Casein Lactose-Glycation of the Maillard-Type Attenuates the Anti-Inflammatory Potential of Casein Hydrolysate to IEC-6 Cells with Lipopolysaccharide Stimulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Regents and Materials
2.2. Sample Preparation
2.3. Assays of Protein and Lactose Content as Well as the Degree of Hydrolysis
2.4. Cell Line and Cell Culture
2.5. Assay of Cell Viability with or without LPS Exposure
2.6. Assay of Cytokine Secretion
2.7. Assay of Protein Expression
2.8. Statistical Analysis
3. Results
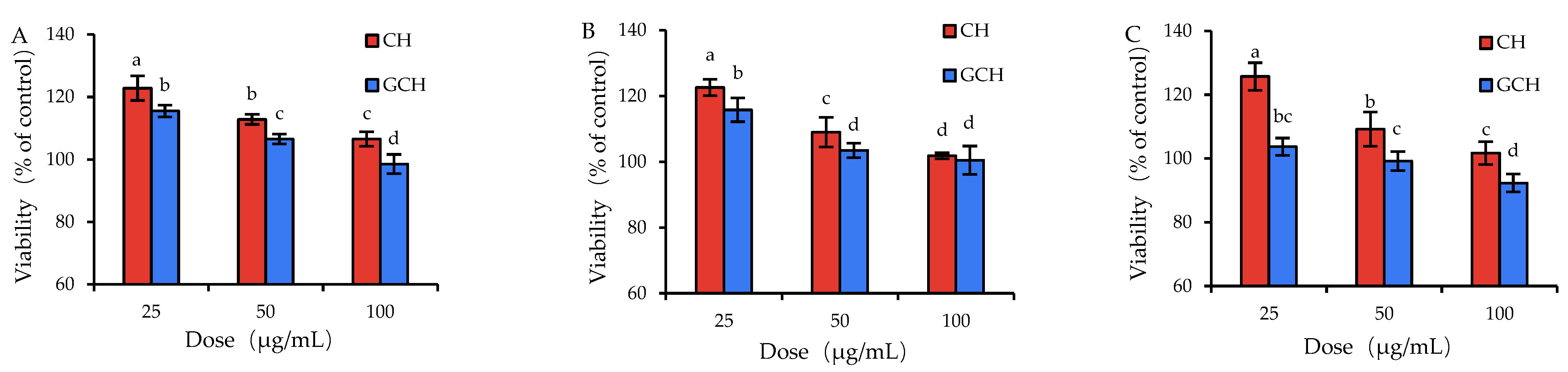
3.1. Effect of the Two Hydrolysates on the Growth Performance of IEC-6 Cells
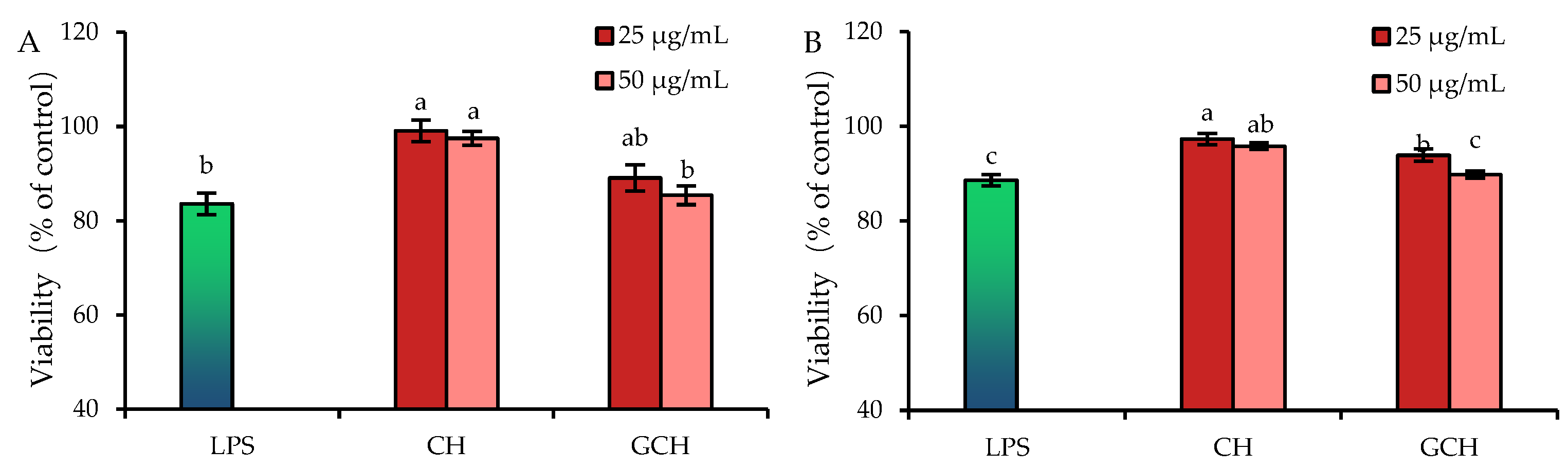
3.2. Effect of the Two Hydrolysates on the LPS-Induced Cellular Injury
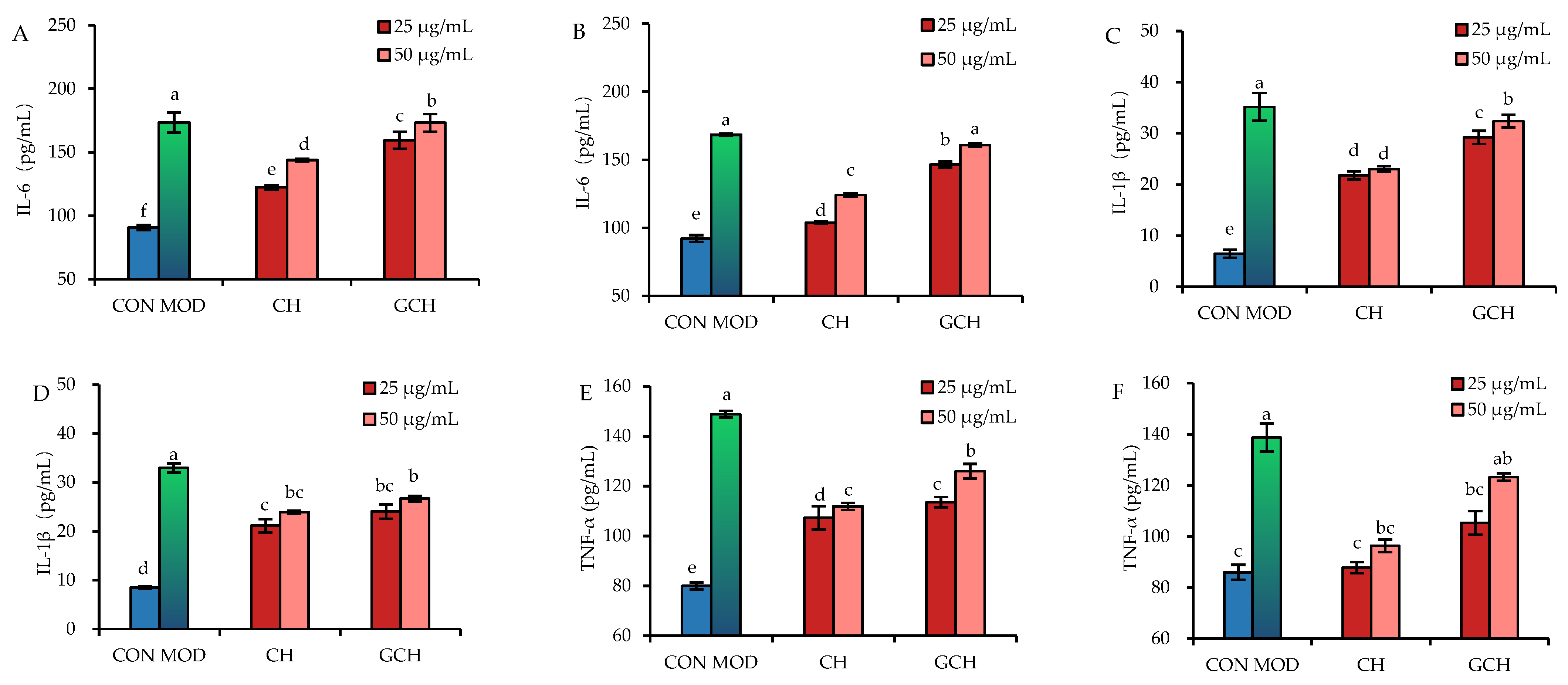
3.3. Effect of the Two Hydrolysates on the Secretion of Three Inflammatory Mediators
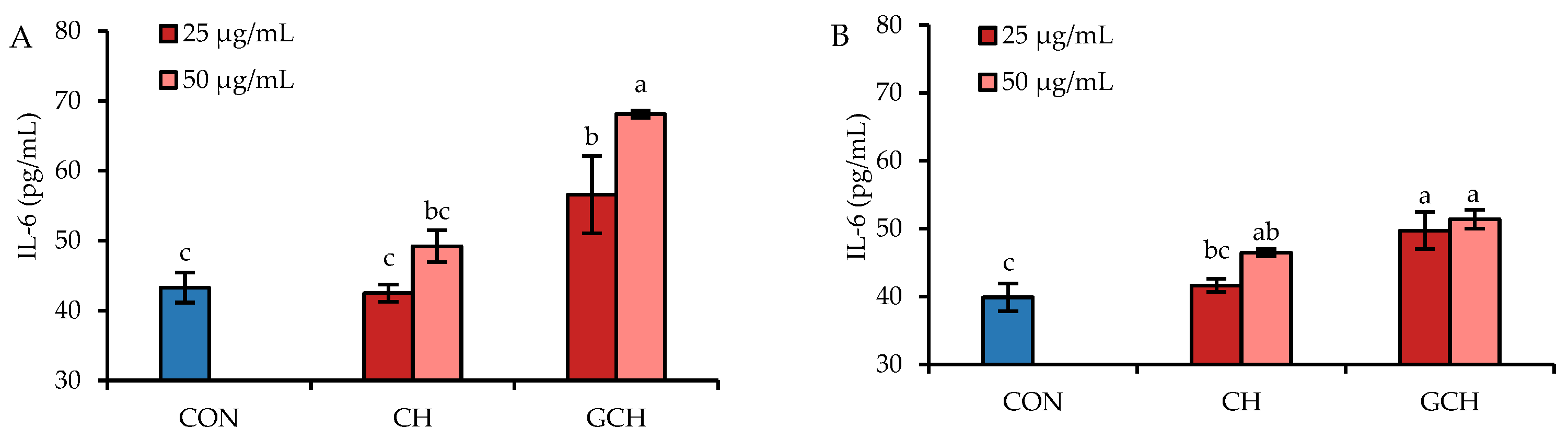
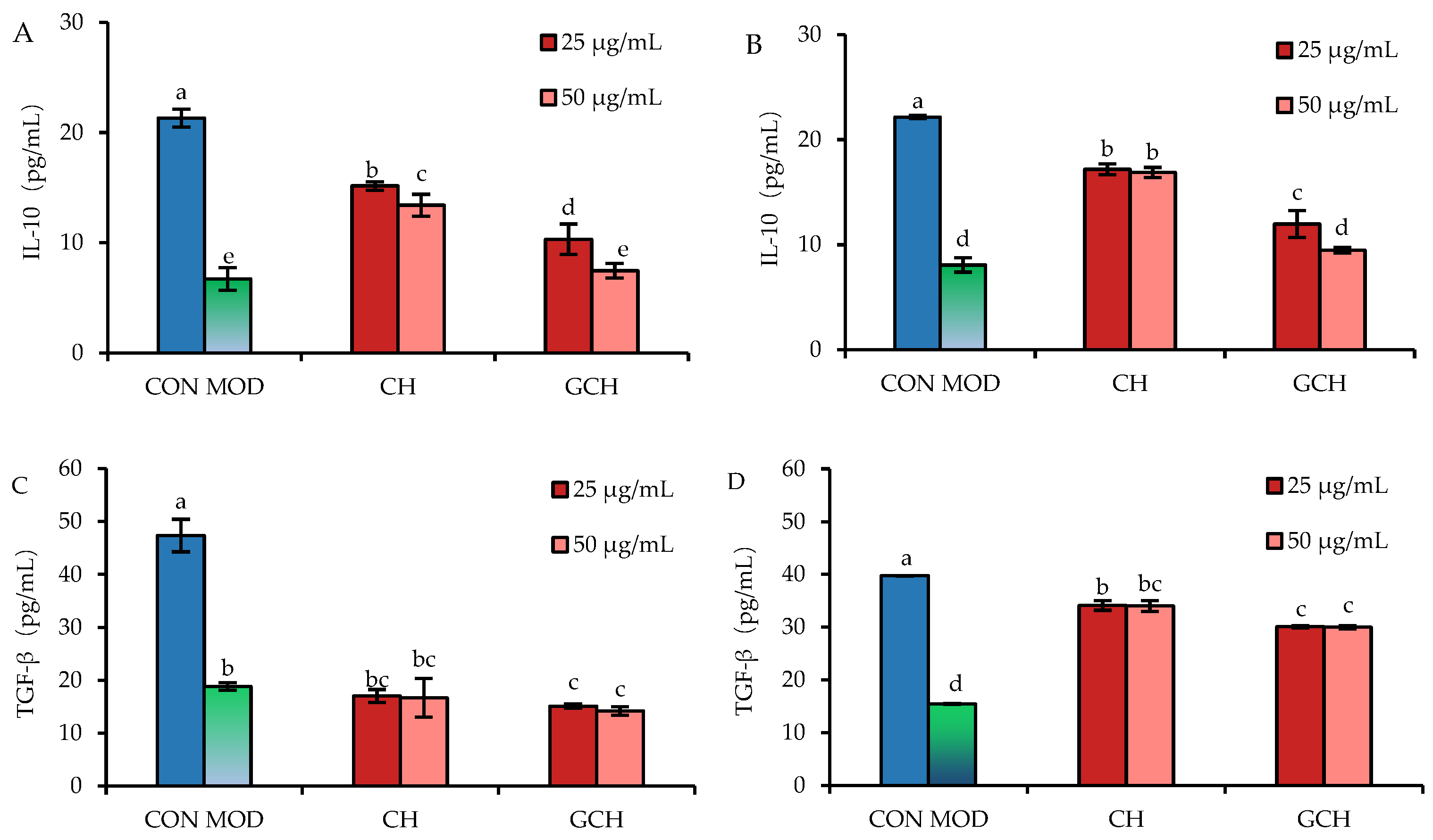
3.4. Effect of the Two Hydrolysates on the Secretion of Two Anti-Inflammatory Mediators
3.5. Expression Changes in Signaling Pathway-Related Proteins in IEC-6 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheru, L.; Saylor, C.F.; Lo, J. Gastrointestinal barrier breakdown and adipose tissue inflammation. Curr. Obes. Rep. 2019, 8, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Okumura, R.; Takeda, K. Roles of intestinal epithelial cells in the maintenance of gut homeostasis. Exp. Mol. Med. 2017, 49, e338. [Google Scholar] [CrossRef] [PubMed]
- Figueroa-Lozano, S.; Valk-Weeber, R.L.; van Leeuwen, S.S.; Dijkhuizen, L.; de Vos, P. Dietary N-glycans from bovine lactoferrin and TLR modulation. Mol. Nutr. Food Res. 2018, 62, e1700389. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.H. Lipopolysaccharide: Basic biochemistry, intracellular signaling, and physiological impacts in the gut. Intest. Res. 2014, 12, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Murakami, A.; Ohigashi, H. Targeting NOX, INOS and COX-2 in inflammatory cells: Chemoprevention using food phytochemicals. Int. J. Cancer 2007, 121, 2357–2363. [Google Scholar] [CrossRef]
- Chang, M.; Chang, L.; Chang, H.M.; Chang, F. Intestinal and extraintestinal cancers associated with inflammatory bowel disease. Clin. Color. Cancer 2018, 17, 29–37. [Google Scholar] [CrossRef]
