Comparative Pharmacokinetic of Curcuminoids Formulations with an Omega-3 Fatty Acids Monoglyceride Carrier: A Randomized Cross-Over Triple-Blind Study
Abstract
1. Introduction
2. Materials and Methods
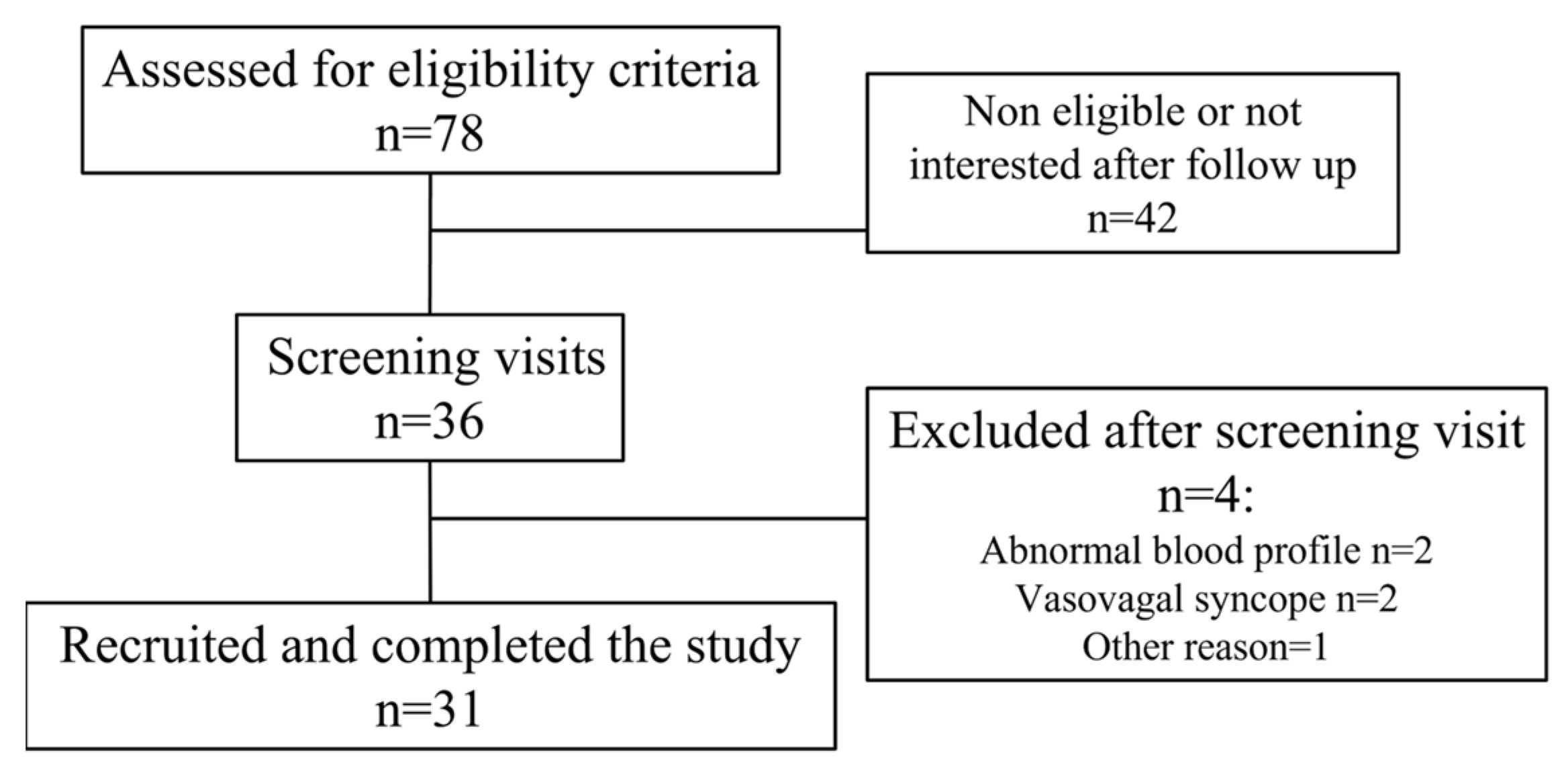
2.1. Study Participants
2.2. Study Products
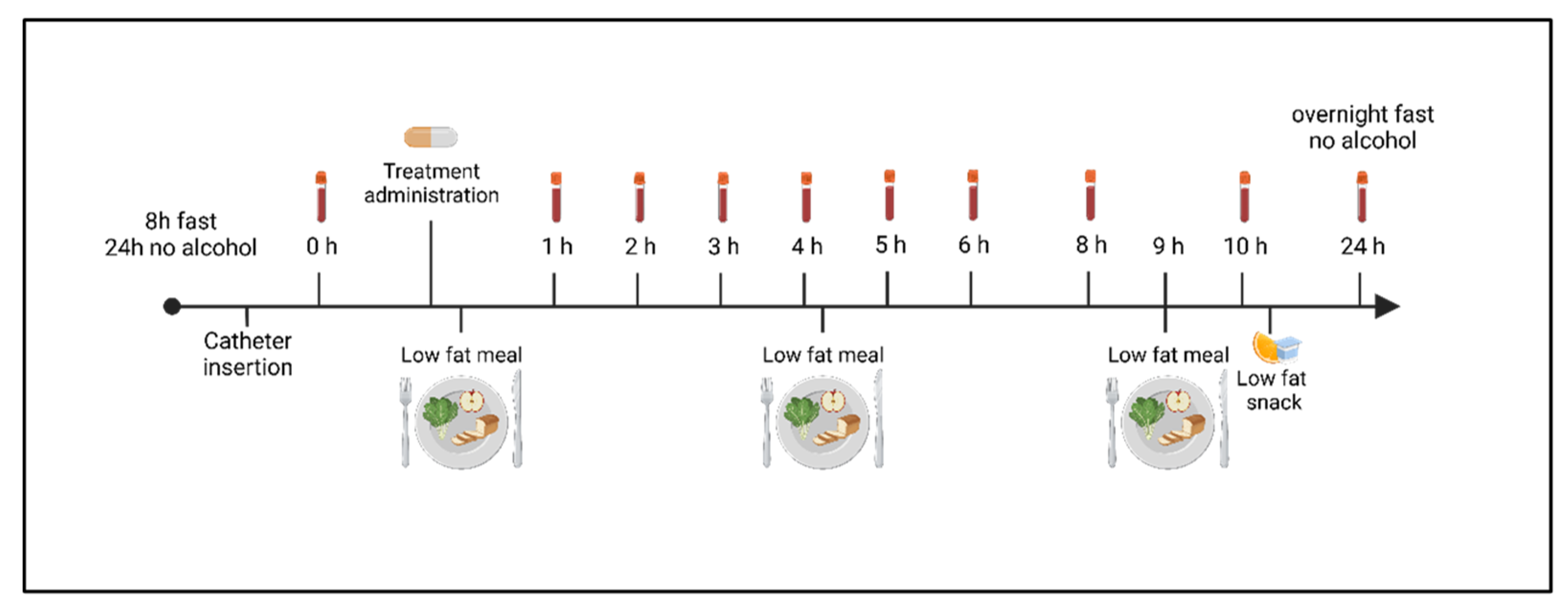
2.3. Study Design
2.4. Curcuminoids Extraction from the Plasma
2.5. Curcuminoids Analysis
2.6. Statistical Analysis
3. Results
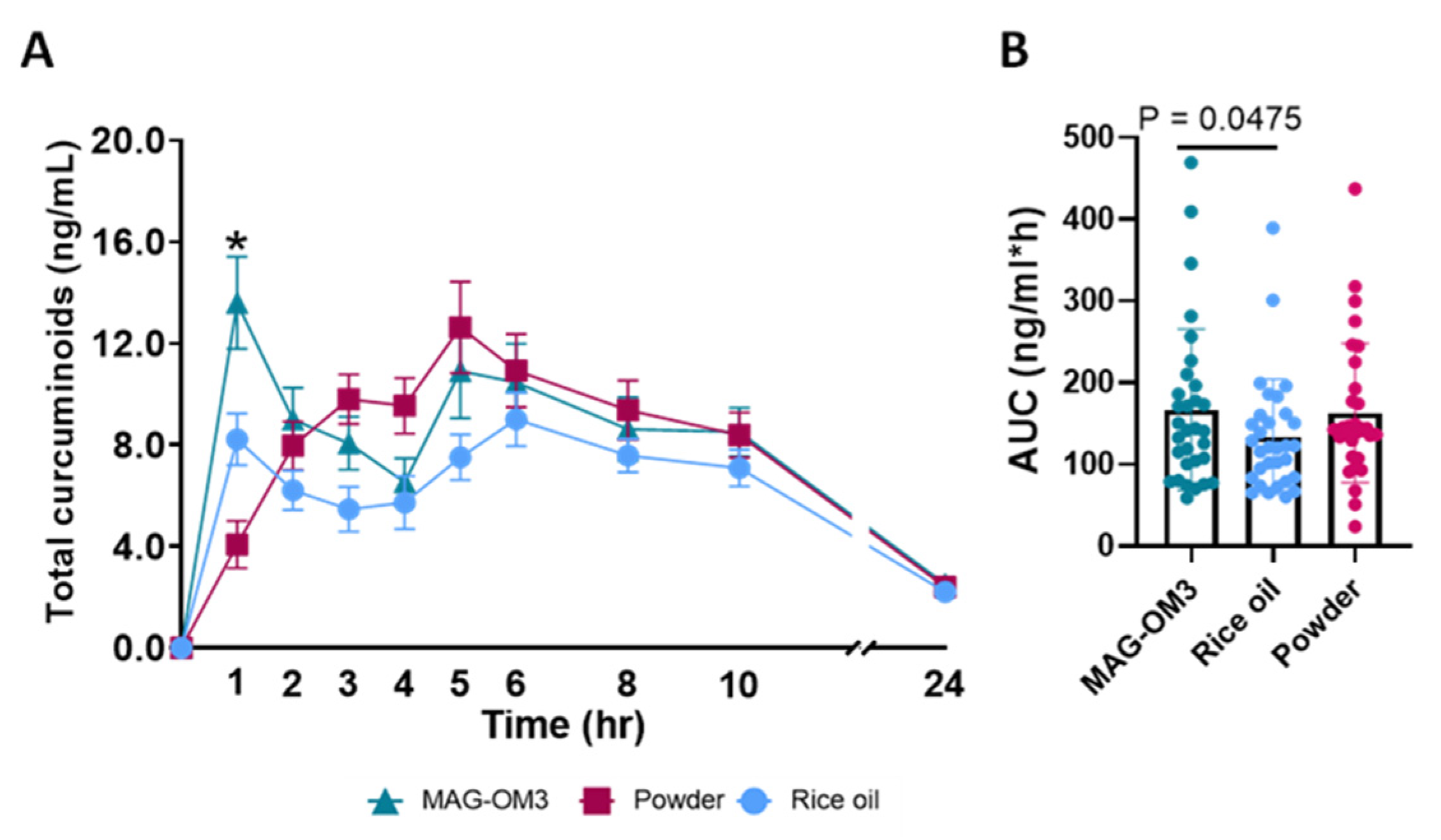
3.1. Primary Outcomes
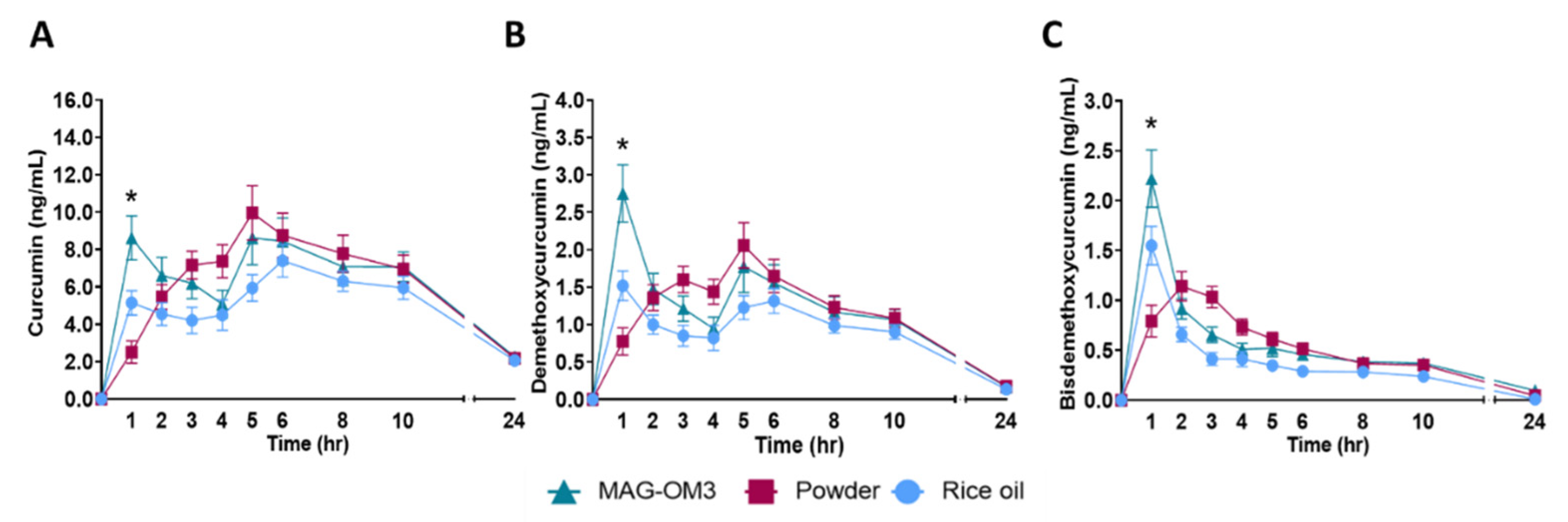
3.2. Secondary Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hatcher, H.; Planalp, R.; Cho, J.; Torti, F.M.; Torti, S.V. Curcumin: From ancient medicine to current clinical trials. Cell. Mol. Life Sci. 2008, 65, 1631–1652. [Google Scholar] [CrossRef] [PubMed]
- Niranjan, A.; Singh, S.; Dhiman, M.; Tewari, S.K. Biochemical Composition of Curcuma longa L. Accessions. Anal. Lett. 2013, 46, 1069–1083. [Google Scholar] [CrossRef]
- Cuomo, J.; Appendino, G.; Dern, A.S.; Schneider, E.; McKinnon, T.P.; Brown, M.J.; Togni, S.; Dixon, B.M. Comparative Absorption of a Standardized Curcuminoid Mixture and Its Lecithin Formulation. J. Nat. Prod. 2011, 74, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.-M.; Jialal, I.; Devaraj, S. Epigenetic regulation of high glucose-induced proinflammatory cytokine production in monocytes by curcumin. J. Nutr. Biochem. 2011, 22, 450–458. [Google Scholar] [CrossRef]
