Obesity Contributes to Inflammation in Patients with IBS via Complement Component 3 and C-Reactive Protein
Abstract
1. Introduction
2. Materials and Methods
2.1. Population
2.2. Measurement of Body Mass Index
2.3. Serum Collection and Storage
2.4. Serum Immunological Determinations
2.5. Statistical Analysis
3. Results
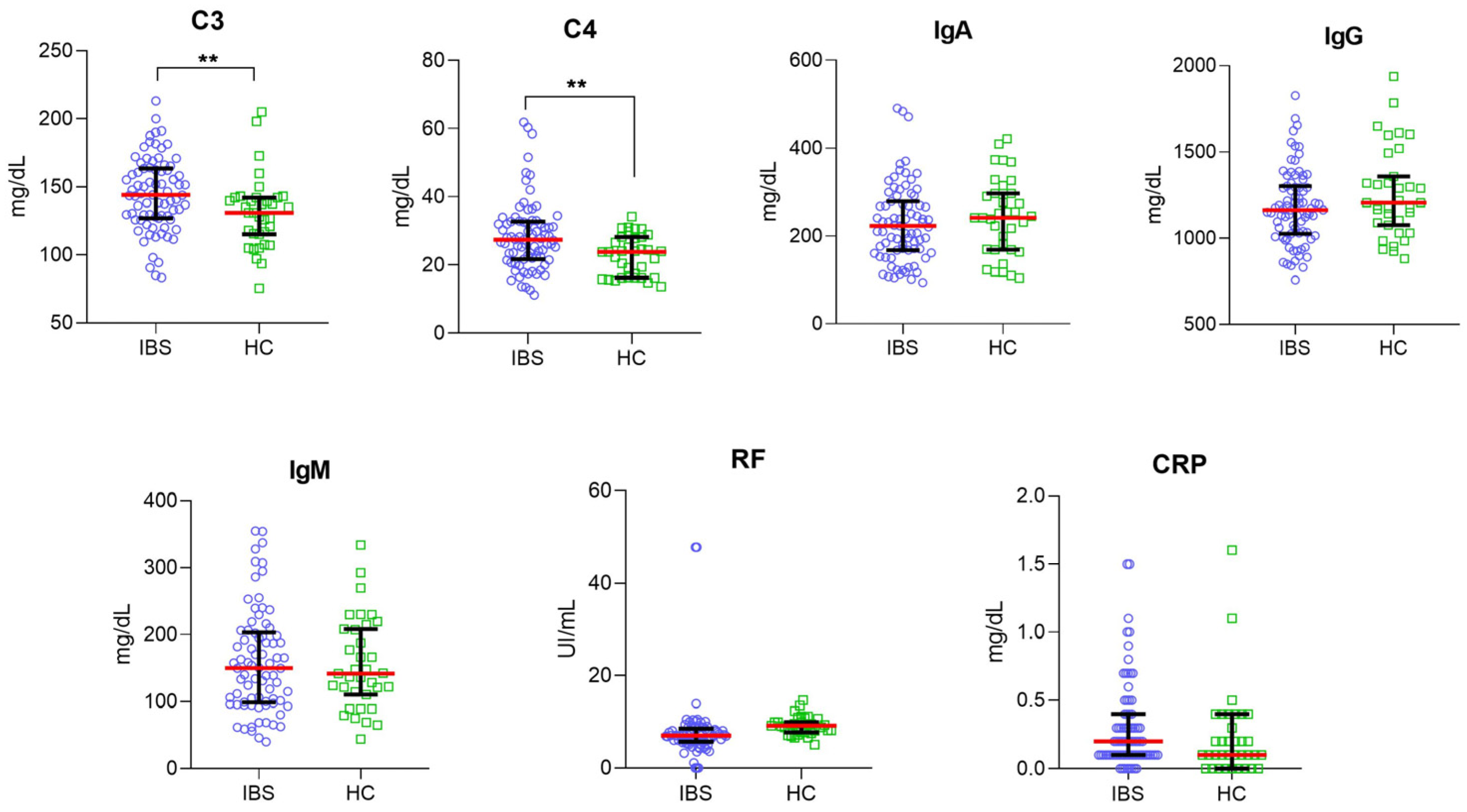
3.1. Higher Serum Levels of C3 and C4 in IBS Patients Than in HC Group
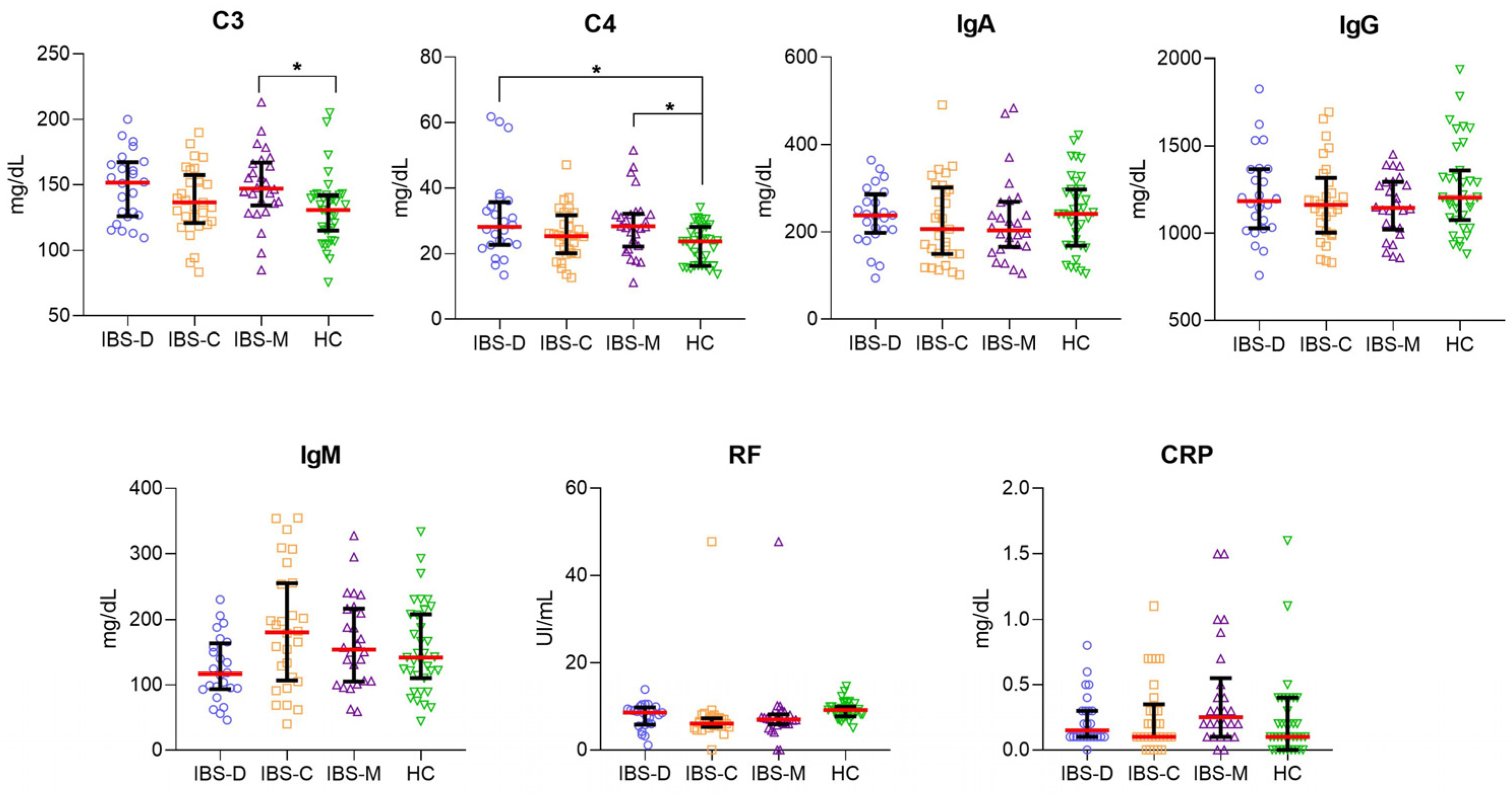
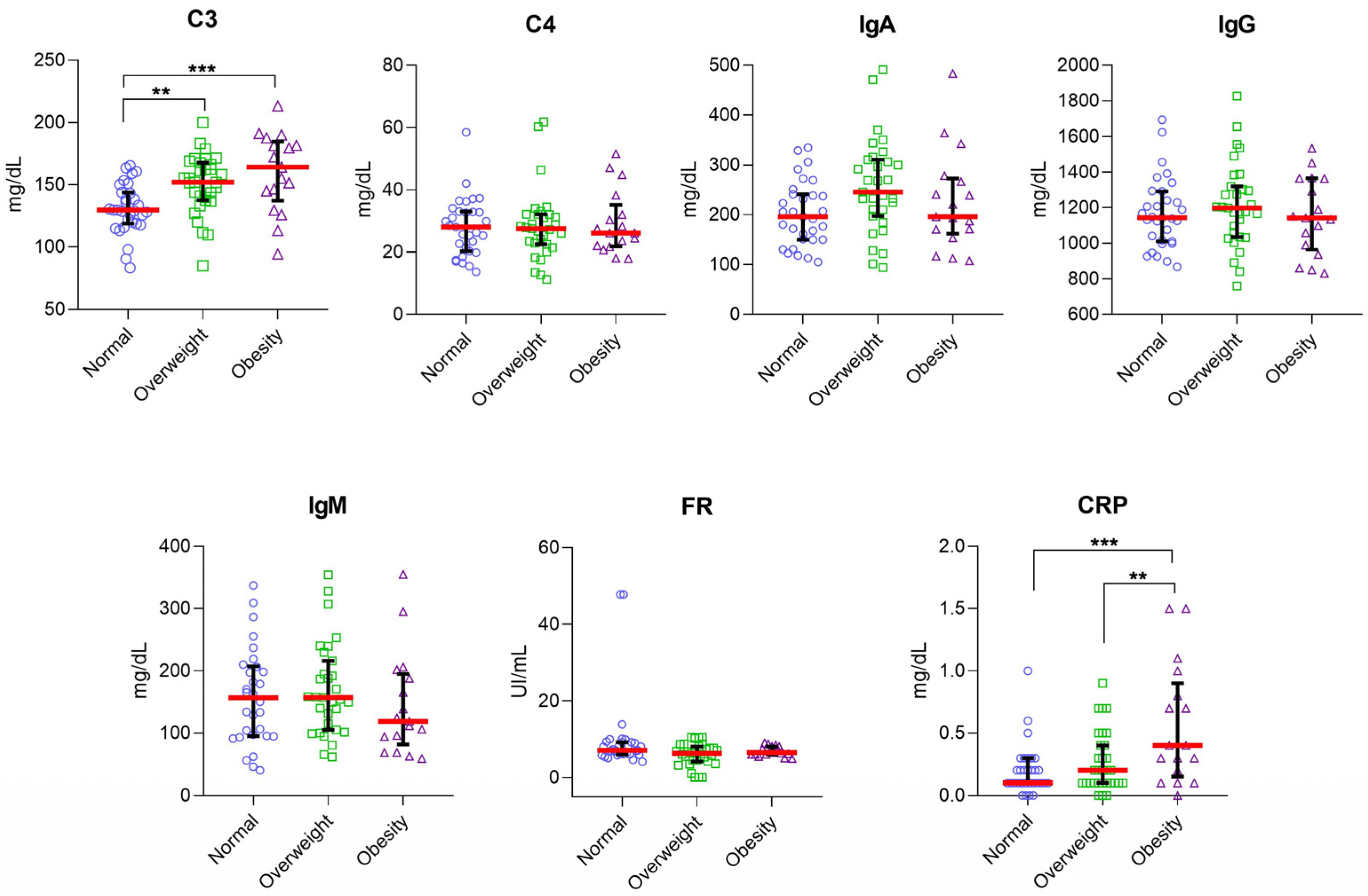
3.2. Higher Serum Levels of C3 and C4 in IBS Subgroups Than in HC Group
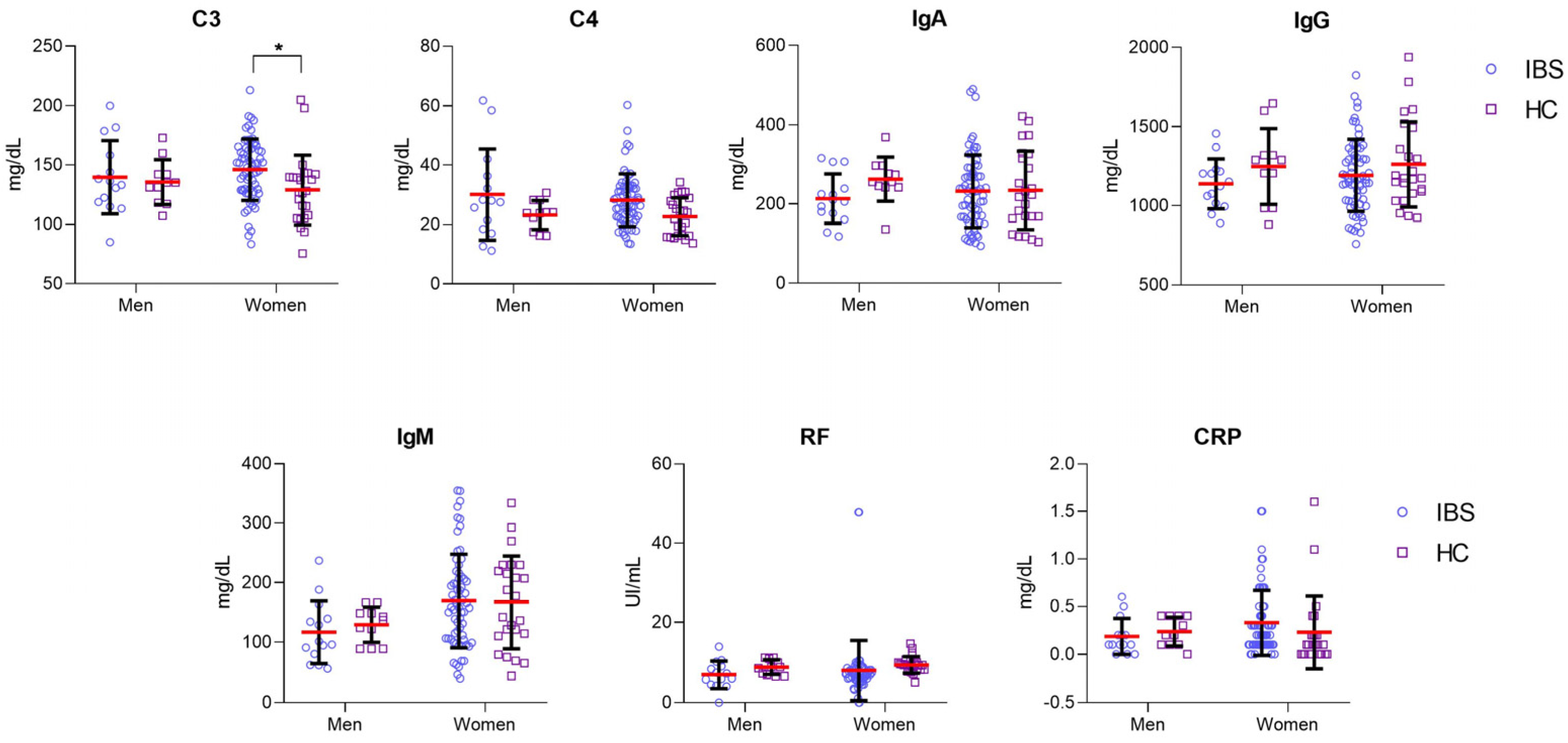
3.3. Higher Serum Levels of C3 in IBS Women Than in HC Women
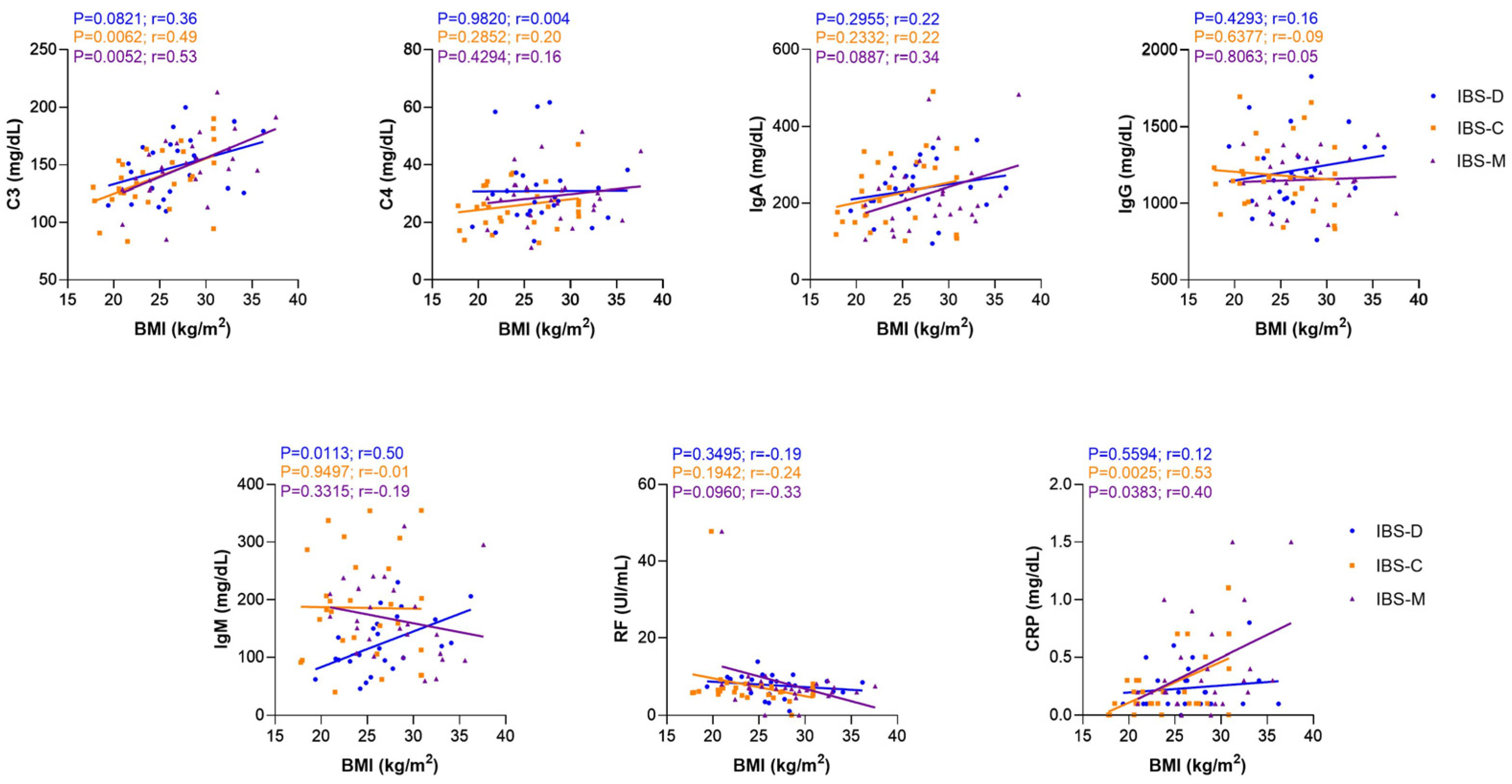
3.4. Correlation Analysis between C3, C4, IgA, IgG, IgM, RF, and CRP Serum Levels and Body Mass Index in IBS and HC Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ford, A.C.; Sperber, A.D.; Corsetti, M.; Camilleri, M. Irritable bowel syndrome. Lancet 2020, 396, 1675–1688. [Google Scholar] [CrossRef] [PubMed]
- Spiller, R.; Aziz, Q.; Creed, F.; Emmanuel, A.; Houghton, L.; Hungin, P.; Jones, R.; Kumar, D.; Rubin, G.; Trudgill, N.; et al. Guidelines on the irritable bowel syndrome: Mechanisms and practical management. Gut 2007, 56, 1770–1798. [Google Scholar] [CrossRef] [PubMed]
- Weaver, K.R.; Melkus, G.D.; Henderson, W.A. Irritable Bowel Syndrome. Am. J. Nurs. 2017, 117, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Saha, L. Irritable bowel syndrome: Pathogenesis, diagnosis, treatment, and evidence-based medicine. World J. Gastroenterol. 2014, 20, 6759–6773. [Google Scholar] [CrossRef] [PubMed]
- Occhipinti, K.; Smith, J.W. Irritable bowel syndrome: A review and update. Clin. Colon Rectal Surg. 2012, 25, 46–52. [Google Scholar] [CrossRef]
