A Meta-Epidemiological Study of Positive Results in Clinical Nutrition Research: The Good, the Bad and the Ugly of Statistically Significant Findings
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
2.4. Stratification of Results Based on the Statistical Significance Level
2.5. Data Analyses
3. Results
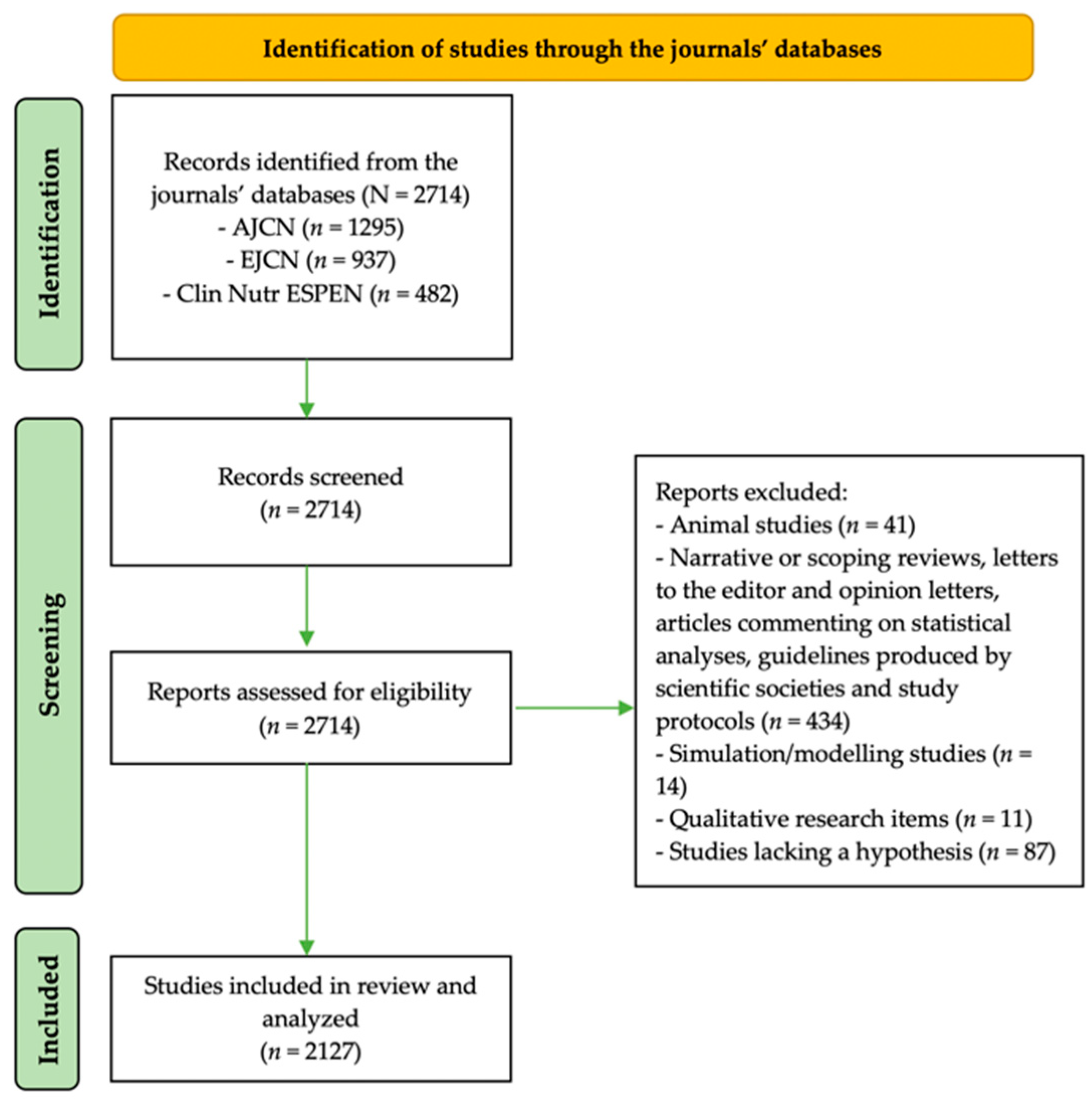
3.1. Search Results
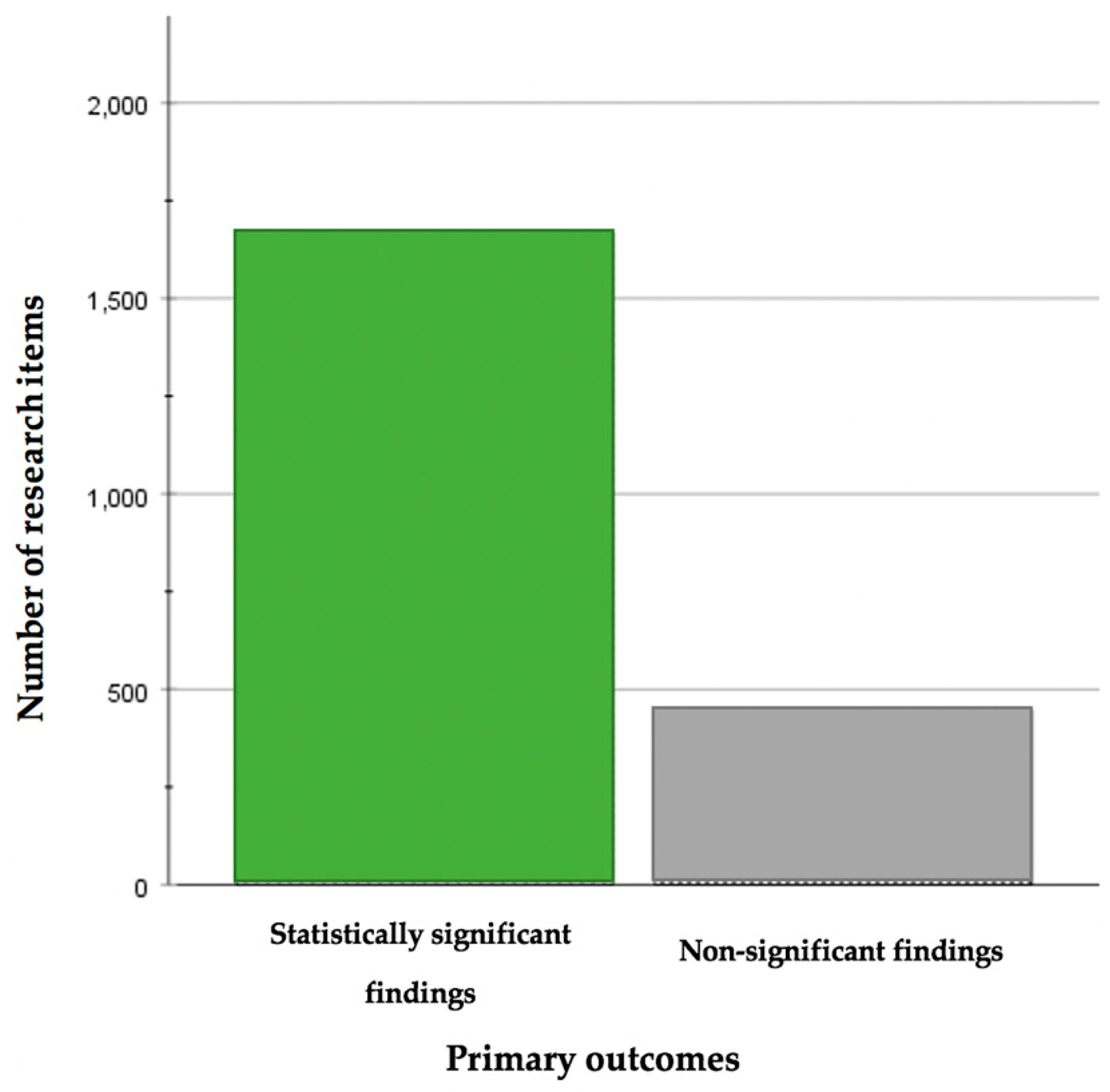
3.2. Significant Findings
3.3. Positive Findings according to Funding Sources
3.4. Positive Findings according to Sample Size
3.5. Positive Findings among Studies with a Published Protocol
3.6. Positive Findings in Studies Adjusting for the Energy Intake of Participants
4. Discussion
4.1. Adjusting for the EI of Participants in Nutrition Research
4.2. Nutrition Research and Industry Funding
4.3. Publication of the Research Protocol and Adherence to the Reporting Guidelines
4.4. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mozaffarian, D.; Forouhi, N.G. Dietary guidelines and health—Is nutrition science up to the task? BMJ 2018, 360, k822. [Google Scholar] [CrossRef] [PubMed]
- Grammatikopoulou, M.G.; Nigdelis, M.P.; Theodoridis, X.; Gkiouras, K.; Tranidou, A.; Papamitsou, T.; Bogdanos, D.P.; Goulis, D.G. How fragile are Mediterranean diet interventions? A research-on-research study of randomised controlled trials. BMJ Nutr. Prev. Health 2021, 4, 115–131. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, J.P. Implausible results in human nutrition research. BMJ 2013, 347, f6698. [Google Scholar] [CrossRef]
- Cox, L.A. Modernizing the Bradford Hill criteria for assessing causal relationships in observational data. Crit. Rev. Toxicol. 2018, 48, 682–712. [Google Scholar] [CrossRef] [PubMed]
- Murad, M.H.; Asi, N.; Alsawas, M.; Alahdab, F. New evidence pyramid. BMJ Evid.-Based Med. 2016, 21, 125–127. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, J.D.; Ioannidis, J.P. Is everything we eat associated with cancer? A systematic cookbook review. Am. J. Clin. Nutr. 2013, 97, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Lampropoulou, M.A.; Grammatikopoulou, M.G.; Theodoridis, X.; Katsaridis, S.; Bobora, D.; Patsatsi, A.; Haidich, A.-B.; Goulis, D.G. Clinical Trials of Vitamin Supplements: Are They Meeting the European Medicines Agency Prompt Dissemination Regulation? Dietetics 2022, 1, 114–123. [Google Scholar] [CrossRef]
- Schwab, T. Dietary disclosures: How important are non-financial interests? BMJ 2018, 361, k1451. [Google Scholar] [CrossRef]
- Ioannidis, J.P.A.; Trepanowski, J.F. Disclosures in Nutrition Research. JAMA 2018, 319, 547. [Google Scholar] [CrossRef]
- Ioannidis, J.P.A. The Challenge of Reforming Nutritional Epidemiologic Research. JAMA 2018, 320, 969. [Google Scholar] [CrossRef]