- Sun, M.; He, C.; Cong, Y.; Liu, Z. Regulatory immune cells in regulation of intestinal inflammatory response to microbiota. Mucosal Immunol. 2015, 8, 969–978. [Google Scholar] [CrossRef]
- Ponce de Leon-Rodriguez, M.D.C.; Guyot, J.P.; Laurent-Babot, C. Intestinal in vitro cell culture models and their potential to study the effect of food components on intestinal inflammation. Crit. Rev. Food Sci. Nutr. 2019, 59, 3648–3666. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Zhao, Y.; Yao, Y.; Xu, M.S.; Du, H.Y.; Wu, N.; Tu, Y.G. Isolation and identification of peptides from simulated gastrointestinal digestion of preserved egg white and their anti-inflammatory activity in TNF-alpha-induced Caco-2 cells. J. Nutr. Biochem. 2019, 63, 44–53. [Google Scholar] [CrossRef]
- Webb, D.R. Animal models of human disease: Inflammation. Biochem. Pharmacol. 2014, 87, 121–130. [Google Scholar] [CrossRef]
- Hu, X.Y.; Yu, Q.; Hou, K.Y.; Ding, X.M.; Chen, Y.; Xie, J.H.; Nie, S.P.; Xie, M.Y. Regulatory effects of Ganoderma atrum polysaccharides on LPS-induced inflammatory macrophages model and intestinal-like Caco-2/macrophages co-culture inflammation model. Food Chem. Toxicol. 2020, 140, e111321. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, N.F.; Kan, J.; Zhang, X.; Wu, X.N.; Sun, R.; Tang, S.X.; Liu, J.; Qian, C.L.; Jin, C.H. Structural characterization of water-soluble polysaccharide from Arctium lappa and its effects on colitis mice. Carbohydr. Polym. 2019, 213, 89–99. [Google Scholar] [CrossRef]
- Kim, H.; Castellon-Chicas, M.J.; Arbizu, S.; Talcott, S.T.; Drury, N.L.; Smith, S.; Mertens-Talcott, S.U. Mango (Mangifera indica L.) polyphenols: Anti-inflammatory intestinal microbial health benefits, and associated mechanisms of actions. Molecules 2021, 26, 2732. [Google Scholar] [CrossRef]
- Wang, K.; Jin, X.L.; Li, Q.Q.; Sawaya, A.C.H.F.; Le Leu, R.K.; Conlon, M.A.; Wu, L.M.; Hu, F.L. Propolis from different geographic origins suppress intestinal inflammation in a model of DSS-induced cilitis is associated with decreased Bacteroides spp. in the gut. Mol. Nutr. Food Res. 2018, 62, e1800080. [Google Scholar] [CrossRef]
