Higher Body Fat but Similar Phase Angle Values in Patients with the Classical Form of Congenital Adrenal Hyperplasia in Comparison to a Control Group
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Ethical Aspects
2.2. Study Participants
2.3. Anthropometry
2.4. Body Composition
2.5. Bioelectrical Impedance Analysis
2.6. Glucocorticoid Therapy
2.7. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Speiser, P.W. Congenital adrenal hyperplasia owing to 21-hydroxylase deficiency. Endocrinol. Metab. Clin. N. Am. 2001, 30, 31–59. [Google Scholar] [CrossRef]
- Speiser, P.W.; Arlt, W.; Auchus, R.J.; Baskin, L.S.; Conway, G.S.; Merke, D.P.; Meyer-Bahlburg, H.F.L.; Miller, W.L.; Murad, M.H.; Oberfield, S.E.; et al. Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2018, 103, 4043–4088. [Google Scholar] [CrossRef] [PubMed]
- Reisch, N. Review of Health Problems in Adult Patients with Classic Congenital Adrenal Hyperplasia due to 21-Hydroxylase Deficiency. Exp. Clin. Endocrinol. Diabetes 2019, 127, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Mooij, C.F.; Kroese, J.M.; Claahsen-Van Der Grinten, H.L.; Tack, C.J.; Hermus, A.R.M.M. Unfavourable trends in cardiovascular and metabolic risk in paediatric and adult patients with congenital adrenal hyperplasia? Clin. Endocrinol. 2010, 73, 137–146. [Google Scholar] [CrossRef]
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Gomez, J.M.; Heitmann, B.L.; Kent-Smith, L.; Melchior, J.-C.; Pirlich, M.; et al. Bioelectrical impedance analysis—Part I: Review of principles and methods. Clin. Nutr. 2004, 23, 1226–1243. [Google Scholar] [CrossRef] [PubMed]
- Campa, F.; Toselli, S.; Mazzilli, M.; Gobbo, L.A.; Coratella, G. Assessment of Body Composition in Athletes: A Narrative Review of Available Methods with Special Reference to Quantitative and Qualitative Bioimpedance Analysis. Nutrients 2021, 13, 1620. [Google Scholar] [CrossRef] [PubMed]
- Di Vincenzo, O.; Marra, M.; Sacco, A.M.; Pasanisi, F.; Scalfi, L. Bioelectrical impedance (BIA)-derived phase angle in adults with obesity: A systematic review. Clin. Nutr. 2021, 40, 5238–5248. [Google Scholar] [CrossRef] [PubMed]
- Genton, L.; Mareschal, J.; Norman, K.; Karsegard, V.L.; Delsoglio, M.; Pichard, C.; Graf, C.; Herrmann, F.R. Association of phase angle and running performance. Clin. Nutr. ESPEN 2020, 37, 65–68. [Google Scholar] [CrossRef]
- Piccoli, A.; Rossi, B.; Pillon, L.; Bucciante, G. A new method for monitoring body fluid variation by bioimpedance analysis: The RXc graph. Kidney Int. 1994, 46, 534–539. [Google Scholar] [CrossRef]