- Henrotin, Y.; Priem, F.; Mobasheri, A. Curcumin: A new paradigm and therapeutic opportunity for the treatment of osteoarthritis: Curcumin for osteoarthritis management. SpringerPlus 2013, 2, 56. [Google Scholar] [CrossRef] [PubMed]
- Sandur, S.K.; Pandey, M.K.; Sung, B.; Ahn, K.S.; Murakami, A.; Sethi, G.; Limtrakul, P.; Badmaev, V.; Aggarwal, B.B. Curcumin, demethoxycurcumin, bisdemethoxycurcumin, tetrahydrocurcumin and turmerones differentially regulate anti-inflammatory and anti-proliferative responses through a ROS-independent mechanism. Carcinogenesis 2007, 28, 1765–1773. [Google Scholar] [CrossRef] [PubMed]
- Lao, C.D.; Ruffin, M.T., 4th; Normolle, D.; Heath, D.D.; Murray, S.I.; Bailey, J.M.; Boggs, M.E.; Crowell, J.; Rock, C.L.; Brenner, D.E. Dose escalation of a curcuminoid formulation. BMC Complement. Altern. Med. 2006, 6, 10. [Google Scholar] [CrossRef]
- Dutta, A.K. Novel Drug Delivery Systems to Improve Bioavailability of Curcumin. J. Bioequivalence Bioavailab. 2014, 6, 1–9. [Google Scholar] [CrossRef]
- Kelloff, G.J.; Crowell, J.A.; Hawk, E.T.; Steele, V.E.; Lubet, R.A.; Boone, C.W.; Covey, J.M.; Doody, L.A.; Omenn, G.S.; Greenwald, P.; et al. Strategy and planning for chemopreventive drug development: Clinical development plans II. J. Cell. Biochem. Suppl. 1996, 26, 54–71. [Google Scholar] [CrossRef]