- Tenore, G.C.; Pagano, E.; Lama, S.; Vanacore, D.; Di Maro, S.; Maisto, M.; Capasso, R.; Merlino, F.; Borrelli, F.; Stiuso, P.; et al. Intestinal anti-inflammatory effect of a peptide derived from gastrointestinal digestion of buffalo (Bubalus bubalis) mozzarella cheese. Nutrients 2019, 11, 610. [Google Scholar] [CrossRef]
- Zhong, L.; Ma, N.; Wu, Y.L.; Zhao, L.Y.; Ma, G.M.; Pei, F.; Hu, Q.H. Characterization and functional evaluation of oat protein isolate-Pleurotus ostreatus β-glucan conjugates formed via Maillard reaction. Food Hydrocoll. 2018, 87, 459–469. [Google Scholar] [CrossRef]
- Nie, X.H.; Xu, D.; Zhao, L.M.; Meng, X.H. Antioxidant activities of chicken bone peptide fractions and their Maillard reaction products: Effects of different molecular weight distributions. Int. J. Food Prop. 2017, 20, S457–S466. [Google Scholar] [CrossRef]
- Chung, Y.C.; Yeh, J.Y.; Tsai, C.F. Antibacterial characteristics and activity of water-soluble chitosan derivatives prepared by the Maillard reaction. Molecules 2011, 16, 8504–8514. [Google Scholar] [CrossRef]
- Hong, X.; Meng, J.; Lu, R.R. Improvement of ACE inhibitory activity of casein hydrolysate by Maillard reaction with xylose. J. Sci. Food Agric. 2015, 95, 66–71. [Google Scholar] [CrossRef]
- Karnjanapratum, S.; O’Callaghan, Y.C.; Benjakul, S.; O’Brien, N.M. In vitro cellular bioactivities of Maillard reaction products from sugar-gelatin hydrolysate of unicorn leatherjacket skin system. J. Funct. Food 2016, 23, 87–94. [Google Scholar] [CrossRef]
- Hernandez-Hernandez, O.; Sanz, M.L.; Kolida, S.; Rastall, R.A.; Moreno, F.J. In vitro fermentation by human gut bacteria of proteolytically digested caseinomacropeptide nonenzymatically glycosylated with prebiotic carbohydrates. J. Agric. Food Chem. 2011, 59, 11949–11955. [Google Scholar] [CrossRef] [PubMed]
- Goulart, P.; Alves, J.; Magalhaes, M.; Lima, L.; Meyer, L. Purification of polyphenoloxidase from coffee fruits. Food Chem. 2003, 83, 7–11. [Google Scholar] [CrossRef]
- Hillman, M.; Westrom, B.; Aalaei, K.; Erlanson-Albertsson, C.; Wolinski, J.; Lozinska, L.; Sjoholm, I.; Rayner, M.; Landin-Olsson, M. Skim milk powder with high content of Maillard reaction products affect weight gain, organ development and intestinal inflammation in early life in rats. Food Chem. Toxicol. 2019, 125, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.J.; Zhao, J.S.; Qu, W.T.; Zhang, Y.X.; Jia, B.P.; Fan, Z.Y.; He, Q.H.; Li, J.X. Accumulation and effects of dietary advanced glycation end products on the gastrointestinal tract in rats. Int. J. Food Sci. Technol. 2018, 53, 2273–2281. [Google Scholar] [CrossRef]
