positive group.
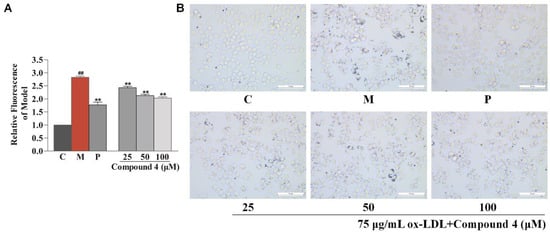
Figure 6.
Inhibitory effects of compound 4 against lipid drops accumulation in ox-LDL-treated RAW264.7 cells measured by Oil Red O staining. (A) Oil Red O content evaluated by isopropanol extraction. (B) Oil Red O staining images of RAW264.7 cells (magnification 400×). Results are expressed as the mean ± SD (n = 3). Model group was subject to 75 μg/mL ox-LDL. Positive group was subject to 25 μM simvastatin. ## p < 0.01 vs. control group; ** p < 0.01 vs. model group.
To further explore the anti-atherosclerotic activity of compounds 3, 3a, 4, and 4a, the total cholesterol (TC) and free cholesterol (FC) levels in foamy macrophages were assessed by ELISA (Shanghai Keshun Science and Technology Co., Ltd., Shanghai, China). As shown in Figure 7, compounds 3, 3a, 4, and 4a significantly decreased the levels of TC and FC in ox-LDL-treated RAW264.7 cells in a dose-dependent way (p < 0.01 vs. model group). In addition, all compounds were investigated for their ability to inhibit lipopolysaccharide-induced NO production in RAW264.7 cells. The results showed that none of the compounds had an anti-inflammatory effect. Thus, it can be inferred that the safflower sesquiterpenoids do not exert their anti-atherogenic effects through the anti-inflammatory pathway []. Unfortunately, no further investigations on the molecular mechanisms were carried out due to the limited sample quantities of the isolates.
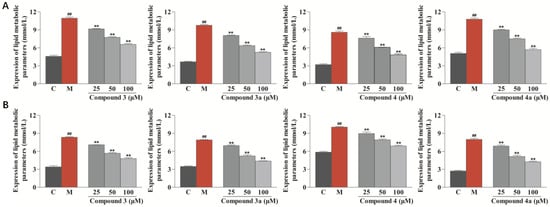
Figure 7.
Effects of compounds 3, 3a, 4, and 4a on the contents of TC and FC in ox-LDL-treated RAW264.7 cells evaluated by ELISA. (A) Effects of compounds 3, 3a, 4, and 4a on the intracellular content of TC. (B) Effects of compounds 3, 3a, 4, and 4a on the intracellular content of FC. Results are expressed as the mean ± SD (n = 3). Model group was subject to 75 μg/mL ox-LDL. ## p < 0.01 vs. control group; ** p < 0.01 vs. model group.
3. Experimental Section
3.1. General Experimental Procedures
General experimental instruments and materials are shown in Text S1, Supplementary Materials.
3.2. Plant Material
The florets of C. tinctorius were gathered from Luole Village, Sichuan Province, China. The sample was authenticated by Dr. Ji-hai Gao (Chengdu University of TCM, Chengdu, China). A voucher specimen (No. CT-20180608) was placed at the State Key Laboratory of Southwestern Chinese Medicine Resources, Chengdu University of TCM.