- Loftsson, T.; Jarho, P.; Másson, M.; Järvinen, T. Cyclodextrins in drug delivery. Expert Opin. Drug Deliv. 2005, 2, 335–351. [Google Scholar] [CrossRef]
- Bisht, S.; Maitra, A. Systemic delivery of curcumin: 21st century solutions for an ancient conundrum. Curr. Drug Discov. Technol. 2009, 6, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Grynkiewicz, G.; Ślifirski, P. Curcumin and curcuminoids in quest for medicinal status. Acta Biochim. Pol. 2012, 59, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Jamwal, R. Bioavailable curcumin formulations: A review of pharmacokinetic studies in healthy volunteers. J. Integr. Med. 2018, 16, 367–374. [Google Scholar] [CrossRef]
- Atal, C.K.; Dubey, R.K.; Singh, J. Biochemical basis of enhanced drug bioavailability by piperine: Evidence that piperine is a potent inhibitor of drug metabolism. J. Pharmacol. Exp. Ther. 1985, 232, 258–262. [Google Scholar]
- Bhardwaj, R.K.; Glaeser, H.; Becquemont, L.; Klotz, U.; Gupta, S.K.; Fromm, M.F. Piperine, a Major Constituent of Black Pepper, Inhibits Human P-glycoprotein and CYP3A4. J. Pharmacol. Exp. Ther. 2002, 302, 645–650. [Google Scholar] [CrossRef]
- Chevalier, L.; Plourde, M. Comparison of pharmacokinetics of omega-3 fatty acid supplements in monoacylglycerol or ethyl ester in humans: A randomized controlled trial. Eur. J. Clin. Nutr. 2021, 75, 680–688. [Google Scholar] [CrossRef]