- Brown, A.W.; Aslibekyan, S.; Bier, D.; Ferreira da Silva, R.; Hoover, A.; Klurfeld, D.M.; Loken, E.; Mayo-Wilson, E.; Menachemi, N.; Pavela, G.; et al. Toward more rigorous and informative nutritional epidemiology: The rational space between dismissal and defense of the status quo. Crit. Rev. Food Sci. Nutr. 2021, 40, 8398. [Google Scholar] [CrossRef] [PubMed]
- McCambridge, J.; Witton, J.; Elbourne, D.R. Systematic review of the Hawthorne effect: New concepts are needed to study research participation effects. J. Clin. Epidemiol. 2014, 67, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Pryer, J.A.; Vrijheid, M.; Nichols, R.; Kiggins, M.; Elliott, P. Who are the “low energy reporters” in the Dietary and Nutritional Survey of British Adults? Int. J. Epidemiol. 1997, 26, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Rhee, J.J.; Sampson, L.; Cho, E.; Hughes, M.D.; Hu, F.B.; Willett, W.C. Comparison of methods to account for implausible reporting of energy intake in epidemiologic studies. Am. J. Epidemiol. 2015, 181, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Macdiarmid, J.; Blundell, J. Assessing dietary intake: Who, what and why of under-reporting. Nutr. Res. Rev. 1998, 11, 231–253. [Google Scholar] [CrossRef]
- Willett, W.C.; Howe, G.R.; Kushi, L.H. Adjustment for total energy intake in epidemiologic studies. Am. J. Clin. Nutr. 1997, 65, 1220S–1228S. [Google Scholar] [CrossRef]
- Willett, W. Nutritional Epidemiology; Oxford University Press: Oxford, UK, 2012; ISBN 9780199754038. [Google Scholar]
- Tomova, G.D.; Arnold, K.F.; Gilthorpe, M.S.; Tennant, P.W.G. Adjustment for energy intake in nutritional research: A causal inference perspective. Am. J. Clin. Nutr. 2022, 115, 189–198. [Google Scholar] [CrossRef]
- Ferreira, J.C.; Patino, C.M. Tipos de desfecho em pesquisa clínica. J. Bras. Pneumol. 2017, 43, 5. [Google Scholar] [CrossRef]
- Vetter, T.R.; Mascha, E.J. Defining the Primary Outcomes and Justifying Secondary Outcomes of a Study: Usually, the Fewer, the Better. Anesth. Analg. 2017, 125, 678–681. [Google Scholar] [CrossRef]
- Martínez-López, E.; Pérez-Guerrero, E.E.; Torres-Carrillo, N.M.; López-Quintero, A.; Betancourt-Núñez, A.; Gutiérrez-Hurtado, I.A. Methodological Aspects in Randomized Clinical Trials of Nutritional Interventions. Nutrients 2022, 14, 2365. [Google Scholar] [CrossRef]
- Petersen, K.S.; Kris-Etherton, P.M.; Mccabe, G.P.; Raman, G.; Miller, J.W.; Maki, K.C. Perspective: Planning and Conducting Statistical Analyses for Human Nutrition Randomized Controlled Trials: Ensuring Data Quality and Integrity. Adv. Nutr. 2021, 12, 1610–1624. [Google Scholar] [CrossRef] [PubMed]