3.3. Extraction and Isolation
The florets of C. tinctorius (50 kg) were decocted twice with 12 times the quantity of H2O for 1 h each time. The extract was concentrated under reduced pressure in a big rotary evaporator (10 L) at 55 °C to produce a residue (16 kg). The residue was separated on a D-101 macroporous adsorbent resin (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) column using a gradient of H2O, 20%, 50%, 70%, and 90% EtOH to afford five fractions (A–E). Fraction D (66.7 g) was performed on a silica gel column (Yantai Institute of Chemical Technology, Yantai, China) and eluted with a gradient of CH2Cl2/MeOH (1:0–0:1) to afford 14 major fractions (D1–D14). D7 (6 g) was chromatographed on RP-MPLC eluted with MeOH/H2O (20:80–100:0) to obtain 10 subfractions (D7-1–D7-10). Six sub-subfractions (D7-3-1–D7-3-6) were obtained from D7-3 by silica gel column chromatography with a gradient elution system of CH2Cl2/MeOH (50:1–1:1). D7-3-1 was further separated into D7-3-1-1–D7-3-1-7 by column chromatography over Toyopearl HW-40F (Tosoh Corp., Tokyo, Japan) using 50% MeOH as the eluent. Compound 4 (3.5 mg) was derived from D7-3-1-2 by RP semipreparative HPLC (75% MeOH in H2O). D7-3-1-3 was further separated by TLC (CH2Cl2/MeOH, 10:1), followed by RP semipreparative HPLC (75% MeOH in H2O), to obtain compound 5 (2 mg). Separation of D7-3-1-4 by semipreparative HPLC (MeOH/H2O, 75:25) yielded compounds 1 (0.8 mg) and 2 (0.7 mg). D7-3-2 was separated by silica gel column chromatography and eluted with a gradient elution of CH2Cl2/MeOH (200:1–1:1) to yield D7-3-2-1–D7-3-2-6. From D7-3-2-5, compound 3 (3.0 mg) was obtained by TLC using CH2Cl2–MeOH (10:1) as the developing solvent.
3.4. Spectroscopic Data
(−)-(1R,4S,9S,11R)-Caryophyll-8(13)-en-14-ol-5-one (1): colorless oil; − 4.2 (c 0.02, MeOH); IR νmax 3437, 2957, 2925, 2855, 1699, 1657, 1629, 1454, 1376, 1039 cm−1; ECD (MeCN) λmax (Δε) 209 (+3.70), 282 (+0.47), 326 (+0.11) nm; 1H NMR (CD3OD, 600 MHz) and 13C NMR (CD3OD, 150 MHz) data, see Table 1 and Table 2; (+)-HR-ESI-MS m/z 259.1666 [M+Na]+ (calcd for C15H24O2Na, 259.1674). The original 1H NMR, 13C NMR, DEPT, HSQC, 1H-1H COSY, HMBC, NOESY, IR, and (+)-HR-ESI-MS spectra are shown in Figures S6–S14, Supplementary Materials.
(+)-(1R,4R,9S,11R)-Caryophyll-8(13)-en-14-ol-5-one (2): colorless oil; + 7.14 (c 0.03, MeOH); IR νmax 3444, 3420, 2957, 2928, 2855, 1699, 1629, 1451, 1378, 1083, 1034, 888 cm−1; ECD (MeCN) λmax (Δε) 207 (+7.25), 295 (+1.62); 1H NMR (CD3OD, 600 MHz) and 13C NMR (CD3OD, 150 MHz) data, see Table 1 and Table 2; (+)-HR-ESI-MS m/z 259.1666 [M+Na]+ (calcd for C15H24O2Na, 259.1674). The original 1H NMR, 13C NMR, DEPT, HSQC, 1H-1H COSY, HMBC, NOESY, 1D NOE, IR, and (+)-HR-ESI-MS spectra are shown in Figures S15–S24, Supplementary Materials.
(−)-(3Z,1R,5S,8S,9S,11R)-5,8-Epoxycaryophyll-3-en-14-O-β-D-glucopyranoside (3): colorless oil; − 15.0 (c 0.02, MeOH); IR νmax 3400, 2964, 2923, 2865, 1637, 1454, 1417, 1381, 1078, 1039 cm−1; 1H NMR (CD3OD, 600 MHz) and 13C NMR (CD3OD, 150 MHz) data, see Table 1 and Table 2; (+)-HR-ESI-MS m/z 421.2196 [M+Na]+ (calcd for C21H34O7Na, 421.2202). The original 1H NMR, 13C NMR, DEPT, HSQC, 1H-1H COSY, HMBC, NOESY, 1D NOE, IR, and (+)-HR-ESI-MS spectra are shown in Figures S25–S34, Supplementary Materials.