- Barbara, G.; De Giorgio, R.; Stanghellini, V.; Cremon, C.; Corinaldesi, R. A role for inflammation in irritable bowel syndrome? Gut 2002, 51 (Suppl. S1), i41–i44. [Google Scholar] [CrossRef]
- Hughes, P.A.; Zola, H.; Penttila, I.A.; Blackshaw, L.A.; Andrews, J.M.; Krumbiegel, D. Immune activation in irritable bowel syndrome: Can neuroimmune interactions explain symptoms? Am. J. Gastroenterol. 2013, 108, 1066–1074. [Google Scholar] [CrossRef]
- Schneck, A.S.; Anty, R.; Tran, A.; Hastier, A.; Amor, I.B.; Gugenheim, J.; Iannelli, A.; Piche, T. Increased Prevalence of Irritable Bowel Syndrome in a Cohort of French Morbidly Obese Patients Candidate for Bariatric Surgery. Obes. Surg. 2016, 26, 1525–1530. [Google Scholar] [CrossRef]
- Fysekidis, M.; Bouchoucha, M.; Bihan, H.; Reach, G.; Benamouzig, R.; Catheline, J.M. Prevalence and co-occurrence of upper and lower functional gastrointestinal symptoms in patients eligible for bariatric surgery. Obes. Surg. 2012, 22, 403–410. [Google Scholar] [CrossRef]
- Bouchoucha, M.; Fysekidis, M.; Julia, C.; Airinei, G.; Catheline, J.M.; Reach, G.; Benamouzig, R. Functional Gastrointestinal Disorders in Obese Patients. The Importance of the Enrollment Source. Obes. Surg. 2015, 25, 2143–2152. [Google Scholar] [CrossRef]
- Wexler, D.J.; Hu, F.B.; Manson, J.E.; Rifai, N.; Meigs, J.B. Mediating effects of inflammatory biomarkers on insulin resistance associated with obesity. Obes. Res. 2005, 13, 1772–1783. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Aros, S.; Locke, G.R., 3rd; Camilleri, M.; Talley, N.J.; Fett, S.; Zinsmeister, A.R.; Melton, L.J., 3rd. Obesity is associated with increased risk of gastrointestinal symptoms: A population-based study. Am. J. Gastroenterol. 2004, 99, 1801–1806. [Google Scholar] [CrossRef] [PubMed]