- Altman, D.G.; Royston, P. The cost of dichotomising continuous variables. Br. Med. J. 2006, 332, 1080. [Google Scholar] [CrossRef]
- Altman, D.G. Categorizing Continuous Variables. In Wiley StatsRef: Statistics Reference Online; John Wiley & Sons, Ltd.: Chichester, UK, 2014. [Google Scholar]
- Krzywinski, M.; Altman, N. Points of significance: Comparing samples-part II. Nat. Methods 2014, 11, 355–356. [Google Scholar] [CrossRef]
- Rhee, J.J.; Cho, E.; Willett, W.C. Energy-adjustment of nutrient intakes is preferable to adjustment using body weight and physical activity in epidemiologic analyses. Public Health Nutr. 2014, 17, 1060. [Google Scholar] [CrossRef] [PubMed]
- Jakes, R.W.; Day, N.E.; Luben, R.; Welch, A.; Bingham, S.; Mitchell, J.; Hennings, S.; Rennie, K.; Wareham, N.J. Adjusting for energy intake—What measure to use in nutritional epidemiological studies? Int. J. Epidemiol. 2004, 33, 1382–1386. [Google Scholar] [CrossRef] [PubMed]
- Spiegelman, D. Commentary: Correlated errors and energy adjustment--where are the data? Int. J. Epidemiol. 2004, 33, 1387–1388. [Google Scholar] [CrossRef] [PubMed]
- Larrick, B.M.; Dwyer, J.T.; Erdman, J.W.; D’aloisio, R.F.; Jones, W. An Updated Framework for Industry Funding of Food and Nutrition Research: Managing Financial Conflicts and Scientific Integrity. J. Nutr. 2022, 152, 1812–1818. [Google Scholar] [CrossRef] [PubMed]
- Teicholz, N. The scientific report guiding the US dietary guidelines: Is it scientific? BMJ 2015, 351, h4962. [Google Scholar] [CrossRef]
- Rao, A. Industry-funded research and bias in food science. Quant. Mark. Econ. 2022, 20, 39–67. [Google Scholar] [CrossRef]
- Nestle, M. Food industry funding of nutrition research: The relevance of history for current debates. JAMA Intern. Med. 2016, 176, 1685–1686. [Google Scholar] [CrossRef]
- Navarrete-Muñoz, E.M.; Tardón, A.; Romaguera, D.; Martínez-González, M.Á.; Vioque, J. La financiación de la industria alimentaria y la investigación epidemiológica sobre nutrición y salud. Gac. Sanit. 2018, 32, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, A.; Holland, T.J.; Bero, L.A. Food industry sponsorship of academic research: Investigating commercial bias in the research agenda. Public Health Nutr. 2018, 21, 3422–3430. [Google Scholar] [CrossRef] [PubMed]
- Sacks, G.; Riesenberg, D.; Mialon, M.; Dean, S.; Cameron, A.J. The characteristics and extent of food industry involvement in peer-reviewed research articles from 10 leading nutrition-related journals in 2018. PLoS ONE 2020, 15, e0243144. [Google Scholar] [CrossRef] [PubMed]
- Neuhouser, M.L. Reply to BC Johnston and GH Guyatt. Am. J. Clin. Nutr. 2020, 111, 1108–1109. [Google Scholar] [CrossRef] [PubMed]