(−)-(3Z,1R,5S,8S,9S,11R)-5,8-Epoxycaryophyll-3-en-14-ol (3a): colorless oil; − 4.8 (c 0.04, MeOH); ECD (MeCN) λmax (Δε) 211 (+0.29), 251 (+0.05) nm; 1H NMR (CD3OD, 600 MHz) data, see Table 1. (+)-HR-ESI-MS m/z 259.1667 [M+Na]+ (calcd for C15H24O2Na, 259.1674). The original 1H NMR and (+)-HR-ESI-MS spectra are shown in Figures S35 and S36, Supplementary Materials.
(+)-(1S,7R,10S)-Guai-4-en-3-one-11-O-β-D-fucopyranoside (4): colorless oil; +29.4 (c 0.02, MeOH); IR νmax 3403, 2923, 2852, 1676, 1623, 1464, 1386, 1172, 1065 cm−1; 1H NMR (CD3OD, 600 MHz) and 13C NMR (CD3OD, 150 MHz) data, see Table 2 and Table 3; (+)-HR-ESI-MS at m/z 405.2244 [M+Na]+ (calcd for C21H34O6Na, 405.2253). The original 1H NMR, 13C NMR, DEPT, HSQC, 1H-1H COSY, HMBC, NOESY, IR, and (+)-HR-ESI-MS spectra are shown in Figures S37–S45, Supplementary Materials.
(+)-(1S,7R,10S)-Guai-4-en-11-ol-3-one (4a): colorless oil; + 26.7 (c 0.03, MeOH); ECD (MeCN) λmax (Δε) 234 (+1.54), 318 (−0.19), 324 (−0.20) nm; 1H NMR (CD3OD, 600 MHz) data, see Table 3. (+)-HR-ESI-MS at m/z 259.1673 [M+Na]+, (calcd for C15H24O2Na, 259.1668). The original 1H NMR and (+)-HR-ESI-MS spectra are shown in Figures S46 and S47, Supplementary Materials.
(−)-(2R,5R,10R)-Vetispir-6-en-8-one-11-O-β-D-fucopyranoside (5): colorless oil; − 28.2 (c 0.04, MeOH); IR νmax 3303, 2972, 2925, 1665, 1467, 1381, 1170, 1070, 990 cm−1; 1H NMR (CD3OD, 600 MHz) and 13C NMR (CD3OD, 150 MHz) data, see Table 2 and Table 3; (+)-HR-ESI-MS at m/z 405.2250 [M+Na]+ (calcd for C21H34O6Na 405.2253). The original 1H NMR, 13C NMR, DEPT, HSQC, 1H-1H COSY, HMBC, NOESY, IR, and (+)-HR-ESI-MS spectra are shown in Figures S48–S56, Supplementary Materials.
(−)-(2R,5R,10R)-Vetispir-6-en-11-ol-8-one (5a): colorless oil; − 15.0 (c 0.02, MeOH); ECD (MeCN) λmax (Δε) 235 (−1.74) nm; 1H NMR (CD3OD, 600 MHz) data, see Table 3. (+)-HR-ESI-MS at m/z 259.1673 [M+Na]+ (calcd for C15H24O2Na, 259.1674). The original 1H NMR and (+)-HR-ESI-MS spectra are shown in Figures S57 and S58, Supplementary Materials.
3.5. Enzymatic Hydrolysis of Compounds 3–5
Snailase (20 mg) was used to hydrolyze compound 3 in water (5.0 mL) for 48 h at 37 °C. The aglycone (compound 3a, 0.8 mg) was extracted by EtOAc (4 × 5 mL) in a liquid–liquid extractor and purified by HPLC. Compounds 4 (2 mg) and 5 (1.2 mg) were enzymatically hydrolyzed as above to obtain compounds 4a (0.8 mg) and 5a (0.5 mg).