- Shao, X.; Bor, G.; Al-Hosayni, S.; Salentinig, S.; Yaghmur, A. Structural characterization of self-assemblies of new omega-3 lipids: Docosahexaenoic acid and docosapentaenoic acid monoglycerides. Phys. Chem. Chem. Phys. 2018, 20, 23928–23941. [Google Scholar] [CrossRef] [PubMed]
- Cuenoud, B.; Rochat, I.; Gosoniu, M.; Dupuis, L.; Berk, E.; Jaudszus, A.; Mainz, J.; Hafen, G.; Beaumont, M.; Cruz-Hernandez, C. Monoacylglycerol Form of Omega-3s Improves Its Bioavailability in Humans Compared to Other Forms. Nutrients 2020, 12, 1014. [Google Scholar] [CrossRef]
- Chevalier, L.; Vachon, A.; Plourde, M. Pharmacokinetics of Supplemental Omega-3 Fatty Acids Esterified in Monoglycerides, Ethyl Esters, or Triglycerides in Adults in a Randomized Crossover Trial. J. Nutr. 2021, 151, 1111–1118. [Google Scholar] [CrossRef]
- Luis, P.B.; Kunihiro, A.G.; Funk, J.L.; Schneider, C. Incomplete Hydrolysis of Curcumin Conjugates by β-Glucuronidase: Detection of Complex Conjugates in Plasma. Mol. Nutr. Food Res. 2020, 64, e1901037. [Google Scholar] [CrossRef]
- Stohs, S.J.; Chen, O.; Ray, S.D.; Ji, J.; Bucci, L.R.; Preuss, H.G. Highly Bioavailable Forms of Curcumin and Promising Avenues for Curcumin-Based Research and Application: A Review. Molecules 2020, 25, 1397. [Google Scholar] [CrossRef] [PubMed]
- Sample Size Calculator. Available online: https://clincalc.com/stats/samplesize.aspx (accessed on 26 August 2022).