- Lauber, K.; McGee, D.; Gilmore, A.B. Commercial use of evidence in public health policy: A critical assessment of food industry submissions to global-level consultations on non-communicable disease prevention. BMJ Glob. Health 2021, 6, e006176. [Google Scholar] [CrossRef]
- Harcombe, Z. Designed by the food industry for wealth, not health: The “Eatwell Guide”. Br. J. Sports Med. 2017, 51, 1730–1731. [Google Scholar] [CrossRef][Green Version]
- Nestle, M. Perspective: Challenges and Controversial Issues in the Dietary Guidelines for Americans, 1980–2015. Adv. Nutr. 2018, 9, 150. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; Harcombe, Z.; O’Keefe, J.H. Problems with the 2015 Dietary Guidelines for Americans: An Alternative. Mo. Med. 2016, 113, 93. [Google Scholar]
- Herman, J. Saving U.S. dietary advice from conflicts of interest. Food Drug Law J. 2010, 65, 285–316. [Google Scholar]
- Harcombe, Z.; Baker, J.S.; DiNicolantonio, J.J.; Grace, F.; Davies, B. Original research article: Evidence from randomised controlled trials does not support current dietary fat guidelines: A systematic review and meta-analysis. Open Hear. 2016, 3, 409. [Google Scholar] [CrossRef]
- Harcombe, Z. Dietary fat guidelines have no evidence base: Where next for public health nutritional advice? Br. J. Sports Med. 2017, 51, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Collin, J.; Wright, A.; Hill, S.; Smith, K. Conflicted and confused? Health harming industries and research funding in leading UK universities. BMJ 2021, 374, n1657. [Google Scholar] [CrossRef] [PubMed]
- Welch, R.W.; Antoine, J.M.; Berta, J.L.; Bub, A.; De Vries, J.; Guarner, F.; Hasselwander, O.; Hendriks, H.; Jäkel, M.; Koletzko, B.V.; et al. Guidelines for the design, conduct and reporting of human intervention studies to evaluate the health benefits of foods. Br. J. Nutr. 2011, 106, S3–S15. [Google Scholar] [CrossRef]
- Schulz, K.F.; Altman, D.G.; Moher, D. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomized trials. Ann. Intern. Med. 2010, 152, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Lachat, C.; Hawwash, D.; Ocké, M.C.; Berg, C.; Forsum, E.; Hörnell, A.; Larsson, C.; Sonestedt, E.; Wirfält, E.; Åkesson, A.; et al. Strengthening the Reporting of Observational Studies in Epidemiology—Nutritional Epidemiology (STROBE-nut): An Extension of the STROBE Statement. PLoS Med. 2016, 13, e1002036. [Google Scholar] [CrossRef]
- Munezero, E.; Behan, N.A.; Diaz, S.G.; Neumann, E.-M.; MacFarlane, A.J. Poor Reporting Quality in Basic Nutrition Research: A Case Study Based on a Scoping Review of Recent Folate Research in Mouse Models (2009–2021). Adv. Nutr. 2022, 12, 56. [Google Scholar] [CrossRef]