- Piccoli, A.; Codognotto, M.; Piasentin, P.; Naso, A. Combined evaluation of nutrition and hydration in dialysis patients with bioelectrical impedance vector analysis (BIVA). Clin. Nutr. 2014, 33, 673–677. [Google Scholar] [CrossRef]
- Norman, K.; Stobäus, N.; Pirlich, M.; Bosy-Westphal, A. Bioelectrical phase angle and impedance vector analysis—Clinical relevance and applicability of impedance parameters. Clin. Nutr. 2012, 31, 854–861. [Google Scholar] [CrossRef]
- Di Somma, S.; Lukaski, H.C.; Codognotto, M.; Peacock, W.F.; Fiorini, F.; Aspromonte, N.; Ronco, C.; Santarelli, S.; Lalle, I.; Autunno, A.; et al. Consensus paper on the use of BIVA (Bioeletrical Impendance Vector Analysis) in medicine for the management of body hydration. Emerg. Care J. 2011, 7, 6–14. [Google Scholar] [CrossRef]
- de Oliveira, N.M.; Langer, R.D.; de Lemos-Marini, S.H.V.; Guerra-Júnior, G.; Gonçalves, E.M. Bioelectrical Impedance Phase Angle and Its Determinants in Patients with Classic Congenital Adrenal Hyperplasia. J. Am. Coll. Nutr. 2021, 41, 407–414. [Google Scholar] [CrossRef]
- Borges, J.H.; de Oliveira, D.M.; de Lemos-Marini, S.H.V.; Geloneze, B.; Gonçalves, E.M.; Guerra-Júnior, G. Fat Distribution and Lipid Profile of Young Adults with Congenital Adrenal Hyperplasia Due to 21-Hydroxylase Enzyme Deficiency. Lipids 2021, 56, 101–110. [Google Scholar] [CrossRef]
- Borges, J.H.; Santoro, R.I.; de Oliveira, D.M.; Lemos-Marini, S.H.V.; Geloneze, B.; Guerra-Júnior, G.; Gonçalves, E.M. Cardiovascular dysfunction risk in young adults with congenital adrenal hyperplasia caused by 21-hydroxylase enzyme deficiency. Int. J. Clin. Pract. 2021, 75, 14233. [Google Scholar] [CrossRef]
- de Araujo, M.; Sanches, M.; Suzuki, L.A.; Guerra, G.; Farah, S.; de Mello, M.P. Molecular analysis of CYP21 and C4 genes in Brazilian families with classical form of steroid 21 hydroxilase deficiency. Braz. J. Med. Biol. Res. 1996, 29, 1–13. [Google Scholar]
- Lau, I.; Soardi, S.; de Lemos-Marini, S.H.V.; Guerra, G.; Baptista, M.T.; de Mello, M.P. H28+C insertion in the CYP21 gene: A novel frameshift mutation in a Brazilian patient with the classical form of 21-hydroxylase deficiency. J. Clin. Endocrinolol. 2001, 86, 5877–5880. [Google Scholar] [CrossRef][Green Version]
- Paulino, L.C.; Araujo, M.; Guerra, G.; Marini, S.H.; De Mello, M.P. Mutation distribution and CYP21/C4 locus variability in Brazilian families with the classical form of the 21-hydroxilase deficiency. Acta. Paediatr. 1999, 88, 275–283. [Google Scholar] [CrossRef]