- Alkhowaiter, S.; Alotaibi, R.M.; Alwehaibi, K.K.; Aljohany, A.; Alruhaimi, B.; Almasaad, M.; Alshammari, S.A.; Alsahafi, M.A. The Effect of Body Mass Index on the Prevalence of Gastrointestinal Symptoms Among a Saudi Population. Cureus 2021, 13, e17751. [Google Scholar] [CrossRef] [PubMed]
- Lisker, R.; Ramirez, E.; Briceno, R.P.; Granados, J.; Babinsky, V. Gene frequencies and admixture estimates in four Mexican urban centers. Hum. Biol. 1990, 62, 791–801. [Google Scholar]
- Weir, C.B.; Jan, A. BMI Classification Percentile and Cut off Points; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef]
- Ellulu, M.S.; Patimah, I.; Khaza’ai, H.; Rahmat, A.; Abed, Y. Obesity and inflammation: The linking mechanism and the complications. Arch. Med. Sci. 2017, 13, 851–863. [Google Scholar] [CrossRef]
- Moreno-Navarrete, J.M.; Fernandez-Real, J.M. The complement system is dysfunctional in metabolic disease: Evidences in plasma and adipose tissue from obese and insulin resistant subjects. Semin. Cell Dev. Biol. 2019, 85, 164–172. [Google Scholar] [CrossRef]
- Maggio, C.A.; Pi-Sunyer, F.X. Obesity and type 2 diabetes. Endocrinol. Metab. Clin. 2003, 32, 805–822. [Google Scholar] [CrossRef]
- Piche, M.E.; Tchernof, A.; Despres, J.P. Obesity Phenotypes, Diabetes, and Cardiovascular Diseases. Circ. Res. 2020, 126, 1477–1500. [Google Scholar] [CrossRef]
- Monteiro, R.; Azevedo, I. Chronic inflammation in obesity and the metabolic syndrome. Mediat. Inflamm. 2010, 2010, 289645. [Google Scholar] [CrossRef]
- Cleven, L.; Krell-Roesch, J.; Nigg, C.R.; Woll, A. The association between physical activity with incident obesity, coronary heart disease, diabetes and hypertension in adults: A systematic review of longitudinal studies published after 2012. BMC Public Health 2020, 20, 726. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Kountouras, J.; Mantzoros, C.S. Obesity and nonalcoholic fatty liver disease: From pathophysiology to therapeutics. Metabolism 2019, 92, 82–97. [Google Scholar] [CrossRef] [PubMed]