- Kanukula, R.; McKenzie, J.E.; Bero, L.; Dai, Z.; McDonald, S.; Kroeger, C.M.; Korevaar, E.; Page, M.J. Methods used to select results to include in meta-analyses of nutrition research: A meta-research study. J. Clin. Epidemiol. 2022, 142, 171–183. [Google Scholar] [CrossRef]
- Myers, E.F.; Parrott, J.S.; Cummins, D.S.; Splett, P. Funding Source and Research Report Quality in Nutrition Practice-Related Research. PLoS ONE 2011, 6, e28437. [Google Scholar] [CrossRef]
- Altman, D.G. Statistics and ethics in medical research. Misuse of statistics is unethical. Br. Med. J. 1980, 281, 1182–1184. [Google Scholar] [CrossRef]
- International Committee of Medical Journal Editors. Recommendations for the Conduct, Reporting, Editing, and Publication of Scholarly Work in Medical Journals. Zhonghua Gan Zang Bing Za Zhi 2014, 22, 781–791. [Google Scholar]
- Moher, D. Reporting guidelines: Doing better for readers. BMC Med. 2018, 16, 233. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, J.P. We need more randomized trials in nutrition—Preferably large, long-term, and with negative results. Am. J. Clin. Nutr. 2016, 103, 1385–1386. [Google Scholar] [CrossRef] [PubMed]
- Stampfer, M. Observational epidemiology is the preferred means of evaluating effects of behavioral and lifestyle modification. Control. Clin. Trials 1997, 18, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Taouktsi, N.; Papageorgiou, S.T.; Tousinas, G.; Papanikolopoulou, S.; Grammatikopoulou, M.G.; Giannakoulas, G.; Goulis, D.G. Fragility of cardiovascular outcome trials (CVOTs) examining nutrition interventions among patients with diabetes mellitus: A systematic review of randomized controlled trials. Hormones (Athens) 2022, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Vitolins, M.Z.; Case, T.L. What Makes Nutrition Research So Difficult to Conduct and Interpret? Diabetes Spectr. 2020, 33, 113–117. [Google Scholar] [CrossRef]
- Trepanowski, J.F.; Ioannidis, J.P.A. Perspective: Limiting Dependence on Nonrandomized Studies and Improving Randomized Trials in Human Nutrition Research: Why and How. Adv. Nutr. 2018, 9, 367–377. [Google Scholar] [CrossRef]
- Lichtenstein, A.H.; Petersen, K.; Barger, K.; Hansen, K.E.; Anderson, C.A.M.; Baer, D.J.; Lampe, J.W.; Rasmussen, H.; Matthan, N.R. Perspective: Design and Conduct of Human Nutrition Randomized Controlled Trials. Adv. Nutr. 2021, 12, 4–20. [Google Scholar] [CrossRef]
- Weaver, C.M.; Lichtenstein, A.H.; Kris-Etherton, P.M. Perspective: Guidelines Needed for the Conduct of Human Nutrition Randomized Controlled Trials. Adv. Nutr. 2021, 12, nmaa083. [Google Scholar] [CrossRef]