- Soardi, F.C.; Barbaro, M.; Lau, I.F.; Lemos-Marini, S.H.V.; Baptista, M.T.M.; Guerra-Junior, G.; Wedell, A.; Lajic, S.; de Mello, M.P. Inhibition of CYP21A2 enzyme activity caused by novel missense mutations identified in Brazilian and Scandinavian patients. Clin. Endrocrinolo. Metab. 2008, 93, 2416–2420. [Google Scholar] [CrossRef]
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Gómez, J.M.; Heitmann, B.L.; Kent-Smith, L.; Melchior, J.-C.; Pirlich, M.; et al. Bioelectrical impedance analysis—Part II: Utilization in clinical practice. Clin. Nutr. 2004, 23, 1430–1453. [Google Scholar] [CrossRef]
- Baumgartner, R.N.; Chumlea, W.C.; Roche, A.F. Bioelectric phase angle and body composition. Am. J. Clin. Nutr. 1988, 48, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, A. BIVA Software 2002; Department of Medical and Surgical Sciences, University of Padova: Padova, Italy, 2002; pp. 1–17. [Google Scholar]
- Liu, D.; Ahmet, A.; Ward, L.; Krishnamoorthy, P.; Mandelcorn, E.D.; Leigh, R.; Brown, J.P.; Cohen, A.; Kim, H. A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy Asthma Clin. Immunol. 2013, 9, 30. [Google Scholar] [CrossRef] [PubMed]
- DuBois, D.; DuBois, E. A formula to estimate the approximate surface area if height and weight be known. Arch. Intern. Med. 1916, 862, 303–311; discussion 312–313. [Google Scholar]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Ryabets-Lienhard, A.; Dao-Tran, A.; Mittelman, S.D.; Gilsanz, V.; Schrager, S.M.; Geffner, M.E. Increased abdominal adiposity in adolescents and young adults with classical congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J. Clin. Endocrinol. Metab. 2015, 100, E1153–E1159. [Google Scholar] [CrossRef] [PubMed]
- Arlt, W.; Willis, D.S.; Wild, S.H.; Krone, N.; Doherty, E.J.; Hahner, S.; Han, T.; Carroll, P.V.; Conway, G.S.; Rees, A.; et al. Health status of adults with congenital adrenal hyperplasia: A cohort study of 203 patients. J. Clin. Endocrinol. Metab. 2010, 95, 5110–5121. [Google Scholar] [CrossRef] [PubMed]
- Sari, C.I.; Eikelis, N.; Head, G.A.; Schlaich, M.; Meikle, P.; Lambert, G.; Lambert, E. Android Fat Deposition and Its Association With Cardiovascular Risk Factors in Overweight Young Males. Front. Physiol. 2019, 10, 1162. [Google Scholar] [CrossRef]
- Ariyawatkul, K.; Tepmongkol, S.; Aroonparkmongkol, S.; Sahakitrungruang, T. Cardio-metabolic risk factors in youth with classical 21-hydroxylase deficiency. Eur. J. Pediatr. 2017, 176, 537–545. [Google Scholar] [CrossRef]