- Kolb, R.; Sutterwala, F.S.; Zhang, W. Obesity and cancer: Inflammation bridges the two. Curr. Opin. Pharmacol. 2016, 29, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Ricklin, D.; Hajishengallis, G.; Yang, K.; Lambris, J.D. Complement: A key system for immune surveillance and homeostasis. Nat. Immunol. 2010, 11, 785–797. [Google Scholar] [CrossRef]
- Merle, N.S.; Church, S.E.; Fremeaux-Bacchi, V.; Roumenina, L.T. Complement System Part I—Molecular Mechanisms of Activation and Regulation. Front. Immunol. 2015, 6, 262. [Google Scholar] [CrossRef]
- Ricklin, D.; Reis, E.S.; Mastellos, D.C.; Gros, P.; Lambris, J.D. Complement component C3—The “Swiss Army Knife” of innate immunity and host defense. Immunol. Rev. 2016, 274, 33–58. [Google Scholar] [CrossRef]
- Andoh, A.; Fujiyama, Y.; Sumiyoshi, K.; Bamba, T. Local secretion of complement C3 in the exocrine pancreas: Ductal epithelial cells as a possible biosynthetic site. Gastroenterology 1996, 110, 1919–1925. [Google Scholar] [CrossRef]
- Andoh, A.; Kinoshita, K.; Rosenberg, I.; Podolsky, D.K. Intestinal trefoil factor induces decay-accelerating factor expression and enhances the protective activities against complement activation in intestinal epithelial cells. J. Immunol. 2001, 167, 3887–3893. [Google Scholar] [CrossRef]
- Al Haj Ahmad, R.M.; Al-Domi, H.A. Complement 3 serum levels as a pro-inflammatory biomarker for insulin resistance in obesity. Diabetes Metab. Syndr. Clin. Res. Rev. 2017, 11 (Suppl. S1), S229–S232. [Google Scholar] [CrossRef]
- Phieler, J.; Garcia-Martin, R.; Lambris, J.D.; Chavakis, T. The role of the complement system in metabolic organs and metabolic diseases. Semin. Immunol. 2013, 25, 47–53. [Google Scholar] [CrossRef]