- Wingrove, K.; Lawrence, M.A.; Machado, P.; Stephens, L.D.; McNaughton, S.A. Using the Hierarchies of Evidence Applied to Lifestyle Medicine (HEALM) Approach to Assess the Strength of Evidence on Associations between Dietary Patterns and All-Cause Mortality. Nutrients 2022, 14, 4340. [Google Scholar] [CrossRef]
| Study Type | N | Positive Outcomes | |||
|---|---|---|---|---|---|
| Primary | p Value | Secondary | p Value | ||
| All journals | 2127 | 1667 | 1440 | ||
| SRs/ΜAs/ΜR | 210 (9.9%) | 152 (9.1%) | <0.001 | 125 (8.7%) | 0.009 |
| RCTs | 587 (27.6%) | 428 (25.7%) | 389 (27.0%) | ||
| Cohorts | 649 (30.5%) | 531 (31.9%) | 443 (30.8%) | ||
| Cross-sectional/non-RCTs | 681 (32.0%) | 556 (33.4%) | 483 (33.5%) | ||
| AJCN | 1020 | 786 | 676 | ||
| SRs/ΜAs/ΜR | 104 (10.2%) | 77 (9.8%) | 0.234 | 64 (9.5%) | 0.710 |
| RCTs | 386 (37.8%) | 288 (38.6%) | 254 (37.6%) | ||
| Cohorts | 323 (31.7%) | 258 (32.8%) | 215 (31.8%) | ||
| Cross-sectional/non-RCTs | 207 (20.3%) | 163 (20.7%) | 143 (21.2%) | ||
| EJCN | 720 | 561 | 474 | ||
| SRs/ΜAs/ΜR | 74 (10.3%) | 48 (8.6%) | 0.001 | 42 (8.9%) | 0.269 |
| RCTs | 122 (16.9%) | 85 (15.2%) | 83 (17.5%) | ||
| Cohorts | 229 (31.8%) | 187 (33.3%) | 146 (30.8%) | ||
| Cross-sectional/non-RCTs | 295 (41.0%) | 241 (43.0%) | 203 (42.8%) | ||
| Clin Nutr ESPEN | 387 | 320 | 290 | ||
| SRs/ΜAs/ΜR | 32 (8.3%) | 27 (8.4%) | 0.003 | 19 (6.6%) | 0.002 |
| RCTs | 79 (20.4%) | 55 (17.2%) | 52 (17.9%) | ||
| Cohorts | 97 (25.1%) | 86 (26.9%) | 82 (28.3%) | ||
| Cross-sectional/non-RCTs | 179 (46.3%) | 152 (47.5%) | 137 (47.2%) | ||
| Funding Type | N | Outcomes | |||
|---|---|---|---|---|---|
| Primary | p Value | Secondary | p Value | ||
| All journals | 2127 | 1667 | 1440 | ||
| Academic | 255 (10.6%) | 158 (9.5%) | 0.001 | 148 (10.3%) | 0.259 |
| Industry | 178 (8.4%) | 127 (7.6%) | 121 (8.4%) | ||
| Organizations | 1.189 (55.9%) | 948 (56.9%) | 800 (55.6%) | ||
| None disclosed | 299 (14.1%) | 236 (14.2%) | 197 (13.7%) | ||
| None | 236 (11.1%) | 198 (11.9%) | 174 (12.1%) | ||
| AJCN | 1020 | 786 | 676 | ||
| Academic | 96 (9.4%) | 67 (8.5%) | 0.320 | 65 (9.6%) | 0.941 |
| Industry | 82 (8.0%) | 61 (7.8%) | 54 (8.0%) | ||
| Organizations | 741 (72.6%) | 578 (73.5%) | 491 (72.6%) | ||
| None disclosed | 19 (1.9%) | 14 (1.8%) | 11 (1.6%) | ||
| None | 82 (8.05) | 66 (8.4%) | 55 (8.1%) | ||
| EJCN | 720 | 561 | 474 | ||
| Academic | 81 (11.3%) | 58 (10.3%) | 0.036 | 50 (10.5%) | 0.467 |
| Industry | 59 (8.2%) | 39 (7.0%) | 40 (8.4%) | ||
| Organizations | 331 (46.0%) | 267 (47.65) | 222 (46.8%) | ||
| None disclosed | 225 (31.3%) | 175 (31.25) | 143 (30.2%) | ||
| None | 24 (3.3%) | 22 (3.95) | 19 (4.0%) | ||
| Clin Nutr ESPEN | 387 | 320 | 290 | ||
| Academic | 48 (12.4%) | 33 (10.3%) | 0.022 | 33 (11.4%) | 0.992 |
| Industry | 37 (9.6%) | 27 (8.4%) | 27 (9.3%) | ||