3.6. ECD Calculation
The details of ECD calculation of compounds 1, 2, and 3a–5a are shown in Texts S2–S6, Figures S1–S5, and Tables S1–S5 Supplementary Materials).
3.7. Cell Viability Assay
RAW264.7 cells were grown in DMEM supplemented with 10% FBS under a humidified environment at 37 °C and 5% CO2. The cells were planted in 96-well plates (3 × 104 cells/well) and incubated with compounds 1–5 and 3a–5a (25, 50, and 100 μM) for one day. Then, the cells were treated by CCK-8 solution (10 μL/well) and incubated for 1 h. Under 450 nm, the absorbance of each well was tested to evaluate the viability of the cells.
3.8. Nile Red Staining
RAW264.7 cells were placed in 96-well plates (3 × 104 cells/well). Then, the cells were treated with ox-LDL (75 μg/mL), together with compounds 1–5 and 3a–5a (25, 50, and 100 μM). After incubation for 24 h, the cells were kept for 30 min at 37 °C with 4% paraformaldehyde, then rinsed with PBS. The cells were hatched with freshly prepared Nile Red staining solution (2 μg/mL) for 30 min. Finally, PBS was used to wash the cells, and the fluorescence intensity was measured at 530 nm/590 nm.
3.9. Oil Red O Staining
RAW264.7 cells were placed in 24-well plates (3 × 105 cells/well). Then, the cells were treated with ox-LDL (75 μg/mL) and compound 4 (25, 50, and 100 μM). After incubation for 24 h, the cells were kept for 30 min at 37 °C with 4% paraformaldehyde, followed by rinsing with PBS. The cells were washed with 50% isopropanol for 20 s, and incubated with fresh-filtered Oil Red O solution (60% saturated Oil Red O/40% deionized water) for 30 min. The cells were washed with alternate rinses of 50% isopropanol and PBS to remove the floating colors and photographed for observation by fluorescence microscopy. Finally, the cells were washed with isopropanol, and the absorbance was observed at 550 nm.
3.10. ELISA
RAW264.7 cells (1 × 106/well) were placed into six-well culture plates and treated by the above method. The contents of TC and FC were measured using the ELISA kits.
4. Conclusions
In conclusion, five new sesquiterpenoids were isolated from safflower, including caryophyllane-type, guaiane-type, and vetispirane-type sesquiterpenoids. The sesquiterpenoid types characterized herein have not been reported from C. tinctorius, and compounds 4 and 5 are rare sesquiterpenoid fucopyranosides. The sesquiterpenoids showed significant anti-atherosclerosis activity by decreasing the contents of lipid, TC, and FC in ox-LDL-treated RAW264.7 cells. Altogether, these new sesquiterpenoids not only enrich the diversity of sesquiterpenoids in safflower but also explain the effective material basis of safflower in treating AS.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/nu14245348/s1, Figure S1: ωB97XD/DGDZVP optimized 21 conformers of (1R,4S,9S,11R)-1, Figure S2: ωB97XD/DGDZVP optimized 18 conformers of (1R,4R,9S,11R)-2,Figure S3: ωB97XD/DGDZVP optimized 9 conformers of (1R,5S,8S,9S,11R)-3a, Figure S4: ωB97XD/DGDZVP optimized 8 conformers of (1S,7R,10S)-4a, Figure S5: ωB97XD/DGDZVP optimized 15 conformers of (2R,5R,10R)-5a, Figure S6: The 1H NMR spectrum of compound 1 in CD3OD, Figure S7: The 13C NMR spectrum of compound 1 in CD3OD, Figure S8: The DEPT spectrum of compound 1 in CD3OD, Figure S9: The HSQC spectrum of compound 1 in CD3OD, Figure S10: The 