- Schiborr, C.; Kocher, A.; Behnam, D.; Jandasek, J.; Toelstede, S.; Frank, J. The oral bioavailability of curcumin from micronized powder and liquid micelles is significantly increased in healthy humans and differs between sexes. Mol. Nutr. Food Res. 2014, 58, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Semalty, A.; Semalty, M.; Rawat, M.S.M.; Franceschi, F. Supramolecular Phospholipids-Polyphenolics Interactions: The PHYTOSOME Strategy to Improve the Bioavailability of Phytochemicals. Fitoterapia 2010, 81, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, H.; Sunagawa, Y.; Takahashi, K.; Imaizumi, A.; Fukuda, H.; Hashimoto, T.; Wada, H.; Katanasaka, Y.; Kakeya, H.; Fujita, M.; et al. Innovative Preparation of Curcumin for Improved Oral Bioavailability. Biol. Pharm. Bull. 2011, 34, 660–665. [Google Scholar] [CrossRef]
- Kothaplly, S.; Alukapally, S.; Nagula, N.; Maddela, R. Superior Bioavailability of a Novel Curcumin Formulation in Healthy Humans Under Fasting Conditions. Adv. Ther. 2022, 39, 2128–2138. [Google Scholar] [CrossRef] [PubMed]
- Gota, V.S.; Maru, G.B.; Soni, T.G.; Gandhi, T.R.; Kochar, N.; Agarwal, M.G. Safety and Pharmacokinetics of a Solid Lipid Curcumin Particle Formulation in Osteosarcoma Patients and Healthy Volunteers. J. Agric. Food Chem. 2010, 58, 2095–2099. [Google Scholar] [CrossRef]
- Schwartzberg, L.S.; Navari, R.M. Safety of Polysorbate 80 in the Oncology Setting. Adv. Ther. 2018, 35, 754–767. [Google Scholar] [CrossRef]
- Rhodes, A.; Eastwood, J.B.; Smith, S.A. Early Acute Hepatitis with Parenteral Amiodarone: A Toxic Effect of the Vehicle? Gut 1993, 34, 565–566. [Google Scholar] [CrossRef]
- Curran, B.J.; Havill, J.H. Hepatic and renal failure associated with amiodarone infusion in a patient with hereditary fructose intolerance. Crit. Care Resusc. 2002, 4, 112–115. [Google Scholar]
- Purpura, M.; Lowery, R.P.; Wilson, J.M.; Mannan, H.; Münch, G.; Razmovski-Naumovski, V. Analysis of different innovative formulations of curcumin for improved relative oral bioavailability in human subjects. Eur. J. Nutr. 2017, 57, 929–938. [Google Scholar] [CrossRef]
- PubChem Cellulose, Microcrystalline. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/14055602 (accessed on 2 September 2022).
- Xu, J.; Tan, X.; Chen, L.; Li, X.; Xie, F. Starch/microcrystalline cellulose hybrid gels as gastric-floating drug delivery systems. Carbohydr. Polym. 2019, 215, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Nsor-Atindana, J.; Chen, M.; Goff, H.D.; Zhong, F.; Sharif, H.R.; Li, Y. Functionality and nutritional aspects of microcrystalline cellulose in food. Carbohydr. Polym. 2017, 172, 159–174. [Google Scholar] [CrossRef] [PubMed]
- PubChem Maltodextrin-Dextrose Equivalent 10–15. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/68229136 (accessed on 2 September 2022).