- Vlaicu, S.I.; Tatomir, A.; Boodhoo, D.; Vesa, S.; Mircea, P.A.; Rus, H. The role of complement system in adipose tissue-related inflammation. Immunol. Res. 2016, 64, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Esterbauer, H.; Krempler, F.; Oberkofler, H.; Patsch, W. The complement system: A pathway linking host defence and adipocyte biology. Eur. J. Clin. Investig. 1999, 29, 653–656. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chung, E.K.; Zhou, B.; Blanchong, C.A.; Yu, C.Y.; Fust, G.; Kovacs, M.; Vatay, A.; Szalai, C.; Karadi, I.; et al. Diversity in intrinsic strengths of the human complement system: Serum C4 protein concentrations correlate with C4 gene size and polygenic variations, hemolytic activities, and body mass index. J. Immunol. 2003, 171, 2734–2745. [Google Scholar] [CrossRef] [PubMed]
- Schiller, L.R.; Pardi, D.S.; Sellin, J.H. Chronic Diarrhea: Diagnosis and Management. Clin. Gastroenterol. Hepatol. 2017, 15, 182–193.e183. [Google Scholar] [CrossRef]
- Zhang, J.; Ye, J.; Ren, Y.; Zuo, J.; Dai, W.; He, Y.; Tan, M.; Song, W.; Yuan, Y. Intracellular activation of complement C3 in Paneth cells improves repair of intestinal epithelia during acute injury. Immunotherapy 2018, 10, 1325–1336. [Google Scholar] [CrossRef]
- Wang, H.; Liu, M. Complement C4, Infections, and Autoimmune Diseases. Front. Immunol. 2021, 12, 694928. [Google Scholar] [CrossRef]
- Timpson, N.J.; Nordestgaard, B.G.; Harbord, R.M.; Zacho, J.; Frayling, T.M.; Tybjaerg-Hansen, A.; Smith, G.D. C-reactive protein levels and body mass index: Elucidating direction of causation through reciprocal Mendelian randomization. Int. J. Obes. 2011, 35, 300–308. [Google Scholar] [CrossRef]