- Van Dongen, K.C.W.; Linkens, A.M.A.; Wetzels, S.M.W.; Wouters, K.; Vanmierlo, T.; van de Waarenburg, M.P.H.; Scheijen, J.; de Vos, W.M.; Belzer, C.; Schalkwijk, C.G. Dietary advanced glycation endproducts (AGEs) increase their concentration in plasma and tissues, result in inflammation and modulate gut microbial composition in mice; evidence for reversibility. Food Res. Int. 2021, 147, e110547. [Google Scholar] [CrossRef]
- Van der Lugt, T.; Vrolijk, M.F.; Bovee, T.F.H.; van Leeuwen, S.P.J.; Vonsovic, S.; Hamers, A.; Opperhuizen, A.; Bast, A. Gastrointestinal digestion of dietary advanced glycation endproducts increases their pro-inflammatory potential. Food Funct. 2021, 12, 6691–6696. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, X.H. Influence of the Maillard-type caseinate glycation with lactose on the intestinal barrier activity of the caseinate digest in IEC-6 cells. Food Funct. 2019, 10, 2010–2021. [Google Scholar] [CrossRef]
- Wang, X.P.; Zhao, X.H. Using an enzymatic galactose assay to detect lactose glycation extents of two proteins caseinate and soybean protein isolate via the Maillard reaction. J. Sci. Food Agric. 2017, 97, 2617–2622. [Google Scholar] [CrossRef]
- Lynch, J.M.; Barbano, D.M. Kjeldahl nitrogen analysis as a reference method for protein determination in dairy products. J. AOAC Int. 1999, 82, 1389–1398. [Google Scholar] [CrossRef]
- Church, F.C.; Swaisgood, H.E.; Porter, D.H.; Catignani, G.L. Spectrophotometric assay using o-phthaldialdehyde for determination of proteolysis in milk and isolated milk proteins. J. Dairy Sci. 1983, 66, 1219–1227. [Google Scholar] [CrossRef]
- Fu, Y.; Zhao, X.H. In vitro responses of hFOB1.19 cells towards chum salmon (Oncorhynchus keta) skin gelatin hydrolysates in cell proliferation, cycle progression and apoptosis. J. Funct. Foods. 2013, 5, 279–288. [Google Scholar] [CrossRef]