- Buttet, M.; Traynard, V.; Tran, T.T.T.; Besnard, P.; Poirier, H.; Niot, I. From fatty-acid sensing to chylomicron synthesis: Role of intestinal lipid-binding proteins. Biochimie 2014, 96, 37–47. [Google Scholar] [CrossRef] [PubMed]
- D’Aquila, T.; Hung, Y.-H.; Carreiro, A.; Buhman, K.K. Recent discoveries on absorption of dietary fat: Presence, synthesis, and metabolism of cytoplasmic lipid droplets within enterocytes. Biochim. Biophys. Acta 2016, 1861, 730–747. [Google Scholar] [CrossRef]
- Bays, H.E.; Ballantyne, C.M.; Kastelein, J.J.; Isaacsohn, J.L.; Braeckman, R.A.; Soni, P.N. Eicosapentaenoic Acid Ethyl Ester (AMR101) Therapy in Patients With Very High Triglyceride Levels (from the Multi-center, plAcebo-controlled, Randomized, double-blINd, 12-week study with an open-label Extension [MARINE] Trial). Am. J. Cardiol. 2011, 108, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Backes, J.; Anzalone, D.; Hilleman, D.; Catini, J. The clinical relevance of omega-3 fatty acids in the management of hypertriglyceridemia. Lipids Health Dis. 2016, 15, 118. [Google Scholar] [CrossRef]
- Clark, R.; Johnson, R. Malabsorption Syndromes. Nurs. Clin. North Am. 2018, 53, 361–374. [Google Scholar] [CrossRef]
- Peng, Y.; Ao, M.; Dong, B.; Jiang, Y.; Yu, L.; Chen, Z.; Hu, C.; Xu, R. Anti-Inflammatory Effects of Curcumin in the Inflammatory Diseases: Status, Limitations and Countermeasures. Drug Des. Dev. Ther. 2021, 15, 4503–4525. [Google Scholar] [CrossRef]
- Limketkai, B.N.; Wolf, A.; Parian, A.M. Nutritional Interventions in the Patient with Inflammatory Bowel Disease. Gastroenterol. Clin. N. Am. 2018, 47, 155–177. [Google Scholar] [CrossRef]
- Rao, C.V. Regulation of cox and lox by curcumin. In The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease; Advances in experimental medicine and biology; Aggarwal, B.B., Surh, Y.-J., Shishodia, S., Eds.; Springer: Boston, MA, USA, 2007; Volume 595, pp. 213–226. ISBN 978-0-387-46400-8. [Google Scholar]
- Goel, A.; Boland, C.; Chauhan, D.P. Specific inhibition of cyclooxygenase-2 (COX-2) expression by dietary curcumin in HT-29 human colon cancer cells. Cancer Lett. 2001, 172, 111–118. [Google Scholar] [CrossRef]
- Mohan, M.; Hussain, M.A.; Khan, F.A.; Anindya, R. Symmetrical and un-symmetrical curcumin analogues as selective COX-1 and COX-2 inhibitor. Eur. J. Pharm. Sci. 2021, 160, 105743. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Omega-3 polyunsaturated fatty acids and inflammatory processes: Nutrition or pharmacology? Br. J. Clin. Pharmacol. 2013, 75, 645–662. [Google Scholar] [CrossRef] [PubMed]
- Bhagavan, H.N.; Chopra, R.K. Plasma coenzyme Q10 response to oral ingestion of coenzyme Q10 formulations. Mitochondrion 2007, 7, S78–S88. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.E.; Parks, E.J. Postprandial metabolism of meal triglyceride in humans. Biochim. Biophys. Acta 2012, 1821, 721–726. [Google Scholar] [CrossRef]
- Pravst, I.; Rodriguez Aguilera, J.C.; Cortes Rodriguez, A.B.; Jazbar, J.; Locatelli, I.; Hristov, H.; Žmitek, K. Comparative Bioavailability of Different Coenzyme Q10 Formulations in Healthy Elderly Individuals. Nutrients 2020, 12, 784. [Google Scholar] [CrossRef]