- Schoepfer, A.M.; Trummler, M.; Seeholzer, P.; Seibold-Schmid, B.; Seibold, F. Discriminating IBD from IBS: Comparison of the test performance of fecal markers, blood leukocytes, CRP, and IBD antibodies. Inflamm. Bowel Dis. 2008, 14, 32–39. [Google Scholar] [CrossRef]
- Kaplan, M.H.; Volanakis, J.E. Interaction of C-reactive protein complexes with the complement system. I. Consumption of human complement associated with the reaction of C-reactive protein with pneumococcal C-polysaccharide and with the choline phosphatides, lecithin and sphingomyelin. J. Immunol. 1974, 112, 2135–2147. [Google Scholar]
- Giefing-Kroll, C.; Berger, P.; Lepperdinger, G.; Grubeck-Loebenstein, B. How sex and age affect immune responses, susceptibility to infections, and response to vaccination. Aging Cell 2015, 14, 309–321. [Google Scholar] [CrossRef]
- Boeckxstaens, G.E.; Drug, V.; Dumitrascu, D.; Farmer, A.D.; Hammer, J.; Hausken, T.; Niesler, B.; Pohl, D.; Pojskic, L.; Polster, A.; et al. Phenotyping of subjects for large scale studies on patients with IBS. Neurogastroenterol. Motil. 2016, 28, 1134–1147. [Google Scholar] [CrossRef] [PubMed]
- Talley, N.J. Irritable bowel syndrome: Definition, diagnosis and epidemiology. Best Pract. Res. Clin. Gastroenterol. 1999, 13, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Roved, J.; Westerdahl, H.; Hasselquist, D. Sex differences in immune responses: Hormonal effects, antagonistic selection, and evolutionary consequences. Horm. Behav. 2017, 88, 95–105. [Google Scholar] [CrossRef]
- Spector, D.; Zachary, J.B.; Sterioff, S.; Millan, J. Painful crises following renal transplantation in sickle cell anemia. Am. J. Med. 1978, 64, 835–839. [Google Scholar] [CrossRef]