1H-1H COSY spectrum of compound 1 in CD3OD, Figure S11: The HMBC spectrum of compound 1 in CD3OD, Figure S12: The NOESY spectrum of compound 1 in CD3OD, Figure S13: The IR spectrum of compound 1, Figure S14: The (+)-HR-ESI-MS spectroscopic data of compound 1, Figure S15: The 1H NMR spectrum of compound 2 in CD3OD, Figure S16: The 13C NMR spectrum of compound 2 in CD3OD, Figure S17: The DEPT spectrum of compound 2 in CD3OD, Figure S18: The HSQC spectrum of compound 2 in CD3OD, Figure S19: The 1H-1H COSY spectrum of compound 2 in CD3OD, Figure S20: The HMBC spectrum of compound 2 in CD3OD, Figure S21: The NOESY spectrum of compound 2 in CD3OD, Figure S22: The 1D NOE spectrum of compound 2 in CD3OD, Figure S23: The IR spectrum of compound 2, Figure S24: The (+)-HR-ESI-MS spectroscopic data of compound 2, Figure S25: The 1H NMR spectrum of compound 3 in CD3OD, Figure S26: The 13C NMR spectrum of compound 3 in CD3OD, Figure S27: The DEPT spectrum of compound 3 in CD3OD, Figure S28: The HSQC spectrum of compound 3 in CD3OD, Figure S29: The 1H-1H COSY spectrum of compound 3 in CD3OD, Figure S30: The HMBC spectrum of compound 3 in CD3OD, Figure S31: The NOESY spectrum of compound 3 in CD3OD, Figure S32: The 1D NOE spectrum of compound 3 in CD3OD, Figure S33: The IR spectrum of compound 3, Figure S34: The (+)-HR-ESI-MS spectroscopic data of compound 3, Figure S35: The 1H NMR spectrum of compound 3a in CD3OD, Figure S36: The (+)-HR-ESI-MS spectroscopic data of compound 3a, Figure S37: The 1H NMR spectrum of compound 4 in CD3OD, Figure S38: The 13C NMR spectrum of compound 4 in CD3OD, Figure S39: The DEPT spectrum of compound 4 in CD3OD, Figure S40: The HSQC spectrum of compound 4 in CD3OD, Figure S41: The 1H-1H COSY spectrum of compound 4 in CD3OD, Figure S42: The HMBC spectrum of compound 4 in CD3OD, Figure S43: The NOESY spectrum of compound 4 in CD3OD, Figure S44: The IR spectrum of compound 4, Figure S45: The (+)-HR-ESI-MS spectroscopic data of compound 4, Figure S46: The 1H NMR spectrum of compound 4a in CD3OD, Figure S47: The (+)-HR-ESI-MS spectroscopic data of compound 4a, Figure S48: The 1H NMR spectrum of compound 5 in CD3OD, Figure S49: The 13C NMR spectrum of compound 5 in CD3OD, Figure S50: The DEPT spectrum of compound 5 in CD3OD, Figure S51: The HSQC spectrum of compound 5 in CD3OD, Figure S52: The 1H-1H COSY spectrum of compound 5 in CD3OD, Figure S53: The HMBC spectrum of compound 5 in CD3OD, Figure S54: The NOESY spectrum of compound 5 in CD3OD, Figure S55: The IR spectrum of compound 5, Figure S56: The (+)-HR-ESI-MS spectroscopic data of compound 5, Figure S57: The 1H NMR spectrum of compound 5a in CD3OD, Figure S58: The (+)-HR-ESI-MS spectroscopic data of compound 5a, Table S1: Energy analysis for the conformers of (1R,4S,9S,11R)-1, Table S2: Energy analysis for the conformers of (1R,4R,9S,11R)-2, Table S3: Energy analysis for the conformers of (1R,5S,8S,9S,11R)-3a, Table S4: Energy analysis for the conformers of (1S,7R,10S)-4a; Table S5: Energy analysis for the conformers of (2R,5R,10R)-5a, Text S1: General experimental details, Text S2: ECD calculation of compound 1, Text S3: ECD calculation of compound 2, Text S4: ECD calculation of compound 3a, Text S5: ECD calculation of compound 4a, Text S6: ECD calculation of compound 5a, Supplementary References [,,,,].