| Organizations | 117 (30.2%) | 103 (32.2%) | 87 (30.0%) | ||
| None disclosed | 55 (14.2%) | 47 (14.7%) | 43 (14.8%) | ||
| None | 130 (33.6%) | 110 (34.4%) | 100 (34.5%) | ||
| Funding Source | N | Outcomes | |||
|---|---|---|---|---|---|
| Primary | p Value | Secondary | p Value | ||
| All journals | 2127 | 1667 | 1440 | ||
| Academic | 151 (7.1%) | 118 (7.1%) | 0.673 | 97 (6.7%) | 0.002 |
| Industry | 116 (5.5%) | 85 (5.1%) | 82 (5.7%) | ||
| Organizations | 831 (39.1%) | 653 (39.2%) | 541 (37.6%) | ||
| None disclosed | 346 (16.3%) | 272 (16.3%) | 227 (15.8%) | ||
| None | 683 (32.15) | 539 (32.3%) | 493 (34.2%) | ||
| AJCN | 1020 | 786 | 676 | ||
| Academic | 87 (8.5%) | 67 (8.5%) | 0.815 | 52 (7.7%) | 0.291 |
| Industry | 73 (7.2%) | 54 (6.9%) | 49 (7.2%) | ||
| Organizations | 575 (56.4%) | 450 (57.3%) | 377 (55.8%) | ||
| None disclosed | 63 (6.2%) | 48 (6.1%) | 40 (5.9%) | ||
| None | 222 (21.8%) | 167 (21.2%) | 158 (23.4%) | ||
| EJCN | 720 | 561 | 474 | ||
| Academic | 40 (5.6%) | 29 (5.2%) | 0.614 | 27 (5.7%) | 0.384 |
| Industry | 31 (4.3%) | 21 (3.7%) | 21 (4.4%) | ||
| Organizations | 196 (27.2%) | 155 (27.6%) | 126 (26.6%) | ||
| None disclosed | 228 (31.7%) | 177 (31.6%) | 144 (30.4%) | ||
| None | 225 (31.3%) | 179 (31.9%) | 156 (32.9%) | ||
| Clin Nutr ESPEN | 387 | 320 | 290 | ||
| Academic | 24 (6.2%) | 22 (6.9%) | 0.674 | 18 (6.2%) | 0.102 |
| Industry | 12 (3.1%) | 10 (3.1%) | 12 (4.1%) | ||
| Organizations | 60 (15.5%) | 48 (15.0%) | 38 (13.1%) | ||
| None disclosed | 55 (14.2%) | 47 (14.7%) | 43 (14.8%) | ||
| None | 236 (61.0) | 193 (60.3%) | 179 (61.7%) | ||
| Age of Participants | N | Outcomes | |||
|---|---|---|---|---|---|
| Primary | p Value | Secondary | p Value | ||
| All journals | 2127 | 2114 | 1440 | ||
| Adults | 1651 (77.6%) | 1293 (77.6%) | 0.924 | 1140 (79.2%) | 0.203 |
| Minors | 333 (15.7%) | 260 (15.6%) | 210 (14.6%) | ||
| Unspecified sample age | 35 (1.6%) | 27 (1.6%) | 19 (1.3%) | ||
| Mixed adults and minors | 108 (5.1%) | 87 (5.2%) | 71 (4.9%) | ||
| AJCN | 1020 | 786 | 676 | ||
| Adults | 807 (79.1%) | 619 (78.8%) | 0.753 | 549 (81.2%) | 0.020 |
| Minors | 154 (15.1%) | 120 (15.3%) | 90 (13.3%) | ||
| Unspecified sample age | 8 (0.8%) | 6 (0.8%) | 2 (0.3%) | ||
| Mixed adults and minors | 51 (5.0%) | 41 (5.2%) | 35 (5.2%) | ||
| EJCN | 720 | 561 | 474 | ||
| Adults | 540 (75.0%) | 428 (76.3%) | 0.273 | 358 (75.5%) | 0.677 |
| Minors | 127 (17.6%) | 93 (16.6%) | 82 (17.3%) | ||
| Unspecified sample age | 14 (1.9%) | 9 (1.6%) | 7 (1.5%) | ||
| Mixed adults and minors | 39 (5.4%) | 31 (5.5%) | 27 (5.7%) | ||
| Clin Nutr ESPEN | 387 | 320 | 290 | ||
| Adults | 304 (78.6%) | 246 (76.9%) | 0.366 | 233 (80.3%) | 0.048 |
| Minors | 52 (13.4%) | 47 (14.7%) | 38 (13.1%) | ||