- Merke, D.P.; Chrousos, G.P.; Eisenhofer, G.; Weise, M.; Keil, M.F.; Rogol, A.D.; Van Wyk, J.J.; Bornstein, S.R. Adrenomedullary Dysplasia and Hypofunction in Patients with Classic 21-Hydroxylase Deficiency. N. Engl. J. Med. 2000, 343, 1362–1368. [Google Scholar] [CrossRef]
- Völkl, T.M.K.; Simm, D.; Körner, A.; Rascher, W.; Kiess, W.; Kratzsch, J.; Dörr, H.G. Does an altered leptin axis play a role in obesity among children and adolescents with classical congenital adrenal hyperplasia due to 21-hydroxylase deficiency? Eur. J. Endocrinol. 2009, 160, 239–247. [Google Scholar] [CrossRef][Green Version]
- Chumlea, W.C.; Guo, S.S. Bioelectrical Impedance and Body Composition: Present Status and Future Directions. Nutr. Rev. 1994, 52, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Bosy-westphal, A.; Danielzik, S.; Dorhofer, R.; Later, W.; Wiese, S.; Muller, M. Phase Angle From Bioelectrical Impedance Analysis: Population Reference Values by Age, Sex, and Body Mass Index. J. Parenter. Enter. Nutr. 2006, 30, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Barbosa-Silva, M.C.G.; Barros, A.J.D.; Wang, J.; Heymsfield, S.B.; Pierson, R.N. Bioelectrical impedance analysis: Population reference values for phase angle by age and sex. Am. J. Clin. Nutr. 2005, 82, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Borges, J.H.; de Oliveira, D.M.; de Lemos-Marini, S.H.V.; Geloneze, B.; Guerra-Júnior, G.; Gonçalves, E.M. Normal bone health in young adults with 21-hydroxylase enzyme deficiency undergoing glucocorticoid replacement therapy. Osteoporos. Int. 2021, 33, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Lukaski, H.C.; Vega Diaz, N.; Talluri, A.; Nescolarde, L. Classification of hydration in clinical conditions: Indirect and direct approaches using bioimpedance. Nutrients 2019, 11, 809. [Google Scholar] [CrossRef]
- Selberg, O.; Selberg, D. Norms and correlates of bioimpedance phase angle in healthy human subjects, hospitalized patients, and patients with liver cirrhosis. Eur. J. Appl. Physiol. 2002, 86, 509–516. [Google Scholar] [CrossRef]
- Lukaski, H.; Piccoli, A. Bioelectrical impedance vector analysis for assessment of hydration in physiological states and clinical conditions. In Handbook of Anthropometry; Springer: New York, NY, USA, 2012; pp. 287–305. [Google Scholar] [CrossRef]
- Moore, F.; McMurray, J.; Parker, H.; Ball, M.; Boyden, C. The Body Cell Mass and Its Supporting Environment. In Body Composition in Health and Disease; Saunders: Philadelphia, PA, USA, 1963. [Google Scholar]