- Wang, X.P.; Zhao, X.H. Prior lactose glycation of caseinate via the Maillard reaction affects in vitro activities of the pepsin-trypsin digest toward intestinal epithelial cells. J. Dairy Sci. 2017, 100, 5125–5138. [Google Scholar] [CrossRef]
- Luisa, A.; Gaspar, C.; de Goes-Favoni, S.P. Action of microbial transglutaminase (MTGase) in the modification of food proteins: A review. Food Chem. 2015, 171, 315–322. [Google Scholar] [CrossRef]
- Shi, J.; Fu, Y.; Zhao, X.H.; Lametsch, R. Glycation sites and bioactivity of lactose-glycated caseinate hydrolysate in lipopolysaccharide-injured IEC-6 cells. J. Dairy Sci. 2021, 104, 1351–1363. [Google Scholar] [CrossRef]
- Oh, N.S.; Young Lee, J.; Lee, H.A.; Joung, J.Y.; Shin, Y.K.; Kim, S.H.; Kim, Y.; Lee, K.W. Chemical characteristics and enhanced hepatoprotective activities of Maillard reaction products derived from milk protein-sugar system. J. Dairy Sci. 2016, 99, 947–958. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, L.; Lan, Q.Y.; Li, M.L.; Wu, D.T.; Chen, H.; Liu, Y.W.; Lin, D.R.; Qin, W.; Zhang, Z.Q.; et al. Protein glycosylation: A promising way to modify the functional properties and extend the application in food system. Crit. Rev. Food Sci. Nutr. 2019, 59, 2506–2533. [Google Scholar] [CrossRef]
- Tazawa, S.; Katayama, S.; Hirabayashi, M.; Yamaguchi, D.; Nakamura, S. Improvement of surface functionalities, including allergenicity attenuation, of whole buckwheat protein fraction by Maillard-type glycation with dextran. Prev. Nutr. Food Sci. 2014, 19, 327–332. [Google Scholar] [CrossRef]
- Nasri, M. Protein hydrolysates and biopeptides: Production, biological activities, and applications in foods and health benefits. A review. Adv. Food Nutr. Res. 2017, 81, 109–159. [Google Scholar] [CrossRef]
- Mengíbar, M.; Miralles, B.; Heras, Á. Use of soluble chitosans in Maillard reaction products with β-lactoglobulin. Emulsifying and antioxidant properties. LWT 2017, 75, 440–446. [Google Scholar] [CrossRef]
- Hamdani, A.M.; Wani, I.A.; Bhat, N.A.; Siddiqi, R.A. Effect of guar gum conjugation on functional, antioxidant and antimicrobial activity of egg white lysozyme. Food Chem. 2018, 240, 1201–1209. [Google Scholar] [CrossRef]