| Unspecified sample age | 13 (3.4%) | 12 (3.8%) | 10 (3.4%) | ||
| Mixed adults and minors | 18 (4.7%) | 15 (4.7) | 9 (3.1%) | ||
| Published Protocols | N | Outcomes | |||
|---|---|---|---|---|---|
| Primary | p Value | Secondary | p Value | ||
| All journals | 797 | 580 | 514 | ||
| Yes | 566 (71.0%) | 417 (71.9%) | 0.427 | 377 (73.3%) | 0.203 |
| No | 231 (29.0%) | 163 (28.1%) | 137 (26.7%) | ||
| SRs/ΜAs/ΜR | 210 | 152 | 125 | ||
| Yes | 68 (32.4%) | 51 (33.6%) | 0.544 | 44 (35.2%) | 0.428 |
| No | 142 (67.6%) | 101 (66.4%) | 81 (64.8%) | ||
| RCTs | 587 | 428 | 389 | ||
| Yes | 498 (84.8%) | 366 (85.5%) | 0.436 | 333 (85.6%) | 0.613 |
| No | 89 (15.2%) | 62 (14.5%) | 56 (14.4%) | ||
| Adjustment for the EI of Participants | N | Outcomes | |||
|---|---|---|---|---|---|
| Primary | p Value | Secondary | p Value | ||
| Items published in all journals | 531 | 421 | 374 | ||
| Yes | 181 (34.1%) | 137 (32.5%) | 0.095 | 130 (34.8%) | 0.719 |
| No | 350 (65.9%) | 284 (67.5%) | 244 (65.2%) | ||
| Cohort studies | 302 | 234 | 204 | ||
| Yes | 114 (37.7%) | 79 (33.8%) | 0.006 | 75 (36.8%) | 0.638 |
| No | 188 (62.3%) | 155 (66.2%) | 129 (63.2%) | ||
| Cross-sectional studies | 229 | 187 | 170 | ||
| Yes | 67 (29.3%) | 58 (31.0%) | 0.284 | 55 (32.4%) | 0.138 |
| No | 162 (70.7%) | 129 (69.0%) | 115 (67.6%) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gkiouras, K.; Choleva, M.-E.; Verrou, A.; Goulis, D.G.; Bogdanos, D.P.; Grammatikopoulou, M.G. A Meta-Epidemiological Study of Positive Results in Clinical Nutrition Research: The Good, the Bad and the Ugly of Statistically Significant Findings. Nutrients 2022, 14, 5164. https://doi.org/10.3390/nu14235164
Gkiouras K, Choleva M-E, Verrou A, Goulis DG, Bogdanos DP, Grammatikopoulou MG. A Meta-Epidemiological Study of Positive Results in Clinical Nutrition Research: The Good, the Bad and the Ugly of Statistically Significant Findings. Nutrients. 2022; 14(23):5164. https://doi.org/10.3390/nu14235164
Chicago/Turabian StyleGkiouras, Konstantinos, Maria-Eleftheria Choleva, Aikaterini Verrou, Dimitrios G. Goulis, Dimitrios P. Bogdanos, and Maria G. Grammatikopoulou. 2022. "A Meta-Epidemiological Study of Positive Results in Clinical Nutrition Research: The Good, the Bad and the Ugly of Statistically Significant Findings" Nutrients 14, no. 23: 5164. https://doi.org/10.3390/nu14235164
APA StyleGkiouras, K., Choleva, M.-E., Verrou, A., Goulis, D. G., Bogdanos, D. P., & Grammatikopoulou, M. G. (2022). A Meta-Epidemiological Study of Positive Results in Clinical Nutrition Research: The Good, the Bad and the Ugly of Statistically Significant Findings. Nutrients, 14(23), 5164. https://doi.org/10.3390/nu14235164