- Dittmar, M.; Reber, H. New equations for estimating body cell mass from bioimpedance parallel models in healthy older Germans. Am. J. Physiol.-Endocrinol. Metab. 2001, 281, 1005–1014. [Google Scholar] [CrossRef]
- Francisco, R.; Matias, C.N.; Santos, D.A.; Campa, F.; Minderico, C.S.; Rocha, P.; Heymsfield, S.B.; Lukaski, H.; Sardinha, L.B.; Silva, A.M. The predictive role of raw bioelectrical impedance parameters in water compartments and fluid distribution assessed by dilution techniques in athletes. Int. J. Environ. Res. Public Health 2020, 17, 759. [Google Scholar] [CrossRef]
- Nescolarde, L.; Yanguas, J.; Lukaski, H.; Alomar, X.; Rosell-Ferrer, J.; Rodas, G. Localized bioimpedance to assess muscle injury. Physiol. Meas. 2013, 34, 237–245. [Google Scholar] [CrossRef]
- Castizo-Olier, J.; Irurtia, A.; Jemni, M.; Carrasco-Marginet, M.; Fernández-García, R.; Rodríguez, F.A. Bioelectrical impedance vector analysis (BIVA) in sport and exercise: Systematic review and future perspectives. PLoS ONE 2018, 13, e0197957. [Google Scholar] [CrossRef]
- Rashid, S.; Lewis, G.F. The mechanisms of differential glucocorticoid and mineralocorticoid action in the brain and peripheral tissues. Clin. Biochem. 2005, 38, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, A.; Pillon, L.; Dumler, F. Impedance vector distribution by sex, race, body mass index, and age in the United States: Standard reference intervals as bivariate Z scores. Nutrition 2002, 18, 153–167. [Google Scholar] [CrossRef] [PubMed]

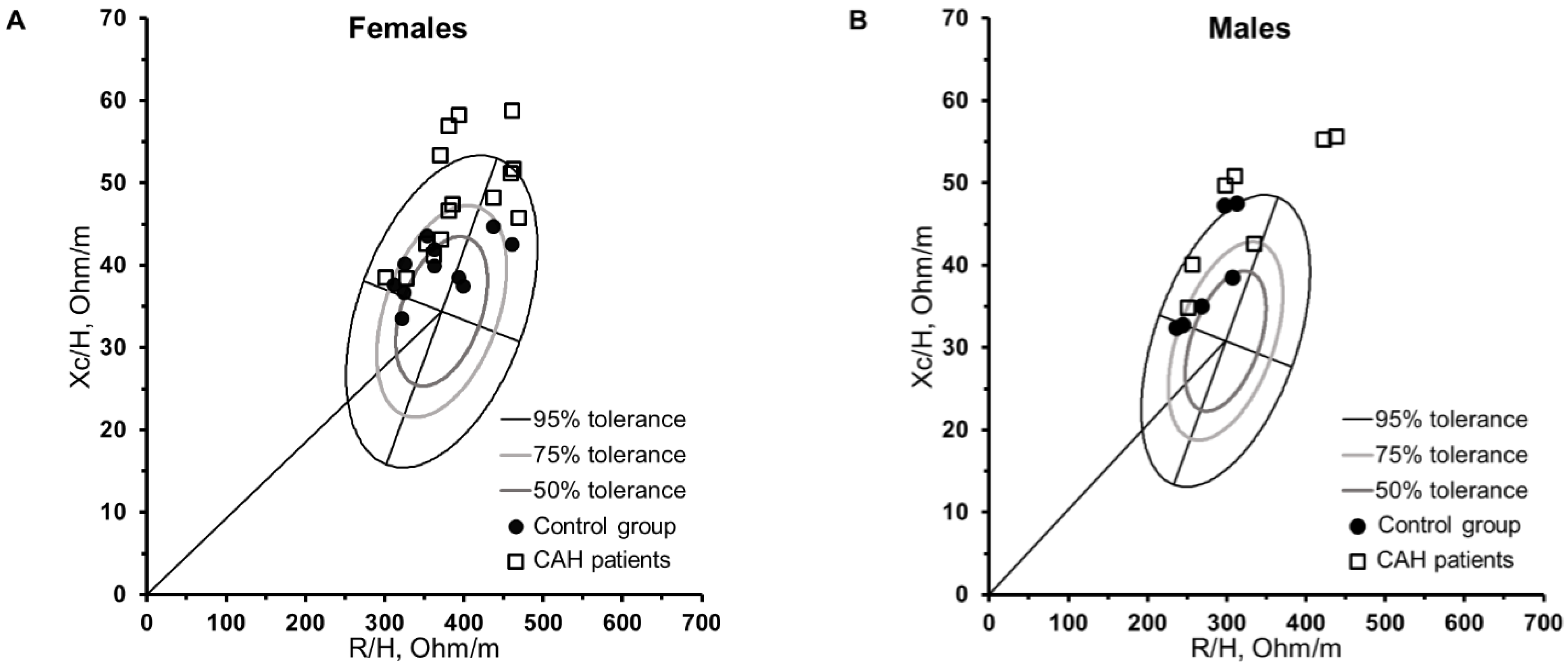
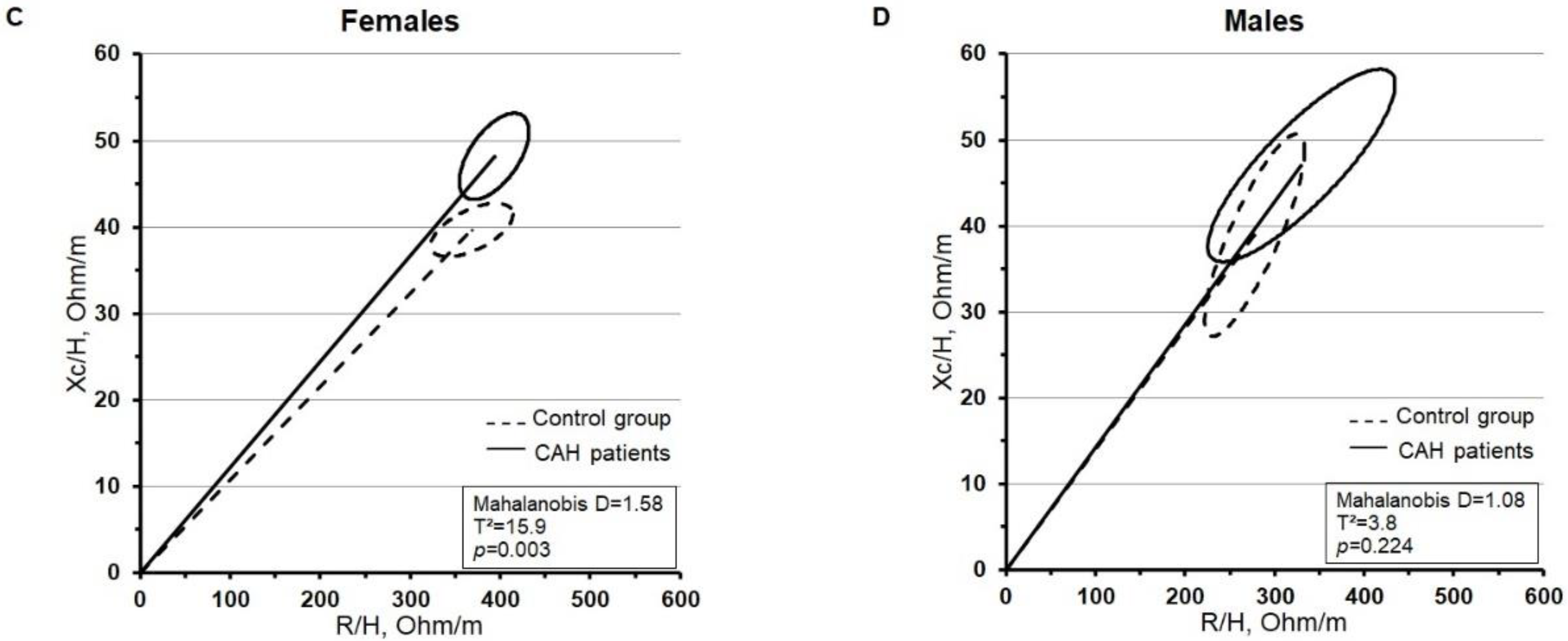
| Females | Males | |||||||
|---|---|---|---|---|---|---|---|---|
| Control (n = 11) | Patients (n = 15) | Control (n = 6) | Patients (n = 7) | |||||
| Mean ± SD | Mean ± SD | p-Value | E.S | Mean ± SD | Mean ± SD | p-Value | E.S | |
| DGT (years) | 22.3 ± 3.6 | 19.1 ± 4.3 | ||||||
| HDE (mg/m2/day) | 13.2 ± 4.8 | 12.5 ± 2.9 | ||||||
| Age (years) | 27.0 ± 2.5 | 22.9 ± 3.7 | 0.004 | 1.23 | 24.4 ± 2.3 | 23.8 ± 4.5 | 0.771 | 0.15 |
| Weight (kg) | 58.7 ± 8.9 | 60.8 ± 13.6 | 0.654 | 0.17 | 71.0 ± 10.8 | 65.4 ± 12.9 | 0.415 | 0.44 |
| Height (cm) | 161.3 ± 7.1 | 154.8 ± 7.0 | 0.029 | 0.89 | 173.7 ± 7.6 | 160.0 ± 8.7 | 0.012 | 1.55 |
| BMI (kg/m2) | 22.6 ± 3.0 | 25.3 ± 4.8 | 0.106 | 0.65 | 23.4 ± 1.7 | 25.5 ± 4.6 | 0.319 | 0.54 |
| %FM | 31.6 ± 5.6 | 37.2 ± 6.3 | 0.027 | 0.91 | 20.3 ± 4.9 | 29.4 ± 8.9 | 0.048 | 1.15 |
| LST (kg) | 37.81 ± 5.5 | 35.5 ± 4.8 | 0.270 | 0.43 | 53.9 ± 7.9 | 43.7 ± 8.9 | 0.052 | 1.12 |
| LSTI (kg/m2) | 14.5 ± 1.3 | 14.9 ± 2.1 | 0.610 | 0.20 | 17.8 ± 1.3 | 17.0 ± 2.7 | 0.518 | 0.34 |