Author Contributions
Conceptualization, C.P. and L.X.; methodology, L.L., Y.G. and Y.F.; investigation, L.L., J.L., X.L. and H.S.; validation, J.L. and G.W.; writing—original draft, L.L.; writing—review and editing, L.X.; supervision, C.P. and L.X.; project administration, L.X.; funding acquisition, C.P. and L.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (NNSFC; Grant Nos. 82022072 and U19A2010), the Fok Ying Tung Education Foundation (Grant No. 171037), the “Xinglin Scholar” Plan of Chengdu University of Traditional Chinese Medicine (Grant No. XKTD2022006), and the Innovation Team and Talents Cultivation Program of the National Administration of Traditional Chinese Medicine (Grant No. ZYYCXTD-D-202209).
Data Availability Statement
The data presented in this study are available in the Supplementary Materials or can be provided by the authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cao, H.; Jia, Q.; Yan, L.; Chen, C.; Xing, S.; Shen, D. Quercetin suppresses the progression of atherosclerosis by regulating MST1-mediated autophagy in ox-LDL-induced RAW264.7 macrophage foam cells. Int. J. Mol. Sci. 2019, 20, 6093. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Guo, N.; Cao, G.; Zhou, J.; Yuan, Z. Molecular analysis of curcumin-induced polarization of murine RAW264.7 macrophages. J. Cardiovasc. Pharmacol. 2014, 63, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jia, Q.; Zhang, Y.; Wei, J.; Liu, P. Taoren honghua drug attenuates atherosclerosis and plays an anti-inflammatory role in ApoE knock-out mice and RAW264.7 cells. Front. Pharmacol. 2020, 11, 1070. [Google Scholar] [CrossRef]
- Yu, B.; Zhang, G.; Jin, L.; Zhang, B.; Yan, D.; Yang, H.; Ye, Z.; Ma, T. Inhibition of PAI-1 activity by toddalolactone as a mechanism for promoting blood circulation and removing stasis by Chinese herb Zanthoxylum nitidum var. tomentosum. Front. Pharmacol. 2017, 8, 489. [Google Scholar] [CrossRef]
- Yuan, R.; Shi, W.L.; Xin, Q.Q.; Chen, K.J.; Cong, W.H. Holistic regulation of angiogenesis with chinese herbal medicines as a new option for coronary artery disease. Evid.-Based Complement. Altern. Med. 2018, 2018, 3725962. [Google Scholar] [CrossRef]
- Bai, Y.; Lu, P.; Han, C.; Yu, C.; Chen, M.; He, F.; Yi, D.; Wu, L. Hydroxysafflor yellow A (HSYA) from flowers of Carthamus tinctorius L. and its vasodilatation effects on pulmonary artery. Molecules 2012, 17, 14918–14927. [Google Scholar] [CrossRef]
- Wang, H.; Liu, J.; Yang, Y.; Cao, Q.; Huo, X.; Ma, S.; Hu, J.; Pavalko, F.M.; Liu, Q. Hydroxy-safflower yellow A inhibits the TNFR1-mediated classical NF-κB pathway by inducing shedding of TNFR1. Phytother. Res. 2016, 30, 790–796. [Google Scholar] [CrossRef]
- Yu, S.Y.; Lee, Y.J.; Kim, J.D.; Kang, S.N.; Lee, S.K.; Jang, J.Y.; Lee, H.K.; Lim, J.H.; Lee, O.H. Phenolic composition, antioxidant activity and anti-adipogenic effect of hot water extract from safflower (Carthamus tinctorius L.) seed. Nutrients 2013, 5, 4894–4907. [Google Scholar] [CrossRef]