- Choghakhori, R.; Abbasnezhad, A.; Amani, R.; Alipour, M. Sex-Related Differences in Clinical Symptoms, Quality of Life, and Biochemical Factors in Irritable Bowel Syndrome. Dig. Dis. Sci. 2017, 62, 1550–1560. [Google Scholar] [CrossRef] [PubMed]
- Gaya da Costa, M.; Poppelaars, F.; van Kooten, C.; Mollnes, T.E.; Tedesco, F.; Wurzner, R.; Trouw, L.A.; Truedsson, L.; Daha, M.R.; Roos, A.; et al. Age and Sex-Associated Changes of Complement Activity and Complement Levels in a Healthy Caucasian Population. Front. Immunol. 2018, 9, 2664. [Google Scholar] [CrossRef]
- Ferri, J.; Navarro, I.; Alabadi, B.; Bosch-Sierra, N.; Benito, E.; Civera, M.; Ascaso, J.F.; Martinez-Hervas, S.; Real, J.T. Gender differences on oxidative stress markers and complement component C3 plasma values after an oral unsaturated fat load test. Clín. Investig. Arterioscler. 2020, 32, 87–93. [Google Scholar] [CrossRef]
- Karkhaneh, M.; Qorbani, M.; Mohajeri-Tehrani, M.R.; Hoseini, S. Association of serum complement C3 with metabolic syndrome components in normal weight obese women. J. Diabetes Metab. Disord. 2017, 16, 49. [Google Scholar] [CrossRef]
- Aguilera-Lizarraga, J.; Hussein, H.; Boeckxstaens, G.E. Immune activation in irritable bowel syndrome: What is the evidence? Nat. Rev. Immunol. 2022, 22, 674–686. [Google Scholar] [CrossRef]
- Burns, G.L.; Talley, N.J.; Keely, S. Immune responses in the irritable bowel syndromes: Time to consider the small intestine. BMC Med. 2022, 20, 115. [Google Scholar] [CrossRef]

| IBS-Rome IV (n = 79) | Healthy Controls (n = 35) | p Value | |
|---|---|---|---|
| Sex | |||
| Women, n (%) | 65 (82.27) | 24 (68.57) | 0.1403 † |
| Men, n (%) | 14 (17.73) | 11 (31.43) | |
| Age (mean, SD) | 32.72 ± 13.28 | 35.24 ± 10.86 | 0.9151 * |
| Body mass index (mean, SD) | 26.26 ± 4.5 | 26.10 ± 4.66 | 0.617 * |
| Length of time of symptom progression (mean in months, SD) | 82.47 ± 91.64 | — | — |