| %FMAndroid | 1.7(1.0–3.1) a | 2.8(1.0–4.4) a | 0.025 | 0.92 | 1.7(0.7–2.1) a | 2.8(0.8–3.9) a | 0.088 | 0.77 |
| %FMGynoid | 6.4 ± 0.9 | 7.9 ± 1.2 | 0.233 | 0.37 | 3.3 ± 1.0 | 5.2 ± 1.8 | 0.048 | 1.15 |
| BIA Parameters | Females | Males | ||||||
|---|---|---|---|---|---|---|---|---|
| Control (n = 11) | Patients (n = 15) | p-Value | E.S | Control (n = 6) | Patients (n = 7) | p-Value | E.S | |
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |||||
| R (Ω) | 592.7 ± 68.2 | 610.4 ± 91.6 | 0.095 | 0.21 | 480.2 ± 40.8 | 503.2 ± 95.4 | 0.346 | 0.51 |
| R/H (Ω/m) | 362.5 (311.7–461.0) a | 380.4 (300.3–468.7) a | 0.226 | 0.48 | 282.6 (235.7–312.8) a | 309.8 (251.1–437.6) a | 0.146 | 0.39 |
| Xc (Ω) | 64.0 (57.0–68.0) a | 74.0 (57.0–95.0) a | 0.002 | 0.58 | 64.5 (59.0–78.0) a | 75.0 (56.0–88.0) a | 0.206 | 0.70 |
| Xc/H (Ω/m) | 39.7 ± 3.3 | 48.2 ± 6.8 | 0.001 | 1.47 | 38.9 ± 6.9 | 47.0 ± 8.0 | 0.078 | 1.00 |
| PhA (°) | 6.2 ± 0.7 | 7.1 ± 0.8 | 0.013 | 1.03 | 8.0 ± 0.7 | 8.3 ± 1.0 | 0.590 | 0.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Oliveira, N.M.; Langer, R.D.; Valente Lemos-Marini, S.H.; de Oliveira, D.M.; Geloneze, B.; Guerra-Júnior, G.; Gonçalves, E.M. Higher Body Fat but Similar Phase Angle Values in Patients with the Classical Form of Congenital Adrenal Hyperplasia in Comparison to a Control Group. Nutrients 2022, 14, 5184. https://doi.org/10.3390/nu14235184
de Oliveira NM, Langer RD, Valente Lemos-Marini SH, de Oliveira DM, Geloneze B, Guerra-Júnior G, Gonçalves EM. Higher Body Fat but Similar Phase Angle Values in Patients with the Classical Form of Congenital Adrenal Hyperplasia in Comparison to a Control Group. Nutrients. 2022; 14(23):5184. https://doi.org/10.3390/nu14235184
Chicago/Turabian Stylede Oliveira, Núbia Maria, Raquel David Langer, Sofia Helena Valente Lemos-Marini, Daniel Minutti de Oliveira, Bruno Geloneze, Gil Guerra-Júnior, and Ezequiel Moreira Gonçalves. 2022. "Higher Body Fat but Similar Phase Angle Values in Patients with the Classical Form of Congenital Adrenal Hyperplasia in Comparison to a Control Group" Nutrients 14, no. 23: 5184. https://doi.org/10.3390/nu14235184
APA Stylede Oliveira, N. M., Langer, R. D., Valente Lemos-Marini, S. H., de Oliveira, D. M., Geloneze, B., Guerra-Júnior, G., & Gonçalves, E. M. (2022). Higher Body Fat but Similar Phase Angle Values in Patients with the Classical Form of Congenital Adrenal Hyperplasia in Comparison to a Control Group. Nutrients, 14(23), 5184. https://doi.org/10.3390/nu14235184