- Maneesai, P.; Prasarttong, P.; Bunbupha, S.; Kukongviriyapan, U.; Kukongviriyapan, V.; Tangsucharit, P.; Prachaney, P.; Pakdeechote, P. Synergistic antihypertensive effect of Carthamus tinctorius L. extract and captopril in L-NAME-induced hypertensive rats via restoration of eNOS and AT1R expression. Nutrients 2016, 8, 122. [Google Scholar] [CrossRef]
- Jeong, E.H.; Yang, H.; Kim, J.; Lee, K.W. Safflower seed oil and its active compound acacetin inhibit UVB-induced skin photoaging. J. Microbiol. Biotechnol. 2020, 30, 1567–1573. [Google Scholar] [CrossRef]
- Ju, G.; Liu, Y. Study of safflower on blood lactate concentration and exercise function of mice after exercise. Afr. J. Biotechnol. 2011, 10, 9148–9152. [Google Scholar] [CrossRef][Green Version]
- Hong, H.; Lim, J.M.; Kothari, D.; Kwon, S.H.; Kwon, H.C.; Han, S.; Kim, S. Antioxidant properties and diet-related α-glucosidase and lipase inhibitory activities of yogurt supplemented with safflower (Carthamus tinctorius L.) petal extract. Food Sci. Anim. Resour. 2021, 41, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Mani, V.; Lee, S.; Yeo, Y.; Hahn, B. A metabolic perspective and opportunities in pharmacologically important safflower. Metabolites 2020, 10, 253. [Google Scholar] [CrossRef] [PubMed]
- Tso, P.; Caldwell, J.; Lee, D.; Boivin, G.P.; DeMichele, S.J. Comparison of growth, serum biochemistries and n-6 fatty acid metabolism in rats fed diets supplemented with high-gamma-linolenic acid safflower oil or borage oil for 90 days. Food Chem. Toxicol. 2012, 50, 1911–1919. [Google Scholar] [CrossRef]
- Yue, S.J.; Qu, C.; Zhang, P.X.; Tang, Y.P.; Jin, Y.; Jiang, J.S.; Yang, Y.N.; Zhang, P.C.; Duan, J.A. Carthorquinosides A and B, quinochalcone C-glycosides with diverse dimeric skeletons from Carthamus tinctorius. J. Nat. Prod. 2016, 79, 2644–2651. [Google Scholar] [CrossRef]
- Zhang, H.; Duan, C.P.; Luo, X.; Feng, Z.M.; Yang, Y.N.; Zhang, X.; Jiang, J.S.; Zhang, P.C. Two new quinochalcone glycosides from the safflower yellow pigments. J. Asian Nat. Prod. Res. 2020, 22, 1130–1137. [Google Scholar] [CrossRef]
- Yue, S.; Tang, Y.; Xu, C.; Li, S.; Zhu, Y.; Duan, J.A. Two new quinochalcone C-glycosides from the florets of Carthamus tinctorius. Int. J. Mol. Sci. 2014, 15, 16760–16771. [Google Scholar] [CrossRef]
- Zhou, X.; Tang, L.; Xu, Y.; Zhou, G.; Wang, Z. Towards a better understanding of medicinal uses of Carthamus tinctorius L. in traditional Chinese medicine: A phytochemical and pharmacological review. J. Ethnopharmacol. 2014, 151, 27–43. [Google Scholar] [CrossRef]
- Cazarolli, L.H.; Kappel, V.D.; Pereira, D.F.; Moresco, H.H.; Brighente, I.M.; Pizzolatti, M.G.; Silva, F.R. Anti-hyperglycemic action of apigenin-6-C-β-fucopyranoside from Averrhoa carambola. Fitoterapia 2012, 83, 1176–1183. [Google Scholar] [CrossRef]
- Suzuki, R.; Okada, Y.; Okuyama, T. A new flavone C-glycoside from the style of Zea mays L. with glycation inhibitory activity. Chem. Pharm. Bull. 2003, 51, 1186–1188. [Google Scholar] [CrossRef]