| Weight | |||
| Underweight, n (%) | 0 | 0 | |
| Normal, n (%) | 31 (39.24) | 18 (51.42) | 0.2313 † |
| Overweight, n (%) | 31 (39.24) | 8 (22.85) | |
| Obesity, n (%) | 17 (21.52) | 9 (25.71) | |
| IBS subtype | |||
| IBS-C, n (%) | 29 (36.71) | — | — |
| IBS-M, n (%) | 26 (32.91) | — | — |
| IBS-D, n (%) | 24 (30.38) | — | — |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomas-Dupont, P.; Velázquez-Soto, H.; Izaguirre-Hernández, I.Y.; Amieva-Balmori, M.; Triana-Romero, A.; Islas-Vázquez, L.; Jiménez-Martínez, M.d.C.; Remes-Troche, J.M. Obesity Contributes to Inflammation in Patients with IBS via Complement Component 3 and C-Reactive Protein. Nutrients 2022, 14, 5227. https://doi.org/10.3390/nu14245227
Thomas-Dupont P, Velázquez-Soto H, Izaguirre-Hernández IY, Amieva-Balmori M, Triana-Romero A, Islas-Vázquez L, Jiménez-Martínez MdC, Remes-Troche JM. Obesity Contributes to Inflammation in Patients with IBS via Complement Component 3 and C-Reactive Protein. Nutrients. 2022; 14(24):5227. https://doi.org/10.3390/nu14245227
Chicago/Turabian StyleThomas-Dupont, Pablo, Henry Velázquez-Soto, Irma Yadira Izaguirre-Hernández, Mercedes Amieva-Balmori, Arturo Triana-Romero, Lorenzo Islas-Vázquez, María del Carmen Jiménez-Martínez, and José María Remes-Troche. 2022. "Obesity Contributes to Inflammation in Patients with IBS via Complement Component 3 and C-Reactive Protein" Nutrients 14, no. 24: 5227. https://doi.org/10.3390/nu14245227
APA StyleThomas-Dupont, P., Velázquez-Soto, H., Izaguirre-Hernández, I. Y., Amieva-Balmori, M., Triana-Romero, A., Islas-Vázquez, L., Jiménez-Martínez, M. d. C., & Remes-Troche, J. M. (2022). Obesity Contributes to Inflammation in Patients with IBS via Complement Component 3 and C-Reactive Protein. Nutrients, 14(24), 5227. https://doi.org/10.3390/nu14245227