- Beaulieu, S.; Vachon, A.; Plourde, M. Women have higher levels of CoQ10 than men when supplemented with a single dose of CoQ10 with monoglycerides omega-3 or rice oil and followed for 48 h: A crossover randomised triple blind controlled study. J. Nutr. Sci. 2022, 11, e2. [Google Scholar] [CrossRef]
- Shoba, G.; Joy, D.; Joseph, T.; Majeed, M.; Rajendran, R.; Srinivas, P.S. Influence of Piperine on the Pharmacokinetics of Curcumin in Animals and Human Volunteers. Planta Med. 1998, 64, 353–356. [Google Scholar] [CrossRef]
- Chung, H.; Yoon, S.H.; Cho, J.-Y.; Yeo, H.K.; Shin, D.; Park, J.-Y. Comparative pharmacokinetics of Theracurmin, a highly bioavailable curcumin, in healthy adult subjects. Int. J. Clin. Pharmacol. Ther. 2021, 59, 684–690. [Google Scholar] [CrossRef]
| Curcuminoids + MAG-OM3 Supplement (mg/Capsule) | Curcuminoids + Rice Oil Supplement (mg/Capsule) | Curcuminoids Powder Supplement (mg/Capsule) | |||||
|---|---|---|---|---|---|---|---|
| Curcuminoids | Mean | SD | Mean | SD | Mean | SD | p-Value |
| Curcumin | 147.6 | 3.4 | 148.6 | 8.2 | 128.8 | 5.8 | 0.0839 |
| Bisdemethoxycurcumin (BDMC) | 5.8 | 0.3 | 5.7 | 0.6 | 5.7 | 0.4 | 0.9945 |
| Desmethoxycurcumin (DMC) | 34.7 | 0.7 | 34.9 | 2.2 | 31.5 | 1.6 | 0.2044 |
| Total Curcuminoids | 188.0 | 4.3 | 189.2 | 11.0 | 166.1 | 3.9 | 0.3287 |
| Curcuminoids + MAG-OM3 Supplement (mg/Capsule) | Curcuminoids + Rice Oil Supplement (mg/Capsule) | Curcuminoids Powder Supplement (mg/Capsule) | ||||
|---|---|---|---|---|---|---|
| Fatty Acids | Mean | SD | Mean | SD | Mean | SD |
| C8:0 | 0.4 | 0.7 | 0.5 | 0.8 | 0.0 | 0.0 |
| C10:0 | 1.0 | 1.0 | 0.6 | 1.1 | 0.0 | 0.0 |
| C14:0 | 2.5 | 0.2 | 2.8 | 0.1 | 0.0 | 0.0 |
| C16:0 | 18.9 | 0.6 | 161.9 | 4.6 | 5.9 | 0.3 |
| C16:1 n-7 | 1.8 | 0.2 | 1.5 | 0.1 | 0.0 | 0.0 |
| C17:1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| C18:0 | 12.9 | 1.0 | 17.9 | 0.5 | 4.3 | 0.2 |
| C18:1 n-9 | 24.5 | 1.6 | 327.3 | 14.5 | 0.0 | 0.0 |
| C18:1 n-7 | 5.6 | 0.5 | 7.1 | 0.3 | 0.0 | 0.0 |
| C18:2 n-6 | 13.8 | 11.1 | 259.2 | 11.0 | 2.5 | 0.1 |
| C18:3 n-6 | 1.1 | 1.0 | 2.7 | 0.4 | 0.0 | 0.0 |
| C18:3 n-3 | 2.1 | 0.2 | 7.3 | 0.3 | 0.0 | 0.0 |
| C20:0 | 3.0 | 0.2 | 6.4 | 0.3 | 0.0 | 0.0 |
| C20:1 | 14.4 | 1.3 | 4.0 | 0.2 | 0.0 | 0.0 |
| C20:2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| C20:3 n-6 | 1.2 | 2.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| C20:4 n-6 | 19.8 | 2.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| C20:3 n-3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| C20:5 n-3 | 372.9 | 36.6 | 0.0 | 0.0 | 0.0 | 0.0 |
| C22:0 | 0.0 | 0.0 | 2.4 | 0.1 | 0.0 | 0.0 |
| C22:1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| C24:0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| C22:5 n-6 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| C22:5 n-3 | 26.3 | 2.7 | 0.0 | 0.0 | 0.0 | 0.0 |
| C22:6 n-3 | 153.5 | 15.9 | 0.0 | 0.0 | 0.0 | 0.0 |
| Total concentration | 675.7 | 78.8 | 801.6 | 34.3 | 12.8 | 0.6 |
| Anthropometric Characteristics | Total (n = 31) | Male (n = 16) | Female (n = 15) | p-Value |
|---|---|---|---|---|
| Age (years) | 27.6 ± 6.0 | 28.9 ± 6.8 | 26.3 ± 5.0 | 0.2346 |
| BMI (kg/m2) | 25.1 ± 3.8 | 25.8 ± 3.0 | 24.4 ± 4.4 | 0.3290 |