- Barrero, A.F.; Haïdour, A.; Sedqui, A.; Mansour, A.I.; Rodríguez-García, I.; López, A.; Muñoz-Dorado, M. Saikosaponins from roots of Bupleurum gibraltaricum and Bupleurum spinosum. Phytochemistry 2000, 54, 741–745. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Jia, Z.; Cates, R.G.; Li, D.; Owen, N.L. Triterpenoid saponins from Clinopodium chinensis. J. Nat. Prod. 1995, 58, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Eskander, J.; Lavaud, C.; Harakat, D. Steroidal saponins from the leaves of Beaucarnea recurvata. Phytochemistry 2011, 72, 946–951. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.P.; Su, J.H.; Yeh, H.C.; Ahmed, A.F.; Dai, C.F.; Wu, Y.C.; Sheu, J.H. Novel norhumulene and xeniaphyllane-derived terpenoids from a formosan soft coral Sinularia gibberosa. Chem. Pharm. Bull. 2009, 57, 162–166. [Google Scholar] [CrossRef][Green Version]
- Li, X.R.; Liu, J.; Peng, C.; Zhou, Q.M.; Liu, F.; Guo, L.; Xiong, L. Polyacetylene glucosides from the florets of Carthamus tinctorius and their anti-inflammatory activity. Phytochemistry 2021, 187, 112770. [Google Scholar] [CrossRef]
- Tsui, W.Y.; Brown, G. Acid-catalysed rearrangement of caryophyllene oxide. J. Chem. Soc. Perkin Trans. 1996, 1, 2507–2509. [Google Scholar] [CrossRef]
- Yu, Y.; Gao, H.; Dai, Y.; Xiao, G.K.; Zhu, H.J.; Yao, X.S. Guaiane-type sesquiterpenoid glucosides from Gardenia jasminoides Ellis. Magn. Reason. Chem. 2011, 49, 258–261. [Google Scholar] [CrossRef]
- Taglialatela-Scafati, O.; Pollastro, F.; Cicione, L.; Chianese, G.; Bellido, M.L.; Munoz, E.; Ozen, H.C.; Toker, Z.; Appendino, G. STAT-3 inhibitory bisabolanes from Carthamus glaucus. J. Nat. Prod. 2012, 75, 453–458. [Google Scholar] [CrossRef]
- Yin, X.; Liu, Y.; Pan, J.; Ye, H.L.; Sun, Y.; Zhao, D.Y.; Kuang, H.X.; Yang, B.Y. Melongenaterpenes A-L, vetispirane-type sesquiterpenoids from the roots of Solanum melongena. J. Nat. Prod. 2019, 82, 3242–3248. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, B.; Guo, R.; Li, S.; Xu, Y. Enhancement in efferocytosis of oxidized low-density lipoprotein-induced apoptotic RAW264.7 cells through Sirt1-mediated autophagy. Int. J. Mol. Med. 2014, 33, 523–533. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, P.; Ye, J. Lipid homeostasis and the formation of macrophage-derived foam cells in atherosclerosis. Protein Cell 2012, 3, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Gotō, H.; Ōsawa, E. Corner flapping: A simple and fast algorithm for exhaustive generation of ring conformations. J. Am. Chem. Soc. 1989, 111, 8950–8951. [Google Scholar] [CrossRef]
- Gotō, H.; Ōsawa, E. An efficient algorithm for searching low-energy conformers of cyclic and acyclic molecules. J. Chem. Soc. Perkin Trans. 1993, 2, 187–198. [Google Scholar] [CrossRef]
- Gaussian 16, Revision B.01; Gaussian, Inc.: Wallingford, CT, USA, 2006.
- Liu, Y.; Liu, F.; Qiao, M.M.; Guo, L.; Chen, M.H.; Peng, C.; Xiong, L. Curcumanes A and B, two bicyclic sesquiterpenoids with significant vasorelaxant activity from Curcuma longa. Org. Lett. 2019, 21, 1197–1201. [Google Scholar] [CrossRef]
- Spec Dis, Version 1.71; University of Würzburg: Würzburg, Germany, 2017.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).