- Teodorowicz, M.; Swiatecka, D.; Savelkoul, H.; Wichers, H.; Kostyra, E. Hydrolysates of glycated and heat-treated peanut 7S globulin (Ara h 1) modulate human gut microbial proliferation, survival and adhesion. J. Appl. Microbiol. 2014, 116, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Birlouez-Aragon, I.; Saavedra, G.; Tessier, F.J.; Galinier, A.; Ait-Ameur, L.; Lacoste, F.; Niamba, C.N.; Alt, N.; Somoza, V.; Lecerf, J.M. A diet based on high-heat-treated foods promotes risk factors for diabetes mellitus and cardiovascular diseases. Am. J. Clin. Nutr. 2010, 91, 1220–1226. [Google Scholar] [CrossRef] [PubMed]
- Corzo-Martínez, M.; Hernandez-Hernandez, O.; Villamiel, M.; Rastall, R.A.; Moreno, F.J. In vitro bifidogenic effect of Maillard-type milk protein-galactose conjugates on the human intestinal microbiota. Int. Dairy J. 2013, 31, 127–131. [Google Scholar] [CrossRef]
- Gu, F.L.; Kin, J.M.; Abbas, S.; Zhang, X.M.; Xia, S.Q.; Chen, Z.X. Structure and antioxidant activity of high molecular weight Maillard reaction products from casein-glucose. Food Chem. 2010, 120, 505–511. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, X.H. In vitro immuno-modulatory ability of tryptic caseinate hydrolysate affected by prior caseinate glycation using the Maillard reaction or transglutaminase. Food Agric. Immunol. 2017, 28, 1029–1045. [Google Scholar] [CrossRef]
- Wang, X.P.; Zhao, X.H. Lactose glycation of the Maillard-type impairs the benefits of caseinate digest to the weaned rats for intestinal morphology and serum biochemistry. Foods 2021, 10, 2014. [Google Scholar] [CrossRef]
- Murray, P.J. The primary mechanism of the IL-10-regulated anti inflammatory response is to selectively inhibit transcription. Proc. Natl. Acad. Sci. USA 2005, 102, 8686–8691. [Google Scholar] [CrossRef]
- Laveti, D.; Kumar, M.; Hemalatha, R.; Sistla, R.; Naidu, V.G.M.; Talla, V.; Verma, V.; Kaur, N.; Nagpal, R. Anti-inflammatory treatments for chronic diseases: A review. Inflamm. Allergy Drug Targets 2013, 12, 349–361. [Google Scholar] [CrossRef]
- Pandey, M.K.; Sung, B.; Kunnumakkara, A.B.; Sethi, G.; Chaturvedi, M.M.; Aggarwal, B.B. Berberine modifies cysteine 179 of I kappa B alpha kinase, suppresses nuclear factor-kappa B-regulated antiapoptotic gene products, and potentiates apoptosis. Cancer Res. 2008, 68, 5370–5379. [Google Scholar] [CrossRef]
- Cargnello, M.; Roux, P.P. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol. Mol. Biol. Rev. 2011, 75, 50–83. [Google Scholar] [CrossRef]