| Plasma TG (mmol/L) | 0.87 ± 0.51 | 0.91 ± 0.49 | 0.84 ± 0.54 | 0.4062 |
| Plasma HDL-C (mmol/L) | 1.39 ± 0.34 | 1.39 ± 0.35 | 1.40 ± 0.33 | 0.9659 |
| Plasma LDL-C (mmol/L) | 2.58 ± 0.70 | 2.68 ± 0.77 | 2.48 ± 0.62 | 0.4462 |
| Plasma glucose (mmol/L) | 4.64 ± 0.54 | 4.81 ± 0.54 | 4.45 ± 0.50 | 0.0691 |
| HbA1c (%) | 5.08 ± 0.30 | 5.13 ± 0.32 | 5.03 ± 0.28 | 0.4017 |
| Curcuminoid | Formulation 2 | Tmax (h) | p-Value 3 | Cmax (ng/mL) | p-Value | C24 h (ng/mL) | p-Value | C1 h (ng/mL) | p-Value | AUC 0–24 h (ng/mL*h) | p-Value | AUC 0–6 h (ng/mL*h) | p-Value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Curcumin | MAG-OM3 | 4.2 ± 0.6 | 0.2502 | 12.9 ± 1.6 | 0.1152 | 2.3 ± 0.3 | 0.9675 | 8.6 ± 1.2 A | <0.0001 | 134.3 ± 14.3 | 0.0307 | 39.3 ± 4.4 A | 0.0337 |
| Rice oil | 5.1 ± 0.5 | 9.1 ± 0.9 | 2.1 ± 0.2 | 5.2 ± 0.7 A | 110.1 ± 10.5 | 28.0 ± 3.3 B | |||||||
| Powder | 5.8 ± 0.7 | 12.3 ± 1.3 | 2.2 ± 0.2 | 2.5 ± 0.6 B | 132.0 ± 12.5 | 36.8 ± 4.1 | |||||||
| Demethoxy-curcumin (DMC) | MAG-OM3 | 2.3 ± 0.4 A | 0.0009 | 3.3 ± 0.5 A | 0.0102 | 0.2 ± 0.1 | 0.8440 | 2.8 ± 2.1 A | <0.0001 | 22.6 ± 2.7 | 0.0576 | 8.9 ± 1.0 A | 0.0113 |
| Rice oil | 3.5 ± 0.5 AB | 2.0 ± 0.2 B | 0.1 ± 0.1 | 1.5 ± 1.1 B | 17.7 ± 1.8 | 6.1 ± 0.7 B | |||||||
| Powder | 4.5 ± 0.4 B | 2.5 ± 0.3 AB | 0.2 ± 0.1 | 0.8 ± 1.0 B | 22.2 ± 2.2 | 8.1 ± 0.9 AB | |||||||
| Bisdemethoxy-curcumin (BDMC) | MAG-OM3 | 1.1 ± 0.1 A | <0.0001 | 2.3 ± 0.3 A | 0.0066 | 0.10 ± 0.03 | 0.8465 | 2.2 ± 1.6 A | <0.0001 | 10.0 ± 1.0 | 0.1590 | 5.1 ± 0.5 A | 0.0063 |
| Rice oil | 1.1 ± 0.1 A | 1.6 ± 0.2 B | 0.01 ± 0.06 | 1.6 ± 1.1 B | 7.1 ± 0.8 | 3.5 ± 0.4 B | |||||||
| Powder | 1.8 ± 0.2 B | 1.5 ± 0.1 B | 0.05 ± 0.04 | 0.8 ± 0.9 B | 9.3 ± 0.8 | 4.6 ± 0.4 AB | |||||||
| Total Curcuminoids | MAG-OM3 | 3.4 ± 0.5 | 0.1681 | 17.7 ± 2.3 | 0.0939 | 2.5 ± 0.4 | 0.7283 | 13.6 ± 1.8 A | <0.0001 | 166.8± 17.8 A | 0.0297 | 53.3 ± 5.9 A | 0.0230 |
| Rice oil | 4.3 ± 0.5 | 11.9 ± 1.1 | 2.2 ± 0.3 | 8.2 ± 1.0 B | 134.0 ± 12.7 B | 37.6 ± 4.3 B | |||||||
| Powder | 4.8 ± 0.4 | 15.7 ± 1.6 | 2.4 ± 0.3 | 4.1 ± 0.9 C | 163.1 ± 15.3 AB | 49.5 ± 5.4 AB |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguilera, E.C.; Vachon, A.; Plourde, M. Comparative Pharmacokinetic of Curcuminoids Formulations with an Omega-3 Fatty Acids Monoglyceride Carrier: A Randomized Cross-Over Triple-Blind Study. Nutrients 2022, 14, 5347. https://doi.org/10.3390/nu14245347
Aguilera EC, Vachon A, Plourde M. Comparative Pharmacokinetic of Curcuminoids Formulations with an Omega-3 Fatty Acids Monoglyceride Carrier: A Randomized Cross-Over Triple-Blind Study. Nutrients. 2022; 14(24):5347. https://doi.org/10.3390/nu14245347
Chicago/Turabian StyleAguilera, Ester Cisneros, Annick Vachon, and Mélanie Plourde. 2022. "Comparative Pharmacokinetic of Curcuminoids Formulations with an Omega-3 Fatty Acids Monoglyceride Carrier: A Randomized Cross-Over Triple-Blind Study" Nutrients 14, no. 24: 5347. https://doi.org/10.3390/nu14245347
APA StyleAguilera, E. C., Vachon, A., & Plourde, M. (2022). Comparative Pharmacokinetic of Curcuminoids Formulations with an Omega-3 Fatty Acids Monoglyceride Carrier: A Randomized Cross-Over Triple-Blind Study. Nutrients, 14(24), 5347. https://doi.org/10.3390/nu14245347