- Guha, S.; Majumder, K. Structural-features of food-derived bioactive peptides with anti-inflammatory activity: A brief review. J. Food Biochem. 2019, 43, e12531. [Google Scholar] [CrossRef]
- Yang, Y.R.; Huang, C.H.; Lin, X.; Wu, Y.; Ouyang, W.J.; Tang, L.Y.; Ye, S.H.; Wang, Y.H.; Li, W.; Zhang, X.B.; et al. 0.005% Preservative-free latanoprost induces dry eye-like ocular surface damage via promotion of inflammation in mice. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3375–3384. [Google Scholar] [CrossRef]
- Popov, S.V.; Ovodova, R.G.; Golovchenko, V.V.; Popova, G.Y.; Viatyasev, F.V.; Shashkov, A.S.; Ovodov, Y.S. Chemical composition and anti-inflammatory activity of a pectic polysaccharide isolated from sweet pepper using a simulated gastric medium. Food Chem. 2011, 124, 309–315. [Google Scholar] [CrossRef]
- Ha, S.K.; Park, H.Y.; Eom, H.; Kim, Y.; Choi, I. Narirutin fraction from citrus peels attenuates LPS-stimulated inflammatory response through inhibition of NF-κB and MAPKs activation. Food Chem. Toxicol. 2012, 50, 3498–3504. [Google Scholar] [CrossRef]
- Jiang, F.; Guan, H.N.; Liu, D.Y.; Wu, X.; Fan, M.C.; Han, J.C. Flavonoids from sea buckthorn inhibit the lipopolysaccharide-induced inflammatory response in RAW264.7 macrophages through the MAPK and NF-κB pathways. Food Funct. 2017, 8, 1313–1322. [Google Scholar] [CrossRef]
- Ott, C.; Jacobs, K.; Haucke, E.; Navarrete Santos, A.; Grune, T.; Simm, A. Role of advanced glycation end products in cellular signaling. Redox Biol. 2014, 2, 411–429. [Google Scholar] [CrossRef]
- Kong, X.Y.; Yang, M.; Guo, J.; Feng, Z.C. Effects of bovine lactoferrin on rat intestinal epithelial cells. J. Pediatr. Gastroenterol. Nutr. 2020, 70, 645–651. [Google Scholar] [CrossRef]
- Shi, Y.J.; Zhao, X.H. Impact of the plastein reaction of casein hydrolysates in the presence of exogenous amino acids on their anti-inflammatory effect in the lipopolysaccharide-stimulated macrophages. Foods 2022, 11, 196. [Google Scholar] [CrossRef]
- Van der Lugt, T.; Antje, R.; Weseler, A.R.; Gebbink, W.A.; Vrolijk, M.F.; Opperhuizen, A.; Bast, A. Dietary advanced glycation endproducts induce an inflammatory response in human macrophages in vitro. Nutrients 2018, 10, 1868. [Google Scholar] [CrossRef]
- Chen, N.; Wang, L.; Zhang, Q.; Zhao, X.H.; Shi, J. Casein oligochitosan-glycation by transglutaminase enhances the anti-inflammatory potential of casein hydrolysates to the lipopolysaccharide-stimulated IEC-6 cells. Nutrients 2022, 14, 686. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, X.H. Effect of caseinate glycation with oligochitosan and transglutaminase on intestinal barrier function of the tryptic caseinate digest in IEC-6 cells. Food Funct. 2019, 10, 652–664. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhao, X.H. Chemical features of the oligochitosan-glycated caseinate digest and its enhanced protection on barrier function of the acrylamide-injured IEC-6 cells. Food Chem. 2019, 290, 246–254. [Google Scholar] [CrossRef] [PubMed]
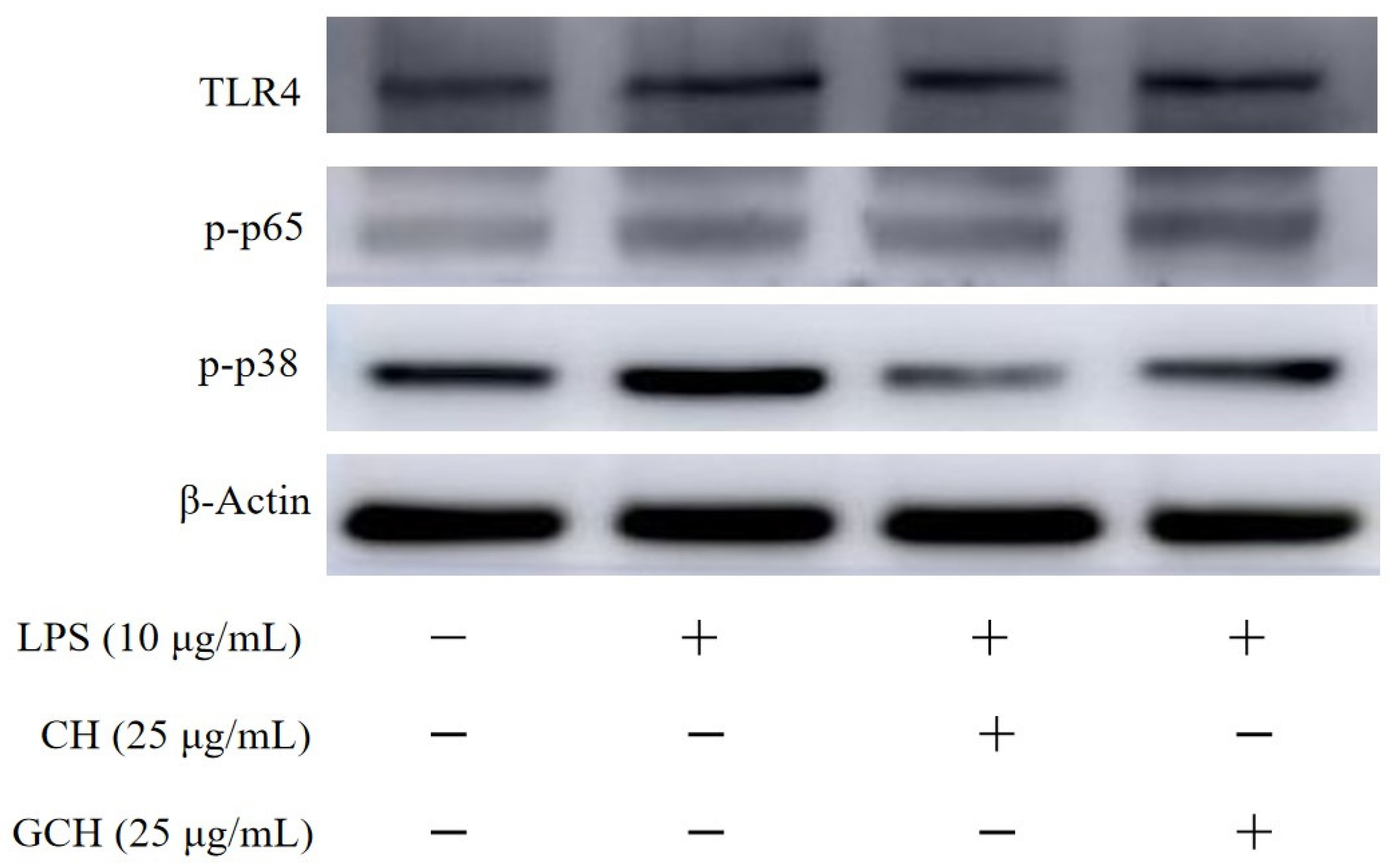
| CON | MOD | CH | GCH | |
|---|---|---|---|---|
| TLR4:β-actin | 0.58 ± 0.02 | 0.75 ± 0.03 | 0.74 ± 0.05 | 0.88 ± 0.02 |
| p-p65:β-actin | 0.53 ± 0.02 | 0.60 ± 0.02 | 0.57 ± 0.04 | 0.73 ± 0.05 |
| p-p38:β-actin | 0.73 ± 0.01 | 1.01 ± 0.05 | 0.50 ± 0.01 | 0.74 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, N.; Fu, Y.; Wang, Z.-X.; Zhao, X.-H. Casein Lactose-Glycation of the Maillard-Type Attenuates the Anti-Inflammatory Potential of Casein Hydrolysate to IEC-6 Cells with Lipopolysaccharide Stimulation. Nutrients 2022, 14, 5067. https://doi.org/10.3390/nu14235067
Chen N, Fu Y, Wang Z-X, Zhao X-H. Casein Lactose-Glycation of the Maillard-Type Attenuates the Anti-Inflammatory Potential of Casein Hydrolysate to IEC-6 Cells with Lipopolysaccharide Stimulation. Nutrients. 2022; 14(23):5067. https://doi.org/10.3390/nu14235067
Chicago/Turabian StyleChen, Na, Yu Fu, Zhen-Xing Wang, and Xin-Huai Zhao. 2022. "Casein Lactose-Glycation of the Maillard-Type Attenuates the Anti-Inflammatory Potential of Casein Hydrolysate to IEC-6 Cells with Lipopolysaccharide Stimulation" Nutrients 14, no. 23: 5067. https://doi.org/10.3390/nu14235067
APA StyleChen, N., Fu, Y., Wang, Z.-X., & Zhao, X.-H. (2022). Casein Lactose-Glycation of the Maillard-Type Attenuates the Anti-Inflammatory Potential of Casein Hydrolysate to IEC-6 Cells with Lipopolysaccharide Stimulation. Nutrients, 14(23), 5067. https://doi.org/10.3390/nu14235067







